Abstract
Neuregulin-1 (NRG1) plays an important role in neural development, synapse formation and synaptic plasticity by activating ErbB receptor tyrosine kinases. Although ligand-induced endocytosis has been shown to be important for many receptor tyrosine kinases, whether NRG1 signaling depends on ErbB endocytosis remains controversial. Here we provide evidence that ErbB4, a prominent ErbB protein in the brain, becomes internalized in NRG1-stimulated neurons. The induced ErbB4 endocytosis requires its kinase activity. Remarkably, inhibition of ErbB endocytosis attenuates NRG1-induced activation of Erk and Akt in nuerons. These observations indicate a role of ErbB endocytosis in NRG1 signaling in neurons.
Keywords: ErbB4, tyrosine kinase, neuregulin, internalization, endocytosis, biotinylation, neurons, schizophrenia
Introduction
Neuregulins (NRGs) are a family of proteins containing an epidermal growth factor (EGF)-like motif. NRG1, a most extensively studied NRG, has been implicated in neural development including neuron differentiation, migration, neurite outgrowth, and synapse formation[1, 2]. NRG1 activate transmembrane tyrosine kianses of the ErbB family. It interacts with ErbB3 and ErbB4 whereas the ligand for ErbB2 remains unclear. On the other hand, the kinase activity of ErbB2 and ErbB4 is increased upon NRG stimulation whereas ErbB3 has an impaired kinase domain [3]. Of the three ErbB proteins, ErbB4 is particular interesting because it has implicated in various steps during neural development including neuronal migration and neurite outgrowth [4, 5]. In adult brains, ErbB4 is localized at the postsynaptic density (PSD), presumably via interacting with PSD-95 [6-8]. These observations suggest a role of NRG1 in regulating synaptic plasticity. In deed, NRG1 can suppress LTP induction at Schaffer collateral-CA1 synapses in the hippocampus without affecting basal synaptic transmission [8]. Subsequently, NRG1 was shown to reduce whole-cell NMDA receptor currents in pyramidal neurons of prefrontal cortex, to decrease NMDA receptor-mediated EPSCs in prefrontal cortex slices[9, 10]. Recent studies indicate that the NRG1 gene is a candidate gene in schizophrenia [11-13] and abnormal NRG1/ErbB4 signaling is detected in postmortem brains of patients with schizophrenia [14].
Upon activation by NRGs, ErbBs form homo- and heterodimers and become phosphorylated at tyrosine residues in the carboxyl terminal region [15]. Subsequently, they recruit adapter proteins activate downstream signaling pathways including PI3 kinase and Erk [16]. Activation of PI3 kinase and Erk has been shown to be required for NRG1 function, for example, regulation of neurite extension and arborization in cultured hippocampal neurons[17], Schwann cell survival[18], and NMDA receptor transmission [8, 9]
Unlike EGF receptors whose endocytosis is necessary for subsequent signaling [19], ErbB proteins were thought to be impaired in internalization [20]. In recent studies, however, we showed that ErbB kinases become endocytosed in heterologous expression systems and in muscle cells and the ligand-stimulated endocytosis is necessary for NRG1 activation of Erk [21]. In this present study, we showed that ErbB proteins were internalized in neurons upon NRG1 stimulation. We characterized the effects of inhibiting ErbB kinase activity and endocytosis on NRG signaling. Our data indicate that ligand-dependent ErbB endocytosis is necessary for NRG activation of Erk and PI3 kinase in neurons.
Materials and methods
Materials
Antibodies used were: ErbB2 (sc-284), ErbB3 (sc-285), and ErbB4 (sc-283) from Santa Cruz Biotechnology (Santa Cruz, CA); phospho-Erk (Thr202/Tyr204, #9101), phospho-Akt (Ser473, #4051), and Akt (#2966) from Cell Signaling Technology (Beverly, MA); HRP-conjugated secondary antibodies (GH9596614) were from Pierce (Rockford, IL). Streptavidin agarose-beads were from Molecular Probes (Eugene, OR). EZ-Link sulfo-NHS-biotin was from Pierce. Chemicals used were: Monodansylcadavenrine (MDC, D4008) from Sigma; AG1478 and AG789 from Calbiochem (San Diego, CA). Neuregulin used was a recombinant polypeptide containing the entire EGF domain of the β-type neuregulin-1 (rHRGβ177-244, NRG1) [22].
Neuron culture
Hippocampal neurons were cultured as previously described [8]. In brief, hippocampi were dissected from embryonic 18 (E18) rats, digested with 0.25% trypsin-EDTA at 37°C for 15 min and dissociated with a fire-polished Pasteur pipette in the plating medium (DMEM/F-12 containing Earle’s salts with 10% fetal bovine serum, 0.5% glucose, 1 mM sodium pyruvate, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin). Neurons were plated at a density of 100-200 neurons/mm2 onto glass coverslips coated with poly-D-lysine for low density culture and of 4×106 neurons/60-mm dishes coated with poly-D-lysine for high density culture. After cell attachment to the substrate (3-4 hr after plating), neurons were changed to the neuronal culture medium (neurobasal medium with 1×B27 supplement, 25 μM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin).
Image analysis
Alexa488-labeled NRG1 was prepared as described previously[21]. Neurons were incubated with 5 nM Alexa 488-NRG1 in the artificial cerebrospinal fluid (ACSF) (in mM: 119 NaCl, 26 NaHCO3, 1 NaH2PO4, 11 D-glucose, 2.5 KCl, 4CaCl2, 4MgCl2, and 1.25 NaHPO4) for 30 min on ice. Neurons were washed with cold ACSF, fixed and imaged. In some experiments, endocytosis was initiated by switching neurons to 37°C neuron growth medium. Images were taken from live neurons on a heated stage at 37°C. In some experiments, neurons were coincubated with 100-fold excess of non-labeled NRG1 at 4°C. Images were captured by water-immersion objective on an inverted confocal microscope (Zeiss Axiovert 200M).
Biotinylation assays
Endocytosis of ErbB proteins was assayed using cleavable biotin as described previously with modification [21]. Control and chemical-pretreated neurons were washed with ACSF at 37°C, cooled gradually to 4°C, and incubated with 1.5 ml of 1.5 mg/ml NHS-SS-biotin with gentle shaking at 4°C for 1 hr. After washing, neurons were switched to the neuronal culture medium and incubated at 37°C with or without NRG1 (5 nM). At indicated times of incubation, neurons were cooled to 4°C and un-endocytosed surface biotin was cleaved by incubating in the glutathione cleavage buffer (50 mM glutathione, 75 mM NaCl, 10 mM EDTA, 1% BSA and 0.075 N NaOH). Neurons were lysed in the modified RIPA buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxylate, 1 mM EDTA, and protease inhibitors. Lysates were cleared by centrifugation at 10,000 g for 10 min at 4°C and incubated with 70 μl of 50% streptavidin beads at 4°C overnight. Bead-associated proteins were subjected to western blot analysis. The intensity of bands was quantified using NIH image 1.63 software.
Western blotting analysis
Neuronal lysates were subjected to SDS-PAGE and western analysis as described previously [21]. Immunoactivity was detected by enhanced chemilluminescence and quantified by densitometry using NIH image 1.63 software. Levels of phospho-Erk1/2 and phospho-Akt were normalized to total Erk1/2 and Akt, respectively.
Data analysis
Data were presented as mean ± SEM. Data were analyzed by one-way ANOVA followed by Dunnett’s test. Values of P < 0.05 were considered significant.
Results
ErbB2 and ErbB4 were internalized in NRG1-stimulated neurons
To determine whether ErbB proteins undergo endocytosis in neurons, hippocampal neurons were incubated with NHS-SS-biotin at 4°C for 1 hr to label surface proteins. Un-bound NHS-SS-biotin was washed off and neurons were incubated at 37°C to initiate internalization. Biotinylated proteins remained on cell surface was debiotinylated by cleaving the NHS-SS-biotin disulfide bond in the cleavage buffer containing glutathione. Neurons were lysed, and internalized biotinylated proteins were purified by streptavidin beads, and subjected to western blot analysis using indicated antibodies. Cortical mature neurons express mainly ErbB2 and ErbB4 and the level of ErbB3 was barely detectable (data not shown). Internalization of ErbB proteins at 4°C was minimal (time 0, Fig. 1A and 1C). Both ErbB2 and ErbB4 were detectable in precipitates with streptavidin-beads in control group, suggesting basal endocytosis in the absence of NRG1. By contrast, however, the amounts of endocytosed ErbB2 and ErbB4 were dramatically increased in NRG1-stimulated neurons. Note that total amounts of ErbB2 and ErbB4 in neuronal lysates remained constant during the experimental period. These results suggest that NRG1 stimulates ErbB2 and ErbB4 endocytosis without altering the level of total protein in neurons. We quantified the amount of endocytosed ErbB4 by comparing to a calibration curve. At 60 min, almost 50% of ErbB4 became endocytosed in stimulated neurons (Fig. 1B).
Fig.1.
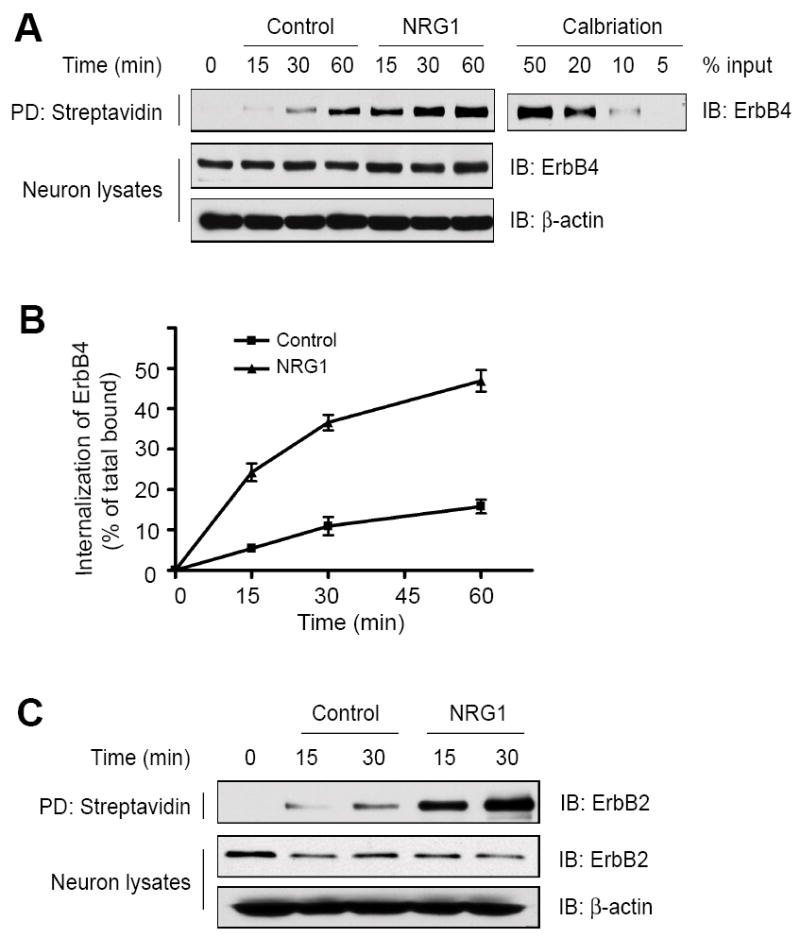
NRG1 induces ErbB internalization in neurons. Cell surface proteins were labeled by NHS-SS-biotin at 4°C before initiation of ErbB receptors internalization at 37°C. After incubation at 37°C for indicated times, the remaining biotin on surface proteins was removed by glutathione. Control and NRG1 stimulated neurons were lysed and internalized biotinylated proteins were purified with streptavidin beads. Shown are western blots of ErbB4 (A) and ErbB2 (C) associated with the beads as well as in neuron lysates. Internalization measured in cells debiotinylated without initiation of internalization (0 min); spontaneous ErbB receptor internalization measured in absence of NRG1 (Control); ErbB receptor internalization induced by NRG1 (NRG1). PD, pull down. (B) shows quantitative analysis of data in A by using the NIH Image 1.63 software. The amount of internalized proteins was calibrated with a standard curve of total biotinylated proteins. Data shown were mean ± SEM (n = 3).
Visualization of neuronal ErbB endocytosis by Alexa 488-NRG1
To further investigate NRG1-induced ErbB endocytosis, we sought to visualize the internalized proteins by Alexa488-NRG1. This fluorescence dye-labeled NRG1 became co-endocytosed with ErbB4 in HEK293 cells[21]. Hippocampal neurons were incubated with Alexa488-NRG1 on ice to label surface ErbB proteins and after unbound Alexa488-NRG1 was washed, neurons were incubated at 37°C for endocytosis to occur. As shown in Fig. 2A, Alexa488-NRG1 remained on surface of neurons when incubated at 4°C. Little if any was detectable in the cytoplasm of the soma. By contrast, however, neurons after incubation at 37°C for 30 min showed numerous fluorescent puncta, typical of endocytotic particles, in the soma of neurons (Fig. 2B). Such staining pattern was observed in neurons incubated with Alexa488-NRG1 at 4°C, and was abolished by plus 100-fold excess non-labeled NRG1 (Fig. 2C). These observations are in line with the notion that ErbB proteins were endocytosed upon NRG1 stimulation.
Fig. 2.
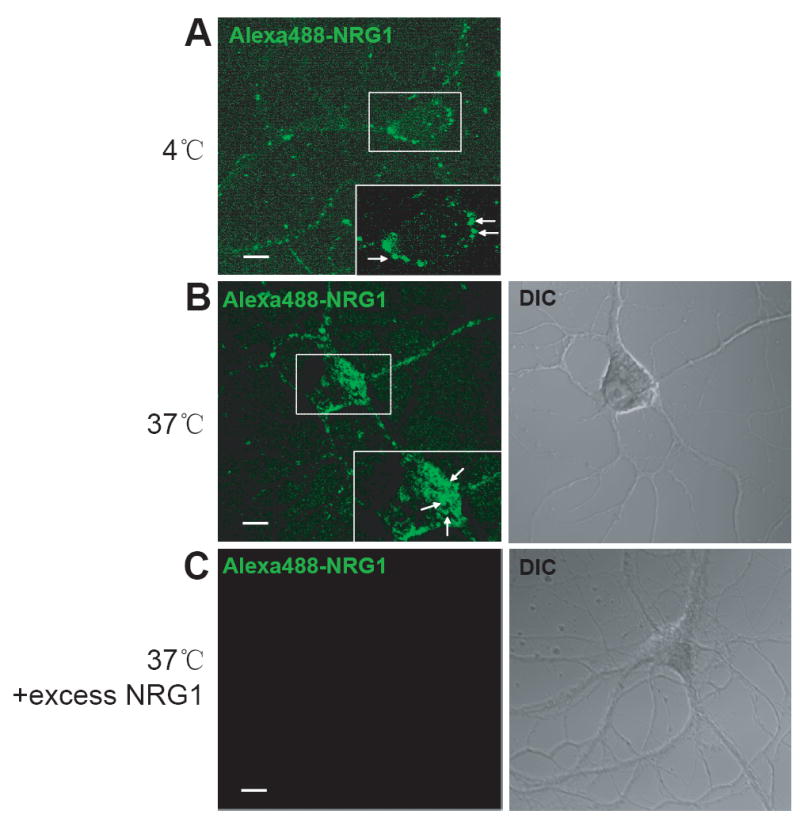
Visualization of ErbB endocytosis by Alexa488-labeled NRG1. Hippocampal neurons were incubated with Alexa488-labeled NRG1 on ice in the absence (A and B) or presence of excess unlabeled NRG1 (C). Neurons were fixed (A) or switched to 37°C for 30 min to allow for receptor internalization (B and C). Neurons were examined under a confocal microscopy. Scale bar, 10 μm.
Dependence of ErbB endocytosis on kinase activation
ErbB proteins are tyrosine kinases. The kinase activity of receptor tyrosine kinases has been shown to be necessary for endocytosis[23, 24]. We determined whether NRG1-induced endocytosis of neuronal ErbB proteins is regulated by tyrosine kinase. This was achieved by pretreating neurons with AG879 and AG1478, specific inhibitors of ErbB2 and ErbB4, respectively. Treatment of neurons with 5 μM AG879 or AG1478 inhibits tyrosine phosphorylation of ErbB2 or ErbB4 [25, 26]. Interestingly, ErbB4 endocytosis was reduced in neurons treated with either of the inhibitors. Quantitatively, ErbB4 internalization was inhibited by 82.7 ± 6.7% by AG879 and by 74.9 ± 4.2% by AG1478. It was almost abolished in neurons treated with both AG879 and AG1478 (Fig. 3B). These results were interpreted as evidence for a necessary role of tyrosine kinase activity of ErbB proteins in internalization.
Fig. 3.
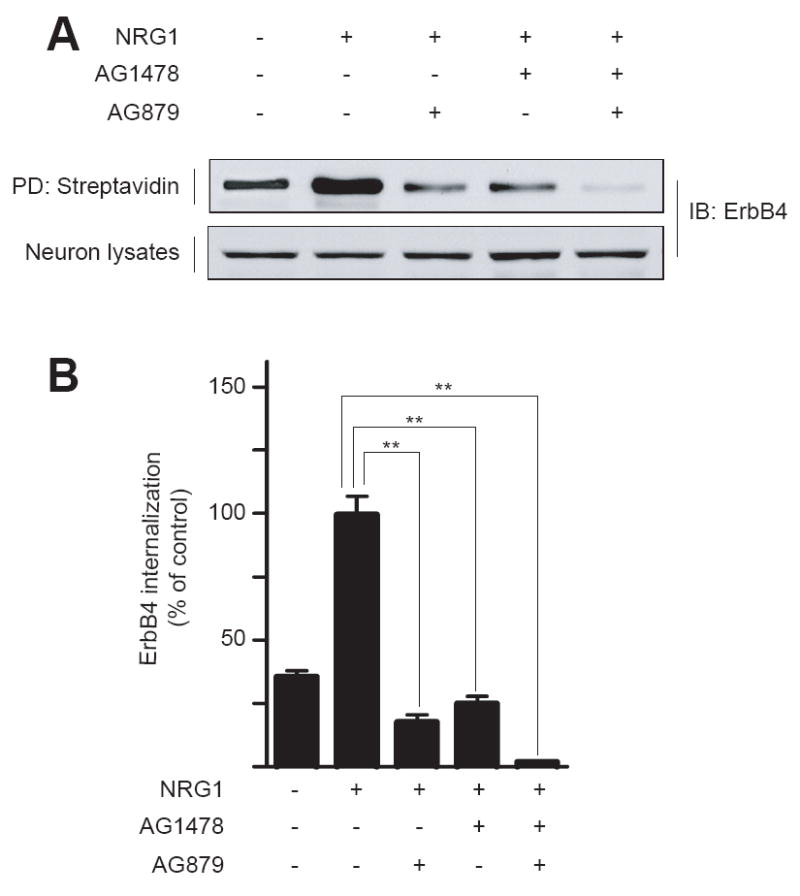
Dependence of NRG1-induced ErbB4 internalization on tyrosine kinase activity. High-density hippocampal neurons were pretreated without or with 5 μM AG879 and/or AG1478 for 20 min prior to stimulation with NRG1 for 30 min. ErbB4 internalization was measured as in Fig. 1. Shown were representative western blots (A) and quantitative analysis (B). NRG1-stimulated internalization was taken as 100%. Data were shown as mean ± SEM (n = 3). **, P < 0.01.
Inhibition of ErbB endocytosis attenuates NRG1 signaling
Is ErbB internalization necessary for neuregulin signaling? To address this question, we explored the consequence of inhibiting ErbB endocytosis in neurons. Receptor endocytosis is thought to be mediated by clathrin-dependent internalization[19], which can be inhibited by monodansylcadavenrine (MDC). Treatment with MDC has been shown to block internalization of various receptors in a variety of cell types [27]. Because NRG1 stimulation has been shown to activate Erk and PI3 kinase [6, 8, 21], we studied the effects of MDC on NRG1-activation of Erk and Akt, a downstream kinase of PI3 kinase. As shown in Fig. 4, NRG1-induced ErbB4 endocytosis in neurons treated with 50 μM MDC was inhibited in comparison with neurons treated with vehicle (0.01% acetic acid), suggesting that ErbB endocytosis may be mediated by clathrin-dependent mechanisms. In control neurons, NRG1 stimulation increased phospho-Erk and phospho-Akt, in agreement with previous reports [6, 8, 21]. Remarkably, NRG1-elicited activation of Erk and Akt was inhibited in neurons treated with 50 μM MDC. The activation of both Erk and Akt became more transient and returned to basal level more rapidly in MDC-treated neurons than control (Fig. 5). Note that the total amount of Akt and Erk was unchanged during the experiment. Thus MDC inhibited both ErbB endocytosis and activation of Erk and Akt in NRG1-stimulated neurons, suggesting that the ligand-induced ErbB endocytosis is necessary for NRG1 signaling.
Fig. 4.
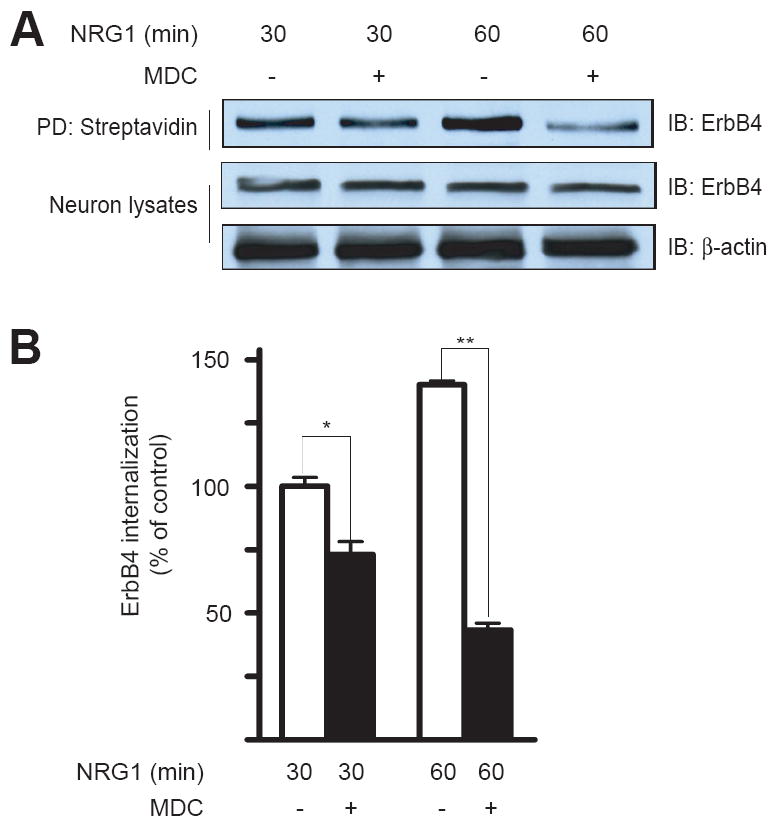
Inhibition of ErbB4 endocytosis by MDC. Neurons were treated with or without MDC for 30 min prior to NRG1 stimulation for indicated times. ErbB4 internalization was measured as in Fig. 1. Shown were representative western blots (A) and quantitative analysis (B). NRG1-stimulated internalization at 30 min was taken as 100%. Data were shown as mean ± SEM (n = 3). *, P < 0.05; **, P < 0.01.
Fig. 5.
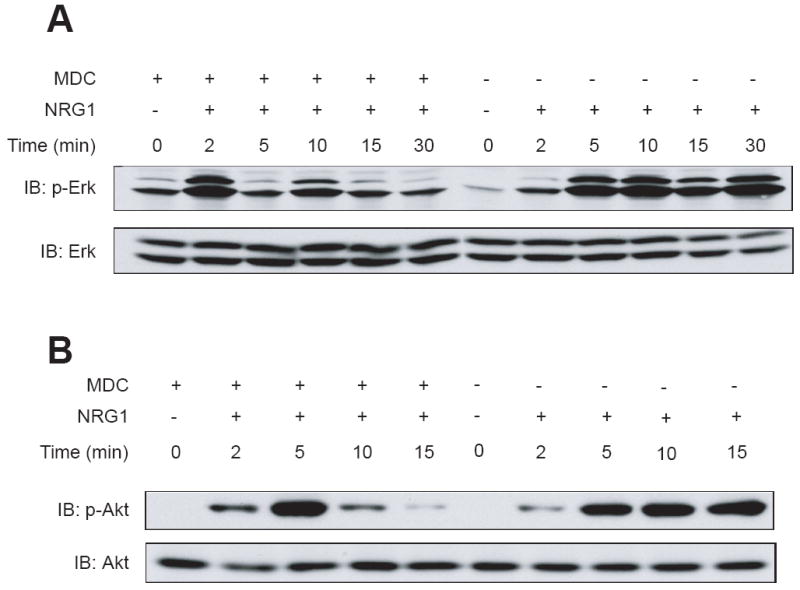
Attenuated NRG1 signaling in MDC-treated neurons. Neurons were treated with or without MDC for 30 min prior to NRG1 stimulation for indicated times. Neurons were lysed and resulting lysates were subjected to western blotting. Top panels, blotting with antibodies against phospho-Erk (A) or phospho-Akt (B). Bottom panels, blotting for total Erk (A) or Akt (B) to indicate equal amounts of proteins analyzed.
Discussion
For many growth factors, intracellular signaling may be terminated by ligand-induced endocytosis of the receptor tyrosine kinases and their subsequent degradation [28]. On the other hand, receptor internalization may be necessary to mediate growth factor-stimulated signaling. For example, endocytosis of activated Trk receptors is necessary for some biological functions of neurotrophins in neurons [29-31]. Internalized Trk kinases could remain tyrosine phosphorylated and active, with extracellular domains interacting with co-internalized growth factors inside signaling endosomes. This mechanism is thought to be key to sustained activation of Erk and PI3 kinase [32, 33]. In terms of NRG1 signaling, however, ErbB proteins were thought to be impaired in endocytosis[20]. The termination of NRG/ErbB signaling was thought to be mediated by proteolytic cleavage of ErbB proteins in the plasma membrane.
In the current study, we demonstrate that ErbB4 and ErbB2 undergo rapid endocytosis in neurons upon NRG1 stimulation. Alexa488-NRG1 was detected in neuronal soma within 30 min after NRG1 stimulation. Using quantitative biotinylation assays, we showed that almost 50% of ErbB4 became internalized within 60 min of stimulation. ErbB endocytosis was clearly stimulated by NRG1 and required the tyrosine kinase activity. Functionally, inhibition of ErbB endocytosis attenuated NRG1-induced activation of Erk and Akt in nuerons. Most noticeably, active Erk and Akt were unable to sustain at elevated levels. These observations are in agreement with our recent studies in HEK293 and muscle cells [21]. Together, they indicate a role of ErbB endocytosis in NRG1 signaling in neurons.
Acknowledgments
This work was supported by grants from NIH (L. Mei and W.C. Xiong). R.S.W. was supported in part by a Korea Research Foundation Grant (MOEHRD, KRF-2004-214- H00004).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 2.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Schaefer G, Akita RW, Sliwkowski MX. A discrete three-amino acid segment (LVI) at the C-terminal end of kinase-impaired ErbB3 is required for transactivation of ErbB2. J Biol Chem. 1999;274:859–866. doi: 10.1074/jbc.274.2.859. [DOI] [PubMed] [Google Scholar]
- 4.Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 5.Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, Huang YZ, Pitcher GM, Valtschanoff JG, Ma YH, Feng LY, Lu B, Xiong WC, Salter MW, Weinberg RJ, Mei L. Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J Neurosci. 2003;23:3164–3175. doi: 10.1523/JNEUROSCI.23-08-03164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 9.Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukui N, Muratake T, Kaneko N, Amagane H, Someya T. Supportive evidence for neuregulin 1 as a susceptibility gene for schizophrenia in a Japanese population. Neurosci Lett. 2006;396:117–120. doi: 10.1016/j.neulet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Bio l Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Stefansson H, Thorgeirsson TE, Gulcher JR, Stefansson K. Neuregulin 1 in schizophrenia: out of Iceland. Mol Psychiatry. 2003;8:639–640. doi: 10.1038/sj.mp.4001384. [DOI] [PubMed] [Google Scholar]
- 14.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 15.Earp HS, Dawson TL, Li X, Yu H. Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with imp lications for breast cancer research. Breast Cancer Res Treat. 1995;35:115–132. doi: 10.1007/BF00694752. [DOI] [PubMed] [Google Scholar]
- 16.Chausovsky A, Waterman H, Elbaum M, Yarden Y, Geiger B, Bershadsky AD. Molecular requirements for the effect of neuregulin on cell spreading, motility and colony organization. Oncogene. 2000;19:878–888. doi: 10.1038/sj.onc.1203410. [DOI] [PubMed] [Google Scholar]
- 17.Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1beta induces neurite extension and arborization in cultured hippocampal neurons. Mol Cell Neurosci. 2004;27:379–393. doi: 10.1016/j.mcn.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Tennekoon GI, Birnbaum M, Marchionni MA, Rutkowski JL. Neuregulin signaling through a PI3K/Akt/Bad pathway in Schwann cell survival. Mol Cell Neurosci. 2001;17:761–767. doi: 10.1006/mcne.2000.0967. [DOI] [PubMed] [Google Scholar]
- 19.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 20.Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 21.Yang XL, Huang YZ, Xiong WC, Mei L. Neuregulin-induced expression of the acetylcholine receptor requires endocytosis of ErbB receptors. Mol Cell Neurosci. 2005;28:335–346. doi: 10.1016/j.mcn.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Holmes WE, Sliwkowski MX, Akita RW, Henzel WJ, Lee J, Park JW, Yansura D, Abadi N, Raab H, Lewis GD, et al. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 23.Nagappan G, Lu B. Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci. 2005;28:464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Gilboa L, Ben-Levy R, Yarden Y, Henis YI. Roles for a cytoplasmic tyrosine and tyrosine kinase activity in the interactions of Neu receptors with coated pits. J Biol Chem. 1995;270:7061–7067. doi: 10.1074/jbc.270.13.7061. [DOI] [PubMed] [Google Scholar]
- 25.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Brattain MG. Synergy of epidermal growth factor receptor kinase inhibitor AG1478 and ErbB2 kinase inhibitor AG879 in human colon carcinoma cells is associated with induction of apoptosis. Cancer Res. 2005;65:5848–5856. doi: 10.1158/0008-5472.CAN-04-3509. [DOI] [PubMed] [Google Scholar]
- 27.Schutze S, Machleidt T, Adam D, Schwandner R, Wiegmann K, Kruse ML, Heinrich M, Wickel M, Kronke M. Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J Biol Chem. 1999;274:10203–10212. doi: 10.1074/jbc.274.15.10203. [DOI] [PubMed] [Google Scholar]
- 28.Sorkin A, Waters CM. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharyya A, Watson FL, Bradlee TA, Pomeroy SL, Stiles CD, Segal RA. Trk receptors function as rapid retrograde signal carriers in the adult nervous system. J Neurosci. 1997;17:7007–7016. doi: 10.1523/JNEUROSCI.17-18-07007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beattie EC, Howe CL, Wilde A, Brodsky FM, Mobley WC. NGF signals through TrkA to increase clathrin at the plasma membrane and enhance clathrin-mediated membrane trafficking. J Neurosci. 2000;20:7325–7333. doi: 10.1523/JNEUROSCI.20-19-07325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]


