Abstract
We evaluated whether abnormal frequency composition of the resting state electroencephalogram (EEG) in schizophrenia was associated with genetic liability for the disorder by studying first-degree biological relatives of schizophrenia patients. The study included a data-driven method for defining EEG frequency components and determined the specificity of resting state EEG frequency abnormalities by assessing schizophrenia patients, bipolar disorder patients, and relatives of both patient groups. Schizophrenia patients and their relatives, but not bipolar patients or their relatives, exhibited increased high-frequency activity (beta) providing evidence for disturbances in resting state brain activity being specific to genetic liability for schizophrenia. Schizophrenia patients exhibited augmented low-frequency EEG activity (delta, theta), while bipolar disorder patients and the 2 groups of relatives generally failed to manifest similar low-frequency EEG abnormalities. The Val158Met polymorphism for the catechol-O-methyl transferase (COMT) gene was most strongly associated with delta and theta activity in schizophrenia patients. Met homozygote schizophrenia patients exhibited augmented activity for the 2 low-frequency bands compared with control subjects. Excessive high-frequency EEG activity over frontal brain regions may serve as an endophenotype that reflects cortical expression of genetic vulnerability for schizophrenia. Low-frequency resting state EEG anomalies in schizophrenia may relate to disorder-specific pathophysiology in schizophrenia and the influence of the COMT gene on tonic dopamanergic function.
Keywords: electroencephalography, endophenotype, catechol-O-methyl transferase
Introduction
Investigations of the genetic basis for mental disorders have increasingly focused on defining alternative phenotypes that are more directly tied to the genetic underpinnings of psychopathology than clinical diagnoses. Endophenotypes are alternative phenotypes reflecting internal phenomena of organisms that ideally define elements of mental disorders proximal to effects of genes that cause psychopathology.1,2 Because endophenotypes reflect predisposing genes, rather than the actual occurrence of the disorder, they are observable in unaffected family members of the affected individual. For an endophenotype to be a specific indicator of genes predisposing a particular disorder, it should be absent in individuals with other disorders and biological relatives of these individuals. Recent genetic work in schizophrenia and bipolar disorder suggests that some genes may confer risk for both conditions,3–5 and therefore endophenotypes may be evident across the 2 disorders. Thus, contrasting genetic effects in schizophrenia and bipolar disorder by examining endophenotypic indices will inform which elements of the disorders are shared or separable. Clarification of the diagnostic specificity of genetic effects may shape future definitions of mental disorders and guide individualization of interventions.
The electrical activity of the brain of an individual at rest is generally stable and heritable.6–11 Researchers have examined the frequency composition of the resting state electroencephalogram (EEG) and identified augmented low-frequency activity in individuals with schizophrenia.8,12–14 Several investigations have also revealed resting state EEG abnormalities in unaffected relatives of individuals with schizophrenia.9,15–17 Nonetheless, no studies have used whole-head EEG to test whether resting state EEG abnormalities are specific to schizophrenia patients and their relatives and absent in individuals with bipolar disorder and their relatives. To determine whether the frequency composition of resting EEG may operate as an endophenotype for schizophrenia, we examined the resting state brain electrical activity of schizophrenia patients, first-degree relatives of schizophrenia patients, bipolar disorder patients, first-degree relatives of bipolar patients, and nonpsychiatric control subjects. We utilized a data-driven method to decompose the frequency characteristics of the resting EEG and examined frequencies across the head that fell into delta, theta, alpha, beta, and gamma bands.
There is preliminary evidence that resting state functional brain abnormalities may mark genetic liability for schizophrenia.9,16 The few studies to date that have directly tested whether individuals with genetic liability for schizophrenia (ie, first-degree biological relatives) exhibit resting state abnormalities provide some evidence for augmented delta and theta,15 reduced frequency of the peak alpha,16 and augmented beta activity.15,17 These studies included a small number of scalp sites,16 only recorded during the eyes-closed condition,15–17 or have yielded inconsistent findings.15–17 To fully examine the assertion that augmented high-frequency beta activity reflects genetic predisposition for schizophrenia,12 it is necessary to test whether individuals with genetic liability for other mental disorders show similar EEG abnormalities. Clementz et al16 conducted the only known study that directly tested whether the association of genetic liability with EEG abnormalities was specific to schizophrenia. Beyond a gender-specific reduction in peak alpha frequency for schizophrenia relatives, the study failed to reveal EEG frequency abnormalities in either relatives of individuals with schizophrenia or bipolar disorder. Because Clementz et al16 were limited to central recording sites, they lacked the ability to detect abnormalities across the scalp.
Stable resting state functional brain abnormalities in schizophrenia may be indicative of frontal lobe pathology.13,14,18–20 Decreases in resting state frontal blood flow and metabolism have been shown to be a robust finding in schizophrenia.21 Recently, Fujimoto et al22 reported decreases in relative glucose metabolism in schizophrenia patients in the frontal cortex in addition to primary sensory regions and the anterior cingulate cortex. EEG studies have revealed augmented low-frequency activity over anterior regions20,23,24 that have been associated with indices reflective of frontal lobe pathology.14 Augmented low-frequency EEG activity has also been associated with nonwinter births and smooth pursuit eye movement dysfunction, suggesting a relationship with genetically influenced pathophysiology in schizophrenia rather than environmental etiologic factors.14,19 Magnetoencephalography studies have also revealed augmented slow wave activity in schizophrenia patients and have localized such activity to frontal, temporal, and parietal brain regions.25,26 Despite older studies with limited numbers of EEG recording sites indicating resting state abnormalities may not be specific to schizophrenia,27,28 recent evidence points to diagnostic variations in the brain regions where EEG abnormalities are most apparent.24,29,30
The present study sought to: (1) provide a data-driven characterization of the frequency composition and scalp topography of the resting state EEG in both eyes-open and closed conditions, (2) determine whether resting state low-frequency EEG abnormalities seen in schizophrenia were evident in another form of severe psychopathology (ie, bipolar disorder), and (3) test whether high-frequency activity (ie, beta and gamma) abnormalities were evident in first-degree relatives of schizophrenia and bipolar disorder patients. We also sought to determine whether the Val158Met polymorphism for the catechol-O-methyl transferase (COMT) gene was associated with EEG abnormalities found in schizophrenia patients. The polymorphism has been associated with schizophrenia and therefore may be related to EEG abnormalities noted in the disorder.
Methods
Participants
Forty-eight schizophrenia patients, 61 first-degree biological relatives of schizophrenia patients, 30 bipolar disorder patients (9 with a history of psychotic symptoms), and 38 first-degree biological relatives of bipolar disorder patients were included in data analysis. Table 1 presents the characteristics of participants subject to analyses. We recruited stable psychiatric outpatients from the Minneapolis VA Medical Center and community mental health agencies and screened them for exclusion criteria. Biological relatives of participating patients were contacted and invited to participate. Seventy-nine demographically similar control participants were recruited via postings in the medical center, community libraries, fitness centers, and fraternal organization newsletters. The vast majority of participants were of European ancestry. In the schizophrenia group, 42 participants reported one or both parents were of Western European descent, and 9 reported one of both parents of Eastern European descent. The remaining ancestries for schizophrenia patients were 1 Latino, 2 Asian, 3 Native American, and 5 African-American. In the bipolar disorder group, 30 participants reported Western European ancestry of one or both parents, 6 reported Eastern European ancestry, and 2 were of Native American descent. Sixty-six of the control subjects reported Western European ancestry of one or both parents, 14 were of Eastern European descent, 2 were Asian, 3 were Native American, 5 were African-American, and 3 were other or unreported.
Table 1.
Characteristics of Participants
| Variable | Schizophrenia Patients (n = 48) | Relatives of Schizophrenia Patients (n = 61) | Control Subjects (n = 79) | Bipolar Patientsa (n = 30) | Relatives of Bipolar Patients (n = 38) |
| Age (years) | 44.8 (9.6)b | 50.0 (10.1)c | 43.7 (15.1) | 44.5 (9.5) | 45.7 (15.9) |
| % Female | 18.8b,c | 59.0 | 46.8 | 16.7c,d | 39.5 |
| Education (years) | 13.9 (2.6)c | 14.6 (2.3) | 15.1 (2.4) | 15.1 (2.2) | 14.1 (2.9) |
| Estimated IQ | 97.4 (12.5)b,c | 105.7 (13.2) | 109.9 (12.5) | 110.7 (15.1) | 110.0 (11.9) |
| CPZ equivalent | 747.7 (606) (n = 44) | NA | NA | 237.5 (170) (n = 4) | NA |
| BPRS total score | 41.8 (11.4) | NA | NA | 35.6 (8.6) | NA |
| SPQ total score | NA | 14.2 (9.6)c | 10.6 (7.0) | NA | 14.8 (14.4) |
| Schizophrenia spectrum disorder | 100% | 8.2% | 1.3% | 6.7% | 10.5% |
| Personality disorder | 0% | 3.3% | 2.5% | 0% | 7.9% |
| Lifetime affective disorder | 8.3% | 34.4% | 0% | 100% | 34.2% |
| Lifetime substance dependence | 14.6% | 18.0% | 3.8% | 0% | 7.9% |
Note: Data are presented as mean (standard deviation). Estimated IQ was derived from the formula of Brooker and Cyr52 using Vocabulary and Block Design subtests. IQ, intelligence quotient; CPZ Equivalent, average chlorpromazine equivalent dosage for those taking antipsychotic medications; BPRS, Brief Psychiatric Rating Scale33; SPQ, Schizotypal Personality Questionnaire38; NA, not applicable.
Nine bipolar patients had past histories of psychosis.
Schizophrenia patients different from relatives of schizophrenia group mean (P < .05).
Different from control group mean (P < .05).
Bipolar disorder patients different from relatives of bipolar disorder group mean (P < .05).
We excluded potential schizophrenia and bipolar disorder participants if they had English as a second language, charted IQ less than 70 or a diagnosis of mental retardation, current alcohol or drug abuse or dependence, past drug dependence, a current or past central nervous system disease or condition, a medical condition or disease with likely significant central nervous system effects, history of head injury with skull fracture or loss of consciousness of greater than 20 min, a physical problem that would render study measures difficult or impossible to administer or interpret (eg, blindness, hearing impairment, paralysis in upper extremities), an age less than 18 or greater than 59, significant tardive dyskinesia as indicated by a Dyskinesia Identification System: Condensed User Scale, or been adopted. Research staff identified first-degree biological relatives of patients by completing a pedigree from the patient's report. Interested relatives completed a telephone interview to determine their demographic and medical characteristics and were only excluded if they had a physical problem that would render study measures impossible to measure or were younger than age 18 or older than age 68 (We compared participants with and without past histories of alcohol dependence and found no significant effects of past alcohol dependence on EEG variables. When examining past alcohol dependence within each of the studied groups, a significant effect emerged within the schizophrenia patient group such that patients with a past history of alcohol dependence exhibited a decrease in eyes-open gamma over temporal recording sites [F(1,38) = 5.21, P = .03]. We performed the same analyses for past histories of illicit drug dependence and found participants with past histories illicit drug dependence exhibited reduced eyes-open slow beta over the temporal region [F(1,181) = 5.67, P = .02] and eyes-open gamma over temporal region [F(1,181) = 6.86, P = .01], and a trend effect emerged for eyes-open gamma over the frontal region [F(1,181) = 3.45, P = .06]. The deviations associated with past substance dependence were in the opposite general direction of EEG abnormalities observed in schizophrenia patients and their relatives.). In addition to exclusion criteria applied to patient groups, control participants were excluded for personal or familial histories of psychotic symptoms or affective disorders as defined by the Diagnostic and Statistical Manual, Fourth Edition (DSM-IV).31 Because efforts were taken to maximally describe the electrophysiological characteristics of families from which schizophrenia and bipolar probands came, we did not exclude family members with histories of psychopathology. Table 1 presents information on lifetime prevalence of mental disorders in the relatives and control subjects. All participants completed an informed consent process, and approval for the study protocol was given by the Institutional Review Boards of the Minneapolis VA Medical Center and the University of Minnesota.
Diagnostic information was obtained by trained doctoral-level clinical psychologists who completed the Diagnostic Interview for Genetic Studies32 (DIGS) with each patient. From the DIGS and supplemental questions, the interviewer made symptom ratings of patients using the 24-item version of the Brief Psychiatric Rating Scale.33 Relatives and control participants completed the Structured Clinical Interview for DSM-IV Axis I Disorders34 (SCID-I), the Structured Interview for Schizotypy35 (SIS), and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders36 (SCID-II) when indicated by the SCID-II Personality Questionnaire.37 Relatives and control subjects also completed the Schizotypal Personality Questionnaire (SPQ)38 in order to measure schizotypal characteristics. Lifetime Axis I and II diagnoses for participants were determined by doctoral-level psychologists and trained advanced graduate students through a consensus process consistent with published guidelines,39 involving review of SCID-I, SCID-II, SIS, medical history, and family informant material. For additional details on clinical assessment and diagnostic procedures, see previous publications.40,41 Operational Criteria Checklist for Psychotic Illness42 (OPCRIT) ratings were also used to characterize lifetime mood and psychotic symptomatology by computing scores on 5 major dimensions of psychosis (positive symptoms, negative symptoms, mania, depression, and disorganization).43 We recorded medications taken by the schizophrenia and bipolar disorder patients. Forty-six schizophrenia patients were taking an antipsychotic medication, 12 were prescribed an antiparkisonian agent, 25 were on an antidepressant, 1 was on a mood stabilizer, 3 were taking antianxiety medications, 5 were on anticonvulsants, and 36 were taking a novel antipsychotic. Four bipolar patients were taking an antipsychotic medication, 1 was taking an anti-Parkinsonian agent, 6 were taking an antidepressant, 6 were taking mood stabilizers, 4 were taking antianxiety medication, 10 were on anticonvulsants, and 4 were taking novel antipsychotic medication (We tested EEG variables for effects of medications. Significant effects were found when grouping schizophrenia patients as either on or off novel antipsychotic medication. When comparing Region by Band by Condition by Medication Status, there was a main effect for Medication Status [F(1,30) = 10.37, P = .003], and pairwise comparisons revealed those who were taking novel antipsychotics exhibited increased theta amplitude, low- and high-alpha components, and slow beta. The average chlorpromazine equivalents for schizophrenia patients on conventional (mean = 1011.1; SD = 935.9) and novel (mean = 680.0; SD = 485.5) antipsychotics were not significantly different [t(42) = 1.03, P = .33]. The only other medication test approaching significance was found when comparing schizophrenia patients on or off anti-Parkinsonian agents in an analysis of Region, Band, and Medication Status. A Medication Status trend emerged F(1,33) = 3.84, P = .06], and pairwise comparisons revealed that those taking anti-Parkinsonian agents exhibited an increase in slow alpha. No other tests for medication effects on EEG variables emerged as significant or trending toward significance, including tests of associations with chlorpromazine equivalents.).
COMT Data Processing
We determined the Val158Met COMT genotype for each individual by a restriction fragment length polymorphism technique. Whole blood was collected on FTA Matrix specimen collection cards (Whatman). Punches from the FTA blood cards were then prepared for polymerase chain reaction (PCR) analysis according to Whatman FTA protocol. The washed punch was used directly for PCR amplification. Primers for PCR amplification spanned the COMT Val158Met polymorphism (single nucleotide polymorphism rs4680) (forward primer 5′ tactgtggctactcagctgtgc 3′, reverse primer 5′ gtgaacgtggtgtgaacacc 3′). Amplification was carried out as described by Bergman-Jungestrom and Wingren.44 PCRs were initially denatured at 94°C for 3 min followed by 39 cycles of denaturation at 93°C for 45 s, annealing at 55°C for 1 min and extension at 72°C for 1 min with a final 4 min extension at 72°C. The PCR products were digested with NlaIII (New England Biolabs, Ipswich, MA) for 3 h at 37°C followed by incubation at 60°C for 20 min to denature the enzyme. The digestion was then separated by polyacrylamide gel electrophoresis and the digestion products visualized by staining with ethidium bromide. The COMT val allele has a G at position 1947 yielding a 114 base pair fragment after digestion with NlaIII, whereas the COMT Met allele has an A at this position which allows digestion of the 114 base pair fragment into 2 products of 96 and 18 base pairs.
Electrophysiological Data Collection and Reduction
EEGs were obtained with an elastic electrode cap fitted with 27 tin electrodes over scalp locations conforming to the 10–20 International System. Electrodes were filled with conductive gel, and scalp sites were abraded to reduce impedances to less than 5 kΩ. EEG signals were digitized at the rate of 500 Hz with 0.05 Hz low-frequency and 100 Hz high-frequency filters. Vertical and horizontal electro-oculograms were monitored by electrodes placed above and below the right eye and on the left and right temples, respectively. EEGs were collected in 2 different resting conditions. In the eyes-closed condition, participants were instructed to sit relaxed with their eyes closed. In the eyes-open condition, participants were instructed to sit and relax while viewing a dot to keep their eyes fixated. Both conditions lasted 3 min, and participants were instructed to remain still and to avoid excessive eye movements throughout.
After data acquisition, EEG recordings were subsampled to 128 Hz using the Matlab resample subcommand (which applies an anti-aliasing filter before downsampling). The data were segmented into 4-s epochs overlapping by 50%, rereferenced to linked ears, and baseline corrected relative to the median value of the entire epoch before applying a Hamming window. A 60-Hz band-stop filter was applied. Epochs were excluded if activity at any point exceeded a ±200 μV threshold (relative to the median value across the epoch) for all EEG channels and electro-oculogram sites (to control for artifacts). To additionally eliminate the influence of slow rolling eye movements, we submitted only data above 2 Hz to the principal components analyses (PCAs). Participants were included if EEG data totaled at least 30 s after epochs tagged for artifact were removed, which has been shown to yield reliable spectral components6 (In the eyes-closed condition, we excluded 1 schizophrenia patient for having less than 30 s worth of valid EEG data. In the eyes-open condition, we excluded 9 schizophrenia patients, 3 bipolar disorder patients, 1 control, 5 schizophrenia relatives, and 1 bipolar disorder relative for having less than 30 s worth of EEG data free of artifacts.). Frequency power (μV2) was computed by conducting fast-Fourier transforms. The square root of frequency-power was taken to yield a rectified frequency-amplitude (μV) measure. Participants’ data were then averaged for each condition and electrode. Using traditional definitions of bands for characterizing EEG frequency spectra,45,46 we excluded participants if frequency-amplitude exceeded 4 SDs of the grand mean for a particular component (ie, delta, theta, slow alpha, fast alpha, slow beta, fast beta, gamma) at any scalp region (ie, aggregated electrodes; anterior [FP1, FP2, F7, F3, Fz, F4, F8], central [C3, Cz, C4], parietal [P7, P3, Pz, P4, P8], temporal [FT9, FT7, FT10, FT8, T7, T8, TP9, TP7, TP10, TP8], and occipital [O1, O2]). (Because of outlier status in the eyes-closed condition, we excluded 8 schizophrenia patients, 9 bipolar disorder patients, 3 controls, 2 schizophrenia relatives, and 1 bipolar disorder relative; in the eyes-open condition, we excluded 3 schizophrenia patients, 3 bipolar disorder patients, 6 controls, 5 schizo-phrenia relatives, and 3 bipolar disorder relatives. Participants generally were excluded for one condition and not the other [4 schizophrenia patients were excluded in both conditions and 2 bipolar disorder patients] and were not excluded from both conditions for within-within condi-tion analyses in order to maximize statistical power for describing EEG abnormalities in the groups.) Remaining data were subjected to PCAs to define spectral components. Because variation in beta frequency power is largely independent of variation of power in delta, theta, and alpha bands,13 we performed separate decompositions for beta/gamma frequencies (13–50 Hz) and frequencies below beta (2–12.875 Hz) for each condition. (A second reason for separate decompositions for higher and lower frequencies is that the power of EEG oscillations [with the exception of the alpha band] generally drops at a rate reciprocal to the frequency. Thus, because of their relatively low amplitude, beta/gamma components may be underrepresented when analyzed in conjunction with lower frequency bands.) Covariance matrices were computed, and components were extracted and rotated using varimax criteria. The weighted orthogonal components (weighted from −1 to 1) were projected against the original spectra yielding EEG spectral values for each independent component. Our analyses focused on means for each weighted principal component for low- and high-frequency ranges, allowing components to overlap but characterizing the covarying activity that defined each component.
Statistical Analyses
To examine group differences in resting state EEG frequency composition, the scalp topography of EEG components, and differences in eyes-closed and eyes-open conditions, we conducted a series of multivariate analyses of covariance (MANCOVA) for schizophrenia patients and their relatives, as well as for bipolar patients and their relatives. Two omnibus tests included 2 between-subjects factors (Group: schizophrenia patient, first-degree relative of schizophrenia patient, nonpsychiatric control or bipolar patient, first-degree relative of bipolar patient, nonpsychiatric control; Gender) and 3 within-subjects factors (Region: anterior, central, parietal, temporal, occipital; Band Component: delta, theta, slow alpha, fast alpha, slow beta, fast beta, gamma; Condition: eyes open, eyes closed). (Gender was not included as a between-subjects factor for analyses of bipolar patients and their relatives because only 5 bipolar woman were included in the sample. In preliminary analyses of 430 bipolar patients and their relatives, gender did not emerge as a significant effect in the MANCOVA.) Group-by-Region MANCOVAs were then conducted separately for each component within each condition, and follow-up contrasts were computed to explain the nature of significant effects. Age was included as a covariate in all MANCOVAs. Nonparametric Wilcoxon tests were used to assess the scalp topography of simple effects. The Wilcoxon pairwise tests were computed by comparing one group to another for each band, condition, and electrode and were displayed as interpolated topographical maps across the scalp for describing scalp topography of Group differences. Pearson correlations were used to assess if there were associations between EEG variables found to be deviant in patients and OPCRIT symptomology variables assessed during diagnostic interviews. We also investigated whether the SPQ factors or SIS symptoms correlated with EEG variables within the groups of relatives. To test associations between COMT and the EEG variables found to be abnormal in schizophrenia, MANCOVAs were conducted with 1 within-subjects factor (Region), 1 between-subjects factor (Genotype: Val/Val patients, Val/Met patients, and Met/Met patients), and Age as a covariate. Significant Genotype by Region interactions were followed-up with 1-way ANOVAs that tested Genotype effects within each scalp region. Bonferroni-adjusted post hoc comparisons were computed to assess which Genotypes differed from each other, and follow-up t-tests were conducted to determine which genotypes were different from the nonpsychiatric control group. Mean frequency amplitude in μV for each PCA-identified component served as continuous dependent measures in each test. All degrees of freedom for the MANCOVAs were Wilks’ Lambda adjusted where applicable and subsequent P values represent the adjusted statistic. We computed η2 values in each MANCOVA as a measure of effect size. Statistical analyses included general linear models, Pearson correlations, and t-tests implemented in the Statistical Package for the Social Sciences (GLM; t-test: SPSS Inc., Chicago, Ill). Wilcoxon rank sum tests for equal medians were implemented in Matlab 6.5 (RANKSUM: Mathworks Inc.).
Results
Principal Components and Topography of Resting State EEG Activity
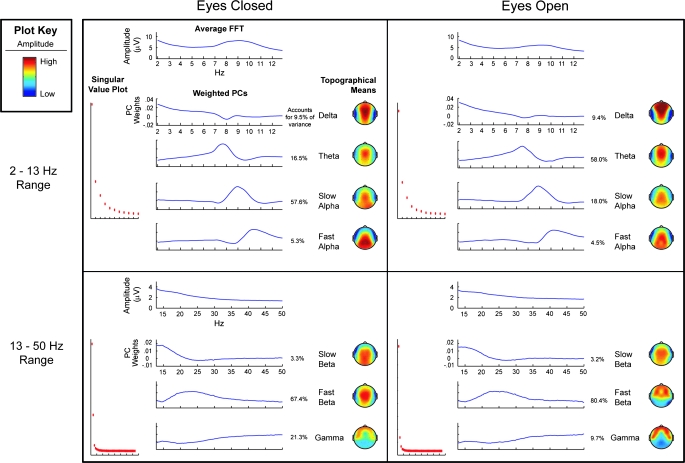
We carried out analyses of all subjects to identify elemental components of resting state brain activity characterized by electroencephalographic recordings. Figure 1 depicts results from 4 PCA decompositions representing the 2 frequency ranges and eyes-closed and eyes-open conditions. Inspection of the singular value plot for the PCA conducted in the 2–12.875 Hz range in the eyes-closed condition supported selection of a 4-factor solution. The singular value plot for eyes-open spectra also suggested a 4-factor solution. As can be seen in figure 1, the 4-factor solutions were similar for the 2 resting EEG conditions. A low-frequency delta component emerged with a peak at 2 Hz and a frontal-central scalp distribution. A theta component also emerged with a peak frequency between 7 and 8 Hz with maximal power over the central scalp. The last 2 components represented alpha frequencies. A slow alpha component defined by a 9-Hz peak was most evident over the central parietal region, and a fast alpha component with a 10-Hz peak exhibited a posterior scalp maximum. The 4 factors generally conformed to traditional definitions of bands used to characterize EEG frequency spectra (cf, Andreassi45 and Davidson et al46).
Fig. 1.
Results from the 4 principal component analyses (PCAs) conducted on the frequency spectra of schizophrenia and bipolar patients, first-degree relatives of each patient group, and nonpsychiatric controls. PCAs were conducted separately for eyes-open and eyes-closed conditions. Separate PCAs were also conducted for low frequencies (ie, 2–12.875 Hz) and high frequencies (ie, 13–50 Hz). Each of the 4 quadrants of the figure represents 1 PCA. The average spectra before conducting the PCA appear at the top of each quadrant. Singular value plots that guided decisions as to the number of factors to extract are displayed on the left. Line graphs plot the principal components-weighted spectra for each component extracted after variamax rotation (ie, extracted orthogonal components were then projected against the average spectra yielding independent component spectra). Topographical maps plot the location on the scalp where activity is most prominent for each component. The variance accounted for by each component is also reported.
Inspection of singular value plots for the decompositions conducted within the beta and gamma frequency ranges suggested 3 components for both conditions. The frequency composition of the 3 components were similar across eyes-closed and eyes-open conditions with one factor most strongly representing frequencies below 20 Hz (slow beta), the second factor representing frequencies between 20 and 30 Hz (fast beta), and a third component representing frequencies between 30 and 50 Hz (gamma). In both resting conditions, slow beta was maximal across central-parietal scalp sites. Fast beta was predominately distributed across central sites in the eyes-closed condition, but in the eyes-open condition it was most prominent at frontal-temporal scalp sites. Gamma was primarily distributed across frontotemporal scalp sites.
Resting State EEG Abnormalities in Schizophrenia Patients and Their Relatives
To determine whether individuals with schizophrenia or people who carry genetic liability for schizophrenia manifest resting state functional brain abnormalities, we conducted analyses of the frequency composition and topography of the derived EEG frequency components in schizophrenia patients and their relatives for eyes-closed and eyes-open rest conditions. The omnibus analysis revealed main effects of Group [F(2,130) = 4.35, P = .01] and Band [F(6,125) = 44.719, P ≤ .001] along with a Group-by-Band interaction [F(12,250) = 1.92, P = .03] and a trend toward a Group-by-Band-by-Region interaction [F(24,107) = 1.31, P = .10], indicating that schizophrenia patients, relatives of schizophrenia patients, and controls differed in the frequency composition of their EEG and that the differences varied by frequency and scalp region. A condition main effect emerged [F(1,130) = 11.68, P ≤ .001], reflecting that the eyes-closed condition elicited larger overall EEG amplitude than the eyes-open condition. Region was also a significant factor [F(4,127) = 24.08, P ≤ .001] along with a Band-by-Region interaction [F(24,107) = 6.95, P ≤ .001].
Given the main effects of Band, Region, and Condition, and several interactions between Band and other factors, we carried out separate MANCOVAs for each frequency component within each condition to elucidate the group effects across scalp regions. Table 2 presents results of statistical tests conducted for each frequency component for each condition. Figure 2 depicts the topography of group amplitude differences for EEG frequency components and follow-up statistical contrasts (For EEG indices showing effects involving group, we carried out analyses to determine the amount of variance accounted for by some subjects coming from the same family. Minimal variance was attributable to family membership, as indicated by intraclass correlations ranging from .02 for eyes-open slow alpha over occipital regions to .18 for eyes-open frontal gamma, with the mean correlation across analyses being .11. Mixed model analyses with family specified as a random variable yielded the same pattern of effects as those reported in table 2.).
Table 2.
Multivariate Tests of Resting EEG Frequency Band Abnormalities in Schizophrenia Patients, First-Degree Relatives of Schizophrenia Patients, Bipolar Disorder Patients, First-Degree Relatives of Bipolar Patients, and Nonpsychiatric Control Groups
| Schizophrenia, First-Degree Relatives, and Controls |
Bipolar, First-Degree Relatives, and Controlsa |
|||||||||||||||
| Eyes Closed |
Eyes Open |
Eyes Closed |
Eyes Open |
|||||||||||||
| Source | dfb,c | F | P Value | η2 | dfb,d | F | P Value | η2 | dfb,e | F | P Value | η2 | dfb,f | F | P Value | η2 |
| Delta | ||||||||||||||||
| Group | 2,152 | 3.19 | .04 | .04 | 2,161 | 4.12 | .02 | .05 | 2,116 | 0.48 | .62 | .01 | 2,130 | 0.43 | .65 | .01 |
| Group by Region | 8,298 | 1.92 | .06 | .05 | 8,316 | 0.50 | .86 | .01 | 8,226 | 1.04 | .41 | .03 | 8,254 | 0.99 | .44 | .03 |
| Theta | ||||||||||||||||
| Group | 2,152 | 4.26 | .02 | .05 | 2,161 | 17.99 | .001g | .18 | 2,116 | 0.38 | .68 | .01 | 2,130 | 2.04 | .13 | .03 |
| Group by Region | 8,298 | 1.69 | .10 | .04 | 8,316 | 2.64 | .008 | .06 | 8,226 | 0.23 | .98 | .01 | 8,254 | 0.69 | .70 | .02 |
| Slow alpha | ||||||||||||||||
| Group | 2,152 | 1.23 | .29 | .02 | 2,161 | 3.56 | .03 | .04 | 2,116 | 1.18 | .31 | .02 | 2,130 | 1.11 | .33 | .02 |
| Group by Region | 8,298 | 1.27 | .26 | .03 | 8,316 | 1.17 | .32 | .03 | 8,226 | 2.01 | .05 | .07 | 8,254 | 0.69 | .70 | .02 |
| Fast alpha | ||||||||||||||||
| Group | 2,152 | 1.04 | .35 | .01 | 2,161 | 1.03 | .36 | .01 | 2,116 | 0.35 | .71 | .01 | 2,130 | 0.70 | .50 | .01 |
| Group by Region | 8,298 | 1.58 | .13 | .04 | 8,316 | 2.12 | .03 | .05 | 8,226 | 1.92 | .06 | .06 | 8,254 | 2.36 | .02 | .07 |
| Slow beta | ||||||||||||||||
| Group | 2,152 | 0.36 | .70 | .01 | 2,161 | 2.68 | .07 | .03 | 2,116 | 0.32 | .73 | .01 | 2,130 | 0.02 | .98 | .00 |
| Group by Region | 8,298 | 0.74 | .65 | .02 | 8,316 | 2.36 | .02 | .06 | 8,226 | 0.79 | .61 | .03 | 8,254 | 1.95 | .05 | .06 |
| Fast beta | ||||||||||||||||
| Group | 2,152 | 0.62 | .54 | .008 | 2,161 | 1.95 | .15 | .02 | 2,116 | 1.66 | .19 | .03 | 2,130 | 1.24 | .29 | .02 |
| Group by Region | 8,298 | 1.67 | .11 | .04 | 8,316 | 3.41 | .001g | .08 | 8,226 | 1.51 | .16 | .05 | 8,254 | 1.05 | .40 | .03 |
| Gamma | ||||||||||||||||
| Group | 2,152 | 0.05 | .95 | .001 | 2,161 | 1.78 | .17 | .02 | 2,116 | 1.16 | .32 | .02 | 2,130 | 3.96 | .02 | .06 |
| Group by Region | 8,298 | 1.54 | .14 | .04 | 8,316 | 2.40 | .02 | .06 | 8,226 | 1.15 | .33 | .04 | 8,254 | 1.41 | .19 | .04 |
Note: MANCOVA results with gender included as a between-subjects factor and age as a covariate.
Gender was not included as a factor for bipolar group analyses because there were only 5 female bipolar patients.
df Wilk's Lambda adjusted where applicable.
Schizophrenia N = 43; relative N = 60; control N = 54.
Schizophrenia N = 40; relative N = 53; control N = 74.
Bipolar N = 29; relative N = 37; control N = 54.
Bipolar N = 26; relative N = 34; control N = 74.
Significant after Bonferroni correction within each condition.
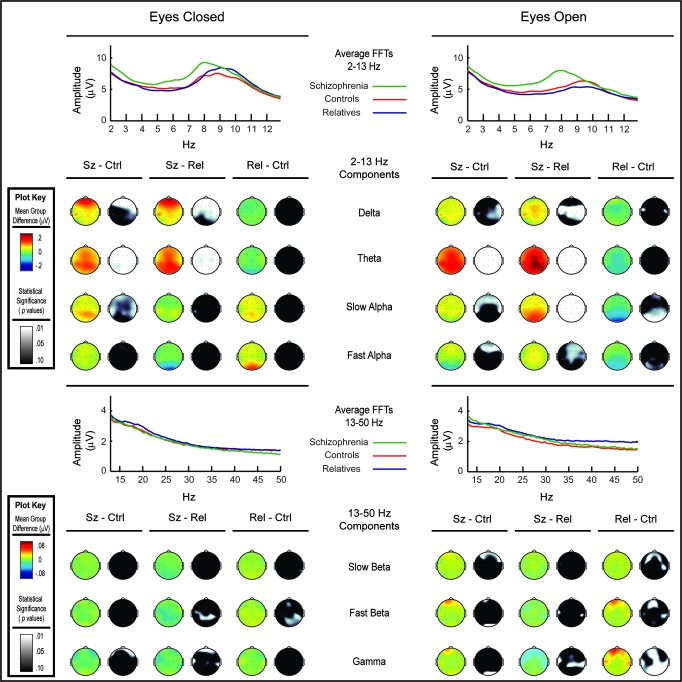
Fig. 2.
Group differences between the schizophrenia patients, their first-degree relatives, and nonpsychiatric controls for each frequency component and reported in frequency amplitude (μV). Results from eyes-closed and eyes-open conditions for low- and high-frequency decompositions are separately displayed. The average spectra for each group are also displayed. Topographical voltage difference maps highlight the amplitude differences between each group. A second set of topographical maps display P values obtained from simple effects statistical tests (ie, Wilcoxon nonparametric analyses).
The delta band component showed Group main effects in both eyes-open and eyes-closed conditions. A trend Group-by-Region interaction was observed in the eyes-closed condition. Follow-up contrasts revealed that group differences were due to elevated delta power in the schizophrenia patients. Figure 2 (top row) depicts schizophrenic patients’ increased amplitude compared with controls and their relatives, particularly at anterior sites in the eyes-closed condition.
The theta band component also showed main effects for Group in both eyes-open and eyes-closed conditions. A trend Group-by-Region interaction was observed in the eyes-closed condition, and a Group-by-Region interaction was observed in the eyes-open condition. Follow-up analyses revealed that group differences resulted from schizophrenia patients having increased theta power in both eyes-closed and eyes-open conditions. Figure 2 (row 2) highlights the schizophrenic patients’ increased amplitude compared with controls and their relatives, which is significant across the scalp in each condition, but maximal at posterior sites.
Slow alpha showed a main effect for Group in the eyes-open condition. Simple effects (see figure 2, row 3) revealed that at anterior sites, the schizophrenia patients had increased slow alpha amplitude compared with controls. Relatives of schizophrenia patients had a decrease in slow alpha power over occipital brain regions compared with controls and a decrease over the entire scalp when compared with the patients. Fast alpha showed a Group-by-Region interaction in the eyes-open condition. Follow-up analyses revealed that schizophrenia patients deviated from nonpsychiatric control subjects in their fast alpha activity by exhibiting increased amplitude, and their relatives trended toward a reduction in amplitude. Simple effects for the eyes-open condition (see figure 2, row 4) highlighted the patients’ increased amplitude at anterior sites compared with controls and increased amplitude at central sites compared with their relatives, while the relatives showed a slight decrease in amplitude over occipital sites.
The slow beta component for the eyes-open condition showed a trend toward a Group effect and a significant Group-by-Region interaction. Simple effects (see figure 2, row 5) revealed an increase in amplitude for the relatives compared with controls at frontal-temporal sites and an increase in amplitude for the patients compared with controls at the anterior sites. Additional tests for group differences in mean eyes-open slow beta activity at sites over frontal brain regions (ie, FP1, FP2, F7, F8, F3, F5, Fz) revealed that both patients [t(112) = 2.21, P = .03] and relatives [t(124) = 1.97, P = .05] differed from controls, and patients and relatives failed to differ from each other. The fast beta band component showed a Group-by-Region effect in the eyes-open condition. Relatives of schizophrenia patients generally exhibited greater fast beta activity than control subjects. Simple effects analyses (see figure 2, row 6) revealed that in the eyes-open condition, the relatives exhibited an increase in amplitude at anterior sites compared with controls. A t-test for beta activity for all frontal sites also revealed a significant difference between the relatives and controls [t(124) = 2.25, P = .03]. Although simple effects in the eyes-closed condition revealed the relatives had increased fast beta amplitude over right temporal-parietal sites compared with patients and controls, the group difference was not evident in the multivariate analysis. The gamma band component showed a Group-by-Region effect in the eyes-open condition. In the eyes-open condition, the relatives exhibited an increase in amplitude at anterior and temporal sites compared with controls (see figure 2, row 7) (To additionally control for the effects of gender, we carried out analyses with only males. Effects largely paralleled those found when both genders were included. The Group-by-Region effect was significant for eyes-open slow beta [F(8,170) = 2.54, P = .01], and the overall Group effect was nearly significant [F(2,88) = 2.89, P = .06]. Group effects for fast beta largely did not change when only males were included. The Group-by-Region trend for the eyes-closed condition failed to be significant [F(8,172) = 1.55, P = .14], but in the eyes-opened condition the Group-by-Region effect was significant [F(8,170) = 3.70, P = .001].).
Resting State EEG Abnormalities in Bipolar Patients and Their Relatives
To determine whether individuals with bipolar disorder, or those who likely carry genetic liability for bipolar disorder, manifest resting state functional brain abnormalities, we conducted analyses of the frequency composition and topography of EEG activity gathered from bipolar patients and their relatives during eyes-closed and eyes-open rest conditions. The omnibus analysis failed to reveal a main effect for Group, and Group failed to interact with Region, Band, or Condition. Nevertheless, we conducted follow-up MANCOVAs to fully test whether any EEG frequency abnormalities were associated with bipolar disorder. The only main effect for Group was in the eyes-open condition for the gamma component. Relatives of bipolar patients exhibited increased gamma amplitude compared with controls over anterior (control mean = .078, SD = .043; relative mean = .10, SD = .05 [t(106) = 2.17, P = .03]), temporal (control mean = .068, SD = .033; relative mean = .092, SD = .048 [t(106) = 2.64, P = .01]), parietal (control mean = .049, SD = .022; relative mean = .062, SD = .029 [t(106) = 2.28, P = .03]), and occipital regions (control mean = .046, SD = .02; relative mean = .057, SD = .026 [t(106) = 2.15, P = .04]). Relatives also showed increased gamma compared with the patients over temporal (patient mean = .068, SD = .027; relative mean = .092, SD = .048 [t(58) = 2.41, P = .02]) and parietal regions (patient mean = .049, SD = .016; relative mean = .062, SD = .029 [t(58) = 2.12, P = .04]). Of the multiple comparisons, the only one that survived Bonferonni correction was increased eyes-open gamma over temporal sites in relatives of bipolar patients compared with controls.
Despite Group-by-Region interactions that were significant or nearly significant for eyes-closed slow alpha and fast alpha, and eyes-open slow beta components, only modest effects were found. All alpha and slow beta effects in the bipolar disorder analysis failed to survive correction for the multiple comparisons. Thus, no EEG significant abnormalities were observed in bipolar patients, and the only resting state EEG abnormality evident in relatives of bipolar patients was increased gamma band activity during the eyes-open condition.
Symptom and COMT Genotype Associations With Abnormal Resting State EEG Frequency Composition in Schizophrenia
Lifetime negative symptomatology for schizophrenia patients was associated with the presence of theta band abnormalities in schizophrenia patients during the eyes-closed condition. Significant correlations were observed at anterior (r = .43, P = .004), central (r = .43, P = .004), parietal (r = .41, P = .007), temporal (r = .31, P = .04), and occipital (r = .45, P = .003) scalp sites, indicating that greater negative symptomatology predicted augmented theta frequencies. No other symptom indices were associated with frequency abnormalities in schizophrenia. There were no associations between indices of schizotypal characteristics (ie, SIS or SPQ scores) and EEG frequency abnormalities within the relatives of schizophrenia.
Because the COMT gene is a candidate susceptibility gene for schizophrenia that has been associated with abnormalities in brain function in schizophrenia, we carried out analyses to examine whether frequency abnormalities in the disorder were related to the Val158Met polymorphism of the gene. Analyses were focused on those EEG indices that showed differences between schizophrenia patients, their relatives, and control subjects. Table 3 presents mean amplitudes of frequency components showing significant COMT genotype effects for schizophrenia patients. Also reported are results of ANOVAs conducted to test for genotype effects within the schizophrenia patients for each frequency band and scalp region. MANCOVAs of the delta component revealed main effects of Genotype in the eyes-closed [F(2,38) = 8.89, P ≤ .001, η2 = .32] and eyes-open [F(2,35) = 3.85, P = .03, η2 = .18] conditions, indicating that the increased delta activity in schizophrenia patients was associated with the Val158Met polymorphism of the COMT gene. In the eyes-closed condition, a Region-by-Genotype interaction was observed [F(8,70) = 2.64, P = .03, η2 = .21]. Follow-up tests indicated that Met homozygote schizophrenia patients exhibited more delta activity than their Val homozygote and heterozygote counterparts, as well as greater delta activity than controls. Val homozygote and heterozygote patients showed similar levels of eyes-closed delta as control subjects. For the eyes-open condition, Met homozygote patients had more delta activity than heterozygote patients and control subjects, but there were no differences with the Val homozygotes, suggesting that the association was weaker than that observed in the eyes-closed condition.
Table 3.
Low-Frequency EEG components in schizophrenia patients by Val158Met genotype of the catechol-O-methyl transferase (COMT) gene
| COMT Val158Met Genotype |
|||||||
| SZ |
Controls |
||||||
| Val/Val |
Val/Met |
Met/Met |
All Genotypes |
||||
| Condition and Band | Region | Frequency-Amplitude | Frequency-Amplitude | Frequency-Amplitude | ANOVA, Statistic F | Effect Size, η2 | Frequency-Amplitude |
| Eyes-closed delta | Frontal | .44 (.10)a,b | .51 (.16)a,b,c | .71 (.27)b,c | 6.88** | .26 | .44 (.12) |
| Temporal | .31 (.07)a,b | .32 (.09)a,b | .44 (.15)b,c | 5.99** | .23 | .30 (.09) | |
| Central | .47 (.10)a,b | .48 (.13)a,b | .68 (.20)b,c | 8.05*** | .29 | .47 (.16) | |
| Parietal | .41 (.08)a,b | .40 (.10)a,b | .57 (.15)b,c | 8.74*** | .31 | .40 (.13) | |
| Occipital | .42 (.09)a,b | .41 (.11)a,b | .59 (.15)b,c | 8.40*** | .30 | .42 (.16) | |
| Eyes-closed theta | Frontal | .43 (.24) | .32 (.14)a,b | .52 (.16)b,c | 4.62* | .19 | .32 (.22) |
| Temporal | .33 (.18) | .24 (.11)a,b | .37 (.14)b,c | 3.70* | .16 | .23 (.13) | |
| Central | .51 (.28) | .36 (.16)a,b | .61 (.19)b,c | 5.31** | .21 | .37 (.25) | |
| Parietal | .56 (.38) | .34 (.18)a,b | .52 (.18)b,c | 3.61* | .16 | .33 (.22) | |
| Occipital | .57 (.39)c | .36 (.21) | .49 (.14) | 2.50 | .11 | .36 (.29) | |
| Eyes-open slow alpha | Frontal | .21 (.07)a | .19 (.08)a,b | .30 (.10)b,c | 5.71** | .24 | .19 (.09) |
| Temporal | .18 (.07)a | .16 (.08)a,b | .26 (.09)b,c | 4.67* | .21 | .15 (.07) | |
| Central | .26 (.11) | .22 (.09)a,b | .36 (.15)b,c | 5.32** | .23 | .24 (.12) | |
| Parietal | .30 (.14) | .23 (.12)a | .43 (.23)b,c | 5.13* | .22 | .27 (.16) | |
| Occipital | .31 (.18) | .26 (.16)a | .47 (.33) | 2.84 | .14 | .32 (.23) | |
Note: Frequency amplitude data are presented as mean (standard deviation) in microvolts and represent the magnitude of the EEG component derived from the product of the weighted principal components and the frequency spectrum for each subject.
SZ, schizophrenia patients. η2 represents the effect size of genotype from the ANOVA.
Val/Val genotype n = 11 in both conditions, Met/Met genotype n = 11 in both conditions, Val/Met n = 20 in eyes-closed condition, and 17 in eyes open.
Hardy-Weinberg equilibrium: eyes-close chi-square = 0.09, P = .76; eyes-open, chi-square = 0.64, P = .42 *P < .05; **P ≤ .01; ***P ≤ .001.
Differs from SZ Met/Met.
Significant after Bonferroni correction.
Differs from nonpsychatric controls.
A COMT Genotype effect was evident for theta in both eyes-closed [F(2,38) = 4.39, P = .02, η2 = .19] and eyes-open [F(2,35) = 4.40, P = .02, η2 = .20] conditions. Significant Region-by-Genotype interactions were also present for both eyes-closed [F(8,70) = 2.23 P = .03, η2 = .20] and eyes-open [F(8,64) = 2.72, P = .01, η2 = .25] conditions. Although Met homozygote patients only showed greater theta activity than heterozygote patients, Met homozygotes exhibited an increase in theta activity in both conditions compared with controls. Heterozygote and Val homozygote patients did not differ from controls on either condition. There was also a main effect of COMT genotype for the slow alpha component in the eyes-open condition [F(2,35) = 4.49, P = .02, η2 = .20]. Met/Met patients exhibited more slow alpha activity at frontal and temporal sites than Val/Val and Val/Met patients. Met homozygotes also had more slow alpha activity than controls, while Val homozygotes and heterozygotes showed normative levels of slow alpha during eyes open (see table 3). There were no significant COMT genotype effects for high-frequency components, and associations between EEG frequency abnormalities and the Val158Met polymorphism were absent in first-degree relatives of schizophrenia patients.
Discussion
Results indicate that resting state EEG frequency abnormalities are evident in schizophrenia patients and their biological relatives but are largely absent in bipolar disorder patients and their biological relatives. High-frequency resting state EEG abnormalities may serve as an indicator of genetic liability specific to schizophrenia (ie, endophenotype). Relatives of schizophrenia patients exhibited more beta and gamma activity over frontal brain regions than nonpsychiatric control subjects during the eyes-open rest condition. Although relatives of bipolar disorder patients also exhibited excess gamma activity at similar electrode sites in the same condition, augmented beta activity was specific to the relatives of schizophrenia patients. Schizophrenia patients also exhibited increased anterior beta activity. Lower frequency (delta, theta, and alpha) abnormalities were evident in schizophrenia patients, while relatives of schizophrenia patients generally showed normal low-frequency composition of their resting state EEGs. In the eyes-closed condition, schizophrenia patients exhibited augmented delta activity over frontal brain regions, increased theta power over the entire scalp, and increased slow alpha activity over temporal and frontal regions. Similar EEG power abnormalities were evident in schizophrenia patients in the eyes-open condition, but increased delta activity was not focused over frontal brain regions and higher frequency alpha activity was augmented over frontal cortex. Importantly, the Val158Met polymorphism of the COMT gene was associated with low-frequency abnormalities in schizophrenia patients. Met homozygote schizophrenia patients exhibited greater delta, theta, and alpha activity than what was observed in control subjects and schizophrenia patients with COMT Val158Met genotypes containing the Val allele.
Results of the present investigation build on other studies documenting increased resting state high-frequency activity over frontal brain regions in relatives of schizophrenia patients15,17 by demonstrating that beta frequency EEG abnormalities are specific to schizophrenia patients and their relatives as compared individuals with another heritable and severe mental disorder (ie, bipolar disorder). Based on findings in children of parents with schizophrenia, Itil12 proposed that increased high-frequency beta may serve as an indicator of genetic liability for schizophrenia. In a study of siblings’ absent current psychopathology, Winterer et al17 identified augmented beta activity over frontal and temporal areas as well as posterior scalp regions in a subsample of siblings. A second study of unaffected relatives found elevated beta across the scalp in parents of schizophrenia patients and specifically in frontal and temporal scalp sites in younger relatives.15 The present investigation also revealed gamma activity to be most prominent over frontal and temporal regions, and it was these sites where relatives of schizophrenia patients exhibited augmented activity. The only other study examining gamma activity during rest failed to identify abnormalities in patients or siblings; however, the data were derived from an eyes-closed condition and reflected gamma frequencies lower than those included in the data-driven characterization of gamma in the present study.17
Thus, there is growing evidence that augmented beta activity over frontal and temporal brain regions reflects genetic liability for schizophrenia, with the present study indicating that the abnormality may be specific to schizophrenia amongst severe mental disorders and extends to higher frequencies that fall into the gamma range. Increased high-frequency activity in schizophrenia patients and their relatives have been interpreted as possibly reflecting cortical hyperexcitability. Nevertheless, cortical gamma oscillations are thought to be produced independent of external stimulation by GABAergic interneurons that are in mutual inhibition through postsynaptic potentials that oscillate around 40 Hz.47 Hence, gamma is thought to reflect inhibition of cortical neurons. A second theory posits that gamma frequency thalamic oscillations are synchronous with cortical oscillations in the presence of stimuli48; however, given the resting state of subjects in the present study, the current findings may be reflective of inhibitory activity of interneurons while the brain is in “default mode.”49
For several decades, investigators have consistently observed increases in delta and theta activity in schizophrenia patients.8,9,13,14,18–20,24 The present study provides evidence that delta anomalies in schizophrenia are most evident over frontal brain regions in the eyes-closed resting condition. Our findings also point to augmented theta activity in schizophrenia being clearly evident across the scalp regardless of whether the eyes are closed or open during the resting state. Finally, the absence of delta and theta frequency abnormalities in bipolar disorder patients provides evidence for the augmentation of the low EEG frequencies as specific to the pathophysiology of schizophrenia. Because we found the augmentation of delta and theta activity to be only evident in the Met homozygote group of schizophrenia patients, it may be that schizophrenic pathophysiology is related to resting state functional brain abnormalities through the dopamanergic effects of the COMT Val158Met polymorphism. The absence of an association in relatives of schizophrenia may have to do with the mechanism by which the COMT polymorphism is expressed in the development of schizophrenia. A current hypothesis about the influence of COMT on dopamanergic activity in nucleus accumbens and prefrontal cortex includes that the Met allele leads to increased tonic dopamine but reduced phasic dopamine, resulting in negative symptoms and pathophysiologic inflexibility.50 Consistent with this hypothesis, the present study revealed lifetime negative symptomatology in schizophrenia was associated with increased theta band activity. In this sample of schizophrenia patients, we have found the Met allele of the COMT Val158Met polymorphism is also directly associated with greater lifetime negative symptomatology.51
Although alpha activity is typically less prominent when the eyes are open, it was in this condition that the schizophrenia patients showed augmented slow and fast alpha activity over frontal brain regions. Slow alpha activity was also associated with the COMT Val158Met polymorphism with Met homozygote schizophrenia patients exhibiting an elevation in slow alpha activity compared with control subjects and schizophrenia patients of other genotypes. Despite being perhaps the most studied EEG rhythm, the neural basis for alpha activity is unknown. Therefore, it is difficult to conclude much more than that deviant corticothalamic interactions are evident in schizophrenia. Other investigations have yielded decreases in alpha activity in schizophrenia patients, rather than increases13,14,53; however, variable findings may be attributable to whether or not frequency band powers were normalized and differences in sample characteristics such as age.13,14,16,19
There are several limitations to the present study. Nearly all patients were on psychotropic medications. Although our statistical tests indicated that EEG findings were not an artifact of medications, dependent variables could be influenced by medications, thereby diluting effects. We did find those individuals taking novel antipsychotics tended to have increased theta, alpha, and slow beta activity. Thus, this is one possible explanation for schizophrenia patients exhibiting augmented slow alpha activity over frontal regions in the eyes-open condition while relatives of schizophrenia patients showed diminished slow alpha over posterior regions. Another potential study limitation is the relatively low number of females in the schizophrenia and bipolar disorder groups. Although analyses showed that results were not due to variable gender composition across groups, a low number of women limits the generalizability of findings in the patients. Also, inclusion of some individuals with histories of alcohol or substance dependence may confound results; however, analyses indicated that history of dependence failed to be associated with EEG anomalies noted in the study. Unlike reports of beta abnormalities associated with substance dependence,54 the reported augmented beta activity does not appear to be gender specific, is only reported as significant in the eyes-open condition, and is located at frontal-temporal recording sites. Augmented beta rhythms may serve as an indicator for both the risk of alcoholism and schizophrenia, but are separable in scalp topography and the conditions in which they are observed. See Boutros et al55 for a recent review of spectral EEG characteristics as a diagnostic test for schizophrenia.
Finally, in the present study, we used PCA to derive frequency components of resting state EEGs rather than using traditionally identified frequency bands. The identified orthogonal components generally agreed with typically employed frequency cut-offs of bands, but also confirmed the need to separate alpha and beta activity into their slow and fast elements. Specifically, low-frequency EEG components consisted of delta (2–4 Hz maximum), theta (7–8 Hz maximum), slow alpha (8–9 Hz maximum), and fast alpha (10–11 Hz maximum). High-frequency EEG components were slow beta (13–15 Hz maximum), fast beta (23–25 Hz maximum), and gamma (maximal between 35 and 50 Hz). The frequency components were highly similar across eyes-closed and eyes-open conditions, suggesting that the fundamental structure of the EEG is stable across resting state conditions.
To conclude, we found evidence that high-frequency activity in resting state EEGs may function as an endophenotype for schizophrenia. Schizophrenia patients and first-degree biological relatives of schizophrenia patients exhibited increased beta activity in their resting state EEGs during an eyes-open condition. Excessive EEG high frequencies in schizophrenia may relate to anomalies of the prefrontal cortex that are neural manifestations of genetic liability for the disorder. The present study also provides evidence for augmented low frequencies in resting state EEGs of schizophrenia patients as reflective of the disorder's pathophysiology. Consistent with the presence of disorder-specific resting state functional brain abnormalities, schizophrenia patients exhibited increased activity in delta, theta, and alpha ranges, while similar anomalies were absent in bipolar disorder patients. Delta, theta, and alpha EEG frequency abnormalities were also associated with the Met allele of the COMT Val158Met polymorphism and thus supportive of the COMT gene and dopamanergic functions affecting abnormal resting brain states in schizophrenia.
Funding
Department of Veterans Affairs, Medical Research Service, the Mental Illness and Neuroscience Discovery Institute; National Institutes of Mental Health (5R24MH069675) to S.R.S.; Mental Health Patient Service Line at the Veterans Affairs Medical Center, Minneapolis, MN.
Acknowledgments
Portions of this study were presented at the 45th Annual Meeting of the Society for Psychophysiological Research in Lisbon, Portugal, September 2005. We gratefully acknowledge John J. Stanwyck, Sarah M. Sass, and Robb Hunter for collection of electroencephalography data, and Tricia Bender and Dr. Laurie Shekels for genotyping of specimens.
References
- 1.Gottesman II, Gould T. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 2.Braff DL, Freedman R, Shork NJ, Gottesman II. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrettini WH. Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry. 2000;48:531–538. doi: 10.1016/s0006-3223(00)00883-0. [DOI] [PubMed] [Google Scholar]
- 4.Craddock N, O'Donnovan MC, Own MJ. Genes for schizophrenia and Bipolar Disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramon E, Sham PC. The common genetic liability between schizophrenia and bipolar disorder: a review. Curr Psychiatry Rep. 2001;3:332–337. doi: 10.1007/s11920-001-0030-1. [DOI] [PubMed] [Google Scholar]
- 6.Lund TR, Sponheim SR, Iacono WG, Clementz BA. Internal consistency reliability of resting EEG power spectra in schizophrenic and normal subjects. Psychophysiology. 1995;32:66–71. doi: 10.1111/j.1469-8986.1995.tb03407.x. [DOI] [PubMed] [Google Scholar]
- 7.Lykken DT, Tellegen A, Iacono WG. EEG spectra in twins: evidence for a neglected mechanism of genetic determination. Physiol Psychol. 1982;10:60–65. [Google Scholar]
- 8.Iacono WG. Bilateral electrodermal habituation-dishabituation and resting EEG in remitted schizophrenics. J Nerv Ment Dis. 1982;170:91–101. doi: 10.1097/00005053-198202000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Stassen H, Coppola R, Gottesman II, et al. EEG differences in monozygotic twins discordant and concordant for schizophrenia. Psychophysiology. 1999;36:109–117. doi: 10.1017/s0048577299970713. [DOI] [PubMed] [Google Scholar]
- 10.van-Beijsterveldt CEM, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Hum Genet. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- 11.Zietsch BP, Hansen JL, Hansell NK, Geffen GM, Martin NG, Wright MJ. Common and specific genetic influences on EEG power bands delta, theta, alpha, and beta. Biol Psychol. 2007;75:154–164. doi: 10.1016/j.biopsycho.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Itil TM. Qualitative and quantitative EEG findings in schizophrenia. Schizophr Bull. 1977;3:61–79. doi: 10.1093/schbul/3.1.61. [DOI] [PubMed] [Google Scholar]
- 13.Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994;31:37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 14.Sponheim SR, Clementz BA, Iacono WG, Beiser M. Clinical and biological concomitants of resting state EEG power abnormalities in schizophrenia. Biol Psychiatry. 2000;48:1088–1097. doi: 10.1016/s0006-3223(00)00907-0. [DOI] [PubMed] [Google Scholar]
- 15.Alfimova M, Uvarova L. Cognitive peculiarities in relatives of schizophrenic and schizoaffective patients: heritability and resting EEG-correlates. Int J Psychophysiol. 2003;49:201–216. doi: 10.1016/s0167-8760(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 16.Clementz BA, Sponheim SR, Iacono WG, Beiser M. Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology. 1994;31:486–494. doi: 10.1111/j.1469-8986.1994.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 17.Winterer G, Egan MF, Radler T, Hyde T, Coppola R, Weinberger DR. An association between reduced interhemispheric EEG coherence in the temporal lobe and genetic risk for schizophrenia. Schizophr Res. 2001;49:129–143. doi: 10.1016/s0920-9964(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 18.Kahn EM, Weiner RD, Coppola R, Kudler HS, Schultz K. Spectral and topographic analysis of EEG in schizophrenia patients. Biol Psychiatry. 1993;33(4):284–290. doi: 10.1016/0006-3223(93)90296-p. [DOI] [PubMed] [Google Scholar]
- 19.Sponheim SR, Iacono WG, Clementz BA, Beiser M. Season of birth and electroencephalogram power abnormalities in schizophrenia. Biol Psychiatry. 1997;(41):1020–1027. doi: 10.1016/S0006-3223(96)00184-9. [DOI] [PubMed] [Google Scholar]
- 20.Williamson P, Mamelak M. Frontal spectral EEG findings in acutely ill schizophrenics. Biol Psychiatry. 1987;22:1021–1024. doi: 10.1016/0006-3223(87)90011-4. [DOI] [PubMed] [Google Scholar]
- 21.Hill K, Mann L, Laws K, Stephenson C, Nimmo-Smith I, McKenna P. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004;110:243–256. doi: 10.1111/j.1600-0447.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto T, Takeuch K, Matsumoto T, et al. Abnormal glucose metabolism in the anterior cingulate in patients with schizophrenia. Psychiatry Res. 2007;154:49–58. doi: 10.1016/j.pscychresns.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Gattaz WF, Mayer S, Ziegler P, Platz M, Gasser T. Hypofrontality on topographic EEG in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1992;241:328–332. doi: 10.1007/BF02191956. [DOI] [PubMed] [Google Scholar]
- 24.Wuebben Y, Winterer G. Hypofronality—a risk marker related to schizophrenia? Schizophr Res. 2001;48:207–217. doi: 10.1016/s0920-9964(00)00047-5. [DOI] [PubMed] [Google Scholar]
- 25.Fehr T, Kissler J, Moratti S, Wienbruch C, Rockstroh B, Elbert T. Source distribution of neuromagnetic slow waves and MEG-delta activity in schizophrenic patients. Biol Psychiatry. 2001;50:108–116. doi: 10.1016/s0006-3223(01)01122-2. [DOI] [PubMed] [Google Scholar]
- 26.Fehr T, Kissler J, Wienbruch C, et al. Source distribution of neuromagnetic slow-wave activity in schizophrenia patients—effects of activation. Schizophr Res. 2003;63:63–71. doi: 10.1016/s0920-9964(02)00213-x. [DOI] [PubMed] [Google Scholar]
- 27.Shagass C, Roemer RA, Straumanis JJ. Relationship between psychiatric diagnosis and some quantitative EEG variables. Arch Gen Psychiatry. 1982;39:1423–1435. doi: 10.1001/archpsyc.1982.04290120053011. [DOI] [PubMed] [Google Scholar]
- 28.Williamson PC, Kaye H. EEG mapping applications in psychiatric disorders. Can J Psychiatry. 1989;34:680–686. doi: 10.1177/070674378903400710. [DOI] [PubMed] [Google Scholar]
- 29.Mientus S, Gallinat J, Wuebben Y, et al. Cortical hypoactivation during resting EEG in schizophrenics but not in depressives and schizotypal subjects as revealed bu low resolution electromagnetic tomography (LORETA) Psychiatry Res. 2002;116:95–111. doi: 10.1016/s0925-4927(02)00043-4. [DOI] [PubMed] [Google Scholar]
- 30.Weinbruch C, Moratti S, Elbert T, et al. Source distribution of neuromagnetic slow wave activity in schizophrenic and depressive patients. Clin Neurophysiol. 2003;114:2052–2060. doi: 10.1016/s1388-2457(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 32.Nurnberger JI, Jr, Blehar M, Kaufmann C, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–864. [DOI] [PubMed] [Google Scholar]
- 33.Lukoff D, Neuchterlein K, Ventura J. Manual for the expanded brief psychiatric rating scale. Schizophr Bull. 1986;12:594–602. [Google Scholar]
- 34.First M, Spitzer R, Gibbon M, Williams J. Biometric Research Department. New York, NY: New York State Psychiatric Institute; 1996. Structured clinical interview for DSM-IV axis I disorders (SCID-I, research version) [Google Scholar]
- 35.Kendler K, Lieberman J, Walsh D. The structured interview for schizotypy (SIS): a preliminary report. Schizophr Bull. 1989;15:559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- 36.First M, Gibbon M, Spitzer R, Williams J, Benjamin L. New York, NY: Biometric Research Department, New York State Psychiatric Institute; 1997. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II) [Google Scholar]
- 37.Ekselius L, Lindstrom E, Knorring Lv, Bodlund O, Kullgren G. SCID II interviews and the SCID screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1994;90:120–123. doi: 10.1111/j.1600-0447.1994.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 38.Raine A, The SPQ. a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- 39.Leckman J, Sholomskas D, Thompson W, Belanger A, Weissman M. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 40.Sponheim SR, Steele V, McGuire KA. Verbal memory processes in schizophrenia patients and biological relatives of schizophrenia patients: intact implicit memory, impaired explicit recollection. Psychiatry Res. 2004;71(2–3):339–348. doi: 10.1016/j.schres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Sponheim SR, McGuire KA, Stanwyck JJ. Neural anomalies during sustained attention in first-degree biological relatives of schizophrenia patients. Biol Psychiatry. 2006;60:242–252. doi: 10.1016/j.biopsych.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 42.McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychoti illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48(8):764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- 43.Serretti A, Olgiati P. Dimensions of major psychoses: a confirmatory factor analysis of six competing models. Psychiatry Res. 2004;127(1–2):101–109. doi: 10.1016/j.psychres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Bergman-Jungestrom M, Wingren S. Catechol-O-Methyltransferase (COMT) gene polymorphism and breast cancer risk in young women. Br J Cancer. 2001;85(6):859–862. doi: 10.1054/bjoc.2001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andreassi JL. Psychophysiology: Human Behavior and Physiological Response. Mahaw, NJ: Lawrence Erlbaum Associates Inc.; 2000. [Google Scholar]
- 46.Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Tassinary LG, Bernston GG, editors. Handbook of Psychophysiology. 2nd ed. New York, NY: Cambridge University Press; 2000. pp. 27–52. [Google Scholar]
- 47.Traub RD, Jefferys JGR, Whittington MA. Fast Oscillations in Cortical Circuits. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 48.Steriade M, Contreras D. Synchronization of fast (30-40Hz) spontaneous cortical rhythms during brain activation. J Neurosci. 1996;16:392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-o-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric reports. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 51.Goghari VM, Sponheim SR. Associations between the COMT polymorphism and symptom dimensions in schizophrenia. Schizophr Bull. 2007;33:280. [Google Scholar]
- 52.Brooker BH, Cyr JJ. Tables for clinicians to use to convert WAIS-R short forms. J Clin Psychol. 42:983. [Google Scholar]
- 53.Knott V, Labelle A, Mahoney C. Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Psychiatry Res. 2001;30:41–53. doi: 10.1016/s0920-9964(00)00165-1. [DOI] [PubMed] [Google Scholar]
- 54.Rangaswamy R, Porjesz B, Chorlian BD, et al. Resting EEG in offspring of male alcoholics: beta frequencies. Int J Psychophysiol. 2004;51:239–251. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono WG. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008;99:225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]