Abstract
Background
We analyzed a pre-specified hypothesis of the Occluded Artery Trial (OAT), that late percutaneous coronary intervention (PCI) of the infarct-related artery (IRA) would be most beneficial for patients with anterior MI.
Methods
2201 stable high-risk patients with total occlusion of the IRA (793 left anterior descending [LAD]) on days 3 to 28 (minimum 24 hrs) after MI were randomized to PCI and stenting with optimal medical therapy (1101 patients) or to optimal medical therapy alone (1100 patients). The primary end point was a composite of death, recurrent MI, or hospitalization for class IV heart failure.
Results
The 5-year cumulative primary end point rate was more frequent in the LAD group (19.5%) than in the non-LAD group (16.4%) (HR=1.34, 99%CI 1.00–1.81, p=.01). Within the LAD group the HR for the primary end point in the PCI group (22.7%) compared with the medical therapy group (16.4%) was 1.35 (99%CI 0.86–2.13, p=.09), whereas in the non-LAD group the HR for the primary end point in PCI (16.9%) compared with medical therapy (15.8%) was 1.03 (99%CI 0.70–1.52, p=.83) (interaction p=.24). The results were similar when the effect of PCI was assessed in patients with proximal LAD occlusion.
Conclusions
In stable patients, persistent total occlusion of the LAD post MI is associated with a worse prognosis compared with occlusion of the other IRAs. A strategy of PCI of occluded LAD IRA more than 24 hours post MI in stable patients is not beneficial and may increase risk of adverse events in comparison to optimal medical treatment alone.
Introduction
Rapid restoration of blood flow in the infarct-related artery (IRA), a cornerstone of contemporary treatment of acute myocardial infarction (MI), prevents myocardial necrosis and its consequences [1]. However, due to late presentation or failed fibrinolytic therapy, up to one third of patients have persistently occluded IRA after MI [2].
Recently, the Occluded Artery Trial (OAT) demonstrated that percutaneous coronary intervention (PCI) with optimal medical therapy does not reduce the frequency of major adverse events during a 4-year follow-up period compared to optimal medical therapy alone when performed on days 3–28 post MI in stable patients [3].
One of the secondary hypotheses of OAT was that late coronary revascularization of the IRA would be most beneficial for patients with anterior wall infarction [4]. Acute myocardial infarction involving the left anterior descending (LAD) coronary artery, especially its proximal segments, has been associated with a worse prognosis compared to MI involving other coronary arteries [5–7]. The difference is believed to be primarily related to a larger area of myocardium at risk with LAD occlusion, resulting in a greater impairment of left ventricular (LV) function and remodeling. Most of the previous studies have shown that late reperfusion can reduce adverse left ventricular remodeling and preserve LV function [8–11]. This effect was hypothesized to have the greatest impact in patients with the largest area of myocardium at risk. Therefore, a high risk population of patients with post MI occlusion of the LAD, and in particular its proximal segments, would be expected to benefit most from late recanalization.
Consequently, we compared the effect of late opening of LAD and non LAD IRAs on outcomes in stable patients post MI enrolled in OAT [3].
Methods
The design and methods of OAT study have been reported previously [4]. Current analysis included 2201 patients (2166 from the main OAT trial randomized between February 2000 and December 2005 and additional 35 patients enrolled in the extension phase of the OAT-NUC ancillary study in 2006). Eligible patients had a total occlusion of the IRA on days 3–28 (minimum 24 hours) after MI and met at least one of the high risk criteria: ejection fraction (EF) <50% and/or proximal occlusion of the IRA. Exclusion criteria included New York Heart Association (NYHA) class III or IV heart failure (CHF), shock, a serum creatinine concentration ≥2.5 mg per deciliter (221 μmol per liter), angiographically significant left main or three-vessel coronary artery disease, angina at rest, or severe ischemia on stress testing (performed if ischemia was suspected and required in those without infarct zone akinesis or dyskinesis). Patients were randomized to PCI with stenting and optimal medical therapy (1101 patients) or optimal medical therapy alone (1100 patients). Medical management included daily aspirin, anticoagulation if indicated, β-blockers, angiotensin-converting enzyme (ACE) inhibitors and lipid-lowering therapy, unless contraindicated. Thienopyridine therapy was initially recommended for 2 to 4 weeks following bare metal stent (BMS) implantation and 3 to 6 months following drug-eluting stent (DES) deployment. After publication of data supporting longer-term therapy following acute coronary syndrome, clopidogrel was recommended for one year in both groups [12]. Patients randomized to PCI were to undergo the procedure within 24 hours after treatment allocation with stenting of the occluded segment as well as high-grade stenoses in major proximal or distal segments whenever technically feasible. Use of glycoprotein IIb/IIIa was strongly recommended.
Images from the PCI were reviewed at the angiography core laboratory. Cardiac markers (preferably creatine kinase myocardial band or, if not available, troponin I or T or creatine kinase) were to be measured routinely in both groups three times during the first 48 hours after randomization and within 24 hours after PCI in patients assigned to PCI.
Institutional review boards at the participating centers approved the study protocol and all patients provided written informed consent. The project described was supported by Award Numbers U01 HL062509 and U01 HL062511 from the National Heart, Lung, And Blood Institute; Supplemental grant funds and product donations equivalent to <5% of total study cost from: Eli Lilly, Millenium Pharmaceuticals ans Schering Plough, Guidant, Cordis/Johnson and Johnson, Medtronic, Merck and Bristol Myers Squibb Medical Imaging. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final content, which does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
Study end points
The primary end point was defined as time to the first occurrence of MI, hospitalization or short-stay unit treatment of class IV CHF, or death from any cause. Secondary end points included the individual components of the primary end point, NYHA III CHF, stroke, nonprotocol revascularization and reported angina. Mean follow up was 3.2 years.
Statistical analysis
Baseline characteristics of study patients were summarized in terms of frequencies and percentages for categorical variables and by means and standard deviations (SD) for continuous variables with normal distribution. Categorical variables were compared by either Fisher exact or χ2 test and continuous variables by student t-test. The primary analysis was intention-to-treat. The occurrence of the primary and secondary end points in the two treatment groups was compared by the log-rank test. Prespecified subgroup analyses of the primary or secondary outcome were carried out with Cox proportional hazards regression [4]. Additionally, a multivariable Cox proportional hazards model was developed to evaluate the relationship between the baseline characteristics, and occurrence of the primary end point and death alone. All baseline variables entered the multivariable stage, regardless of the significance of the association in the univariable analyses. In order to be sensitive to the likelihood of a Type 1 error after multiple analyses, statistical significance in all tests was set at p<.01. Results are reported with 99% confidence intervals (CI). Statistical testing was performed using the SAS System version 9.1.3 (SAS Institute, Cary, NC).
Results
Baseline characteristics
There were 793 patients (36%) with left anterior descending artery IRA and 1408 patients (64%) with non-LAD as the IRA (left circumflex artery [335, 15%] or right coronary artery [1073, 49%]). Baseline clinical and angiographic characteristics of patients with LAD IRA as compared with non-LAD are presented in Table 1. Patients with LAD IRA compared to non-LAD IRA were older and less frequently male or cigarette smokers with less common history of previous MI or PCI. Patients with LAD IRA were also more likely to have heart failure as demonstrated by higher Killip class during index MI, more frequent NYHA class II and lower LV ejection fraction at randomization. Patients with IRA-LAD had lower frequency of angiographically visible collateral vessels or TIMI flow<1 in IRA as compared with non-LAD. There was no difference in median time between index MI and randomization in relation to LAD and non-LAD IRA. PCI success rates were similar for LAD and non-LAD IRAs. The elevation of cardiac markers within 48h after randomization was similar in both IRA groups.
Table 1.
Baseline clinical, angiographic and post-randomization core laboratory characteristics by infarct related artery
| Characteristic | LAD* N=793 | non-LAD* N=1408 | P |
|---|---|---|---|
| Clinical | |||
|
| |||
| Age – yr (SD) | 59.5±11.2 | 58.1±10.8 | .005 |
|
| |||
| Male sex – no. (%) | 591 (74.5) | 1126 (80.0) | .003 |
|
| |||
| White race – no. (%) | 630 (79.4) | 1133 (80.5) | ns |
|
| |||
| History – no. (%) | |||
| Angina | 188 (23.7) | 307 (21.8) | ns |
| Myocardial infarction | 69 (8.7) | 178 (12.6) | .005 |
| PCI | 19 (2.4) | 86 (6.1) | <.0001 |
| CABG | 0 (0.0) | 9 (0.6) | ns |
| Stroke | 21 (2.6) | 42 (3.0) | ns |
| Peripheral-vessel disease | 24 (3.0) | 59 (4.2) | ns |
| Heart failure | 18 (2.3) | 34 (2.4) | ns |
| Diabetes | 185 (23.3) | 269 (19.1) | ns |
| Hypertension | 393 (49.6) | 678 (48.2) | ns |
| Hypercholesterolemia | 407 (51.3) | 735 (52.2) | ns |
| Renal insufficiency | 12 (1.5) | 18 (1.3) | ns |
| Family history | 310 (39.1) | 573 (40.7) | ns |
|
| |||
| Current cigarette smoker – no. (%) | 260 (32.8) | 599 (42.5) | <.0001 |
|
| |||
| Highest Killip class II–IV during index MI – no.(%) | 189/788 (24.0) | 228/1404 (16.2) | <.0001 |
|
| |||
| NYHA at randomization class II – no. (%) | 165/791 (20.9) | 202/1406 (14.4) | .001 |
|
| |||
| New Q waves – no.(%) | 592 (74.7) | 883 (62.7) | <.0001 |
|
| |||
| ST-segment elevation – no.(%) | 631/766 (82.4) | 779/1360 (57.3) | <.0001 |
|
| |||
| ST-segment elevation or Q-wave or R-wave loss – no. (%) | 749 (94.5) | 1156 (82.1) | <.0001 |
|
| |||
| Thrombolytic therapy during first 24hr after onset of index MI – no. (%) | 174 (21.9) | 250 (17.8) | ns |
|
| |||
| Interval between MI and randomization – days | |||
| Median | 9 | 8 | ns |
| 25th, 75th | 5,17 | 5,16 | |
|
| |||
| Stress test perfomed – no. (%) | 168 (21.2) | 430 (30.5) | <.0001 |
|
| |||
| Ischemia in infarct-related artery territory – no. (%) | |||
| Severe | 0 (0) | 1 (0.2) | ns |
| Moderate | 16 (9.5) | 43 (10.0) | |
| Mild | 46 (27.4) | 134 (31.2) | |
| None | 106 (63.1) | 252 (58.6) | |
|
| |||
| Angiographic | |||
|
| |||
| TIMI flow grade in IRA prior to randomization- no. (%) | N=784 | N=1187 | |
| 0 | 618 (78.8) | 1187 (85.0) | .0002 |
| 1 | 159 (20.3) | 207 (14.8) | |
| 2 | 6 (0.8) | 1 (0.1) | |
| 3 | 1 (0.1) | 1 (0.1) | |
|
| |||
| Collateral vessel present – no. (%) | 656/780 (84.1) | 1266/1393 (90.9) | <.0001 |
|
| |||
| Multivessel disease – no. (%) | 134/784 (17.1) | 245/1399 (17.5) | ns |
|
| |||
| Ejection fraction | |||
| Mean | 42.3±11.5 | 50.7±9.3 | <.0001 |
| <50% – no. (%) | 583/787 (74.1) | 587/1398 (42.0) | <.0001 |
| <40% – no. (%) | 300/787 (38.1) | 149/1398 (10.7) | <.0001 |
|
| |||
| Post-randomization | |||
|
| |||
| PCI successful – no. (%) | 341/383 (89.0) | 612/718 (85.2) | ns |
|
| |||
| Elevation of cardiac marker within 48h after randomization. | 45/709 (6.35) | 86/1255 (6.85) | ns |
CABG – coronary artery bypass grafting, LAD – left anterior descending artery, MI –myocardial infarction, NYHA – New York Heart Association, PCI – percutaneous coronary intervention, TIMI – Thrombolysis in Myocardial Infarction
There was no between group difference in baseline characteristics between those assigned to PCI and medical therapy only for LAD and non-LAD patients (except for a 2 mm Hg higher diastolic blood pressure for the medical therapy only LAD patients, 1.5 mmHg higher diastolic blood pressure for PCI non-LAD patients and 5.1% greater prevalence of diabetes for medical therapy only non-LAD patients).
Medications prescribed at discharge are shown in Table 2. Patients with LAD IRA were more likely prescribed medications to treat heart failure such as angiotensin-converting enzyme inhibitors, digoxin, spironolactone, and diuretic agents. They also were more likely to receive anti-arrhythmic drugs and warfarin. They were less likely to receive sublingual nitrates or lipid lowering-agents.
Table 2.
Medication use by infarct realted artery
| Medication | LAD N=793 | non-LAD N=1408 | P |
|---|---|---|---|
| In-hospital | |||
| Gp IIb/IIIa in first 24 hours | 58 (7.3) | 124 (8.8) | ns |
| Gp IIb/IIIa inhibitors | 268 (33.8) | 512 (36.4) | ns |
| On discharge | |||
| Aspirin – no. (%) | 753 (95.0) | 1352 (96.0) | ns |
| Clopidogrel – no. (%) | 363 (45.8) | 712 (50.6) | ns |
| Ticlopidine – no. (%) | 98 (12.4) | 161 (11.4) | ns |
| Thienopyridine (clopidogrel or ticlopidine) – no. (%)* | 457 (57.6) | 872 (61.9) | ns |
| Warfarin – no. (%) | 187 (23.6) | 28 (2.0) | <.0001 |
| Aspirin and thienopyridine and warfarin – no. (%) | 70 (8.8) | 8 (0.6) | <.0001 |
| Beta blocker – no. (%) | 723 (91.2) | 1209 (85.9) | .0003 |
| Calcium-channel blocker – no. (%) | 34 (4.3) | 95 (6.7) | ns |
| Sublingual nitrate – no. (%) | 205 (25.9) | 447 (31.7) | .004 |
| Long acting nitrate – no. (%) | 189 (23.8) | 309 (21.9) | ns |
| ACE inhibitor or ARB – no. (%) | 700 (88.3) | 1071 (76.1) | <.0001 |
| Diuretic agent – no. (%) | 188 (23.7) | 183 (13.0) | <.0001 |
| Digoxin – no. (%) | 42 (5.3) | 19 (1.3) | <.0001 |
| Spironolactone – no. (%) | 72 (9.1) | 52 (3.7) | <.0001 |
| Insulin – no. (%) | 66 (8.3) | 71 (5.0) | .002 |
| Oral hypoglycemic agent – no. (%) | 117 (14.8) | 181 (12.9) | ns |
| Lipid-lowering agent – no. (%) | 622 (78.4) | 1166 (82.8) | .01 |
| Antiarrhythmic (other than beta-blocker) – no. (%) | 46 (5.8) | 38 (2.7) | .0003 |
ACE – angiotensin converting enzyme, ARB – angiotensin receptor blocker, GP –glycoprotein, LAD – left anterior descending artery
A thienopyridine was prescribed for more then 99% of patients in the PCI group in whom PCI with stenting was successful. A thienopyridine was prescribed for 29% of patients assigned to medical therapy only.
Five-year outcomes within IRA classification and treatment allocation
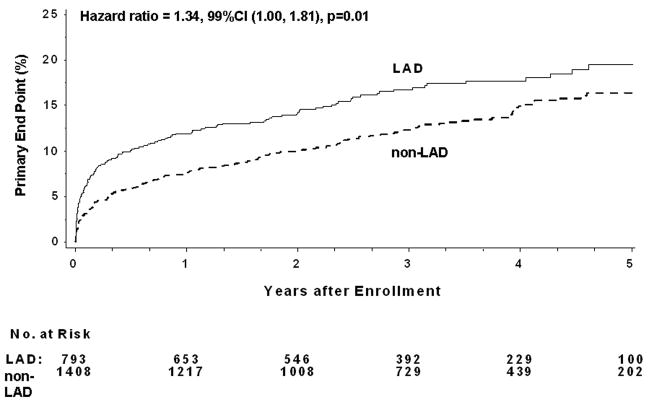
The five-year primary end point was more frequent in LAD (19.5%) than in non-LAD IRA (16.4%) (HR=1.34, 99%CI 1.00–1.81, p=.01) (Table 3, Figure 1). Analysis of secondary outcomes revealed excess of deaths (14.1% vs. 10.4%, HR=1.54, 99%CI 1.05–2.26, p=.003) and hospitalizations for class IV heart failure (6.4% vs. 3.6%, HR=1.93, 99%CI 1.11–3.34, p=.002), but no significant difference in recurrent MIs (5.2% vs. 6.5%, HR=0.91, 99%CI 0.54–1.55, p=.66) in the LAD group compared to the non LAD IRA group. Multivariable Cox analysis revealed that the LAD IRA was not independently associated with the primary end point or death. Factors independently associated with the primary end point were history of heart failure, peripheral vascular disease, diabetes, rales during randomization, decreased EF, time elapsed from MI and lower glomerular filtration rate (GFR). Independent predictors of death were: Killip class>1, history of cerebrovascular disease, angina, history of heart failure, decreased EF, days from MI and low GFR.
Table 3.
5-year outcomes by study groups
| LAD N=793 | non-LAD N=1408 | Cox P-value | |||
|---|---|---|---|---|---|
| No of outcomes | Estimated 5-yr cumulative event rate (%) | No. of outcomes | Estimated 5-yr cumulative event rate (%) | ||
| Centrally adjudicated | |||||
| Primary end point | 131 | 19.5 | 180 | 16.4 | .01 |
| Death from all causes | 84 | 14.1 | 100 | 10.4 | .003 |
| Fatal and nonfatal reinfarction | 35 | 5.2 | 70 | 6.5 | .66 |
| Procedure-related | 4 | 3 | |||
| Not procedure-related | 31 | 67 | |||
| Nonfatal reinfarction | 33 | 4.9 | 66 | 6.2 | .67 |
| NYHA class IV heart failure | 45 | 6.4 | 43 | 3.6 | .002 |
| Cardiovascular death | 63 | 10.2 | 57 | 5.6 | <.001 |
| Death or nonfatal reinfarction | 109 | 17.1 | 158 | 15.4 | .06 |
| NYHA class III or IV heart failure | 61 | 8.5 | 58 | 4.5 | <.001 |
| Death, reinfarction, or NYHA class III or IV heart failure | 142 | 20.8 | 194 | 17.4 | .006 |
| Stroke | 17 | 2.3 | 18 | 1.5 | .11 |
| Site-determined | |||||
| Nonprotocol revascularization | 138 | 19.8 | 247 | 21.1 | .81 |
| Reported angina* | 276 | 41.9 | 488 | 40.4 | .76 |
LAD – left anterior descending artery, NYHA – New York Heart Association
For the reported angina, which required at least one follow-up visit the following denominators were used: LAD – 750, non-LAD – 1364.
Figure 1.
Kaplan-Meier for the primary 5-year endpoint for combined PCI and medical therapy in LAD and non-LAD IRA (log rank test used for comparison between groups).
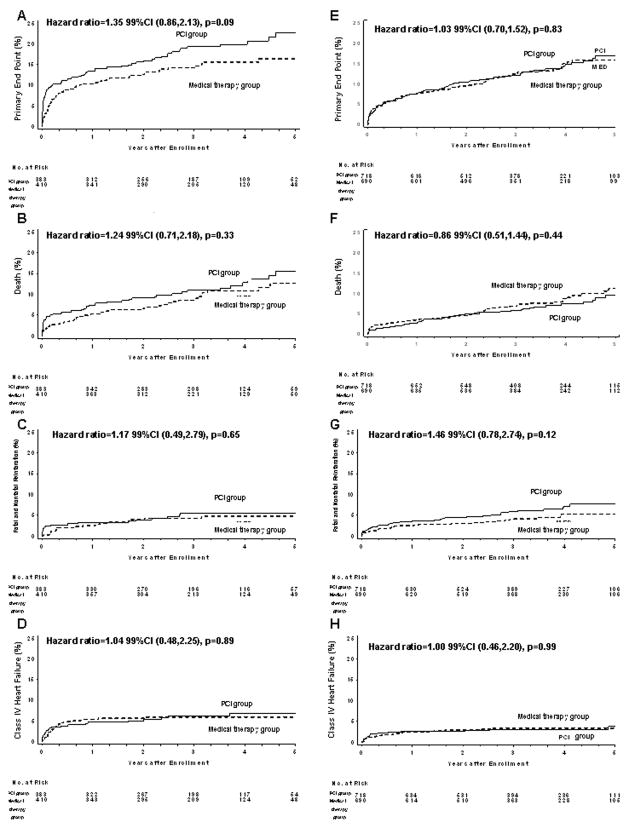
The HR for PCI vs. medical therapy alone for the primary end point was 1.35 (99%CI 0.86–2.13, p=.09) in the LAD group and 1.03 (99%CI 0.70–1.52, p=.83) in the non-LAD group (Table 4, Figure 2) (interaction p=.24). When secondary end points were analyzed, there were no significant differences in all cause deaths, fatal and non-fatal myocardial infarctions and hospitalizations for heart failure or strokes according to treatment allocation in both the LAD and non-LAD. The benefit of PCI on angina and revascularization over 5 year follow-up was more readily apparent in the larger non-LAD group (Table 4).
Table 4.
5-year outcomes by treatment assignment
| LAD | PCI (n=383) | Medical therapy (n=410) | P* | HR* (99%CI) | Inter action P | ||
|---|---|---|---|---|---|---|---|
| No of outcomes | Estimated 5-yr cumulative event rate (%) | No. of outcomes | Estimated 5-yr cumulative event rate (%) | ||||
| Centrally adjudicated | |||||||
| Primary end point | 72 | 22.7 | 59 | 16.4 | .09 | 1.35 (0.86–2.13) | .24 |
| Death from all causes | 45 | 15.6 | 39 | 12.7 | .33 | 1.24 (0.71–2.18) | .22 |
| Fatal and nonfatal reinfarction | 18 | 5.6 | 17 | 4.8 | .65 | 1.17 (0.49–2.79) | .58 |
| Procedure- Related | 4 | 0 | |||||
| Not procedure- related | 14 | 17 | |||||
| Nonfatal reinfarction | 18 | 5.6 | 15 | 4.3 | .42 | 1.32 (0.54–3.25) | .76 |
| NYHA class IV heart failure | 22 | 6.9 | 23 | 6.0 | .89 | 1.04 (0.48–2.25) | .93 |
| Death or nonfatal reinfarction | 60 | 19.2 | 49 | 15.1 | .13 | 1.34 (0.82–2.21) | .39 |
| NYHA class III or IV heart failure | 32 | 9.6 | 29 | 7.5 | .47 | 1.2 (0.62–2.33) | .24 |
| Death, reinfarction, or NYHA class III or IV heart failure | 80 | 24.6 | 62 | 17.1 | .03 | 1.43 (0.92–2.21) | .08 |
| Stroke | 11 | 1.6 | 6 | 3.0 | .30 | 0.59 (0.16–2.18) | .3 |
| Site-determined | |||||||
| Nonprotocol revascularization | 65 | 20.1 | 73 | 19.5 | .71 | 0.94 (0.60–1.46) | .26 |
| Reported angina* | 126 | 39.2 | 150 | 44.6 | .25 | 0.87 (0.64–1.19) | .22 |
| Non-LAD | PCI (n=718) | Medical therapy (n=690) | |||||
| Centrally adjudicated | |||||||
| Primary end point | 93 | 16.9 | 87 | 15.8 | .83 | 1.03 (0.7–1.52) | |
| Death from all causes | 47 | 9.6 | 53 | 11.2 | .44 | 0.86 (0.51–1.44) | |
| Fatal and nonfatal reinfarction | 42 | 7.7 | 28 | 5.2 | .12 | 1.46 (0.78–2.74) | |
| Procedure-Related | 2 | 1 | |||||
| Not procedure-related | 40 | 27 | |||||
| Nonfatal reinfarction | 40 | 7.4 | 26 | 5.0 | .11 | 1.50 (0.78–2.87) | |
| NYHA class IV heart failure | 22 | 3.8 | 21 | 3.4 | .99 | 1.00 (0.46–2.2) | |
| Death or nonfatal reinfarction | 83 | 16.0 | 75 | 14.8 | .62 | 1.08 (0.72–1.63) | |
| NYHA class III or IV heart failure | 26 | 3.9 | 32 | 5.2 | .34 | 0.78 (0.39–1.54) | |
| Death, reinfarction, or NYHA class III or IV heart failure | 97 | 17.4 | 97 | 17.3 | .81 | 0.97 (0.67–1.4) | |
| Stroke | 10 | 1.6 | 8 | 1.3 | .69 | 1.21 (0.36–4.1) | |
| Site-determined | |||||||
| Nonprotocol revascularization | 109 | 18.9 | 138 | 23.4 | .02 | 0.74 (0.53–1.02) | |
| Reported angina** | 220 | 36.3 | 268 | 44.7 | .0003 | 0.72 (0.57–0.91) | |
LAD – left anterior descending artery, NYHA – New York Heart Association
Covariate adjustment of treatment effect (for diabetes and diastolic blood pressure) demonstrated minimal change in the hazard ratios.
For the reported angina, which required at least one follow-up visit the following denominators were used: in the LAD group (PCI – 358, medical therapy – 392) and in the non-LAD (PCI – 694, medical therapy – 670).
Figure 2.
Kaplan-Meier for the primary and secondary 5-year end points, according to treatment assignment (PCI vs. medical therapy) and the intention-to-treat analysis within study groups (LAD, non-LAD) (log-rank test used for comparisons between groups). A–D LAD: A – primary end point, B – death, C – fatal and nonfatal reinfarction, D – class IV heart failure requiring hospitalization or a stay in a short-stay unit; E–H non-LAD: E –primary end point, F – death, G – fatal and nonfatal reinfarction, H – class IV heart failure requiring hospitalization or a stay in a short-stay unit.
The results were similar for the primary outcome when the effect of PCI was assessed in patients with proximal LAD occlusion before the first septal branch (proximal LAD, n=271; PCI vs. medical therapy, HR=1.51, 99%CI 0.72–3.14, p=.15) as well as in patients with occlusion up to second septal branch (modified proximal LAD, n=765; PCI vs. medical therapy, HR=1.35, 99%CI 0.85–2.13, p=.09).
Discussion
Our results confirm that the LAD IRA is a risk indicator among stable patients with persistent total occlusion of the infarct artery over a time horizon of several years post MI. The main reason for the unfavorable outcome in this group of patients compared to other infarct locations was an association with larger index infarctions, as demonstrated by lower EF and higher Killip and NYHA classes. Multivariable modeling for the primary end point and death suggested that higher risk associated with the LAD IRA was mediated by lower ejection fraction and more frequent history of heart failure in those patients.
Contrary to the hypothesis, there was no suggestion that patients with LAD infarction benefited from late opening of the artery in the OAT time window.
Few studies on the role of PCI in the late reperfusion after MI performed have concentrated specifically on patients with anterior infarctions. All of them included a relatively small number of patients in comparison to OAT, which enrolled 793 patients with LAD occlusion and 2201 patients overall. Pizzetti and colleagues showed that coronary angioplasty performed after a mean of 15 days in 67 patients with anterior MI improved LV ejection fraction and reduced a degree of LV dilation at 6 months in comparison to conservative treatment [8]. In another study by Horie et al. 83 patients with anterior MI were randomized to PCI or medical therapy after >24 hours from the onset of symptoms [9]. The trial showed that late PCI leads to smaller end-diastolic and end-systolic volumes at 6 months as well as fewer deaths, recurrent MI, and congestive heart failure at 50 months. Both of those studies however were performed over a decade ago, when patients less frequently received beta-blockers, angiotensin converting enzyme inhibitors or statins in comparison to contemporary standards. The less rigorous medical therapy may have increased the apparent positive effects of the interventional strategy, as all of those drugs have a potential to increase survival [13]. More recently a study by Silva et al. with high use of ACE inhibitors failed to demonstrate that PCI improves LV volumes or decreases infarct size, but did show an increase in LV ejection fraction and a change in the circumferential shortening of the remote segments in the PCI group compared to no-PCI group. The study, which enrolled 36 patients, was not powered to analyze clinical end-points [10].
Only one small trial – The Open Artery Trial (TOAT) performed more recently enrolled 66 patients with clinical characteristics similar to the OAT subgroup in this report (occluded LAD, ejection fraction <50%, mostly one-vessel disease and no ischemia on symptom-limited exercise treadmill test, over 80% of patients receiving beta-blockers and 100% ACE inhibitors) and utilized stents in all PCI patients [14]. Of note, contrary to TOAT, OAT allowed mild or moderate ischemia at randomization. Although not powered for clinical events, TOAT reported an apparent excess of events, including death, MI, stroke, congestive heart failure as well as revascularization at 12 months in patients randomized to PCI. In the same trial the end-systolic and end-diastolic volumes unexpectedly increased more in patients undergoing PCI than in patients treated conservatively.
As demonstrated above previously published clinical trials on late reperfusion in patients with LAD occlusion after MI, except TOAT, reported improvement of LV function measured with LV ejection fraction or volumes. The TOSCA-2 study, an ancillary study of OAT also demonstrated that assignment to PCI is related to smaller increase of LV volume in a subgroup of patients with LV measurements, although this analysis did not compare different IRAs. The current analysis shows that surrogate benefits of late reopening of the IRA are not related to better prognosis in the subset of patients with LAD occlusion treated with PCI, those previously hypothesized to benefit most from the improvement of left ventricular function or prevention of its remodeling.
There was also no difference in outcomes in relation to the location of LAD occlusion [5, 6]. An explanation for that finding may be that there were only a relatively small number of patients with mid or distal LAD occlusion, because OAT entry criteria included a requirement for proximal coronary occlusion (up to second septal branch in the case of the LAD) and/or depressed LVEF.
The percentage of patients with LAD occlusion in OAT (36% of all IRAs) was modestly lower then in primary PCI trials [15]. This difference is most likely because OAT focused on stable patients after MI, while LAD occlusion more often then occlusion of other IRAs leads to haemodynamic instability in the acute phase of MI. Therefore patients with LAD occlusion were less likely to qualify for OAT. This is also supported by the evidence of higher prevalence of collateral vessels in patients with LAD as IRA in OAT in comparison to patients from primary PCI trials. In patients with acute MI, the presence of angiographically detectable collateral vessels reduces the likelihood of hemodynamic instability, especially when LAD is involved in the infarct [7, 15–17].
The analysis has several limitations. Conclusions drawn about the effects of PCI of the LAD in the context of MI pertain only to patients who would qualify for OAT, that is, stable patients with not more than moderate ischemia (mostly none or mild ischemia) who do not have three-vessel CAD. Although the number of patients with LAD occlusion is far greater than all prior studies of late recanalization of the IRA, the power of this subset analysis is lower then 94% observed in the main OAT report [3]. Nevertheless, there was no suggestion of any benefit of PCI for patients with LAD occlusion.
Conclusions
Persistent total occlusion of the LAD is associated with a worse prognosis compared with occlusion of other IRAs. A strategy of PCI of the occluded LAD more than 24 hours post MI in stable patients is not beneficial and may increase risk of adverse events in comparison to optimal medical treatment alone.
The great value of strategies aimed to avoid late presentation of patients with acute MI and a stronger involvement in regional programs of acute MI interventions to achieve rapid restoration of blood flow through IRA either in urban or rural areas should be therefore emphasized.
Footnotes
No conflict of interest.
NIH PubMed Central Policy: We request the journal to acknowledge that the Author retains the right to provide a copy of the final manuscript to the NIH upon acceptance for Journal publication, for public archiving in PubMed Central as soon as possible but no later than 12 months after publication by Journal.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boden WE, Eagle K, Granger CB. Reperfusion strategies in acute ST-segment elevation myocardial infarction: a comprehensive review of contemporary management options. J Am Coll Cardiol. 2007;50:917–929. doi: 10.1016/j.jacc.2007.04.084. [DOI] [PubMed] [Google Scholar]
- 2.Eagle KA, Goodman SG, Avezum A, et al. Practice variation and missed opportunities for reperfusion in ST-segment-elevation myocardial infarction: findings from the Global Registry of Acute Coronary Events (GRACE) Lancet. 2002;359:373–7. doi: 10.1016/S0140-6736(02)07595-5. [DOI] [PubMed] [Google Scholar]
- 3.Hochman JS, Lamas GA, Buller CE, et al. Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:1–13. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Occluded Artery Trial (OAT) Research Group. Design and methodology of the Occluded Artery Trial (OAT) Am Heart J. 2005;150:627–42. doi: 10.1016/j.ahj.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Gheeraert PJ, Henriques JP, De Buyzere ML, et al. Out-of-hospital ventricular fibrillation in patients with acute myocardial infarction: coronary angiographic determinants. J Am Coll Cardiol. 2000;35:144–50. doi: 10.1016/s0735-1097(99)00490-8. [DOI] [PubMed] [Google Scholar]
- 6.Karha J, Murphy SA, Kirtane AJ, et al. Evaluation of the association of proximal coronary culprit artery lesion location with clinical outcomes in acute myocardial infarction. Am J Cardiol. 2003;92:913–918. doi: 10.1016/s0002-9149(03)00969-x. [DOI] [PubMed] [Google Scholar]
- 7.Kandzari DE, Tcheng JE, Gersh BJ, et al. Relationship between infarct artery location, epicardial flow, and myocardial perfusion after primary percutaneous revascularization in acute myocardial infarction. Am Heart J. 2006;151:1288–95. doi: 10.1016/j.ahj.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Pizzetti G, Belotti G, Margonato A, et al. Coronary recanalization by elective angioplasty prevents ventricular dilation after anterior myocardial infarction. J Am Coll Cardiol. 1996;28:837–45. doi: 10.1016/s0735-1097(96)00276-8. [DOI] [PubMed] [Google Scholar]
- 9.Horie H, Takahashi M, Minai K, et al. Long-term beneficial effect of late reperfusion for acute anterior myocardial infarction with percutaneous transluminal coronary angioplasty. Circulation. 1998;98:2377–82. doi: 10.1161/01.cir.98.22.2377. [DOI] [PubMed] [Google Scholar]
- 10.Silva JC, Rochitte CE, Junior JS, et al. Late coronary artery recanalization effects on left ventricular remodelling and contractility by magnetic resonance imaging. Eur Heart J. 2005;26:36–43. doi: 10.1093/eurheartj/ehi011. [DOI] [PubMed] [Google Scholar]
- 11.Dzavik V, Buller CE, Lamas GA, et al. Randomized trial of percutaneous coronary intervention for subacute infarct-related coronary artery occlusion to achieve long-term patency and improve ventricular function: The Total Occlusion Study of Canada (TOSCA)-2 Trial. Circulation. 2006;114:2449–2457. doi: 10.1161/CIRCULATIONAHA.106.669432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinhubl SR, Berger PB, Mann JT, 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–20. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 14.Yousef ZR, Redwood SR, Bucknall CA, et al. Late intervention after anterior myocardial infarction: effects on left ventricular size, function, quality of life, and exercise tolerance. Results of the Open Artery Trial (TOAT Study) J Am Coll Cardiol. 2002;40:869–76. doi: 10.1016/s0735-1097(02)02058-2. [DOI] [PubMed] [Google Scholar]
- 15.Boersma E The Primary Coronary Angioplasty vs. Thrombolysis Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J. 2006;27:779–88. doi: 10.1093/eurheartj/ehi810. [DOI] [PubMed] [Google Scholar]
- 16.Elsman P, van’t Hof AW, de Boer MJ, et al. Role of collateral circulation in the acute phase of ST-segment-elevation myocardial infarction treated with primary coronary intervention. Eur Heart J. 2004;25:854–8. doi: 10.1016/j.ehj.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Nathoe HM, Koerselman J, Buskens E, et al. Determinants and prognostic significance of collaterals in patients undergoing coronary revascularization. Am J Cardiol. 2006;98:31–5. doi: 10.1016/j.amjcard.2006.01.050. [DOI] [PubMed] [Google Scholar]