Abstract
The earliest stages of delusion are characterized by an overabundance of meaningful coincidences impinging on the sufferer's existing worldview. Successive events are seen by him as pointing to, and then confirming, a fundamentally new reality that takes him over and engulfs his everyday life. Research over the last 4 decades has revealed the importance of dopamine (DA), D2 receptors, and the basal ganglia in psychotic thinking. Recent work has implicated the aberrant reward learning initiated by the excess release of striatal DA in the attribution of excessive importance or “salience” to insignificant stimuli and events. But our knowledge of what is happening beyond D2 receptors has remained scant. The gap is especially apparent at the cellular and microcircuit levels, encompassing the plastic changes, which are believed to be essential for new learning, and whose processes may go awry in major mental illness. Now new pharmacological findings are advancing our understanding of information processing and learning within the striatum. DA has an important role in setting the strength of individual striatal connections, but it does not act in isolation. Two other modulator systems are critical, the endocannabinoids and adenosine. Thus, at medium spiny neurons belonging to the indirect pathway, D2 stimulation evokes endocannabinoid-mediated depression of cortical inputs. Adenosine acting at A2A receptors elicits the opposite effect. Remarkably, drugs that target the endocannabinoid and purinergic systems also have pro- or antipsychotic properties. Here, we discuss how the 3 modulators regulate learning within the striatum and how their dysfunction may lead to delusional thinking.
Keywords: psychosis, schizophrenia, dopamine, endocannabinoid, glutamate, GABA
Introduction
“Since time immemorial delusion has been taken as the basic characteristic of madness. To be mad was to be deluded and indeed what constitutes a delusion is one of the basic problems of psychopathology”. Jaspers (1913)
Trainee psychiatrists are well versed in citing the criteria by which beliefs are judged to be delusional. Later, as their practice develops, some questions become less easy to answer. Patients’ relatives often ask about the nature of the illness: “Has something gone wrong in the brain? Are the drugs involved? What does the medicine do?” How does one answer? Possibly, an explanation in terms of “excess dopamine (DA)” might be offered, much in the same way that the physician talks about “narrowing of the arteries.” It has been suggested that psychosis stems from a psychological state of aberrant salience, which itself arises from excessive stimulation of DA D2 receptor proteins in the corpus striatum.1
The present article explores how neuroscience has uncovered the details of information processing within the striatum. Initially, we outline the role of the basal ganglia within the central nervous system as a whole. Next, the focus is on the intricacies of striatal learning. Finally, based on how various small molecules affect cell signaling within the striatum, we describe how neuroscience is beginning to reveal the physical foundations of delusional thinking.
A Brief Tour of the Functional Anatomy of the Basal Ganglia
There is a massive excitatory projection from the whole neocortex and the limbic system into the basal ganglia.2 Information is funneled through the striatum and pallidum/substantia nigra and then ultimately returned to the cortex as positive or negative feedback. Three major parallel loops—motor, cognitive, and affective—have been described. It was initially held that information within different loops was kept distinct, but integration at many levels has now been demonstrated.2,3
The basal ganglia have 2 general roles.4,5 On the one hand, they are crucial for the selection and initiation (termed “embodiment”) of a particular psychomotor behavior.6,7 Assuming that at any one time a mass of different inputs bombards the striatum, it is feasible that the “loudest call wins out,” and competing inputs are suppressed.6,8 On the other hand, the basal ganglia are necessary for associative, categorical, and sequence learning.9,10 In this case, different inputs are combined and laid down as a new memory trace, which serves as the basis for habitual thoughts and behavior.4 Following a normal developmental trajectory, the striatum seamlessly implements these 2 apparently conflicting functions within a single architecture.
Previously, it was assumed that the striatum was the recipient of learning that had already taken place in the cortex. It has been shown however, that for associative learning, modifications in the striatum occurred before those in the cortex, suggesting that the basal ganglia “inform” the higher cortices about new associations rather than the other way around.11,12 Three aspects of basal ganglia–dependent learning are relevant to the formation of delusional beliefs. First, in comparison to systems based in the medial temporal lobe, learning is “slow,” requiring multiple reiterations. Second, once developed however, new traces become strongly ingrained (habitual). Third, basal ganglia–dependent learning is largely implicit (unconscious).5
Cortically derived fibers traverse the striatum as a longitudinally arranged band (figure 1a). Each fiber forms excitatory synapses with thousands of medium spiny neurons (MSNs), of which there are approximately 100 million in humans.2 Individual MSNs receive input from about approximately 20 000 different cortical neurons. In comparison to most other neurons, spiking (action-potential firing) in MSNs is a rare event, requiring the convergent drive of multiple cortical inputs.2,4,13 As the lone striatal output neuron, the MSN is in a pivotal position for the embodiment of behavior. MSN fibers converge on the much smaller population of pallidal/nigral neurons (approximately 600 000) permitting further integration of information from disparate cortical sources.2,4 As well as targeting the output structures of the basal ganglia, recurrent collaterals provide inhibitory input to the dendrites of other MSNs. A “supporting cast” of interneurons and brain stem–derived fibers, including those containing DA, modulate MSNs and their cortical inputs.2
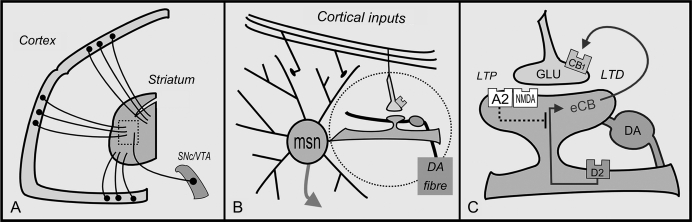
Fig. 1.
Bidirectional Modification of Corticostriatal synapses. Plasticity within the striatum is believed to underlie the development of habitual patterns of thought and behavior. A. The cortex sends a massive projection to the striatum. Neurons in the substantia nigra pars compacta (SNc) and adjacent ventral tegmental area (VTA) provide the neuromodulator dopamine. B. At the medium spiny neuron (MSN), cortical fibers form glutamate synapses with the heads of dendritic spines and dopamine-containing varicosities lie at the spine neck. C. Corticostriatal connections can be strengthened (long-term potentiation, LTP) or weakened (long-term depression, LTD). At MSNs belonging to the indirect pathway, dopamine (DA) acting at D2 receptors promotes LTD, which is mediated via retrograde endocannabinoid (eCB) signaling at presynaptic CB1 receptors. LTP is driven by adenosine at A2A receptors and glutamate N-methyl-D-aspartic acid receptors. Drugs that promote LTD (or block LTP) have propsychotic properties. Drugs promoting LTP are antipsychotic.
The Nuts and Bolts of Striatal Learning
Cortical fibers form glutamate synapses on the spine heads of MSNs while DA-containing varicosities are found at the necks of spines, where they provide modulation (Figure 1b & 1c).2,14 The MSN population is categorized into 2 groups, which express different types of DA receptor: direct pathway MSNs express D1 receptors and indirect pathway MSNs express D2 receptors.2 In the discussion here, we focus mainly on the indirect pathway because D1 antagonists have no discernable antipsychotic properties.15,16
The brain stem DA neurons supplying the striatum have 3 firing modes, tonic (low frequency), phasic (high frequency), and short, silent periods. In tonic mode, extracellular concentrations of DA in the striatum are sufficient to activate D2 but not D1 receptors, whereas D1 receptor activation requires phasic DA release.2 “Real-world” events influence the firing rate of DA neurons. Tonic mode appears to be essential for the embodiment of psychomotor behavior, while phasic DA is believed to provide a training signal.9 A rapid switch into phasic mode is elicited by unexpected reward, predictors of reward, or salient stimuli; a short pause in firing occurs when an anticipated reward fails to materialize.17 It is thought that phasic DA, and pauses in firing, instruct the striatal circuits to update themselves (learn).18,19
Over time, the striatal circuitry is modified by experience. Corticostriatal connections that are strong because they have been reinforced in the past will be more likely to contribute to future psychomotor behaviors. As well as DA, 2 additional modulators, adenosine and the endocannabinoids (eCBs), are critical in striatal learning.14
It has now been shown that bidirectional corticostriatal plasticity occurs at both MSN classes.20 If a cortical volley is successful in triggering a spike in an MSN, then the individual synapses that contributed are “tagged” for change. Cortical inputs that arrive in the immediate aftermath of an MSN spike are similarly tagged. Once a synapse has been tagged, DA's role is in determining the direction of change.20 Crucially, untagged synapses remain unchanged regardless of extracellular DA levels. This ensures that plasticity is confined to active synapses, while the rest of the network remains constant. Active synapses can either be strengthened (long-term potentiation, LTP) or weakened (long-term depression, LTD) depending on which DA receptor type is stimulated.20
Briefly, at tagged synapses belonging to the direct pathway, D1 receptor stimulation is a requirement for LTP. If DA is absent, LTD occurs by default. At tagged synapses belonging to the indirect pathway, the presence of DA at D2 receptors is essential for LTD. Stimulation of the adenosine A2A receptor (which ”substitutes” for the nonexpressed D1 receptor) can overpower LTD, and trigger LTP (Figure 1c).20 The elucidation of these learning rules has considerably advanced our understanding of striatal functioning.
At corticostriatal synapses, LTP and LTD are in dynamic opposition. Under tonic DA conditions LTD appears to prevail, and this applies to both classes of MSN. Corticostriatal LTD is mediated by retrograde eCB signaling.14,20–22 The eCBs are synthesized in, and released from, the dendritic spines of MSNs.23 They act at CB1 receptors on the terminals of cortical inputs, inhibiting the pre-synaptic release of glutamate.14 At MSNs of the indirect pathway, LTD is not just an electrophysiological curiosity, but has clinical implications. In animal models of Parkinson's disease, which deplete DA, LTD is lost. Treatment with anti-parkinsonian drugs (direct D2 agonists) restores LTD and motor functioning. Drugs that inhibit eCB breakdown significantly augmented the anti-parkinsonian benefits of D2 agonists.24 The same framework can be used to describe the mechanisms of pro- and anti-psychotic drugs.
The Mechanisms of Action of Pro- and Antipsychotic Molecules
Drugs that increase the extracellular concentration of DA (cocaine, amphetamines, L-DOPA) can elicit a psychotic reaction. People with a preexisting psychotic illness are especially prone but with repeated “sensitizing” doses; many healthy individuals become transiently psychotic.25 Abi-Dargham et al26 showed that the baseline occupancy of striatal D2 receptors by DA is higher in people with schizophrenia compared with controls. Finally, molecules that block D2 receptors are the mainstay in the treatment of acute and chronic psychoses, but despite their utility, how these drugs work beyond the D2 receptor has remained a mystery.
The downstream effects of D2 stimulation include the release of eCB mediators from the dendritic spines of (indirect pathway) MSNs and corticostriatal LTD.27–30 This mechanism appears to be important for psychosis (figure 1c). Direct agonists at the CB1 receptor, typified by Δ9-tetrahydrocannabinol (THC), are also psychotogenic.31–34 Using functional magnetic resonance imaging, Bhattacharyya et al35 recently showed that the degree of acute psychosis following THC was inversely related to the blood oxygen level–dependent signal in the ventral striatum. Because the eCB system is downstream of DA, it might be predicted that D2 blockers would be ineffective against THC psychosis, and this has been demonstrated.36 But can CB1 blockers inhibit the propsychotic effects of excessive D2 stimulation? In animals, microinjection of the potent CB1 antagonist (SR147778) into the ventral striatum inhibited the expression of behavioral sensitization to methamphetamine.37 Moreover in humans, cannabidiol, which uncouples CB1 receptors from their intracellular effectors (and inhibits adenosine reuptake),38 inhibited L-DOPA–induced psychosis.39
If promotion of (indirect pathway) LTD is associated with propsychotic effects, are enhancements of A2A signaling associated with antipsychotic effects? Molecules such as dipyridamole inhibit the reuptake of adenosine. In patients, dipyridamole was shown to augment the antipsychotic properties of haloperidol.40 In contrast, adenosine receptor antagonists, such as the methylxanthines, can induce a transient exacerbation of psychotic symptoms.41,42
LTP of corticostriatal connections of the indirect pathway depends not only on the presence of adenosine at A2A receptors but also requires activation of glutamate N-methyl-D-aspartic acid (NMDA) receptors. In keeping with the scheme outlined here, drugs that block NMDA receptor channels (ketamine and phencyclidine) are also psychotogenic. However, the converse might not be true. Despite considerable theoretical support, trials of putative antipsychotics designed to directly enhance NMDA channel opening have been disappointing.25
Overall, the simplest explanation at present is that, at corticostriatal synapses belonging to the indirect pathway, drugs promoting LTD are propsychotic, whereas drugs that act via neuromodulatory systems to promote LTP are antipsychotic. Activation of the indirect pathway ultimately returns a negative feedback signal to the cortex.2 The strengthening of negative feedback via LTP at corticostriatal synapses of the indirect pathway might be a vital property of antipsychotic molecules.
Whether the above pharmacological observations are attributable to the actions of adenosine, DA, and eCBs at corticostriatal synapses, as opposed to some other synapse, is unknown. All 3 systems modulate fast transmission and plasticity in the prefrontal cortex (PFC) and limbic system. However, at present, there are no further examples outside the striatum (see below), where the 3 systems show such a high degree of confluence. For instance, unlike the striatum, eCB-dependent LTD in the cortex and hippocampus does not require D2 receptor stimulation.43 Moreover, D2 receptors are in relatively short supply outside of the basal ganglia and compared with the D1 receptor, which predominates, very little is known about how they modulate cells and synapses in the hippocampus and PFC. One exception is the amygdala where induction of LTP in the amygdala-dentate pathway required D2 receptors (but not NMDA receptors).44 Another study showed that amphetamine-induced acute and long-term depression of entorhinal inputs to the amygdala was mediated via retrograde eCB signaling.45 Curiously, however, the long-term effects of amphetamine at this synapse did not require DA (or other monoamine) receptors.45
Competition and Cooperation Within the Striatum
So far, we have considered how DA, adenosine, and the eCBs act in concert in order to “gate” excitatory drive entering the striatum from the cortex. Several lines of evidence indicate that the propsychotic manifestations of excess D2 receptor activity appear to depend on downstream eCB signaling at CB1 receptors.
Importantly, however, the CB1 receptor is also expressed on γ-aminobutyric acid–mediated (GABAergic) inputs to MSNs.46 Moreover, a consistent finding is that the density of CB1 receptors is higher on GABAergic, as opposed to neighboring glutamatergic, terminals.47,48 The effect of CB1 stimulation is depression of GABAergic terminals, which can either be transient or long term.49–52
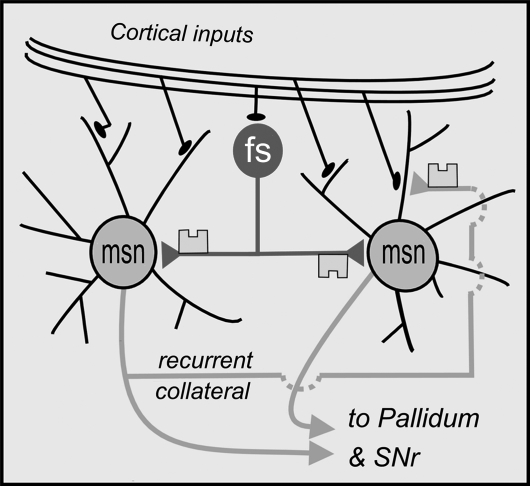
Two distinct types of GABAergic terminal are involved48,51 (figure 2). Firstly, there is a network of fast-spiking, parvalbumin (PV)-containing internurons, whose dendrites are interconnected by gap junctions.2 Their terminals form baskets around the somata of MSNs (figure 2) where they exert a strong, fast GABAergic influence, which decays rapidly. Functionally, they permit groups of MSNs to synchronize their action potentials en masse. Similar PV-containing interneurons are found in the hippocampus and cortex, and their possible dysfunction in schizophrenia has attracted much attention.53 Notably, whereas in the striatum, the terminals of PV-containing interneurons display CB1 receptors; those in the cortex and hippocampus do not.54–57
Fig. 2.
The Role of CB1 Receptors at γ-aminobutyric acid–mediated (GABAergic) synapses in the striatum. Two populations of GABAergic neuron express CB1 receptors at their terminals. Fast-spiking (fs) interneurons synapse on the somata of medium spiny neurons (MSNs). These interneurons are thought to synchronize spike discharges in groups of MSNs. Recurrent collaterals synapse on the dendrites of other MSNs. They are believed to mediate lateral inhibition between competing assemblies of MSNs, in a “winner-takes-all” scenario. Endocannabinoid (eCB)-mediated depression of recurrent collaterals might favor cooperation and new learning, rather than competition between MSNs. In support, recent findings show that eCBs signaling at CB1 receptors in the striatum is essential for habit formation. Excessive, prolonged, or sensitized CB1 receptor signaling might favor connections between logically unrelated ideas.
The second group of GABAergic terminals that express CB1 receptors are the recurrent collaterals of MSNs.48 They form synapses with the dendrites of other MSNs where they provide a relatively weak GABAergic input (figure 2). Recurrent collaterals are important for the competitive/cooperative network functions of the striatum. An assembly of MSNs that is engaged in selecting behavior achieves “dominance” by inhibiting the dendrites of other MSNs (lateral inhibition).4,58,59 If, on the other hand, the task is to form an association between separate psychomotor streams, then it would make sense for the inhibition between (formerly competing, now cooperating) MSNs to be relaxed. Recent work has shown that eCBs acting at CB1 receptors within the striatum are essential for habit formation.60 Furthermore, eCBs have been shown to fine-tune (depress) lateral inhibition at MSN-MSN synapses.51 As part of basal ganglia–dependent learning, CB1 receptor–mediated depression of recurrent collaterals may be crucial in facilitating associations between distinct streams of information.
Remarkably, stimulation of A2A receptors facilitates GABAergic signaling between MSNs.61 Thus, at both excitatory (cortical) and inhibitory (recurrent collateral) inputs to the dendrites of MSNs, A2A and CB1 receptors appear to have diametrically opposing effects.
The Emergence of Delusions?
THC- and stimulant-induced psychoses are dominated by delusional thinking and ideas of reference. In normal physiology, the synthesis and release of eCBs are tightly regulated. Following the administration of THC, CB1 receptor stimulation is prolonged and excessive. Behavioral sensitization to amphetamines/cocaine (a requirement for their propsychotic properties) can be inhibited by specific CB1 antagonists or by CB1 receptor knockout.37,62,63 Significantly, the repeated administration of cocaine has been shown to sensitize GABAergic terminals in the striatum to the effects of exogenously applied CB1 agonists,64 while the sensitivity of glutamatergic inputs from the cortex remained unchanged. This is an intriguing finding, suggesting that CB1 receptors on GABAergic terminals are important for stimulant-induced psychopathology. A previous study had shown that the ability of cocaine to depress intrastriatal GABAergic currents depended on D2 receptors and the activation of retrograde eCB signaling.65
From the available evidence, we conjecture that THC and stimulants relax the mutual inhibition between MSN assemblies to such an extent that associations are formed between coincident psychomotor streams that would otherwise remain separate. The essential concept is that new connections (new meanings) appear for consciousness.
Jaspers held that the delusional experience of reality is a transformation in which the environment offers a world of new meanings. He reasoned that delusion proper stems from the unconscious mind. Here, we have been arguing that delusion emerges in an implicit (unconscious) processing system—the basal ganglia—before being relayed to the cortex. We have focused on the input side, the striatum, describing how its circuitry is impressionable. Several modulatory systems fine-tune the synapses at MSNs, influencing how the higher cortices “talk” to the striatum and how MSNs talk to each other. The pharmacology of a range of drugs with either pro- or anti-psychotic properties shows a remarkable confluence at MSNs. Drugs that strengthen cortical inputs to the indirect pathway have anti-psychotic properties. Conversely, drugs that weaken the same inputs appear to be pro-psychotic. In addition, we speculate that when a drug relaxes lateral inhibition between MSNs, logically opposed thoughts might be interwoven as a new, and abnormal memory trace, which forms the basis of a delusion.
The basal ganglia return their computations to a higher processor. Through re-iteration, the nascent delusion could be elaborated and strengthened. A more mature network might resist the fundamental changes demanded by ‘new connections’ and re-orientate. But, for some people there comes a point when the critical faculty is put into the service of the delusion.66 Beyond this stage a psychiatrist can expend much energy and skill in trying to persuade someone that a drug molecule can re-orientate their belief network for the better.
Funding
Medical Research Council (G0800462 to P.D.M.).
Acknowledgments
We are grateful for the comments and suggestions of Roger Pertwee and Spilios Argyopoulos. Declaration of interest: None.
References
- 1.Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis–linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. Berlin, Heidelberg; New York: Springer-Verlag; 2008. [Google Scholar]
- 3.Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolls ETT, A . Neural networks and Brain Function. Oxford university press; 1997. [Google Scholar]
- 5.Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- 6.Houk JC, Bastianen C, Fansler D, et al. Action selection and refinement in subcortical loops through basal ganglia and cerebellum. Philos Trans R Soc Lond B Biol Sci. 2007;362:1573–1583. doi: 10.1098/rstb.2007.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 8.Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- 9.Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Seger CA. The basal ganglia in human learning. Neuroscientist. 2006;12:285–290. doi: 10.1177/1073858405285632. [DOI] [PubMed] [Google Scholar]
- 11.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 12.Seger CA, Cincotta CM. Dynamics of frontal, striatal, and hippocampal systems during rule learning. Cereb Cortex. 2006;16:1546–1555. doi: 10.1093/cercor/bhj092. [DOI] [PubMed] [Google Scholar]
- 13.Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wickens JR. Synaptic plasticity in the basal ganglia. Behav Brain Res. 2009;199:119–28. doi: 10.1016/j.bbr.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson P, Smith L, Farde L, Harnryd C, Sedvall G, Wiesel FA. Lack of apparent antipsychotic effect of the D1-dopamine receptor antagonist SCH39166 in acutely ill schizophrenic patients. Psychopharmacology. 1995;121:309–316. doi: 10.1007/BF02246068. [DOI] [PubMed] [Google Scholar]
- 16.de Beaurepaire R, Labelle A, Naber D, Jones BD, Barnes TR. An open trial of the D1 antagonist SCH 39166 in six cases of acute psychotic states. Psychopharmacology. 1995;121:323–327. doi: 10.1007/BF02246070. [DOI] [PubMed] [Google Scholar]
- 17.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 18.Schultz W, Tremblay L, Hollerman JR. Changes in behavior-related neuronal activity in the striatum during learning. Trends Neurosci. 2003;26:321–328. doi: 10.1016/S0166-2236(03)00122-X. [DOI] [PubMed] [Google Scholar]
- 19.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neuroscience. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 22.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 25.McKenna P. Schizophrenia and Related Syndromes. Routledge; 2007. [Google Scholar]
- 26.Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 28.Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci U S A. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 32.D'Souza DC, Abi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning [published online ahead of print April 01, 2009] Psychol Med. doi: 10.1017/S0033291709005522. doi:10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- 34.Henquet C, Rosa A, Krabbendam L, et al. An experimental study of catechol-o-methyltransferase val(158)met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology. 2006;31:2748–2757. doi: 10.1038/sj.npp.1301197. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharyya S, Fusar-Poli P, Borgwardt S, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66:442–451. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- 36.D'Souza DC, Braley G, Blaise R, et al. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology. 2008;198:587–603. doi: 10.1007/s00213-007-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang YC, Chen JC. The role of the cannabinoid type 1 receptor and down-stream cAMP/DARPP-32 signal in the nucleus accumbens of methamphetamine-sensitized rats. J Neurochem. 2007;103:2505–2517. doi: 10.1111/j.1471-4159.2007.04981.x. [DOI] [PubMed] [Google Scholar]
- 38.Pertwee RG. The diverse CB(1) and CB(2) receptor pharmacology of three plant cannabinoids: Delta(9)-tetrahydrocannabinol, cannabidiol and Delta(9)-tetrahydrocannabivarin. Br J Pharmacology. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuardi A, Crippa J, Hallak J, et al. Cannabidiol for the treatment of psychosis in Parkinson's disease [published online ahead of print November 21, 2008] J Psychopharmacol. doi: 10.1177/0269881108096519. doi:10.1177/0269881108096519. [DOI] [PubMed] [Google Scholar]
- 40.Akhondzadeh S, Shasavand E, Jamilian H, Shabestari O, Kamalipour A. Dipyridamole in the treatment of schizophrenia: adenosine-dopamine receptor interactions. J Clin Pharm Ther. 2000;25:131–137. doi: 10.1046/j.1365-2710.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- 41.Lucas PB, Pickar D, Kelsoe J, Rapaport M, Pato C, Hommer D. Effects of the acute administration of caffeine in patients with schizophrenia. Biol Psychiatry. 1990;28:35–40. doi: 10.1016/0006-3223(90)90429-6. [DOI] [PubMed] [Google Scholar]
- 42.Zaslove MO, Russell RL, Ross E. Effect of caffeine intake on psychotic in-patients. Br J Psychiatry. 1991;159:565–567. doi: 10.1192/bjp.159.4.565. [DOI] [PubMed] [Google Scholar]
- 43.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-Mediated Synaptic Plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 44.Abe K, Niikura Y, Fujimoto T, Akaishi T, Misawa M. Involvement of dopamine D2 receptors in the induction of long-term potentiation in the basolateral amygdala-dentate gyrus pathway of anesthetized rats. Neuropharmacology. 2008;55:1419–1424. doi: 10.1016/j.neuropharm.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Huang YC, Wang SJ, Chiou LC, Gean PW. Mediation of amphetamine-induced long-term depression of synaptic transmission by CB1 cannabinoid receptors in the rat amygdala. J Neurosci. 2003;23:10311–10320. doi: 10.1523/JNEUROSCI.23-32-10311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kofalvi A, Rodrigues RJ, Ledent C, et al. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci. 2009;29:32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur J Neurosci. 2006;24:2246–2252. doi: 10.1111/j.1460-9568.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- 51.Freiman I, Anton A, Monyer H, Urbanski MJ, Szabo B. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. J Physiol. 2006;575:789–806. doi: 10.1113/jphysiol.2006.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- 53.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 54.Katona I, Sperlagh B, Sik A, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- 56.Bodor AL, Katona I, Nyiri G, et al. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- 58.Fukai T, Tanaka S. A simple neural network exhibiting selective activation of neuronal ensembles: from winner-take-all to winners-share-all. Neural Comput. 1997;9:77–97. doi: 10.1162/neco.1997.9.1.77. [DOI] [PubMed] [Google Scholar]
- 59.Wickens JR, Arbuthnott GW, Shindou T. Simulation of GABA function in the basal ganglia: computational models of GABAergic mechanisms in basal ganglia function. Prog Brain Res. 2007;160:313–329. doi: 10.1016/S0079-6123(06)60018-6. [DOI] [PubMed] [Google Scholar]
- 60.Hilario MR, Clouse E, HH Y, RM C. Endocannabinoid Signaling is Critical for Habit Formation. Front Integr Neurosci. 2007;1:6. doi: 10.3389/neuro.07.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shindou T, Arbuthnott GW, Wickens JR. Actions of adenosine A 2A receptors on synaptic connections of spiny projection neurons in the neostriatal inhibitory network. J Neurophysiol. 2008;99:1884–1889. doi: 10.1152/jn.01259.2007. [DOI] [PubMed] [Google Scholar]
- 62.Corbille AG, Valjent E, Marsicano G, et al. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci. 2007;27:6937–47. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thiemann G, van der Stelt M, Petrosino S, Molleman A, Di Marzo V, Hasenöhrl RU. The role of the CB1 cannabinoid receptor and its endogenous ligands, anandamide and 2-arachidonoylglycerol, in amphetamine-induced behavioural sensitization. Behav Brain Res. 2008;187:289–96. doi: 10.1016/j.bbr.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 64.Centonze D, Rossi S, De Chiara V, et al. Chronic cocaine sensitizes striatal GABAergic synapses to the stimulation of cannabinoid CB1 receptors. Eur J Neurosci. 2007;25:1631–1640. doi: 10.1111/j.1460-9568.2007.05433.x. [DOI] [PubMed] [Google Scholar]
- 65.Centonze D, Battista N, Rossi S, et al. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic transmission. Neuropsychopharmacology. 2004;29:1488–1497. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- 66.Jaspers K. General Psychopathology. Baltimore: The John Hopkins University Press; 1997. [Google Scholar]