Abstract
Clinical observations suggest that the experience of time phenomenology is disturbed in schizophrenia, possibly originating disorders in dynamic cognitive functions such as language or motor planning. We examined the subjective evaluation of temporal structure using an experimental approach involving judgments of simultaneity of simple, visually presented stimuli. We included a priming procedure, ie, a subthreshold presentation of simultaneous or asynchronous stimuli. This allowed us to evaluate the effects of subthreshold synchrony and to check for bias effects, ie, changes in the criteria used by the subjects to rate the stimuli. Primes were adapted to the responses of the subjects. Bias effects were thus expected to yield a change in the efficiency of the prime and to induce a change in the amplitude of the priming effect. Nineteen outpatients with schizophrenia and their individually matched controls participated in the study. In all tests, patients required longer delays between stimuli to detect that they were asynchronous. In other words, they judged stimuli to be synchronous even when their onset was separated by delays of 100 milliseconds and even more in some cases. These results contrasted with preserved effects of subthreshold synchrony. Our findings argue against the hypothesis that the patients’ responses were influenced by biases. We conclude that the subjective evaluation of simultaneity/asynchrony is impaired in schizophrenia, thus leading to impairment in the phenomenology of event-structure coding. The method used in the present study provides a novel approach to the assessment of those disturbances related to time in patients with schizophrenia.
Keywords: time processing, visual perception, psychophysics, consciousness, synchrony
Introduction
Schizophrenia is a pathology with heterogeneous cognitive deficits and symptoms that appear to affect consciousness itself. Clinical observations suggest that the sense of conscious continuity is disturbed in patients,1 indicating a disturbed time phenomenology. A number of recent commentaries have emphasized the explanatory power of the phenomenological approach to schizophrenia1–4: time phenomenology is often considered in the terms described by Husserl, who described mental life as composed of 3 integrated types of moments; the past or “retentional,” the present or “presentational,” and the future or “protentional.” Integration of these moments is necessary to produce the wholistic sense of a continuous present.5
Adoption of Husserl as a framework for understanding disturbed time phenomenology emphasizes the notion that experience of the phenomenological present must be considered as an integral of events in the past and those anticipated to occur in the future. This idea has been developed by Varela6 in relation with neurophysiological constraints. He suggested that present-time consciousness is underpinned by the concurrent activity of multiple neuronal networks acting in concert by means of the synchronization of action potentials. The dynamics of this activity requires time, and as a consequence, the coding of each event has a certain duration and the coding of successive events can overlap. This process would generate the sense of a continuous present rather than of discrete moments. In the light of recent speculation concerning the role of impaired neuronal synchrony in schizophrenia,7–9 it seems promising to consider that one outcome of impaired neuronal synchronization will be an impairment in the ability to maintain coherent or normal time phenomenology. We use here a novel experimental approach to measure variations in the magnitude of intervals of time during which events are judged to occur within a single phenomenological moment.10
Aside from Husserl, natural variation in the temporal extent of the phenomenological present had been put forth by von Baer.11,12 Indeed, von Baer proposed a discrete interval of time that would correspond to a fundamental or elementary perceptual moment. This moment was estimated to have a duration of one-eighteenth of a second, a value discussed in similar terms by von Uexküll.13 This value was corroborated experimentally by von Uexküll's collaborator, Brecher,14 who examined stimuli presented in rapid sequences and found very reliable estimates of simultaneity thresholds (ie, the interval at which perception of a simultaneity gives way to the perception of a succession) at intervals of around 55 milliseconds. “Windows of simultaneity” of this order, while brief, are nonetheless intervals of time extending beyond physical simultaneity. As such, they may be considered equivalent to Husserl's notion of the integral of retentional, presentational, and protentional moments because they contain, simultaneously, events that are physically past and present. While one is unable to phenomenally differentiate past and present events, the same must also hold, at least in principle, for an event that occurs after another, ie, for future events.
It was assumed by von Baer11 that the precise interval defining a window of simultaneity is of biological significance and is representative of the precise psychological and psychomotor timing of the organism in its natural environment. Accordingly, variations in the intervals defining a window of simultaneity may have profound and deleterious effects on the ability to correctly time events. These deleterious effects would further be troublesome for cognitive operations that require precise timing, eg, language, sensory-motor coordination, or motor timing. All these processes have been shown to be impaired in patients15–20: eg, an action as simple as lifting a cup requires the fingers to be opened in time during the reaching movement, its weight and texture estimated before contact, and the cup gripped before it can be lifted correctly. For this sequence to be executed smoothly, the events must succeed each other precisely in time. Thus, a disturbed ability in timing different events may interfere with the smooth execution of even such a simple motor sequence. Previous studies have suggested the reach-to-grip sequence to be impaired in patients.15,16,21,22
In the study described here, we investigated the interval of time over which 2 events would be viewed as a simultaneity by patients with schizophrenia. As far as we know, only 2 studies have explored the ability of patients with schizophrenia to discriminate simultaneous from asynchronous stimuli.23,24 Typically, 2 stimuli are flashed on the screen at different screen locations, and subjects decide whether the 2 stimuli are simultaneous or asynchronous. In a study by Schwartz et al24 but not in that conducted by Foucher et al,23 the stimuli were offset at the same time. This procedure provided the means to avoid an effect of apparent motion between stimuli.25 However, only the study by Foucher et al23 revealed a significant impairment in patients with schizophrenia, which might be explained by the fact that they tested 30 patients, whereas only 10 patients were tested in Schwartz et al's study. The results in study of Foucher et al23 are consistent with our hypothesis that patients have difficulties in discriminating simultaneous from asynchronous stimuli. However, the possibility of biased responding was not eliminated. In the current study, we aimed to examine a plausible impairment in simultaneity judgment in schizophrenia but controlling for possible bias effects. We employed a paradigm developed by Elliott et al26 in which 2 target stimuli, presented at separate monitor locations, change luminance either simultaneously or with an asynchrony. Elliott et al26 also examined the effects of a subthreshold (ie, nondetected) synchrony signal presented within a premask and the effects this signal had on target simultaneity judgments. Synchrony signals within the premask were found to influence those judgments when changes in luminance were separated by very short asynchronies.26 Importantly, using this paradigm, Elliott et al26 found a mean target simultaneity threshold located at 59 milliseconds, which is very close to the value proposed by von Baer11 and that subsequently corroborated by Brecher.14
We considered that judging the simultaneity of 2 events involves the processing of information at multiple levels: the neuronal level, the perception and conscious realization of this information, decisional and response components—all of which may be altered in the pathology. A disturbance at a sensory level27 or at the level of conscious realization28 may both lead to a disturbance in phenomenological time. However, if either decision or responses are influenced, performance might appear to be impaired even if the subjective experience of simultaneity is in fact preserved. Additionally, the use of psychophysical procedures that require repeated measures and long periods of testing may in itself lead to impaired performance in patients. In this case, their responses may come to be based on factors other than their phenomenal experience and thus would be unreliable. The paradigm that was used here provided the means to take into account these different possibilities. Accordingly, we adopted the rationale that priming effects have been shown to be preserved in patients.28–33 We then undertook the task to assess the reliability of simultaneity judgments by evaluating the effects of the subthreshold premask, in which events occurred either synchronously or with a delay (asynchronously) but which could not be reported by observers and which should remain independent of any trends in judgments of phenomenal simultaneity. Under this condition, the synchronous or asynchronous presentation of premask stimuli is set below detection threshold by embedding their presentation within a sequence of flankers. The subsequent task required participants to judge the simultaneity or asynchrony of a second, above threshold change in the luminance of target stimuli. Elliott et al26 have shown that this manipulation induces premask effects in healthy volunteers. If patients have a difficulty in giving an appropriate judgment, leading to patterns of biased responding, then their answer should be more susceptible to the influence of the premask and consequently the premask effect should be stronger in patients.
In sum, it is expected that patients will have difficulty to discriminate simultaneous from asynchronous stimuli and require longer intervals than healthy controls to detect asynchronies, whereas preserved priming effects would be evidence for unbiased answers. Such results, ie, unbiased answers associated with extended thresholds in simultaneity judgments, would be evidence for disturbed phenomenal experience of present time.
Methods
Participants
Participants were 19 stabilized chronic outpatients (6 women and 13 men; mean age = 30.6 years, SD = 6.1; mean level of education = 13.2, SD = 2.5) and 19 controls (6 women and 13 men; mean age = 30.1 years, SD = 6.7; mean level of education = 13.2, SD = 2.6) recruited in the University of Strasbourg Psychiatry Department. Controls, recruited from hospital staff, were individually matched with patients on gender, level of education, and age. Patients and controls were identical on level of education and age (all F's < 1).
The project was approved by the local ethics committee, and informed written consent was obtained, before the study, from each patient and control subject in accordance with the recommendations of the Declaration of Helsinki. All subjects had normal or corrected-to-normal visual acuity.
Psychiatric diagnoses of the patients with schizophrenia and Positive and Negative Syndrome Scale (PANSS) scores were established by a senior psychiatrist from the University Psychiatry Department on the basis of semistructured interviews. Diagnoses fulfilled the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for diagnosis of schizophrenia. The mean scores for the PANSS were 17.5 (SD = 6.9) for the positive subscale, 21.2 (SD = 6.7) for the negative subscale, and 36.7 (SD = 12.7) for the global subscale. The mean total score for the PANSS was 75.4 (SD = 12.7).
The mean age at onset of schizophrenia symptoms was 21.3 years (SD = 3.7); the mean disease duration was 9.2 years (SD = 6.5); and the mean number of hospitalizations was 2.7 (SD = 2). The 19 patients were all receiving long-term neuroleptic treatment, administered in a standard dose (mean dose = 243 mg/day of chlorpromazine or chlorpromazine equivalents, SD = 135).34 Three patients were receiving typical neuroleptics, and 16 were receiving atypical neuroleptics. Two were also receiving antiparkinsonian treatment, one trihexyphenidyl (5 mg), and one tropatepine (10 mg).
Equipment
The experiment was run on a Pentium4 PC equipped with a Cambridge Research Systems (Rochester, Kent, UK) visual stimulus generator, which was programmed in the C programming language using the VSG software library. Visual signals were presented on a Mitsubishi visual display monitor with vertical refresh rate set to 120 Hz. The monitor was calibrated using the Cambridge Research Systems OptiCAL photometer. The distance between the screen and the participants was held constant, at 100 cm, by means of a chinrest. Participants gave their response by pressing the “F” or “J” key on the keyboard, according to the asynchrony or simultaneity of the stimuli, respectively. Stimulus presentation occurred in an environment of low-intensity ambient light (0.1 cd/m2; windows were occluded, and day light did not enter the room).
Stimuli
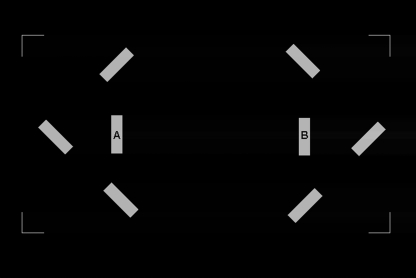
Stimulus presentation was preceded by the presentation of a rectangular orientation frame of corner junctions, displayed for 500 milliseconds. This had the purpose to delimit a 13° × 13° square region at the center of the monitor within which all experimental stimuli were presented. Stimuli were presented following a randomly generated delay of 50–150 milliseconds from trial onset. The stimuli were 2 vertical gray bars, one on the right and one on the left of the center of the monitor; these bars were separated by 5° of visual angle. Each bar subtended 0.5° (horizontal) by 1.5° of visual angle. In order to reduce potential confounds introduced by stimulus transients, luminance was increased gradually for all stimulus onsets within 75 milliseconds Gaussian envelopes. In the main experiment, the bars changed luminance on 2 separate occasions and thus served the dual role of premask and target stimuli. During premask stimulus presentation, 6 flanking bars (hereafter referred to as flankers) were presented around the premask bars. This served as a mask for the first change in luminance. Three of these flankers were positioned around each premask bar (on the external side, above and below); they were separated from the premasks by 2° of visual angle. The flankers were of the same size than both premasks and targets but were oriented pseudorandomly at either 45° or −45° relative to the horizon (figure 1). The flankers increased luminance nonlinearly and then decreased nonlinearly to background luminance over a presentation interval of 75 milliseconds. By contrast, the targets increased luminance in an identical fashion to the premasks (from 0.02 to 12 cd/m2) but then remained on display at the same luminance level until the end of the trial.
Fig. 1.
Illustration of the Flankers Used as Mask During the Initial Increase in Luminance of the 2 Target Bars A and B, ie, During the Premask Effect in the Main Experiment.
Procedure
As a first step, staircase procedures were run to determine 2 independent simultaneity thresholds. In both cases, subjects pressed on the F or J of the keyboard if they judged the bars as asynchronous or simultaneous, respectively. (1) The first staircase procedure was used to determine a lower simultaneity threshold in the absence of flankers. (2) The second staircase procedure was used to determine an upper simultaneity threshold in the presence of flankers. The lower simultaneity threshold represents the minimum interval between changes in bar luminance at which those changes would start to be considered to have occurred asynchronously, in the absence of flankers. The upper simultaneity threshold is located at the maximum interval at which bars are judged to change luminance simultaneously that was still below detection threshold in the presence of flankers. Simultaneity thresholds were indexed relative to the time of onset of the luminance increase and were measured in terms of bar—bar stimulus onset asynchrony (SOA), ie, the delay between time of onset of the 2 bars.
Both lower and upper simultaneity thresholds were determined by using a stochastic approximation adaptive procedure after Treutwein.35 This follows usual psychophysical procedures, in which the 2 stimuli are first displayed with a SOA well above threshold and with SOA being reduced on a trial-by-trial basis until the subject gives a “simultaneous” response. Both lower and upper simultaneity thresholds were determined separately and on at least 2 occasions for each subject.
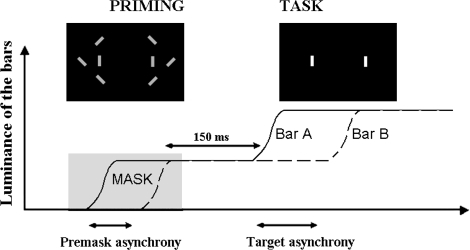
In the main experiment, the premask bars were once again presented within a pseudorandomized sequence of flankers that were rapidly switched on and off at locations flanking the premasks. However, unlike the procedure used to determine the upper simultaneity threshold, the changing luminance of the premask and the concurrent presentation of flanker bars were followed by a second increase in luminance at the location of the premask bars. This second change in (target) luminance was to be judged by participants as being either simultaneous or occurring with an asynchrony. This change in target luminance occurred systematically after the flankers had been switched off and was fully visible. In the main experiment, the SOAs between premask bars were set at 0 milliseconds in the case of a synchronous premask and, in the case of an asynchronous premask, within the range of SOAs circumscribed by the lower and upper thresholds. These SOAs are referred to as “premask asynchronies” in the remainder of the text. The second change in target luminance occurred 150 milliseconds after the change in premask luminance at which time there were no flankers present in the display. Target bars were presented at SOAs ranging from 0 milliseconds (ie, simultaneously) to 92, 184, 276, 368, or 460 milliseconds (depending upon the participant's performance). For each possible range, 12 SOAs were used (the interval between SOAs being a multiple of 8.3 milliseconds). For example, for SOAs ranging from 0 to 92 milliseconds, SOAs were equal to 0, 8, 17, 25, 33, 42, 50, 58, 66, 75, 84, and 92 milliseconds. For SOAs ranging from 0 to 460 milliseconds, these SOAs were multiplied by 5. These SOAs are referred to as “target SOAs” in the remainder of the text. After increasing luminance, the target bars were maintained at the same luminance while participants were asked to judge whether they had changed luminance simultaneously or with an asynchrony (procedure illustrated in figure 2). It was important to adapt target SOAs ranges to each participant's performance in order to make sure that patients were attending to stimuli and followed instructions correctly. High rates of errors at the highest target SOA could be attributed either to a nonspecific difficulty to perform the task or to a real difficulty in discriminating asynchronous from simultaneous stimuli. With the present procedure, it was possible for us to make sure that patients followed the instructions correctly and attended to the stimuli only if the results show that patients reach similar numbers of errors as controls when target SOAs are long enough.
Fig. 2.
Illustration of the Events Occurring During Each Trial in the Main Experiment. The curves represent the increase in luminance of the 2 target bars, A and B. The first increase in luminance is used as a premask and masked by the flankers, as shown in figure 1. The total duration of the prime presentation is adapted to the thresholds derived from the initial staircases, using the following formula: (3 × (lower threshold + upper threshold)/2) + envelope duration. The envelope corresponds to the gradual increase luminance of the bars and has a duration of 75 milliseconds. The premask is said to be asynchronous when the 2 bars do not increase their luminance simultaneously, which is the case presented on the graph. The task of the participant is to decide whether the second increase in luminance is synchronous (SOA = 0 milliseconds) or asynchronous.
Participants were not informed and, although systematically asked, did not report having detected the first change in luminance. This suggests that the flankers had successfully masked the synchrony or asynchrony of the change in premask luminance and that effects of premask synchrony/asynchrony were implicit and not attributable to direct perception. The main experiment required 2 sessions of 5 blocks comprising 92 trials per block. All target SOAs were equally represented in random order, with 40 trials per condition (target SOA and premask). The order of the target bars was also equally represented across conditions and randomized across trials (first bar displayed on the right vs first bar displayed on the left).
Threshold Measurement in the Main Experiment
Preliminary inspection of the data revealed a high false alarm rate in the main experiment, evidenced by the high rate of asynchronous as compared with simultaneous judgments when premask bar SOAs were 0 milliseconds and the target bars were presented simultaneously (36% in controls and 41% in patients; these rates did not differ significantly between groups, F < 1). On this basis, the individual data were submitted to the following probability-based correction26:
where P(0) is the percentage of “simultaneity response” for “subthreshold simultaneity” (ie, a subthreshold SOA = 0). This transformation provides the means to ensure that all “asynchronous responses” taken into account in the following analysis cannot be attributed to false alarms, ie, a biased tendency to provide an “asynchronous response.” Thus, it eliminates the possible problem of a bias toward asynchronous responses. The thresholds were then derived from a linear adjustment between the SOAs and the corrected rate of “simultaneous” responses (rate of simultaneous responses = a × SOA + b). Thresholds were calculated as the SOA corresponding to a rate of 50% simultaneous responses. No subject presented a flat response curve that would correspond to 50% simultaneous responses at all SOAs, ie, random responses. Thus, the rate of 50% simultaneous responses corresponded to an intermediate point between simultaneous and asynchronous responses. Given that the experiments were relatively long in duration, it might have been expected that the judgments of patients were less reliable than controls, ie, the rate of simultaneous responses would have decreased less regularly with target SOAs. This would have led to a lower adjustment rate between target SOAs and simultaneous judgments in patients than in controls. In fact, adjustment rates were good and equivalent in both groups (F < 1), as suggested by the corresponding linear regression coefficients (.83 and .81 in patients compared with .85 and .83 in controls, when the premasks changed luminance synchronously or asynchronously, respectively). These findings indicate that the threshold measurements are at least as reliable in patients as in controls.
Except otherwise stated, the dependent variables were the thresholds.
Results
Initial Staircases
The initial staircases revealed overall longer thresholds in patients than in controls. An analysis of variance (ANOVA) was undertaken with group (patients vs controls) as a between-group variable and with flankers (staircase with vs without flankers) and repetition (first vs second staircase estimates) as within-group variables. This analysis showed a global effect of group, with thresholds being longer in patients than in controls (F1,36 = 9.3, P < .005). There was no main effect of repetition, indicating that the results were stable across sample times (F < 1). The significant group × condition interaction (F1,36 = 6.5, P < .05) revealed that the threshold without flanker was approximately twice as high in the patient group (50 milliseconds) than in the control group (26 milliseconds, F1,36 = 5, P < .05). This result was also observed for the threshold with flankers (125 milliseconds in patients vs 58 milliseconds in controls, F1,36 = 9.4, P < .005).
Main Experiment
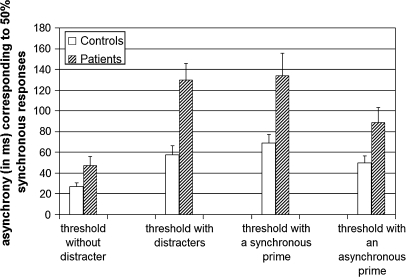
In the main experiment, simultaneity thresholds were overall longer in patients (111 milliseconds) than in controls (59 milliseconds) (F1,36 = 7.1, P < .05), both when the premask was synchronous (134 milliseconds in patients vs 69 milliseconds in controls; F1,36 = 7.6, P < .01) and when the premask was asynchronous (89 milliseconds in patients vs 50 milliseconds in controls; F1,36 = 5.8, P < .05) (figure 3). In both groups, thresholds were longer when the premask bars changed luminance synchronously (101 milliseconds) rather than asynchronously (69 milliseconds: F1,36 = 33, P < .001). The difference between the 2 thresholds was higher in patients (45 milliseconds) than in controls (19 milliseconds), but this was mainly due to 2 patients showing substantially extended thresholds in both conditions (above 170 milliseconds for asynchronous premasks). When these 2 patients were removed from the analysis, the effect of premask condition on the threshold was not significantly different between groups (there was a difference of 33 milliseconds between the 2 thresholds in patients vs 19 milliseconds in controls, F1,34 = 3.2, nonsignificant), but the thresholds were still longer in patients than in controls (89 vs 59 milliseconds, F1,34 = 6, P < .05).
Fig. 3.
Bar Chart of the Different Thresholds Measured During the Protocol and Derived from the Initial Staircases (on the Left: the Threshold Without and With Flankers), and in the Main Experiment (on the Right: the Threshold With a Synchronous and With an Asynchronous Premask), in the 19 Controls (in White) and 19 Patients (Dashed Columns).
Priming Effect and Bias
If patients are biased toward simultaneous responses but perceive asynchrony as efficiently as controls, then the thresholds used for premask bars asynchronies should correspond to those asynchronies perceived by the patients. Hence, the asynchrony of the 2 premask bars should have a greater impact on the patients’ judgments concerning target bars asynchronies, inducing a relatively high proportion of asynchronous responses. If this were the case, asynchronous premasks would bring about a decrease in the rate of simultaneous responses, as compared with synchronous premasks, leading to an artificially increased effect of premask synchrony/asynchrony. In order to test this hypothesis, we distinguished between premasks that changed luminance asynchronously at shorter SOAs within the intervals demarcated by lower and upper simultaneity thresholds and those that changed luminance asynchronously at longer SOAs (3 control participants were excluded from this analysis because there was only one value of premask SOA). As expected, thresholds decreased when the asynchrony between premasks was larger (F1,33 = 20.1, P < .001). (This analysis was performed without the 2 patients with extended thresholds, but similar results were obtained when they were included.) This confirms that when the premask asynchrony approaches visibility subjects are more likely to give an asynchronous response to the change in target luminance. The magnitude of this decrease was identical in patients and controls (the threshold decreased by 15 milliseconds in patients, F1,18 = 8.5, P < .01, and by 14 milliseconds in controls, F1,15 = 17.8, P < .001).
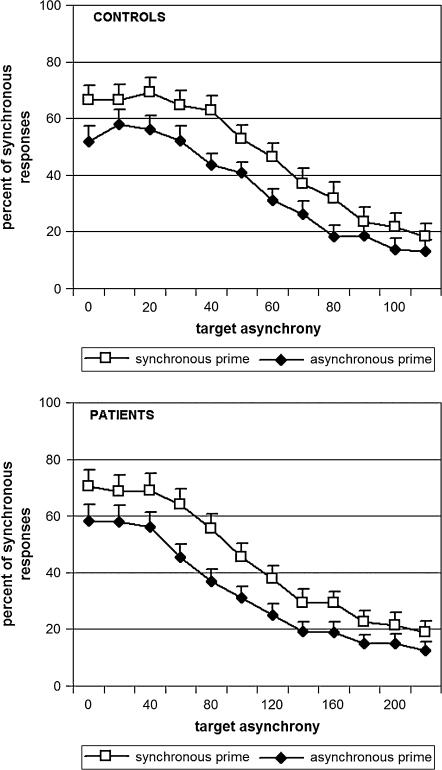
In addition, we compared premask effects for all target SOAs that were used during the experiment by means of an ANOVA with groups as a between-group variable and SOAs (first to twelfth) and the premask (synchronous vs asynchronous) as 2 within-group variables. This time the dependent variable was the rate of “synchronous” responses. Premask effects were identical in patients and in controls with a difference of 11.5% in the rate of synchronous responses when the premask bars changed luminance synchronously as compared with when they changed asynchronously. Comparisons for each measurement (target SOA) yielded similar results (F's < 1.9) (figure 4).
Fig. 4.
Psychometric Curves Averaged Over the Participants in Each Group (19 Controls in the Upper Panel and 19 Patients in the Lower Panel), as a Function of Premask Type (Synchronous in White and Asynchronous in Black). The only difference between the 2 graphs is the abscissa. In patients, the abscissa is twice that observed in controls.
Impact of the Flankers During the Main Experiment
The impact of the flankers during premask bar presentation was evaluated by (1) calculating the difference between the mean threshold observed in the main experiment and the initial threshold measured with the staircase procedure without flankers (ie, the lower simultaneity threshold) and then (2) by dividing this difference by the initial threshold obtained with the staircase procedure without flankers. The results showed that the increase in the threshold was numerically larger but not significantly different in patients (+231%) and in controls (+169%), F < 1. The numerical difference was due to a unique patient who was especially sensitive to the effect of flankers (>+1300%). By excluding this patient from the analysis, results revealed a relative lengthening in threshold of +169% in the patient group, lengthening that was not different from that observed in the control group.
Correlations
There were no significant correlations between performance and clinical ratings or neuroleptic dosage.
Discussion
This study reports consistently longer simultaneity thresholds in patients with schizophrenia relative to controls. This result is unlikely to come about due to a nonspecific factor, such as response bias or difficulty in completing long and tedious experimental procedures. The results are consistent with the hypothesis that patients with schizophrenia are subject to enlarged windows of simultaneity. Our findings are all the more remarkable in that the size of the simultaneity window appears to be very consistent across studies and populations.14,26 With our paradigm, we furthermore demonstrate that the enlarged window of simultaneity in the patients with schizophrenia is not due to a decisional bias effect or an attentional effect induced by the distracting bars.
When stimuli were simultaneous, both controls and patients showed the same bias to respond asynchrony. When stimuli were in fact asynchronous, we used the premask effect to evaluate the subjects’ bias to respond simultaneity. The results in both controls and patients confirm that the largest premask asynchronies, ie, those closer to visibility, do increase the rate of asynchrony judgments. Now, if the lengthened simultaneity thresholds observed in patients in the initial staircase procedures were directly associated to biased responses, then the visibility of the asynchrony between changes in premask bar luminance should be greater because premask asynchronies are determined on the basis of these initial staircase procedures. Bias effects should thus have resulted in larger premask effects in patients than in controls, which was not the case. The normality of the premask effect in patients contrasts with their extended subjective thresholds, suggesting that longer thresholds in patients are not due to the effects of bias. (A decisional bias is usually evaluated by signal detection theory, which is difficult to apply in the present case, given that the situation of simultaneous bar onsets cannot be equated simply to a lack of asynchrony. Indeed, simultaneity can itself induce a specific subjective experience. We conducted the calculations nonetheless, for the SOA 50 milliseconds, that was used in every participant. Biases were found to be identical in both patients and controls.)
Another possible explanation for the impairment observed in patients may have been the use of flankers during the task. The greater distractibility of patients relative to controls has featured in the literature on schizophrenia for nearly 100 years36–38 (although effects of distracters are reduced as compared with controls in some cases39–41). In the present case, a higher distractibility may have impeded patients in focusing on the target bars and thus have artificially lengthened the patients’ thresholds. However, patients showed lengthened thresholds during threshold determination even when there were no flankers. Second, the presence of flankers appeared to induce an increase in the thresholds in all participants—and in equal proportions for patients and controls.
We cannot exclude an effect of treatment, although there was no significant correlation between threshold and treatment dosage. A further caveat may have been difficulty to detect the increase in luminance on the part of the patients or a difficulty to attend to the stimuli due to aberrant eye movements. However, increasing luminance occurred well above detection thresholds, while the task was neither a detection nor a speeded response task. Consequently, performance should be little if at all influenced by impairments in contrast sensitivity.42,43 Additionally, because the increase in luminance is the same at all SOAs, it should have manifest in a global inhibition in target discrimination, irrespective to SOA. This was not the case. In the same fashion, aberrant eye movements would have impaired the detection of an asynchrony irrespective to SOA, thereby resulting in lengthened simultaneity reports at all SOAs: figure 2 shows that the psychometric function differs in patients relative to controls on the abscissa but not in terms of variation in response rates across target SOA. In fact, these functions appear almost identical, while patients do not make more false alarms and have more difficulty in providing a correct answer when the target SOA is above threshold. The quality of the functions in patients shows that the results are reliable in patients and argues against an explanation in terms of a role of impaired contrast detection, aberrant eye movements, or many other nonspecific effect in the present results.
The results may be thus interpreted in terms of a genuine impairment in simultaneity judgments in patients with schizophrenia. There is, to the best of our knowledge, no consensus on the existence of a dedicated brain system for the processing of time and even less so for simultaneity detection.23 This impairment might rather reveal a disorder in generating or maintaining neuronal synchrony, as hypothesized by Varela,6 and leading, in this perspective, to problems in maintaining normal time phenomenology. Elliott et al26 argued that simultaneity detection is brought about by a distributed brain process, potentially including both posterior and anterior circuits. The impairment presented by patients with schizophrenia might thus be related to abnormal neuronal connections as described in patients by some observers (ie, the neuronal disconnectivity hypothesis44–47). This hypothesis is currently under investigation.
Although the relationship between the present pattern of results and known cognitive alterations remains speculative, it is tempting to propose that the timing deficit described in the present work reflects a generalized timing deficit that could influence other mechanisms. One possibility is that several impairments are related through an impaired discrimination of event structure over time. As emphasized in the introduction, distinguishing successive moments might be essential for consciousness to function efficiently. In fact, the existence of a true difficulty to distinguish events in function of time may originate the nonspecific slowing down reported in patients with schizophrenia in many experiments.
A disorder in properly timing event structures may influence language, given that temporal coordination is essential for articulating and linking words in the sentential context. Other impacts are plausible in everyday conditions. Temporal coordination is also essential for sequencing movements and linking auditory or visual events. All these activities require singular events to be distinguished from one another in time, and all these functions are known to be altered in patients with schizophrenia.15–20
Finally, it remains to be studied to which extent the present results are related to known impairments in other paradigms such as masking or duration estimation. The body of existing evidence shows that patients with schizophrenia present a general impairment in evaluating event duration.48–51 Yet, duration evaluation involves a memory component, and it has been suggested that both impairments are related in patients with schizophrenia.49 In addition, the paradigms used in these studies generally concern durations largely above 50 milliseconds, and it is not completely straightforward how elementary time windows are related with the evaluation of longer time intervals.52 Masking paradigms, in contrast, are based on the use of time intervals that are closer to the ones used here. It is now clearly established that patients are impaired at detecting masked information, ie, information followed by a mask after short delays.27,53–59 Using fusion critical tests, it has also been repeatedly shown that patients need larger intervals between 2 consecutive stimuli to discriminate the 2 stimuli.60,61 Most interestingly, such impairments have been observed in patients’ relatives62 and, in patients, to be related with high-level dysfunctions and social interaction.63 However, masking and critical fusion experiments differ from the paradigm used here, in that they involve fusion in the dimensions of both time and space. Critically, the most popular explanation for patients’ impairments in masking or fusion is a prolonged persistence effect of the first stimulus, which leads to fusion in both time and space with the second stimulus.40,64 Persistence means that the sensory signal is processed longer. In our paradigm, stimuli can only be distinguished on the basis of the stimulus onset, but persistence effects cannot have an impact because target bars remain on the screen once appeared. Hence, stimuli cannot be fused in space. In masking experiments, in contrast, signals may be fused in space but still be distinguished from one another in time, on the basis of their onset or offset. For example, subjects may not be able to locate or identify the masked stimulus but may still be able to report that there were 2 successive stimuli. It remains to be explored to which extent an additional difficulty to discriminate 2 successive stimuli through time is involved in the abnormalities observed in the masking paradigm with patients with schizophrenia.
In conclusion, our results show that patients with schizophrenia are impaired in discriminating simultaneous from asynchronous stimuli. This impairment is not due to a bias effect or to attentional disturbances. It may be related to a difficulty to correctly time phenomenological present. This concept has been developed by phenomenologists and would contribute to the rupture in the sense of continuity, as described at a clinical level in patients with schizophrenia. An inability to consciously distinguish successive events is likely to slow down and disturb both the perception of the external world and the production of properly timed thoughts, actions, or speech, at least at a conscious level. Work remains to be done, however, to understand how the deficit described in the present study is related to other deficits and thus to bridge the gap between the present experimental evidence and both clinical and neurophysiological disturbances observed in patients with schizophrenia.
Funding
INSERM (grant to A.G., J.F., M.A.E. from the Centre Hospitalier Régional Universitaire de Strasbourg (API HUS n°3494); Centre de Coopération Universitaire Franco-Bavarois, Munich (grant to M.A.E., A.G., J.F.).
Acknowledgments
The authors report no competing interest.
References
- 1.Fuchs T. The temporal structure of intentionality and its disturbance in schizophrenia. Psychopathology. 2007;40:229–235. doi: 10.1159/000101365. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher S. Neurocognitive models of schizophrenia: a neurophenomenological critique. Psychopathology. 2004;37:8–19. doi: 10.1159/000077014. [DOI] [PubMed] [Google Scholar]
- 3.Vogeley K, Kupke C. Disturbances of time consciousness from a phenomenological and neuroscientific perspective. Schizophr Bull. 2007;33:142–156. doi: 10.1093/schbul/sbl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uhlhaas PJ, Mishara AL. Perceptual anomalies in schizophrenia: integrating phenomenology and cognitive neuroscience. Schizophr Bull. 2007;33:142–156. doi: 10.1093/schbul/sbl047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husserl E. Vorlesungen zur Phaenomenologie des inneren Zeitbewustseins. Halle, Germany: Max Niemeyer Verlag; 1928. [Google Scholar]
- 6.Varela FJ. The specious present: a neurophenomenology of time consciousness. In: Petitot J, Varela FJ, Pachoud B, Roy JM, editors. Naturalizing Phenomenology. Issues in Contemporary Phenomenology and Cognitive Science. Stanford, Calif: Stanford University Press; 1999. pp. 266–314. [Google Scholar]
- 7.Andreasen NC. A unitary model of schizophrenia. Bleuler's “Fragmented Phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 8.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 9.Tononi G, Edelman GM. Schizophrenia and the mechanisms of conscious integration. Brain Res Rev. 2000;31:391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 10.Elliott MA, Shi Z, Kelly SD. A moment to reflect upon perceptual synchrony. J Cogn Neurosci. 2006;18:1663–1665. doi: 10.1162/jocn.2006.18.10.1663. [DOI] [PubMed] [Google Scholar]
- 11.von Baer KE. Welche Auffassung der lebenden Natur ist die richtige Und wie ist diese Auffassung auf die Entomologie anzuwenden? In: von Baer KE, editor. Reden, gehalten in wissenschaftlichen Versammlungen und kleinere Aufsätze vermischten Inhalt. St Petersburg, Russia: H. Schmitzdorf; 1864. pp. 237–284. [Google Scholar]
- 12.Pöppel E. A hierarchical model of temporal perception. Trends Cogn Sci. 1997;1:56–61. doi: 10.1016/S1364-6613(97)01008-5. [DOI] [PubMed] [Google Scholar]
- 13.von Uexküll J. Umwelt und Innenwelt der Tiere. 2. verm. u. verb. Aufl, Berlin: J Springer; 1921. p. 224. [Google Scholar]
- 14.Brecher GA. Die Entstehung und biologische Bedeutung der subjectktiven Zeiteinheit—des Momentes. Z Vgl Physiol. 1932;18:204–243. [Google Scholar]
- 15.Delevoye-Turrell Y, Giersch A, Danion JM. Abnormal sequencing of motor actions in patients with schizophrenia: evidence from grip force adjustments during object manipulation. Am J Psychiatry. 2003;160:134–141. doi: 10.1176/appi.ajp.160.1.134. [DOI] [PubMed] [Google Scholar]
- 16.Delevoye-Turrell Y, Giersch A, Wing A, Danion JM. Fluency deficits in the sequencing of motor actions in schizophrenia. J Abnorm Psychol. 2007;116:56–64. doi: 10.1037/0021-843X.116.1.56. [DOI] [PubMed] [Google Scholar]
- 17.De Gelder B, Vroomen J, Annen L, Masthof E, Hodiamont P. Audio-visual integration in schizophrenia. Schizophr Res. 2003;59:211–218. doi: 10.1016/s0920-9964(01)00344-9. [DOI] [PubMed] [Google Scholar]
- 18.Docherty NM, DeRosa M, Andreasen NC. Communication disturbances in schizophrenia and mania. Arch Gen Psychiatry. 1996;53:358–364. doi: 10.1001/archpsyc.1996.01830040094014. [DOI] [PubMed] [Google Scholar]
- 19.Docherty NM, Strauss ME, Dinzeo TJ, St-Hilaire A. The cognitive origins of specific types of schizophrenic speech disturbances. Am J Psychiatry. 2006;163:2111–2118. doi: 10.1176/ajp.2006.163.12.2111. [DOI] [PubMed] [Google Scholar]
- 20.Manschreck TC, Maher BA, Rucklos ME, Vereen DR, Ader DN. Deficient motor synchrony in schizophrenia. J Abnorm Psychol. 1981;90:321–328. doi: 10.1037//0021-843x.90.4.321. [DOI] [PubMed] [Google Scholar]
- 21.Delevoye-Turrell Y, Giersch A, Danion JM. A deficit in the adjustment of grip force responses in schizophrenia. Neuroreport. 2002;27:1537–1539. doi: 10.1097/00001756-200208270-00010. [DOI] [PubMed] [Google Scholar]
- 22.Delevoye-Turrell Y, Thomas P, Giersch A. Attention for movement production: abnormal profiles in schizophrenia. Schizophr Res. 2006;84:430–432. doi: 10.1016/j.schres.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Foucher JR, Lacambre M, Pham BT, Giersch A, Elliott MA. Low time resolution in schizophrenia. Lengthened windows of simultaneity for visual, auditory and bimodal stimuli. Schizophr Res. 2007;97:118–127. doi: 10.1016/j.schres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz BD, Winstead DK, Walker WG. A corpus callosal deficit in sequential analysis by schizophrenics. Biol Psychiatry. 1984;19:1667–1676. [PubMed] [Google Scholar]
- 25.Larsen A, Madsen KH, Lund TE, Bundesen C. Images of illusory motion in primary visual cortex. J Cogn Neurosci. 2006;18:1174–1180. doi: 10.1162/jocn.2006.18.7.1174. [DOI] [PubMed] [Google Scholar]
- 26.Elliott MA, Shi Z, Sürer F. The effects of subthreshold synchrony on the perception of simultaneity. Psychol Res. 2007;71:687–693. doi: 10.1007/s00426-006-0057-3. [DOI] [PubMed] [Google Scholar]
- 27.Saccuzzo DS, Cadenhead KS, Braff DL. Backward versus forward visual masking deficits in schizophrenic patients: centrally, not peripherally, mediated? Am J Psychiatry. 1996;153:1564–1570. doi: 10.1176/ajp.153.12.1564. [DOI] [PubMed] [Google Scholar]
- 28.Del Cul A, Dehaene S, Leboyer M. Preserved subliminal processing and impaired conscious access in schizophrenia. Arch Gen Psychiatry. 2006;63:1313–1323. doi: 10.1001/archpsyc.63.12.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barch DM, Cohen JD, Servan-Schreiber D, et al. Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. J Abnorm Psychol. 1996;105:592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- 30.Baving L, Wagner M, Cohen R, Rockstroh B. Increased semantic and repetition priming in schizophrenia patients. J Abnorm Psychol. 2001;110:67–75. doi: 10.1037//0021-843x.110.1.67. [DOI] [PubMed] [Google Scholar]
- 31.Condray R, Siegle GJ, Cohen JD, van Kammen DP, Steinhauer SR. Automatic activation of the semantic network in schizophrenia: evidence from event-related potentials. Biol Psychiatry. 2003;54:1134–1148. doi: 10.1016/s0006-3223(03)00699-1. [DOI] [PubMed] [Google Scholar]
- 32.Gras-Vincendon A, Danion JM, Grangé D, et al. Explicit memory, repetition priming and cognitive skill learning in schizophrenia. Schizophr Res. 1994;13:117–126. doi: 10.1016/0920-9964(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 33.Höschel K, Irle E. Emotional priming of facial affect identification in schizophrenia. Schizophr Bull. 2001;27:317–327. doi: 10.1093/oxfordjournals.schbul.a006877. [DOI] [PubMed] [Google Scholar]
- 34.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 35.Treutwein B. Adaptive psychophysical procedures. Vision Res. 1995;35:2503–2522. [PubMed] [Google Scholar]
- 36.Boucart M, Mobarek N, Cuervo C, Danion JM. What is the nature of increased Stroop interference in schizophrenia? Acta Psychol. 1999;101:3–25. doi: 10.1016/s0001-6918(98)00037-7. [DOI] [PubMed] [Google Scholar]
- 37.Kraepelin E. Dementia Praecox and Paraphrenia. Edinburgh, UK: Livingston E and S; 1913. [Google Scholar]
- 38.Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- 39.Dakin S, Carlin P, Hemsley D. Weak suppression of visual context in chronic schizophrenia. Curr Biol. 2005;15:R822–R824. doi: 10.1016/j.cub.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Giersch A, Danion JM, Boucart M, Roeser C, Abenhaim K. Reduced or increased influence of non-pertinent information in patients with schizophrenia? Acta Psychol. 2002;111:171–190. doi: 10.1016/s0001-6918(02)00048-3. [DOI] [PubMed] [Google Scholar]
- 41.Uhlhaas PJ, Silverstein SM, Phillips WA, Lovell PG. Evidence for impaired visual context processing in schizotypy with thought disorder. Schizophr Res. 2004;68:249–260. doi: 10.1016/S0920-9964(03)00184-1. [DOI] [PubMed] [Google Scholar]
- 42.Kéri S, Antal A, Szekeres G, Benedek G, Janka Z. Spatiotemporal visual processing in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14:190–196. doi: 10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- 43.Slaghuis WL. Spatio-temporal luminance contrast sensitivity and visual backward masking in schizophrenia. Exp Brain Res. 2004;156:196–211. doi: 10.1007/s00221-003-1771-3. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. NeuroImage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- 45.Foucher JR, Vidailhet P, Chanraud S, et al. Functional integration in schizophrenia: too little or too much? Preliminary results on fMRI data. NeuroImage. 2005;26:374–388. doi: 10.1016/j.neuroimage.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 46.Kwon JS, O'Donnell BF, Wallenstein GV, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Symond MP, Harris AW, Gordon E, Williams LM. “Gamma synchrony” in first-episode schizophrenia: a disorder in temporal connectivity? Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- 48.Davalos DB, Kisley MA, Freedman R. Behavioral and electrophysiological indices of temporal processing dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 2005;17:517–525. doi: 10.1176/jnp.17.4.517. [DOI] [PubMed] [Google Scholar]
- 49.Elvevåg B, Brown GDA, McCormack T, Vousden JI, Goldberg TE. Identification of tone duration, line length, and letter position: an experimental approach to timing and working memory deficits in schizophrenia. J Abnorm Psychol. 2004;113:509–521. doi: 10.1037/0021-843X.113.4.509. [DOI] [PubMed] [Google Scholar]
- 50.Haggard P, Martin F, Taylor-Clarke M, Jeannerod M, Franck N. Awareness of action in schizophrenia. Neuroreport. 2003;14:1081–1085. doi: 10.1097/01.wnr.0000073684.00308.c0. [DOI] [PubMed] [Google Scholar]
- 51.Volz HP, Nenadic I, Gaser C, Rammsayer T, Hager F, Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport. 2001;12:313–316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- 52.Lavoie P, Grondin S. Information processing limitations as revealed by temporal discrimination. Brain Cogn. 2004;54:198–200. doi: 10.1016/j.bandc.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 53.Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biol Psychiatry. 1996;40:295–298. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 54.Herzog MH, Kopmann S, Brand A. Intact figure-ground segmentation in schizophrenia. Psychiatry Res. 2004;129:55–63. doi: 10.1016/j.psychres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Koelkebeck K, Ohrmann P, Hetzel G, Arolt V, Suslow T. Visual backward masking: deficits in locating targets are specific to schizophrenia and not related to intellectual decline. Schizophr Res. 2005;78:261–268. doi: 10.1016/j.schres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Merritt RD, Balogh DW. Backward masking spatial frequency effects among hypothetically schizotypal individuals. Schizophr Bull. 1989;15:573–583. doi: 10.1093/schbul/15.4.573. [DOI] [PubMed] [Google Scholar]
- 57.Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr Res. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz BD, Tomlin HR, Evans WJ, Ross KV. Neurophysiologic mechanisms of attention: a selective review of early information processing in schizophrenics. Front Biosci. 2001;6:D120–D134. doi: 10.2741/schwartz. [DOI] [PubMed] [Google Scholar]
- 59.Wynn JK, Light CA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz BD, Mallott DB, Winstead DK. Preattentive deficit in temporal processing by chronic schizophrenics. Biol Psychiatry. 1988;23:664–669. doi: 10.1016/0006-3223(88)90049-2. [DOI] [PubMed] [Google Scholar]
- 61.Slaghuis WL, Bishop AM. Luminance flicker sensitivity in positive- and negative-symptom schizophrenia. Exp Brain Res. 2001;138:88–99. doi: 10.1007/s002210100683. [DOI] [PubMed] [Google Scholar]
- 62.Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Forward and backward masking in unaffected siblings of schizophrenic patients. Biol Psychiatry. 2006;59:446–451. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 63.Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–454. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- 64.Slaghuis WL, Curran CE. Spatial frequency masking in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1999;108:42–50. doi: 10.1037//0021-843x.108.1.42. [DOI] [PubMed] [Google Scholar]