Abstract
Background
The relation between plasma lipid levels and Alzheimer disease (AD) and vascular dementia (VaD), and the impact of drugs to lower lipid levels remains unclear.
Objective
To investigate the relation between plasma lipid levels and the risk of AD and VaD and the impact of drugs to lower lipid levels on this relationship.
Design and Setting
Cross-sectional and prospective community-based cohort studies.
Participants
Random sample of 4316 Medicare recipients, 65 years and older, residing in northern Manhattan, NY.
Main Outcome Measures
Vascular dementia and AD according to standard criteria.
Results
Elevated levels of non–high-density lipoprotein (HDL-C) and low-density lipoprotein cholesterol (LDL-C) and decreased levels of HDL-C were weak risk factors for VaD in either cross-sectional or prospective analyses. Higher levels of total cholesterol were associated with a decreased risk of incident AD after adjustment for demographics, apolipoprotein E genotype, and cardiovascular risk factors. Treatment with drugs to lower lipid levels did not change the disease risk of either disorder.
Conclusions
We found a weak relation between non–HDL-C, LDL-C, and HDL-C levels and the risk of VaD. Lipid levels and the use of agents to lower them do not seem to be associated with the risk of AD.
The prevalence of dementia is increasing in western societies, and there are no known measures to prevent or cure it. Conflicting data show that dyslipidemia, a modifiable risk factor, is associated with a higher risk of dementia. Reduced high-density lipoprotein cholesterol (HDL-C)1,2 and apolipoprotein A-I levels,1 as well as increased levels of lipoprotein(a),3 have been observed in vascular dementia (VaD) in some but not all studies.4,5 Contradictory results have been found in studies relating total cholesterol, 6,7 HDL-C,3,8,9 and LDL-C6,8 levels with Alzheimer disease (AD).
Interest in these relationships has been increased by the observation that widely available agents to lower lipid levels, particularly hydroxymethylglutaryl coenzyme A reductase inhibitors (statins), may lower the risk of AD10 or VaD,11 and that cholesterol alters the degradation of the amyloid precursor protein, which plays a major role in the pathogenesis of AD.12 Moreover, cerebrovascular disease, which is associated with dyslipidemia, may be related to the risk of AD.13 A previous report from our group found an association between high levels of total and low-density lipoprotein cholesterol (LDL-C)14 and VaD, but no association of LDL-C levels with AD. Our objectives in this study were to explore these associations in a larger cross-sectional study and a prospective study with longer follow-up and to assess the association between agents to lower lipid levels and dementia.
METHODS
PARTICIPANTS AND SETTING
Participants were enrolled in a longitudinal cohort study by means of random sampling of Medicare recipients 65 years or older residing in northern Manhattan, NY (Washington Heights, Hamilton Heights, and Inwood). The sampling procedures have been described elsewhere. 15 Each participant underwent an in-person interview about general health and function at the time of study entry, followed by a standard assessment of medical history, physical and neurological examination, and a neuropsychological battery.16 Ethnic origin was classified by self-report using the format of the 1990 US Census. 17 Participants were recruited at 2 periods (1992–1994 and 1999–2002). They have been followed up at approximately 18-month intervals with similar assessments at each follow-up. The institutional review board of Columbia Presbyterian Medical Center, New York, NY, approved this study.
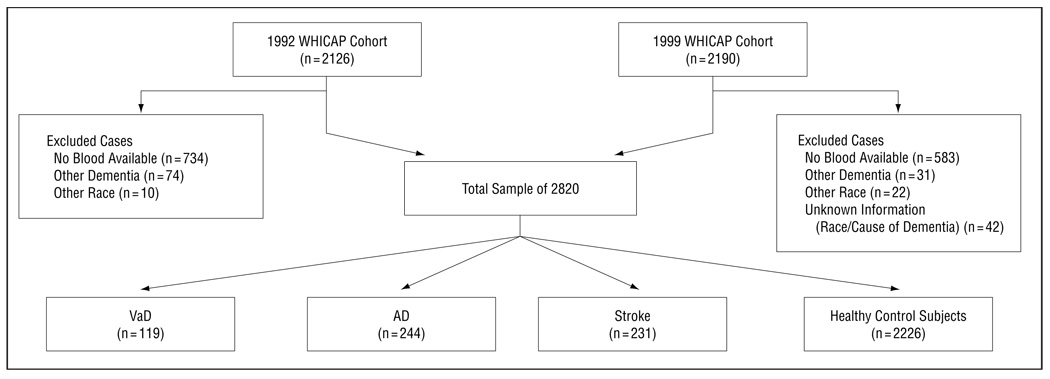
Of the 4316 individuals who underwent clinical assessment at baseline, we excluded data from 1496 individuals in the cross-sectional analysis (34.6%) (Figure 1). Plasma lipid measurements were unavailable in 1317 cases, because lipid levels were obtained during the second follow-up visit. Information about ethnic group and cause of dementia was unknown in 42 cases. One hundred five cases had other causes of dementia (not AD or VaD), and 32 were members of an ethnic group other than African American, white (European American), or Carribean Hispanic. The final analytic sample in the cross-sectional analysis contained 2820 participants.
Figure 1.
Description of the cross-sectional sample. AD indicates Alzheimer disease; VaD, vascular dementia; and WHICAP, Washington Heights–Inwood Columbia Aging Project.
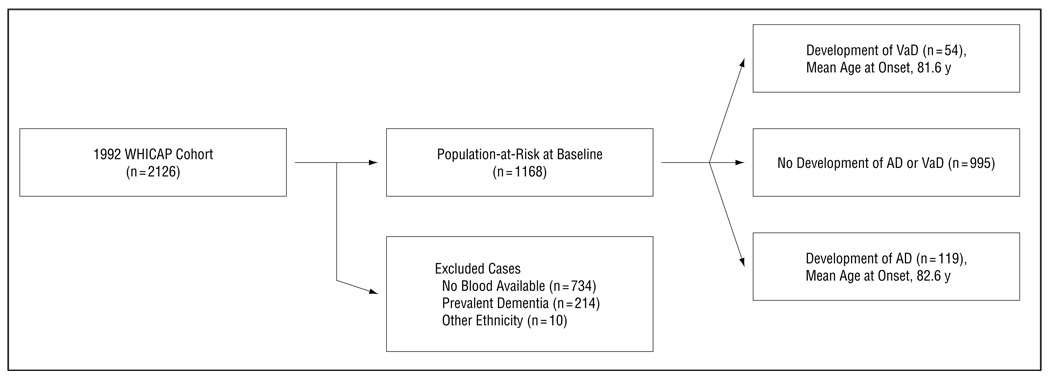
The prospective study included only participants from the 1992 cohort. Of the 2126 subjects who underwent clinical assessment at baseline, we excluded data from 958 individuals (45.1%) (Figure 2). Plasma lipids were unavailable in 734 cases, because lipid levels were obtained during the second follow-up visit. Ten individuals were members of an ethnic group other than African American, white (European American), or Carribean Hispanic. Two hundred fourteen subjects were excluded because of prevalent dementia. The final analytic sample contained 1168 participants.
Figure 2.
Description of the sample used in the prospective study. AD indicates Alzheimer disease; VaD, vascular dementia; and WHICAP, Washington Heights–Inwood Columbia Aging Project.
DIAGNOSIS OF STROKE
Stroke was defined according to the World Health Organization criteria.18 The diagnosis was based on questioning of the participant and/or relatives, supplemented by results of a neurological examination and/or review of medical records. Results of brain imaging were available on 85% of those with VaD.
DIAGNOSIS OF DEMENTIA
The diagnosis of dementia was established on the basis of all available information gathered from the initial and follow-up assessments and medical records. Dementia was determined by consensus at a conference of physicians, neurologists, neuro-psychologists, and psychiatrists. The diagnosis of dementia was based on standard research criteria and required evidence of cognitive decline, including memory impairment, on the neuropsychological test battery and evidence of impairment in social or occupational function (clinical dementia rating,>0.5).19
A diagnosis of VaD was considered for individuals with dementia combined with a history or clinical evidence of stroke and was classified as follows20: (1) stroke-related dementia (eg, new onset of dementia within 3 months of a stroke); (2) dementia due to focal effects of a stroke (eg, dementia resulting from stroke[s] in strategic area[s] whose singular or additive effects accounted for the cognitive impairment); and (3) possible AD with concomitant stroke (eg, progressive dementia associated with a clinical history of stroke in which the temporal relationship could not be established).
The diagnosis of AD was based on the National Institute of Neurological and Cognitive Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria.21
DIABETES MELLITUS, HEART DISEASE, AND HYPERTENSION
Diabetes and hypertension were defined as a history of either disorder at any time during life. At baseline, all participants were asked whether they had a history of diabetes or hypertension. If affirmed, they were asked whether they were under treatment and the specific type of medication. Heart disease was defined as a history of myocardial infarction, congestive heart failure, or angina pectoris at any time during life.
TREATMENT WITH DRUGS TO LOWER LIPID LEVELS
At baseline, all participants were asked if they ever have been treated with drugs to lower lipid levels. If affirmed, they were asked for the specific type of drug.
PLASMA LIPIDS AND APOLIPOPROTEIN E GENOTYPING
Fasting plasma total cholesterol and triglyceride levels were determined at initial assessment using standard enzymatic techniques. High-density lipoprotein cholesterol levels were determined after precipitation of apolipoprotein B–containing lipoproteins with phosphotungstic acid.22 Low-density lipoprotein cholesterol levels were recalculated using the formula of Friedewald et al.23 Apolipoprotein E (APOE) genotypes were determined as described by Hixson and Vernier24 with slight modification.25 We classified persons as homozygous or heterozygous for the APOE ∊4 allele or not having any ∊4 type of allele.
STATISTICAL METHODS
Lipid levels and other potentially relevant factors were compared among individuals with VaD, AD, and stroke and healthy control subjects in the cross-sectional and prospective samples. We used χ2 tests for categorical data and analysis of variance for continuous variables. Because the distribution of HDL-C and triglyceride levels was skewed, we performed logarithmic transformation of these data and repeated the statistical tests.
In the cross-sectional analysis, we included participants of both the 1992 and 1999 cohorts. Logistic regression was used to estimate the odds ratio (OR) of dementia (AD or VaD) associated with plasma lipid levels. Plasma lipid levels were analyzed first as continuous variables and later grouped into quartiles. After adjusting for sex, age, ethnicity, and education, we performed a second model adjusting for body mass index, diabetes mellitus, hypertension, heart disease, and APOE ∊4 genotype. To estimate the effect of treatment to lower lipid levels, separate analyses were performed for treated and not treated individuals.
The prospective study included only participants from the 1992 cohort. Proportional hazard models were used to estimate the association of plasma lipid levels with the incidence of AD and VaD. The time-to-event variable was age at onset of dementia. Data from individuals in whom AD or VaD did not develop, who died, or who were lost to follow-up owing to relocation before development of dementia were censored at the time of their last evaluation.
Information on covariates was obtained at baseline. After adjusting for sex, age, race, and education, we adjusted for body mass index, diabetes mellitus, hypertension, heart disease, and APOE ∊4 genotype in a second model. Separate models were performed for treated and not treated individuals. Data analysis was performed using SPSS version 11.0 software (SPSS Inc, Chicago, Ill).
RESULTS
CROSS-SECTIONAL ANALYSIS
First, we performed a cross-sectional analysis of the 1992 and 1999 cohorts. Lipid levels, demographics, and vascular risk factors were compared among individuals with VaD, AD, and stroke and healthy controls.
The mean±SD age of the sample was 77.2±6.7 years. Of the total sample of 2820, 66.7% were women, 25.7% were white, 32.0% were black, and 42.3% were Hispanic. The median number of years of education was 9. The mean level of total cholesterol was 198.8 mg/dL (5.1 mmol/L); non–HDL-C, 151.4 mg/dL (3.9 mmol/L); HDL-C, 47.4 mg/dL (1.2 mmol/L); triglycerides, 155.9 mg/dL (1.8 mmol/L); and LDL-C, 120.1 mg/dL (3.1 mmol/L). Of the total cohort, 28.7% were heterozygous or homozygous for the APOE ∊4 allele; 19.9% had a history of diabetes, 23.3% had a history of heart disease, and 62.0% had a history of hypertension. Use of agents to lower lipid levels was reported by 477 subjects (16.9%).
Women had higher levels of total, non–HDL-C, HDL-C, and LDL-C than men (Table 1). Hispanic subjects had significantly lower levels of total cholesterol than white subjects. They had lower levels of HDL-C and LDL-C and higher levels of triglycerides than white and black subjects. Black subjects had higher levels of HDL-C and lower levels of non–HDL-C and triglycerides than white subjects.
Table 1.
Comparison of Lipid Levels by Demographics in 2820 Subjects*
| Lipid Levels, mg/dL | |||||
|---|---|---|---|---|---|
| Total Cholesterol | Non–HDL-C Cholesterol |
HDL-C | Triglycerides | LDL-C | |
| Men | 188.34 (38.5) | 145.6 (37.7) | 42.45 (14.1) | 156.28 (89.7) | 114.69 (33.4) |
| Women | 204.15 (39.9)† | 154.2 (39.6)† | 49.97 (14.8)† | 155.71 (83.7) | 122.92 (35.3)† |
| Ethnic group‡ | |||||
| White/non-Hispanic | 201.59 (39.2)† | 154.3 (37.9)† | 47.41 (14.5)† | 155.74 (79.4)† | 122.90 (33.0)† |
| Black/non-Hispanic | 199.56 (38.1) | 147.6 (36.4) | 51.94 (15.7)§ | 128.30 (65.9) | 121.95 (34.0)† |
| Hispanic | 196.74 (42.2) | 152.6 (39.1) | 44.12 (13.8) | 176.89 (96.3)§ | 117.19 (36.5) |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113.
Values are expressed as mean (SD).
Significant at P<.05 vs lowest value within the lipid group, based on analysis of variance for continuous data and χ2 test for categorical data.
Classified by self-report using the format of the 1990 US census.15
Significant at P<.05 vs all lower values within the lipid group, based on analysis of variance for continuous data and χ2 test for categorical data.
The subjects with VaD or AD were significantly older and less educated than individuals with stroke without dementia or than controls (Table 2). The VaD group had significantly more Hispanic than white subjects and the AD group had more Hispanic and black than white subjects. A history of diabetes, heart disease, and hypertension was more frequent in the stroke and VaD groups compared with the control group. The APOE ∊4 genotype was significantly more frequent in the AD group compared with the control group.
Table 2.
Comparison of Characteristics Among Outcome Groups in 2820 Subjects in the Cross-Sectional Analysis*
| Vascular Dementia (n = 119) |
Alzheimer Disease (n = 244) |
Stroke Without Dementia (n = 231)† |
Control Subjects (n = 2226) |
|
|---|---|---|---|---|
| Men | 36 (30.3) | 55 (22.5) | 87 (37.7) | 760 (34.1) |
| Women | 83 (69.7) | 189 (77.5)‡ | 144 (62.3) | 1466 (65.9) |
| Education, mean (SD), y | 6.61 (4.1)‡ | 6.33 (4.3)‡ | 9.85 (4.8) | 9.74 (4.7) |
| Age, mean (SD), y | 80.42 (6.9)‡ | 82.85 (7.3)‡ | 77.66 (6.2) | 76.42 (6.3) |
| BMI, mean (SD) | 26.34 (5.3) | 26.42 (5.7) | 27.25 (5.9) | 27.59 (6.8) |
| Ethnic group§ | ||||
| White/non-Hispanic | 8 (6.7) | 19 (7.8) | 57 (24.7) | 642 (28.8) |
| Black/non-Hispanic | 39 (32.8) | 96 (39.3)‡ | 78 (33.8) | 689 (31.0) |
| Hispanic | 72 (60.5)‡ | 129 (52.9)‡ | 96 (41.6) | 895 (40.2) |
| APOE genotype | ||||
| ∊4/∊4 | 3 (3.4) | 9 (4.4)‡ | 1 (0.7) | 26 (1.8) |
| ∊4/− | 29 (33.3) | 72 (35.5)‡ | 48 (35.0) | 355 (24.2) |
| −/− | 55 (63.2) | 122 (60.1) | 88 (64.2) | 1085 (74.0) |
| Lipid levels, mean (SD), mg/dL | ||||
| Total cholesterol | 199.76 (44.7) | 197.11 (41.2) | 199.48 (45.3) | 198.98 (39.3) |
| Non–HDL-C | 155.3 (44.9) | 149.6 (39.1) | 152.8 (44.7) | 151.3 (38.2) |
| HDL-C | 44.42 (14.2) | 47.49 (15.6) | 46.97 (17.0) | 47.68 (14.7) |
| Triglycerides | 165.03 (83.7) | 147.51 (84.1) | 162.27 (100.3) | 155.67 (84.4) |
| LDL-C | 122.33 (37.9) | 120.11 (35.8) | 119.36 (37.6) | 120.16 (34.4) |
| Diabetes | ||||
| None | 85 (71.4)‡ | 188 (77.0) | 170 (74.6)‡ | 1727 (82.0) |
| Not treated | 9 (7.6)‡ | 13 (5.3) | 15 (6.6) | 71 (3.4) |
| Treated | 25 (21.0)‡ | 43 (17.6) | 43 (18.9)‡ | 308 (14.6) |
| Heart disease | ||||
| None | 82 (68.9)‡ | 198 (81.1) | 142 (62.0)‡ | 1649 (78.3) |
| Not treated | 13 (10.9)‡ | 22 (9.0) | 34 (14.8)‡ | 141 (6.7) |
| Treated | 24 (20.2)‡ | 24 (9.8) | 53 (23.1)‡ | 317 (15.1) |
| Hypertension | ||||
| None | 35 (29.4)‡ | 99 (40.7) | 51 (22.3)‡ | 836 (39.9) |
| Not treated | 25 (21.0)‡ | 38 (15.6) | 35 (15.3) | 231 (11.0) |
| Treated | 59 (49.6) | 106 (43.6) | 143 (62.4)‡ | 1029 (49.1) |
Abbreviations: APOE, apolipoprotein E; BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; minus sign, allele other than the ∊4 type.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113.
Unless otherwise indicated, data are expressed as number (percentage) of subjects. Some percentages are based on an incomplete sample owing to small amounts of missing data. Percentages have been rounded and may not total 100.
Defined according to World Health Organization criteria.16
Significant at P<.05 vs control group, based on analysis of variance for continuous data and χ2 test for categorical data.
Classified by self-report using the format of the 1990 US census.15
Plasma Lipid Levels and the Risk of AD
There was no association between plasma lipid levels and a higher risk of AD (Table 3). Adjustment for demographics, cardiovascular risk factors, and APOE genotype did not change this relation. Treatment with drugs to lower lipid levels was negatively associated with the risk of AD (OR, 0.45; 95% confidence interval [CI], 0.27–0.75; P=.002).
Table 3.
Relation of Plasma Lipids and the Risk of Prevalent AD
| No. (%) of Subjects* | ||||||
|---|---|---|---|---|---|---|
| Lipid, Quartile (Range, mg/dL) |
AD (n = 244) |
Control Subjects (n = 2226) |
B (SE) | P Value | OR (95% CI)† | OR (95% CI)‡ |
| Total cholesterol | ||||||
| 1 (≤172.00) | 72 (29.5) | 552 (24.8) | 1.00 | 1.00 | ||
| 2 (172.01–197.00) | 63 (25.8) | 565 (25.4) | 0.06 (0.20) | .76 | 1.06 (0.72–1.58) | 0.96 (0.61–1.53) |
| 3 (197.01–225.00) | 56 (23.0) | 552 (24.8) | −0.19 (0.21) | .36 | 0.82 (0.55–1.24) | 0.72 (0.44–1.16) |
| 4 (≥225.01) | 53 (21.7) | 557 (25.0) | −0.12 (0.21) | .56 | 0.89 (0.59–1.35) | 0.94 (0.58–1.52) |
| Trend test | P = .37 | P = .52 | ||||
| Non–HDL-C | ||||||
| 1 (≤124.00) | 71 (29.1) | 562 (25.2) | 1.00 | 1.00 | ||
| 2 (124.01–149.00) | 65 (26.6) | 555 (24.9) | 0.12 (0.20) | .54 | 1.13 (0.77–1.66) | 1.15 (0.73–1.81) |
| 3 (149.01–176.00) | 54 (22.1) | 575 (25.8) | −0.12 (0.20) | .57 | 0.89 (0.60–1.33) | 0.88 (0.55–1.41) |
| 4 (≥176.01) | 54 (22.1) | 534 (24.0) | 0.01 (0.21) | .98 | 1.01 (0.67–1.51) | 1.05 (0.65–1.68) |
| Trend test | P = .74 | P = .86 | ||||
| HDL-C | ||||||
| 1 (≤37.00) | 69 (29.2) | 494 (23.4) | 1.00 | 1.00 | ||
| 2 (37.01–45.00) | 46 (19.5) | 555 (26.3) | −0.66 (0.22) | .003 | 0.52 (0.34–0.80) | 0.47 (0.28–0.78) |
| 3 (45.01–55.00) | 51 (21.6) | 513 (24.3) | −0.50 (0.22) | .03 | 0.61 (0.39–0.94) | 0.58 (0.35–0.97) |
| 4 (≥55.01) | 70 (29.7) | 552 (26.1) | −0.30 (0.21) | .16 | 0.74 (0.49–1.13) | 0.66 (0.41–1.08) |
| Trend test | P = .34 | P = .22 | ||||
| Triglycerides | ||||||
| 1 (≤97.00) | 73 (29.9) | 548 (24.8) | 1.00 | 1.00 | ||
| 2 (97.01–135.00) | 65 (26.6) | 536 (24.3) | −0.16 (0.20) | .43 | 0.85 (0.57–1.27) | 0.93 (0.58–1.50) |
| 3 (135.01–191.00) | 50 (20.5) | 578 (26.2) | −0.42 (0.22) | .05 | 0.65 (0.43–1.00) | 0.80 (0.48–1.32) |
| 4 (≥191.01) | 53 (21.7) | 545 (24.7) | −0.19 (0.22) | .37 | 0.82 (0.54–1.26) | 0.95 (0.58–1.56) |
| Trend test | P = .21 | P = .72 | ||||
| LDL-C | ||||||
| 1 (≤96.50) | 69 (28.3) | 550 (24.7) | 1.00 | 1.00 | ||
| 2 (96.51–118.80) | 59 (24.2) | 559 (25.1) | 0.10 (0.21) | .63 | 1.10 (0.74–1.66) | 0.87 (0.54–1.40) |
| 3 (118.81–142.80) | 57 (23.4) | 565 (25.4) | −0.10 (0.21) | .65 | 0.91 (0.60–1.37) | 0.84 (0.52–1.36) |
| 4 (≥142.81) | 59 (24.2) | 552 (24.8) | −0.02 (0.21) | .94 | 1.02 (0.68–1.53) | 1.02 (0.63–1.65) |
| Trend test | P = .83 | P = .99 | ||||
Abbreviations: AD, Alzheimer disease; B, estimated logistic regression coefficient; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113.
Some percentages are based on an incomplete sample owing to small amounts of missing data. Percentages have been rounded and may not total 100.
Adjusted for sex, age, education, and race.
Adjusted for body mass index, apolipoprotein E genotype, diabetes, heart disease, and hypertension.
Plasma Lipid Levels and the Risk of VaD
Levels of both HDL-C and non–HDL-C were associated with the risk of VaD. The prevalence of VaD decreased with higher levels of HDL-C (OR, 0.47; 95% CI, 0.27–0.83; P for trend=.02), whereas it increased with higher levels of non–HDL-C (OR, 1.60; 95% CI, 0.92–2.79; P for trend=.04) (Table 4). The strength of these associations was similar in men and women. Treatment with agents to lower lipid levels was not associated with the risk of prevalent VaD (OR, 0.87; 95% CI, 0.49–1.56; P=.65).
Table 4.
Relation of Plasma Lipids and the Risk of Prevalent VaD
| No. (%) of Subjects* | ||||||
|---|---|---|---|---|---|---|
| Lipid, Quartile | ||||||
| (Range, mg/dL) | VaD (n = 119) |
Control Subjects (n = 2226) |
B (SE) | P Value | OR (95% CI)† | OR (95% CI)‡ |
| Total cholesterol | ||||||
| 1 (≤172.00) | 27 (22.7) | 552 (24.8) | 1.00 | 1.00 | ||
| 2 (172.01–197.00) | 33 (27.7) | 565 (25.4) | 0.42 (0.28) | .13 | 1.52 (0.88–2.62) | 1.39 (0.70–2.78) |
| 3 (197.01–225.00) | 23 (19.3) | 552 (24.8) | 0.06 (0.30) | .83 | 1.07 (0.59–1.93) | 0.80 (0.36–1.77) |
| 4 (≥225.01) | 36 (30.3) | 557 (25.0) | 0.57 (0.28) | .04 | 1.77 (1.03–3.04) | 1.68 (0.83–3.39) |
| Trend test | P = .11 | P = .34 | ||||
| Non–HDL-C | ||||||
| 1 (≤124.00) | 30 (25.2) | 562 (25.1) | 1.00 | 1.00 | ||
| 2 (142.01–149.00) | 21 (17.6) | 555 (24.9) | −0.13 (0.32) | .69 | 0.88 (0.47–1.64) | 0.96 (0.45–2.06) |
| 3 (149.01–176.00) | 34 (28.6) | 575 (25.8) | 0.37 (0.28) | .19 | 1.45 (0.84–2.52) | 1.10 (0.52–2.33) |
| 4 (≥176.01) | 34 (28.6) | 534 (24.0) | 0.47 (0.28) | .10 | 1.60 (0.92–2.79) | 1.55 (0.75–3.19) |
| Trend test | P = .04 | P = .22 | ||||
| HDL-C | ||||||
| 1 (≤37.00) | 42 (36.5) | 494 (23.4) | 1.00 | 1.00 | ||
| 2 (37.01–45.00) | 24 (20.9) | 555 (26.3) | −0.78 (0.28) | .005 | 0.46 (0.27–0.79) | 0.62 (0.31–1.25) |
| 3 (45.01–55.00) | 25 (21.7) | 513 (24.3) | −0.63 (0.28) | .03 | 0.53 (0.31–0.92) | 0.78 (0.38–1.57) |
| 4 (≥55.01) | 24 (20.9) | 552 (26.1) | −0.75 (0.29) | .009 | 0.47 (0.27–0.83) | 0.60 (0.28–1.28) |
| Trend test | P = .02 | P = .27 | ||||
| Triglycerides | ||||||
| 1 (≤97.00) | 22 (18.5) | 548 (24.8) | 1.00 | 1.00 | ||
| 2 (97.01–135.00) | 39 (32.8) | 536 (24.3) | 0.57 (0.29) | .049 | 1.76 (1.00–3.10) | 1.25 (0.61–2.57) |
| 3 (135.01–191.00) | 21 (17.6) | 578 (26.2) | −0.15 (0.33) | .65 | 0.86 (0.46–1.64) | 0.78 (0.35–1.75) |
| 4 (≥191.01) | 37 (31.1) | 545 (24.7) | 0.62 (0.30) | .04 | 1.87 (1.08–3.36) | 1.39 (0.66–2.93) |
| Trend test | P = .23 | P = .62 | ||||
| LDL-C | ||||||
| 1 (≤96.50) | 26 (21.8) | 550 (24.7) | 1.00 | 1.00 | ||
| 2 (96.51–118.80) | 29 (24.4) | 559 (25.1) | 0.28 (0.29) | .32 | 1.33 (0.76–2.33) | 0.93 (0.44–1.93) |
| 3 (118.81–142.80) | 30 (25.2) | 565 (25.4) | 0.29 (0.28) | .31 | 1.33 (0.76–2.32) | 1.20 (0.59–2.44) |
| 4 (≥142.81) | 34 (28.6) | 552 (24.8) | 0.53 (0.28) | .057 | 1.70 (0.98–2.94) | 1.40 (0.68–2.88) |
| Trend test | P = .07 | P = .28 | ||||
Abbreviations: B, estimated logistic regression coefficient; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; VaD, vascular dementia.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113.
Some percentages are based on an incomplete sample owing to small amounts of missing data. Percentages have been rounded and may not total 100.
Adjusted for sex, age, education, and race.
Adjusted for body mass index, apolipoprotein E genotype, diabetes, heart disease, and hypertension.
PROSPECTIVE ANALYSIS
Subsequently, we performed a proportional hazards model of the 1992 cohort. The mean±SD age of the sample was 78.4±6.2 years; 68.3% were women, 20.4% were white, 31.8% were black, and 47.9% were Hispanic. The median number of years of education was 8. The mean level of total cholesterol was 203.1 mg/dL (5.3 mmol/L); non–HDL-C, 156.1 mg/dL (4.0 mmol/L); HDL-C, 47.0 mg/dL (1.2 mmol/L); triglycerides, 185.4 mg/dL (2.1 mmol/L); and LDL-C, 118.9 mg/dL (3.1 mmol/L). Of the total cohort, 27.3% were heterozygous or homozygous for the APOE ∊4 allele, 17.9% had a history of diabetes, 16.1% had a history of heart disease, and 55.1% had a history of hypertension. Use of agents to lower lipid levels was reported by 136 subjects (11.6%). There were 54 cases of incident VaD and 119 cases of incident AD during the 5189 person-years of observation. The mean±SD duration of observation was 4.8±2.9 years.
Individuals in whom AD or VaD developed at follow-up were significantly less educated, older, and more often Hispanic or black than white compared with controls (Table 5). Individuals in whom AD developed had a higher frequency of an APOE ∊4 genotype and significantly lower baseline levels of total cholesterol than controls. Individuals in whom VaD developed were more often women than men, had higher non–HDL-C levels than individuals in whom AD developed or who remained free of dementia, and were more likely to have a history of diabetes and heart disease compared with controls.
Table 5.
Comparison of Characteristics Among Outcome Groups in 1168 Subjects Followed Up Prospectively*
| Incident VaD (n = 54) |
Incident AD (n = 119) |
Control Subjects (n = 856) |
|
|---|---|---|---|
| Men | 11 (20.4)† | 40 (36.6) | 276 (32.2) |
| Women | 43 (79.6)† | 79 (66.4) | 580 (67.8) |
| Education, mean (SD), y | 7.30 (4.0)† | 6.75 (4.6)† | 8.83 (4.6) |
| Age, mean (SD), y | 80.05 (6.6)† | 81.49 (7.2)† | 77.81 (5.9) |
| Body mass index, mean (SD) | 27.29 (6.8) | 27.09 (5.7) | 27.57 (5.5) |
| Ethnic group‡ | |||
| White/non-Hispanic | 3 (5.6)† | 10 (8.4)† | 199 (23.2) |
| Black/non-Hispanic | 21 (38.9)† | 46 (38.7)† | 264 (30.8) |
| Hispanic | 30 (55.6)† | 63 (52.9)† | 393 (45.9) |
| APOE genotype | |||
| ∊4/∊4 | 1 (2.0) | 8 (6.9)† | 13 (1.6) |
| ∊4/− | 17 (33.3) | 30 (25.9) | 203 (24.7) |
| −/− | 33 (64.7) | 78 (67.2)† | 607 (73.8) |
| Lipid levels, mean (SD), mg/dL | |||
| Total cholesterol | 210.44 (35.5) | 194.02 (41.0)† | 204.02 (39.9) |
| Non–HDL-C | 165.11 (39.3)§ | 147.95 (38.9) | 156.53 (40.6) |
| HDL-C | 46.75 (12.2) | 46.59 (14.5) | 47.45 (15.9) |
| Triglycerides | 187.72 (86.8) | 169.62 (77.5) | 186.68 (96.2) |
| LDL-C | 126.42 (32.3) | 112.95 (35.9) | 119.14 (36.3) |
| Diabetes | |||
| None | 37 (68.5)† | 96 (80.7) | 635 (84.7) |
| Not treated | 4 (7.4)† | 5 (4.2) | 29 (3.9) |
| Treated | 13 (24.1)† | 18 (15.1) | 86 (11.5) |
| Heart disease | |||
| None | 41 (75.9)† | 98 (82.4) | 652 (86.9) |
| Not treated | 5 (9.3)† | 5 (4.2) | 28 (3.7) |
| Treated | 8 (14.8)† | 16 (13.4) | 70 (9.3) |
| Hypertension | |||
| None | 23 (42.6) | 58 (48.7) | 345 (46.3) |
| Not treated | 13 (24.1) | 22 (18.5) | 125 (16.8) |
| Treated | 18 (33.3) | 39 (32.8) | 275 (36.9) |
Abbreviations: AD, Alzheimer disease; APOE, apolipoprotein E; BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VaD, vascular dementia; minus sign, allele other than the ∊4 type.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113.
Unless otherwise indicated, data are expressed as number (percentage) of subjects. Some percentages are based on an incomplete sample owing to small amounts of missing data. Percentages have been rounded and may not total 100.
Significant at P<.05 vs control group, based on analysis of variance for continuous data and χ2 test for categorical data.
Classified by self-report using the format of the 1990 US census.15
Significant at P<.05 vs AD group, based on analysis of variance for continuous data and χ2 test for categorical data.
Risk of Incident VaD
The mean age at onset of VaD was 81.6 years. Levels of both LDL-C and non–HDL-C were associated with VaD (Table 6). The risk of VaD increased with increasing quartile of non–HDL-C (hazard ratio [HR], 2.38; 95% CI, 1.05–5.37; P for trend=.04) and LDL-C levels (HR, 2.45; 95% CI, 1.05–5.70; P for trend=.04). The strength of these associations was similar in men and women. Treatment with agents to lower lipid levels was not associated with the risk of incident VaD (HR, 1.45; 95% CI, 0.65–3.28; P=.36).
Table 6.
Relation of Plasma Lipids and the Risk of Incident VaD*
| No. (%) of Subjects | HR (95% CI) | |||
|---|---|---|---|---|
| Lipid, Quartile (Range, mg/dL) |
At-Risk Population | Incident VaD | Model 1† | Model 2‡ |
| Total cholesterol | ||||
| 1 (≤176.00) | 293 (25.1) | 9 (3.1) | 1.00 | 1.00 |
| 2 (176.01–202.50) | 291 (24.9) | 12 (4.1) | 1.05 (0.44–2.51) | 0.78 (0.32–1.94) |
| 3 (202.51–229.00) | 293 (25.1) | 16 (5.5) | 1.68 (0.72–3.96) | 1.57 (0.65–3.79) |
| 4 (≥229.01) | 291 (24.9) | 17 (5.8) | 1.61 (0.70–3.74) | 1.05 (0.42–2.60) |
| Trend test | P = .162 | P = .56 | ||
| Non–HDL-C | ||||
| 1 (≤128.00) | 299 (25.6) | 11 (3.7) | 1.00 | 1.00 |
| 2 (128.01–154.00) | 285 (24.4) | 12 (4.2) | 1.19 (0.48–2.95) | 1.08 (0.42–2.80) |
| 3 (154.01–182.00) | 296 (25.4) | 9 (3.0) | 0.99 (0.37–2.60) | 0.94 (0.34–2.57) |
| 4 (≥182.01) | 285 (24.4) | 21 (7.4) | 2.38 (1.05–5.37) | 2.01 (0.84–4.77) |
| Trend test | P = .04 | P = .13 | ||
| HDL-C | ||||
| 1 (≤36.00) | 266 (24.1) | 11 (4.1) | 1.00 | 1.00 |
| 2 (36.01–45.00) | 309 (27.9) | 15 (4.8) | 1.04 (0.47–2.29) | 0.92 (0.39–2.18) |
| 3 (45.01–55.00) | 260 (23.5) | 10 (3.8) | 0.52 (0.21–1.32) | 0.58 (0.21–1.55) |
| 4 (≥55.01) | 271 (24.5) | 16 (5.9) | 0.84 (0.36–1.95) | 0.81 (0.32–2.05) |
| Trend test | P = .45 | P = .54 | ||
| Triglycerides | ||||
| 1 (≤121.00) | 295 (25.5) | 12 (4.1) | 1.00 | 1.00 |
| 2 (121.01–161.00) | 283 (24.5) | 13 (4.6) | 0.99 (0.44–2.21) | 0.90 (0.38–2.17) |
| 3 (161.01–224.00) | 288 (24.9) | 12 (4.2) | 1.23 (0.54–2.78) | 1.00 (0.40–2.54) |
| 4 (≥224.01) | 290 (25.1) | 16 (5.5) | 1.49 (0.67–3.32) | 1.34 (0.55–3.27) |
| Trend test | P = .28 | P = .47 | ||
| LDL-C | ||||
| 1 (≤94.35) | 290 (24.9) | 8 (2.8) | 1.00 | 1.00 |
| 2 (94.36–117.30) | 291 (25.0) | 12 (4.1) | 1.63 (0.66–4.04) | 1.57 (0.63–3.91) |
| 3 (117.31–142.75) | 291 (25.0) | 14 (4.8) | 1.61 (0.66–3.93) | 1.12 (0.43–2.90) |
| 4 (≥142.76) | 291 (25.0) | 19 (6.5) | 2.45 (1.05–5.70) | 2.07 (0.85–5.06) |
| Trend test | P = .04 | P = .18 | ||
Abbreviations: CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; VaD, vascular dementia.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113.
Cox proportional hazards model, with age at onset as time variable, as described in the text. Some percentages are based on an incomplete sample due to small amounts of missing data.
Adjusted for sex, age, education, and race.
Adjusted for body mass index, apolipoprotein E genotype, diabetes, heart disease, and hypertension.
Risk of AD
The mean age of onset of AD was 82.6 years. Higher levels of total cholesterol were associated with a lower risk of incident AD after adjustment for demographics and body mass index, APOE genotype, diabetes, heart disease, and hypertension (HR, 0.48; 95% CI, 0.26–0.86; P for trend=.04) (Table 7). No other plasma lipid was associated with AD risk. Treatment with agents to lower lipid levels was not associated with the risk of incident AD (HR, 0.88; 95% CI, 0.44–1.76; P=.72).
Table 7.
Relation of Plasma Lipids and the Risk of Incident AD*
| No. (%) of Subjects | HR (95% CI) | |||
|---|---|---|---|---|
| Lipid, Quartile | ||||
| (Range, mg/dL) | At-Risk Population | Incident AD | Model 1† | Model 2‡ |
| Total cholesterol | ||||
| 1 (≤176.00) | 293 (25.1) | 43 (14.7) | 1.00 | 1.00 |
| 2 (176.01–202.50) | 291 (24.9) | 26 (8.9) | 0.63 (0.38–1.04) | 0.58 (0.34–0.97) |
| 3 (202.51–229.00) | 293 (25.1) | 29 (9.9) | 0.85 (0.52–1.40) | 0.82 (0.48–1.41) |
| 4 (≥229.01) | 291 (24.9) | 21 (7.2) | 0.55 (0.32–0.95) | 0.48 (0.26–0.86) |
| Trend test | P = .07 | P = .04 | ||
| Non–HDL-C | ||||
| 1 (≤128.00) | 299 (25.6) | 41 (13.7) | 1.00 | 1.00 |
| 2 (128.01–154.00) | 285 (24.4) | 28 (9.8) | 0.76 (0.48–1.22) | 0.79 (0.48–1.29) |
| 3 (154.01–182.00) | 296 (25.4) | 31 (10.5) | 0.84 (0.53–1.37) | 0.88 (0.54–1.43) |
| 4 (≥182.01) | 285 (24.4) | 19 (6.7) | 0.61 (0.36–1.04) | 0.60 (0.34–1.04) |
| Trend test | P = .11 | P = .11 | ||
| HDL-C | ||||
| 1 (≤36.00) | 266 (24.1) | 29 (10.9) | 1.00 | 1.00 |
| 2 (36.01–45.00) | 309 (27.9) | 27 (8.7) | 0.87 (0.51–1.51) | 0.79 (0.44–1.42) |
| 3 (45.01–55.00) | 260 (23.5) | 31 (11.9) | 0.85 (0.49–1.46) | 0.97 (0.54–1.75) |
| 4 (≥55.01) | 271 (24.5) | 27 (10.0) | 0.72 (0.41–1.26) | 0.70 (0.37–1.32) |
| Trend test | P = .27 | P = .39 | ||
| Triglycerides | ||||
| 1 (≤121.00) | 295 (25.5) | 37 (12.5) | 1.00 | 1.00 |
| 2 (121.01–161.00) | 283 (24.5) | 29 (10.2) | 0.82 (0.50–1.35) | 0.75 (0.44–1.27) |
| 3 (161.01–224.00) | 288 (24.9) | 29 (10.1) | 0.98 (0.58–1.63) | 0.89 (0.51–1.56) |
| 4 (≥224.01) | 290 (25.1) | 23 (7.9) | 0.76 (0.44–1.34) | 0.76 (0.42–1.39) |
| Trend test | P = .48 | P = .50 | ||
| LDL-C | ||||
| 1 (≤94.35) | 290 (24.9) | 36 (12.4) | 1.00 | 1.00 |
| 2 (94.36–117.30) | 291 (25.0) | 32 (11.0) | 1.00 (0.62–1.63) | 0.99 (0.59–1.65) |
| 3 (117.31–142.75) | 291 (25.0) | 26 (8.9) | 0.78 (0.46–1.31) | 0.78 (0.46–1.34) |
| 4 (≥142.76) | 291 (25.0) | 24 (8.2) | 0.88 (0.51–1.51) | 0.80 (0.46–1.40) |
| Trend test | P = .43 | P = .31 | ||
Abbreviations: AD, Alzheimer disease; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113.
Calculated as Cox proportional hazards model, with age at onset as time variable, as described in the text. Some percentages are based on an incomplete sample due to small amounts of missing data.
Adjusted for sex, age, education, and race.
Adjusted for body mass index, apolipoprotein E genotype, diabetes, heart disease, and hypertension.
COMMENT
In our cross-sectional analysis of 2820 subjects, we found that higher non-HDL-C and lower HDL-C levels were associated with a higher risk of VaD but not AD. We also found an association between higher LDL-C levels and a higher risk of VaD that was close to statistical significance, but higher LDL-C levels were not related to AD risk. Treatment with agents to lower lipid levels was negatively associated with the risk of AD but not VaD. In a longitudinal analysis of 1168 subjects (5189 person-years of follow-up), we observed an association between higher LDL-C levels and a higher risk of VaD but not AD, and replicated the association between higher non–HDL-C levels and a higher risk of VaD found in the cross-sectional analysis. Moreover, we found an association between higher cholesterol levels and a lower risk of AD. We did not replicate the association between HDL-C levels and risk of VaD found in the cross-sectional analysis in the prospective analysis. We also did not replicate the negative association of agents to lower lipid levels with the risk of AD found in the cross-sectional analysis.
The causal role of vascular risk factors in different types of dementia has been stressed during the past decade. 26 The sclerosis of small cerebral arteries and arterioles is considered to be responsible for diffuse periventricular white matter abnormalities, which play an important role in the development of VaD.26 Dyslipidemia, a well-established risk factor for ischemic heart disease, has not yet been convincingly demonstrated as a factor associated with brain ischemia, VaD, or AD. For example, several authors observed reference or low levels of total cholesterol or LDL-C in patients with ischemic stroke.26
There are different pathways in which plasma lipids could be associated with the risk of VaD. High concentrations of LDL-C and low levels of HDL-C are known to be independent risk factors for coronary heart disease14 and carotid artery atherosclerosis,27 which in turn may lead to cognitive impairment through cerebral hypoperfusion or embolism.28 Particles of HDL-C might also be linked with small-vessel disease by playing a role in the removal of excess cholesterol from the brain by interaction with APOE and heparan sulfate proteoglycans in the subendothelian space of cerebral microvessels.29 Second, the brain appears to be particularly vulnerable to oxidative lipid damage because of its high content of polyunsaturated fatty acids.30–32 Much evidence suggests that decreased levels of antioxidants such as vitamin E, vitamin A, vitamin C, or serum paraoxonase lead to higher susceptibility to oxidative stress and a higher grade of LDL-C oxidation, and different studies have found evidence of lower levels of antioxidants in patients with VaD.33,34 Moreover, there is evidence that LDL-C peroxidation increases with age.35
The role of dyslipidemia in the development of AD remains unclear. Brain cholesterol alters the degradation of amyloid precursor protein,12 which contributes to the pathogenesis of AD.12 However, brain cholesterol is almost entirely synthesized in situ and not transferred from the plasma into the brain because of the blood-brain barrier.36 Evidence also suggests that plasma cholesterol levels have no effect on brain hydroxymethylglutaryl coenzyme A reductase levels and their activity9 or levels of 24S-hydroxycholesterol, which is a degradation product of brain cholesterol.37 Moreover, reduced and not increased cellular cholesterol levels promote tau phosphorylation in neurons, inhibit dendrite outgrowth and synaptogenesis, and induce neurodegeneration.9
There are different pathways in which statins could lower the risk of dementia. Besides having a lowering effect on plasma lipid levels, they could also lower the risk of dementia by means of their pleiotropic effects.38 They can improve the endothelial function of atherosclerotic vessels by decreasing endothelin 1 and angiotensin II type 1 receptor and increasing nitric oxide.39 A lack of nitric oxide contributes to impaired endothelial function and platelet aggregation and enhances leukocyte adhesion to the endothelium. Moreover, statins are antithrombotic because they decrease plasminogen activator levels and anti-inflammatory because they decrease adhesion molecules. Statins may reduce apoptosis and cellular death by inhibiting the farnesylation of small G proteins, specifically Ras p21.38
Different studies have investigated the relationship between lipid levels and the risk of VaD. Many of them found an association with decreased levels of HDL-C. Zuliani et al29 found lower levels of HDL-C in 60 subjects with VaD compared with 54 controls. Kuriyama et al1 reported lower HDL-C levels in 43 patients with VaD compared with controls, and Muckle and Roy2 found lower HDL-C levels in 5 subjects with VaD compared with 12 patients with AD. Van Exel et al40 found a significant association between decreased HDL-C levels and cognitive impairment. Sacco et al41 found in a study from northern Manhattan, the same community as our sample, that high HDL-C levels were related to a lower risk of stroke, which indirectly supports our findings. The role of LDL-C remains controversial. Like Klich-Raczka et al4 and Paragh et al,33 we found an association between increased LDL-C levels and the risk of VaD in a former study.14 Other studies did not observe an association.40,42
Contradictory results have also been reported in AD. Increased and reduced levels of HDL-C3,8,9 and LDL-C6,8 have been observed to be associated with AD risk. Be-sides Lesser et al,7 who observed an association between high cholesterol levels and the risk of AD, Scacchi et al6 and Kuusisto et al43 found an association between high cholesterol levels and a lower risk of AD.
The role of drugs to lower lipid levels also remains unclear. Besides Hajjar et al,11 who reported an association of these agents with a lower risk of AD and VaD, Muldoon et al44 found a decrease in cognitive function in subjects using statins.
Our results are consistent with those of the studies by Zuliani et al,29 Kuriyama et al,1 and Muckle and Roy,2 which showed an association between low levels of HDL-C and the risk of VaD. They also agree with the findings by Paragh et al33 and Klich-Raczka et al,4 which showed an association between the risk of VaD and high LDL-C levels. Contrary to the results by Hajjar et al,11 we did not find an association between the use of agents to lower lipid levels and the risk of VaD. Unlike the study by Lesser et al,7 we did not observe an association between plasma lipid levels and the risk of AD. We found in the cross-sectional analysis a negative association between the use of statins and the risk of AD, as reported by Hajjar et al,11 but we did not replicate this in the longitudinal study. This discrepancy may be due to confounding by indication in the cross-sectional analyses. That is, persons with AD are not prescribed statins, whereas this confounding does not occur in the longitudinal analysis.
In our study, we observed an unexpected association between high cholesterol levels and a lower risk of AD. A possible explanation for this is the nutritional status of elderly patients in the early, prodromal stages of AD. At this stage, patients show alterations in the energetic profile as weight loss, reduced caloric intake, and increased energy requirement,6 and low cholesterol levels might reflect malnutrition in subjects with prodromal AD.
We found an association between higher LDL-C levels and a higher risk of VaD in the longitudinal study but not in the cross-sectional study. However, the results of the latter were close to statistical significance. We also observed an association between lower HDL-C levels and a higher risk of VaD in the cross-sectional but not the longitudinal study. Because the sample size of the longitudinal study was much smaller, it could be considered to lack statistical power. However, in this study we had 80% power to detect a relative risk of 2.0 in the cross-sectional and prospective analyses. Associations of smaller magnitude may be explained by bias and confounding. 45 In addition, regardless of the power of our data, our analyses with AD as an outcome clearly show that the HRs were close to 1.0. The magnitude of the HRs and the CIs did not suggest an association between higher lipid levels and an increased risk of AD, making it unlikely that our analyses missed meaningful associations owing to lack of power.
Compared with the lipid levels of the population of similar age and sex in the Third National Health and Nutrition Examination Survey (NHANES III), levels of total cholesterol and LDL-C were slightly lower and triglyceride levels were slightly higher in our population, whereas HDL-C levels were similar. However, the NHANES III data sampled white, Mexican American, and African American subjects,46 whereas almost half of our sample consisted of Caribbean Hispanic subjects, who are not represented in the NHANES III population. Thus, NHANES III findings may not be generalizable to our urban sample from northern Manhattan.
The mean limitation of this study is that we had only 1 measurement of lipid levels, which could have led to measurement error and an underestimation of the association between lipid levels and dementia.
CONCLUSIONS
We found that the risk of VaD increases with lower HDL-C levels and higher levels of non–HDL-C and LDL-C in cross-sectional or longitudinal analysis. Our results do not support the hypothesis that the risk of AD is associated with plasma lipid levels. They also do not support the hypothesis that statin use is associated with a lower risk of AD. The relation between HDL-C level and VaD needs further exploration in a larger prospective study.
REFERENCES
- 1.Kuriyama M, Takahashi K, Yamano T, et al. Low levels of serum apolipoprotein A I and A II in senile dementia. Jpn J Psychiatry Neurol. 1994;48:589–593. doi: 10.1111/j.1440-1819.1994.tb03019.x. [DOI] [PubMed] [Google Scholar]
- 2.Muckle TJ, Roy JR. High-density lipoprotein cholesterol in differential diagnosis of senile dementia. Lancet. 1985;1:1191–1193. doi: 10.1016/s0140-6736(85)92866-1. [DOI] [PubMed] [Google Scholar]
- 3.Kuriyama M, Hokezu Y, Togo S, et al. Serum lipids, lipoproteins and apolipoproteins in patients with senile dementia. Nippon Ronen Igakkai Zasshi. 1992;29:559–564. doi: 10.3143/geriatrics.29.559. [DOI] [PubMed] [Google Scholar]
- 4.Klich-Raczka A, Necki M, Wizner B, et al. Vascular dementia and systemic changes. Przegl Lek. 2002;59:269–271. [PubMed] [Google Scholar]
- 5.Wieringa GE, Burlinson S, Rafferty JA, Gowland E, Burns A. Apolipoprotein E genotypes and serum lipid levels in Alzheimer’s disease and multi-infarct dementia. Int J Geriatr Psychiatry. 1997;12:359–362. [PubMed] [Google Scholar]
- 6.Scacchi R, De Bernardini L, Mantuano E, et al. DNA polymorphisms of apolipoprotein B and angiotensin-1–converting enzyme genes and relationships with lipid levels in Italian patients with vascular dementia or Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9:186–190. doi: 10.1159/000017045. [DOI] [PubMed] [Google Scholar]
- 7.Lesser G, Kandiah K, Libow LS, et al. Elevated serum total and LDL cholesterol in very old patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12:138–145. doi: 10.1159/000051248. [DOI] [PubMed] [Google Scholar]
- 8.Kuo YM, Emmerling MR, Bisgaier CL, et al. Elevated low-density lipoprotein in Alzheimer’s disease correlates with brain Aβ 1–42 levels. Biochem Biophys Res Commun. 1998;252:711–715. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- 9.Michikawa M. Cholesterol paradox: is high total or low HDL cholesterol level a risk for Alzheimer’s disease? J Neurosci Res. 2003;72:141–146. doi: 10.1002/jnr.10585. [DOI] [PubMed] [Google Scholar]
- 10.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer’s disease associated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 11.Hajjar I, Schumpert J, Hirth V, Wieland D, Eleazer GP. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol A Biol Sci Med Sci. 2002;57:M414–M418. doi: 10.1093/gerona/57.7.m414. [DOI] [PubMed] [Google Scholar]
- 12.Burns M, Duff K. Cholesterol in Alzheimer’s disease and tauopathy. Ann N Y Acad Sci. 2002;977:367–375. doi: 10.1111/j.1749-6632.2002.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 13.Scheltens P, Kittner B. Preliminary results from an MRI/CT-based database for vascular dementia and Alzheimer’s disease. Ann N Y Acad Sci. 2000;903:542–546. doi: 10.1111/j.1749-6632.2000.tb06411.x. [DOI] [PubMed] [Google Scholar]
- 14.Moroney JT, Tang MX, Berglund J, et al. Low-density lipoprotein cholesterol and the risk of dementia with stroke. JAMA. 1999;282:254–260. doi: 10.1001/jama.282.3.254. [DOI] [PubMed] [Google Scholar]
- 15.Tang MX, Stern Y, Marder K, et al. The APOE ∊4 allele and the risk of Alzheimer’s disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 16.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population: development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49:453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 17.Census of Population and Housing. Summary Tape File 1, Technical Documentation [computer diskette] Washington, DC: Bureau of the Census; 1991. [Google Scholar]
- 18.Hatano S. Experience from a multicenter stroke register: a preliminary report. Bull World Health Organ. 1976;54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 20.Tatemichi TK, Desmond DW, Mayeux R, et al. Dementia after stroke: baseline frequency, risks and clinical features in a hospitalized cohort. Neurology. 1992;42:1185–1193. doi: 10.1212/wnl.42.6.1185. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Virella MF, Stone P, Ellis S, Colwell JA. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23:882–884. [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultra-centrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhAI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 25.Mayeux R, Ottmann R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 26.Ryglewicz D, Rodo M, Kunicki PK, et al. Plasma antioxidant activity and vascular dementia. J Neurol Sci. 2002;203–204:195–197. doi: 10.1016/s0022-510x(02)00290-3. [DOI] [PubMed] [Google Scholar]
- 27.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoprotein A1 and B, and triglycerides with carotid atherosclerosis and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1994;14:1098–1104. doi: 10.1161/01.atv.14.7.1098. [DOI] [PubMed] [Google Scholar]
- 28.Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam study. BMJ. 1994;308:1604–1608. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuliani G, Ble A, Zanca R, et al. Lipoprotein profile in older patients with vascular dementia and Alzheimer’s disease. BMC Geriatr. 2001;1:5. doi: 10.1186/1471-2318-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polidori MC, Mecocci P, Frei B. Plasma vitamin C levels are decreased and correlated with brain damage in patients with intracranial hemorrhage or head trauma. Stroke. 2001;32:898–902. doi: 10.1161/01.str.32.4.898. [DOI] [PubMed] [Google Scholar]
- 31.Braughler JM, Hall ED. Involvement of lipid peroxidation in CNS injury. J Neurotrauma. 1992;9:1–7. [PubMed] [Google Scholar]
- 32.Cao W, Carney JM, Duchon A, Floyd RA, Chevion M. Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci Lett. 1988;88:233–238. doi: 10.1016/0304-3940(88)90132-2. [DOI] [PubMed] [Google Scholar]
- 33.Paragh G, Balla P, Katona E, Seres I, Egerhazi A, Degrell I. Serum paraoxonase activity changes in patients with Alzheimer’s disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci. 2002;252:63–67. doi: 10.1007/s004060200013. [DOI] [PubMed] [Google Scholar]
- 34.Dantoine TF, Debord J, Merle L, Lacroix-Ramiandrisoa H, Bourzeix L, Charmes JP. Paraoxonase 1 activity: a new vascular marker of dementia? Ann N Y Acad Sci. 2002;977:96–101. doi: 10.1111/j.1749-6632.2002.tb04802.x. [DOI] [PubMed] [Google Scholar]
- 35.Napoli C, Abete P, Corso G, et al. Increased low-density lipoprotein peroxidation in elderly men. Coron Artery Dis. 1997;8:129–136. doi: 10.1097/00019501-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Schonknecht P, Lutjohann D, Pantel J, et al. Cerebrospinal fluid 24S-hydroxycholesterol is increased in patients with Alzheimer’s disease compared to healthy controls. Neurosci Lett. 2002;324:83–85. doi: 10.1016/s0304-3940(02)00164-7. [DOI] [PubMed] [Google Scholar]
- 38.Ruocco A, Postiglione A, Santillo M, et al. New possible role of statins in age-related diseases. J Am Geriatr Soc. 2002;50:2099–2100. doi: 10.1046/j.1532-5415.2002.50631.x. [DOI] [PubMed] [Google Scholar]
- 39.Wassmann S, Laufs U, Baumer AT, et al. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1407. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 40.Van Exel E, de Craen AJ, Gussekloo J. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann Neurol. 2002;51:716–721. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- 41.Sacco RL, Benson R, Kargman DE, et al. High-density lipoprotein cholesterol and ischemic stroke in the elderly: the Northern Manhattan Stroke Study. JAMA. 2001;285:2729–2735. doi: 10.1001/jama.285.21.2729. [DOI] [PubMed] [Google Scholar]
- 42.Yoshitake T, Kiyohora Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hi-sayama Study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 43.Kuusisto J, Koivisto K, Mykkanen L, et al. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross-sectional population based study. BMJ. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muldoon MF, Barger SD, Ryan CM, et al. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. 2000;108:538–547. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro S. Bias in the evaluation of low-magnitude associations: an empirical perspective. Am J Epidemiol. 2000;151:946–950. doi: 10.1093/oxfordjournals.aje.a010135. [DOI] [PubMed] [Google Scholar]
- 46.Sundquist J, Winkleby MA, Pudaric S. Cardiovascular disease risk factors among older Black, Mexican-American, and White women and men: an analysis of NHANES III, 1988–1994. J Am Geriatr Soc. 2001;49:109–116. doi: 10.1046/j.1532-5415.2001.49030.x. [DOI] [PubMed] [Google Scholar]