Nuclear imaging is an established clinical molecular imaging modality that offers good sensitivity deep in tissue. However, nuclear imaging is limited by several factors such as time-consuming data acquisition, expensive equipment, exposure to radioactivity, the need for highly skilled personnel, and relatively poor spatial resolution.1 Optical imaging is a relatively new imaging modality that offers real-time, non-radioactive, and, depending on the technique, high-resolution imaging of fluorophores embedded in diseased tissues.2 Of the various optical imaging techniques investigated to date, near-infrared (NIR, 700−900 nm wavelength) fluorescence-based imaging is of particular interest for noninvasive in vivo imaging because of the relatively low tissue absorption, scatter, and minimal autofluorescence of NIR light.3
NIR fluorescence has the potential to provide rapid, inexpensive, and non-radioactive population-based screening for breast cancer.4-6 However, it is unclear whether currently available optical imaging systems have adequate sensitivity and/or resolution to identify breast pathology such as microcalcifications. In this study, we developed a critical reagent for exploring the limits of NIR fluorescence-based breast cancer diagnosis, namely, a simultaneous optical and nuclear contrast agent.
Bisphosphonates (BPs) bind avidly to hydroxyapatite (HA) bone mineral surfaces,7 and have many uses. BP-based radiotracers are used to diagnose osteoblastic bone lesions and to treat bone metastasis associated with breast cancer.8 In addition, contrast agents (CAs) with BPs and phosphonates as the targeting group have been developed for use with nuclear imaging.9, 10 Although, our group11-13 and others14 have explored NIR imaging with BPs, to the best of our knowledge, no BP-based dual modality nuclear-NIR contrast agents have been reported. Dual-labeled targeting imaging agents, such as the one described herein, allow cross validation and direct comparison between nuclear (the gold-standard) and fluorescence optical imaging.
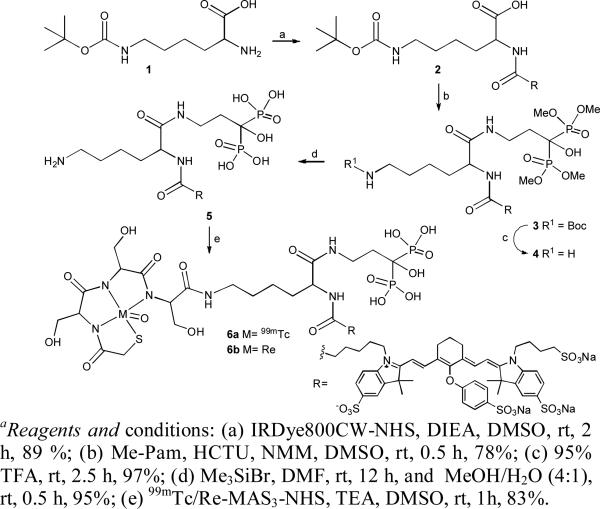
The tri-functional diagnostic agent Pam-Tc/Re-800 was synthesized in 5 chemical steps (Scheme 1), with an overall yield of 53%, from N-ε-t.-Boc-L-lysine 1, Me-Pam,13 MAS3, and IRDye800CW. Primary amine of compound 1 was conjugated with N-hydroxy succinimide ester of IRDye800CW to obtain Lys(t.-Boc)-800CW-carboxylic acid 2. Me-Pam reacted with activated compound 2 in presence of HCTU and NMM to generate Lys(t.-Boc)-800CW-Pam-Me 3. Compound 3 was treated with trifluoroacetic acid and trimethylsilyl bromide to deprotect Boc on primary amine and methylester group on phosphonates, respectively to obtain Lys-800CW-Pam 5. The final molecule Pam-Tc/Re-800 6 was obtained by 99mTc and Re solid-phase pre-labeling strategy15 on compound 5. In all synthetic steps, compounds were purified and characterized by reverse phase preparative HPLC and LCMS.
Scheme 1.a.
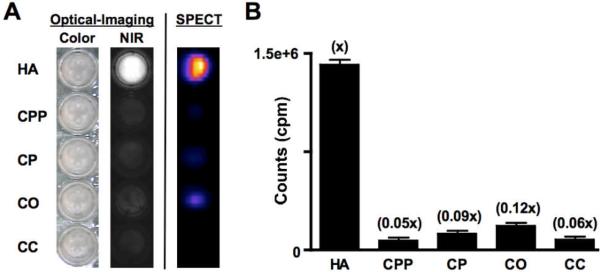
Pam-Tc/Re-800 6 was fully characterized for its radioactivity, spectral properties (Supporting Information), and calcium salt specificity (Figure 1). Specific activity of Pam-Tc-800 6a was greater than or equal to 6,250 Ci/mmol and radiochemical purity was greater than or equal to 98%. Peak absorption (781 nm) and emission (800 nm) of Pam-Re-800 6b are located within the “NIR window,”16 an area of the electromagnetic spectrum that maximizes photon penetration and recovery in living tissue. The extinction coefficient of Pam-Re-800 at 781 nm was 197,000 M−1cm−1 and its quantum yield was 8.9% in PBS. In 100% fetal bovine serum, its quantum yield was 8.7%.
Figure 1.
In vitro specificity of Pam-Tc/Re-800 6 (mixture) for crystals of HA and other calcium salts. (A) Optical and SPECT images are shown. (B) Quantification (mean ± SD) of crystals from (A) using a gamma counter. All measurements (3 independent experiments) were from identically sized and shaped regions of a 96-well plate.
To determine the selectivity and specificity of Pam-Tc/Re-800 for hydroxyapatite (HA), a major mineral component of breast cancer microcalcifications and normal bone, over other calcium salts we incubated HA and Ca-pyrophosphate (CPP), Ca-phosphate (CP), Ca-oxalate (CO) and Ca-carbonate (CC) salts with Pam-Tc/Re-800 containing 200 nCi (99mTc) and 100 nM (Re) in 100 μL PBS for 30 min at RT with constant motion, then washed 4 times with a 100-fold excess of PBS. SPECT/CT and NIR images were acquired for comparison. As shown in Figure 1, Pam-Tc/Re-800 6 has a greater than 8-fold specificity for HA over other calcium salts found in the body, and permits SPECT/CT and NIR fluorescence detection of HA with high sensitivity.
To help develop new screening technology, we have previously published17 the first syngeneic rat model of breast cancer microcalcification based on ectopic expression of adenovirus-expressed bone morphogenetic protein-2 (BMP-2).
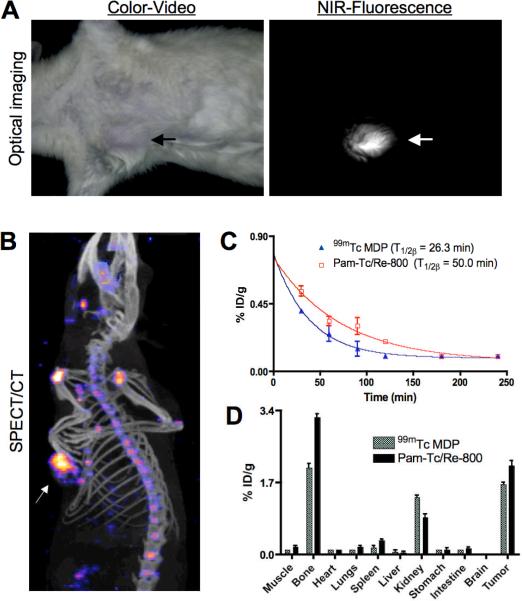
This rat model of breast cancer microcalcification was used to quantify Pam-Tc/Re-800 performance in vivo, with intravenous injection at a dose of 500 μCi (99mTc) and 25 nmol (Re). After 4 h of clearance, simultaneous imaging of breast cancer microcalcifications were performed by both SPECT/CT and a custom intraoperative NIR fluorescence imaging system.18 As shown in Figure 2A, breast cancer microcalcifications were observed using a simple reflectance NIR fluorescence imaging system in the presence of Pam-Re-800. Although fluorescence lifetime imaging was not explored in this study, it is possible that even higher signal-to-background ratios could be achieved with this technique. Optical detection of breast cancer microcalcification was compared to SPECT/CT. As shown in Figure 2B, Pam-Tc-800 provided high sensitivity detection of breast cancer microcalcifications as well as normal bones.
Figure 2.
In vivo imaging of rat breast cancer microcalcification. (A) Intraoperative NIR-fluorescence imaging and (B) SPECT/CT imaging. Arrows mark location of breast cancer microcalcification. (C) Blood clearance and (D) biodistribution of Pam-Tc-800 6a compared to 99mTc MDP. Figure data are representative of 3 independent experiments.
Finally, since the current gold standard for SPECT imaging of calcification is 99mTc-methylene diphosphonate (MDP), we performed a quantitative comparison of Pam-Tc-800 to 99mTc-MDP. Blood clearance T1/2β and results of biodistribution studies at 4 h post injection (p.i.) are shown in Figure 2C and 2D, respectively. The total body clearance at 4 hr p.i. (70−75% ID) was almost identical for 99mTc-MDP and Pam-Tc-800, however, Pam-Tc-800 has a higher uptake in bone and tumor than 99mTc MDP. Activity that was not taken up in the tumor and bone was excreted rapidly via the kidneys into urine. In muscles and all other organs, the activity was low for both compounds. These results suggest that in addition to use as an optical/nuclear contrast agent for validating NIR fluorescence imaging, Pam-Tc/Re-800 is itself a bone-seeking radiopharmaceutical with rapid clearance from soft tissue and slightly higher uptake in skeleton and microcalcified tumors than even 99mTc- MDP.
In conclusion, we have produced an efficient chemical synthesis of a tri-functional, HA-binding molecule, which provides simultaneous imaging by NIR fluorescence and SPECT. Quantitation by SPECT provides the “gold standard” by which NIR fluorescence tomography of breast cancer microcalcifications can now be compared and optimized.
Supplementary Material
Detection of breast cancer microcalcifications using a dual-modality SPECT/NIR fluorescent probe
Kumar R. Bhushan,1 Preeti Misra,1 Fangbing Liu,1 Sanjeev Mathur,1 Robert E. Lenkinski,2 and John V. Frangioni1,2*
1Division of Hematology/Oncology, Department of Medicine, and 2Department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, USA
SUPPORTING INFORMATION
General Experimental Procedures
Reagents: All chemicals and solvents were of American Chemical Society or high-performance liquid chromatography (HPLC) purity and were used as received. Ultra-dry DMSO was purchased from Acros Organics (Geel, Belgium). HPLC grade triethylammonium acetate (TEAA), pH 7 was from Glen Research (Sterling, VA, USA). HPLC grade water was from American Bioanalytic (Natick, MA, USA). HPLC grade Methanol (MeOH) was from Mallinckrodt Baker (Phillipsburg, NJ, USA). The N-hydroxysuccinimide (NHS) ester of IRDye800CW was purchased from LI-COR (Lincoln, NE, USA) and stored in the dark as dry powder, under nitrogen, at −80°C until use. All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA, USA) and Sigma-Aldrich (Saint Louis, MO, USA).
Animals: Animals were used in accordance with an approved institutional protocol. Breast cancer microcalcification was developed in Fischer 344 female rats (Taconic Farms, Germantown, NY) by our previously published methods.1 During experiments, rats were anesthetized, intubated and maintained with 1.5−2% isoflurane/balance O2.
Reverse Phase HPLC (Figure S1): HPLC purification of compounds was performed on a Waters (Milford, MA, USA) prepLC 150 mL fluid handling unit (Waters) equipped with a Symmetry Prep®C18 column (19 × 150 mm, 7 μm particle size), a manual injector (Rheodyme 3725i) and a 2487 dual wavelength absorbance detector (Waters) outfitted with a semi-preparative flow cell. A flow splitter (Upchurch Scientific, Oak Harbor, WA, USA) diverted a portion of the eluate into an evaporative light scatter detector (ELSD, Richards Scientific, Novato, CA, USA) with the nebulizer modified to reduce band broadening at low flow rates while the other part flowed into a fraction collector (Waters, Fraction Collector II). The ELSD was set to 40°C, with a nitrogen pressure at 3.5 bar and a gain of 6. For HPLC mobile phase was solvent A = TEAA, solvent B = Methanol and flow rate was 15 ml/min.
ES-TOF Mass Spectroscopic Analysis (Figure S1): The purity of all compounds was measured using liquid chromatography-mass spectrometry (LCMS) on a Waters system consisting of a 1525 binary HPLC pump with a manual 7725i Rheodyme injector, a 2487 dual wavelength absorbance detector, and a 2475 multi wavelength fluorescence detector (Waters). The column eluate was divided in two using a flow splitter (Upchurch Scientific). A portion of the eluate flowed into an ELSD (Richards Scientific) while the rest flowed into a Micromass LCT TOF-ES spectrometer (Waters) equipped with a Symmetry®C18 (4.6 × 150 mm, 5 μm) reverse-phase HPLC column. For mass spectrometry mobile phase was solvent A = TEAA, solvent B = Methanol, flow rate was 0.5 ml/min, capillary voltage was −3317V, and sample cone voltage was −50V.
Spectral Measurements: Absorbance spectroscopy (Figure S1) was performed in a 1 cm path length quartz cuvette (Starna, Atascadero, CA, USA), mounted in a CUV-ALL-UV four-way cuvette holder (Ocean Optics, Dunedin, FL, USA), and excited with a balanced deuterium-tungsten light source (Ocean Optics) spectrometer with a 1.3 nm resolution from 200 to 850 nm, using 2 μM of NIR fluorophore in the indicated buffer. Fluorescence spectrometry was performed in a three-sided quartz cuvette (Starna) excited with a 5 mW 770 nm laser diode coupled through a 300 μm core diameter, NA 0.22 fiber (Fiberguide Industries, Stirling, NJ, USA). Fluorescence measurements (Figure S1) were made on an HR2000 (Ocean Optics) spectrometer with a 7.6 nm resolution from 350 to 1000 nm, using 2 μM NIR fluorophore in the indicated buffer. QYs of Pam-Re-800 6b in PBS, pH 7.4 or 100% fetal bovine serum were calculated using ICG in DMSO (QY 13%2) as calibration standard, under conditions of matched fluorophore absorbance at the 770 nm laser line.
Figure S1. Analytical and spectral characterization. (A) HPLC of Pam-Tc-800 6a: Fluorescence (top; 770 nm excitation) and gamma emission (bottom). (B) MALDI-TOF m/z of 6b: Calculated C66H85N8O30P2ReS53−: 1878.30, found 1875.02 (C) Absorbance/fluorescence spectra of Pam-Re-800 6b.
Intraoperative NIR Fluorescence Imaging System: The principles of the optical path were published in detail previously.3 Briefly, a zoom lens with a working distance of 18” directs light to a dichroic mirror, where the visible light (400−700 nm) is deflected to a color video camera (Imitech IMC-80F, IMI Technology Co., Seoul, Korea), and the NIR fluorescence light (> 785 nm) is filtered by an emission filter (> 795 nm longpass filter) prior to reaching an NIR sensitive camera (Orca-AG, Hamamatsu, Bridgewater, NJ, USA). The entire optical system is mounted on a shock-resistant aluminum frame, enclosed by a sealed cover measuring 12”W × 12”H × 9”D, and mounted on an adjustable arm that swings out over the surgical field.
NIR excitation light is provided by an array of 10 high-powered (1W) light emitting diodes (LEDs; L-760−66−60, Marubeni Epitex, New York, NY, USA), which are filtered (HHQ750/50x, Chroma Technology, Brattleboro, VT, USA) and focused on the surgical field. White light is provided by an array of four Luxeon III white light diodes (Lumileds, San Jose, CA, USA) from which all NIR wavelengths are filtered out (Filter E680SP, Chroma). The generated fluence rates for NIR excitation and white light range from 0−5 mW/cm2 and 0−1 mW/cm2, respectively.
Spatial resolution at a field-of-view of 20 × 15 cm is 625 μm, and at a field-of-view of 4 × 3 cm is 125 μm. After computer-controlled (LabVIEW) camera acquisition via custom LabVIEW (National Instruments, Austin, TX, USA) software, anatomic (white light) and functional (NIR fluorescence light) images can be displayed separately and merged. To create a single image that displays both anatomy (color video) and function (NIR fluorescence), the NIR fluorescence image is pseudo-colored in lime green and overlaid images are refreshed up to 15 times per second. The entire apparatus is suspended on an articulated arm over the surgical field, thus permitting noninvasive and non-intrusive imaging. Hands-free operation utilizing motorized zoom and focus lenses, a four-pedal footswitch, and a multifunction data acquisition board were described in detail previously.4
Gamma scintigraphy and SPECT/CT: Animals were monitored by gamma radioscintigraphy performed with a Research Digital Camera (Isochem Technologies, Castana, IA, USA) equipped with a 1/2” NaI crystal, 86 photomultiplier tubes, and high-resolution, low-energy lead collimator. Sequential anterior images were collected at specified time 4 h using 512 × 512 image matrix while the animal remained under anesthesia (data not shown).
SPECT/CT (single photon emission computed tomography/computed tomography) scans and image analysis were performed using a rodent scanner (NanoSPECT/CT; Philips). Five-minute static scans were acquired at 4 h after injection. The images were reconstructed by a 2-dimensional ordered-subsets expectation maximum algorithm, and no correction was applied for attenuation or scatter.
Detailed Procedures
Synthesis of Lys(t.-Boc)-800CW-carboxylic acid 2: NHS ester of the NIR fluorophore IRDye800CW (1 mg, 0.91 μmol) and N,N-diisopropylethylamine (0.15 μL, 0.91 μmol) were added at 0°C under nitrogen atmosphere to the N-ε-t.-Boc-L-lysine 1 (0.23 mg, 0.91 μmol) taken in 1 mL anhydrous DMSO. The vortexing continued for 2 h at RT in the dark. The reaction mixture was poured over 4 mL ice-cold water and purified by HPLC. After concentration on an Oasis HLB desalting cartridge and subsequent lyophilization a bright green solid reaction component, Lys(t.-Boc)-800CW-carboxylic acid 2 was obtained (∼1 mg, 89%).
Synthesis of Lys(t.-Boc)-800CW-Pam-Me 3: Me-Pam5 (0.23 mg, 0.81 μmol), HCTU (0.33 mg, 0.81 μmol) and n-methylmorpholine (0.11 μL, 0.98 μmol) were added at RT under N2 atmosphere to Lys(t.-Boc)-800CW-carboxylic acid 2 (1 mg, 0.81 μmol) taken in 0.5 mL anhydrous DMSO. The stirring continued for 0.5 h at RT, the reaction mixture was poured over 2 mL ice-cold water and purified by HPLC. After concentration on an Oasis HLB desalting cartridge and subsequent lyophilization a bright green solid reaction component, Lys(t.-Boc)-800CW-Pam-Me 3 was obtained (0.95 mg, 78%).
Synthesis of Lys-800CW-Pam-Me 4: Lys(t.-Boc)-800CW-Pam-Me 3 (0.95 mg, 0.6 μmol) was taken in 95% trifluoroacetic acid (0.4 mL). The solution was stirred at RT for 2.5 h then removed the acid by N2 stream. After lyophilization, the compound 4 was obtained without further purification as a green powder (0.86 mg, 97 %).
Lys-800CW-Pam 5: Trimethylsilyl bromide (0.39 μL, 3.05 μmol) was added slowly to a solution of Lys-800CW-Pam-Me 4 (0.86 mg, 0.61 μmol) in dry DMF (0.2 mL) at 0°C under nitrogen atmosphere. The reaction mixture was vortexed at RT for 12 h in the dark and solvent was evaporated off to dryness. 0.5 mL of CH3OH-H2O (4:1) was added to yield a bright green solution, which was vortexed for 30 min at RT and purified by HPLC. After concentration on an Oasis HLB desalting cartridge and subsequent lyophilization, a bright green solid reaction component termed Lys-800CW-Pam 5 was obtained (0.78 mg, 95%).
Synthesis of Pam-Tc-800 6a: The N-hydroxysuccinimide (NHS) of the 99mTc-MAS3 was prepared with high radiochemical purity (>99%) and high specific activity in DMSO as described previously.6 For radiolabeling, Lys-800CW-Pam 5 (0.75 nmol) in 20 μL DMSO was conjugated to 500 μL of [99mTcMAS3]-NHS (4 mCi, 0.36 nmol)) in DMSO in presence of triethylamine (0.75 nmol). Constant vortexing at RT was maintained for 1 h. The radiolabeled compound was purified by reverse phase HPLC.
Synthesis of Pam-Re-800 6b: Lys-800CW-Pam 5 (0.60 mg, 0.53 μmol) was taken in 0.2 mL dry DMSO and reacted with 1 equivalent Re-MAS3- NHS in presence of 1.5 equivalent DIEA at RT for 1 h in dark under anhydrous conditions. The reaction mixture was poured over 2 mL ice-cold water and purified by HPLC. After concentration on an Oasis HLB desalting cartridge and subsequent lyophilization a bright green solid reaction component, Pam-Re-800 6b was obtained (0.70 mg, 83%).
Table S1: Preparative HPLC purification and LCMS characterization of compounds.
In Vitro Calcium Salt Specificity Experiments: In a 96-well plate, 5 mg/mL of hydroxyapatite (HA) or the phosphate, oxalate, carbonate, and pyrophosphate salts of calcium were separately incubated with Pam-Tc/Re-800 containing 200 nCi (99mTc) and 100 nM (Re) in 100 μl PBS for 30 min with continuous vortexing at RT. Crystals were washed 4 times with a 100-fold excess of PBS, centrifuged and visualized using the previously described NIR fluorescence imaging system at a fluence rate of 5 mW/cm2. All NIR fluorescence images had identical exposure times and normalizations.
The same crystals were also mounted on SPECT/CT and 5 minute static scans were acquired. The images were reconstructed by a 2-dimensional ordered-subsets expectation maximum algorithm, and no correction was applied for attenuation or scatter.
In control experiments, there was no detectable binding of IRDye800CW carboxylic acid or 99mTc-MAS3 to any of the calcium salts. Also, the possibility of fluorescence quenching of Pam-Tc/Re-800 leading to a false-negative result with non-HA salts was ruled out because absorbance measurements of the supernatant confirmed complete recovery of unbound dye.
In vivo SPECT/CT and NIR fluorescence imaging study: Rats with breast cancer microcalcification tumors were intravenously administered Pam-Tc/Re-800 with a dose of 500 μCi (99mTc) and 25 nmol (Re) per rat in 200 μl saline. Four hours post-injection, rats were imaged with a custom intraoperative NIR fluorescence imaging system.7 The same rats were imaged using nanoSPECT/CT.
For biodistribution, 500 μCi of Pam-Tc-800 in 200 μL of saline was administered intravenously. Blood was collected at 0, 1, 2, 5, 10, 15, 30, 60, 90, 120, 180, and 240 min post-injection from the tail vein using micro-capillary tubes, weighed, and counted on a model 1470 Wallac Wizard (Perkin Elmer, Wellesley, MA, USA) 10-detector gamma counter. Curve fitting was performed using Prism version 4.0a (GraphPad, San Diego, CA, USA) software. For measurement of total body retention and clearance, at 4-h post-injection, we ligated the ureters and urethra with silk sutures, removed the bladder en masse, and combined it with excreted urine and feces before measurement of radioactivity in a dose calibrator.8 The remaining carcass was also measured in a dose calibrator, then the heart, lungs, spleen, liver, kidneys, stomach, intestine, tumor, and brain were resected, washed twice in PBS, weighed, and counted as described above. An additional set of rats with breast tumors were injected with clinically available ∼ 500μCi 99mTc MDP to measure the tumor uptake. Statistical analysis was performed with an unpaired t-test using Prism.
References
1. Liu, F.; Bloch, N.; Bhushan, K. R.; De Grand, A. M.; Tanaka, E.; Solazzo, S.; Mertyna, P. M.; Goldberg, S. N.; Frangioni, J. V.; Lenkinski, R. E. Mol. Imaging 2008, In Press.
2. Benson, C.; Kues, H. A. J. Chem. Eng. Data 1977, 22, 379−383.
3. De Grand, A. M.; Frangioni, J. V. Technol. Cancer Res. Treat. 2003, 2, 553−62.
4. Gioux, S.; De Grand, A. M.; Lee, D. S.; Yazdanfar, S.; Idoine, J. D.; Lomnes, S. J.; Frangioni, J. V. SPIE Proceedings 2005, 6009, 39−48.
5. Bhushan, K. R.; Tanaka, E.; Frangioni, J. V. Angew. Chem. Int. Ed. Engl. 2007, 46, 7969−71.
6. Misra, P.; Humblet, V.; Pannier, N.; Maison, W.; Frangioni, J. V. J. Nucl. Med. 2007, 48, 1379−89.
7. Tanaka, E.; Choi, H. S.; Fujii, H.; Bawendi, M. G.; Frangioni, J. V. Ann. Surg. Oncol. 2006, 13, 1671−81.
8. Misra, P.; Lebeche, D.; Ly, H.; Schwarzkopf, M.; Diaz, G.; Hajjar, R. J.; Schecter, A. D.; Frangioni, J. V. J. Nucl. Med. 2008, 49, 963−9.
Acknowledgment
This work was funded by NIH R01-CA-115296, NIH R01-EB-005805, high-end instrumentation grant NIH S10-RR-023010, and grants from the Lewis Family Fund and the Ellison Foundation.
Footnotes
Supporting Information Available: Experimental procedures and spectroscopic data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Weissleder R, Mahmood U. Radiology. 2001;219:316–33. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 2.Ntziachristos V, Bremer C, Weissleder R. Eur. Radiol. 2003;13:195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 3.Frangioni JV. Curr. Opin. Chem. Biol. 2003;7:626–34. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Alacam B, Yazici B, Intes X, Nioka S, Chance B. Phys. Med. Biol. 2008;53:837–59. doi: 10.1088/0031-9155/53/4/002. [DOI] [PubMed] [Google Scholar]
- 5.Nioka S, Chance B. Technol. Cancer Res. Treat. 2005;4:497–512. doi: 10.1177/153303460500400504. [DOI] [PubMed] [Google Scholar]
- 6.Hawrysz DJ, Sevick-Muraca EM. Neoplasia. 2000;2:388–417. doi: 10.1038/sj.neo.7900118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Beek ER, Lowik CW, Ebetino FH, Papapoulos SE. Bone. 1998;23:437–42. doi: 10.1016/s8756-3282(98)00120-3. [DOI] [PubMed] [Google Scholar]
- 8.Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, Reitsma DJ, Heffernan M, Seaman JJ. Cancer. 2000;88:1082–90. doi: 10.1002/(sici)1097-0142(20000301)88:5<1082::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa K, Mukai T, Arano Y, Ono M, Hanaoka H, Ishino S, Hashimoto K, Nishimura H, Saji H. Bioconjug. Chem. 2005;16:751–7. doi: 10.1021/bc040249w. [DOI] [PubMed] [Google Scholar]
- 10.Lam MGEH, de Klerk JMH, van Rijk PP, Zonnenberg BA. Anti-Cancer Agents Med. Chem. 2007;7:381–397. doi: 10.2174/187152007781058596. [DOI] [PubMed] [Google Scholar]
- 11.Zaheer A, Lenkinski RE, Mahmood A, Jones AG, Cantley LC, Frangioni JV. Nat. Biotechnol. 2001;19:1148–54. doi: 10.1038/nbt1201-1148. [DOI] [PubMed] [Google Scholar]
- 12.Lenkinski RE, Ahmed M, Zaheer A, Frangioni JV, Goldberg SN. Acad. Radiol. 2003;10:1159–64. doi: 10.1016/s1076-6332(03)00253-8. [DOI] [PubMed] [Google Scholar]
- 13.Bhushan KR, Tanaka E, Frangioni JV. Angew. Chem. Int. Ed. Engl. 2007;46:7969–71. doi: 10.1002/anie.200701216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zilberman Y, Kallai I, Gafni Y, Pelled G, Kossodo S, Yared W, Gazit D. J. Orthop. Res. 2008;26:522–30. doi: 10.1002/jor.20518. [DOI] [PubMed] [Google Scholar]
- 15.Misra P, Humblet V, Pannier N, Maison W, Frangioni JV. J. Nucl. Med. 2007;48:1379–89. doi: 10.2967/jnumed.107.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chance B. Ann. N. Y. Acad. Sci. 1998;838:29–45. doi: 10.1111/j.1749-6632.1998.tb08185.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Bloch N, Bhushan KR, De Grand AM, Tanaka E, Solazzo S, Mertyna PM, Goldberg SN, Frangioni JV, Lenkinski RE. Mol. Imaging. 2008 In Press. [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka E, Choi HS, Fujii H, Bawendi MG, Frangioni JV. Ann. Surg. Oncol. 2006;13:1671–81. doi: 10.1245/s10434-006-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detection of breast cancer microcalcifications using a dual-modality SPECT/NIR fluorescent probe
Kumar R. Bhushan,1 Preeti Misra,1 Fangbing Liu,1 Sanjeev Mathur,1 Robert E. Lenkinski,2 and John V. Frangioni1,2*
1Division of Hematology/Oncology, Department of Medicine, and 2Department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, USA
SUPPORTING INFORMATION
General Experimental Procedures
Reagents: All chemicals and solvents were of American Chemical Society or high-performance liquid chromatography (HPLC) purity and were used as received. Ultra-dry DMSO was purchased from Acros Organics (Geel, Belgium). HPLC grade triethylammonium acetate (TEAA), pH 7 was from Glen Research (Sterling, VA, USA). HPLC grade water was from American Bioanalytic (Natick, MA, USA). HPLC grade Methanol (MeOH) was from Mallinckrodt Baker (Phillipsburg, NJ, USA). The N-hydroxysuccinimide (NHS) ester of IRDye800CW was purchased from LI-COR (Lincoln, NE, USA) and stored in the dark as dry powder, under nitrogen, at −80°C until use. All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA, USA) and Sigma-Aldrich (Saint Louis, MO, USA).
Animals: Animals were used in accordance with an approved institutional protocol. Breast cancer microcalcification was developed in Fischer 344 female rats (Taconic Farms, Germantown, NY) by our previously published methods.1 During experiments, rats were anesthetized, intubated and maintained with 1.5−2% isoflurane/balance O2.
Reverse Phase HPLC (Figure S1): HPLC purification of compounds was performed on a Waters (Milford, MA, USA) prepLC 150 mL fluid handling unit (Waters) equipped with a Symmetry Prep®C18 column (19 × 150 mm, 7 μm particle size), a manual injector (Rheodyme 3725i) and a 2487 dual wavelength absorbance detector (Waters) outfitted with a semi-preparative flow cell. A flow splitter (Upchurch Scientific, Oak Harbor, WA, USA) diverted a portion of the eluate into an evaporative light scatter detector (ELSD, Richards Scientific, Novato, CA, USA) with the nebulizer modified to reduce band broadening at low flow rates while the other part flowed into a fraction collector (Waters, Fraction Collector II). The ELSD was set to 40°C, with a nitrogen pressure at 3.5 bar and a gain of 6. For HPLC mobile phase was solvent A = TEAA, solvent B = Methanol and flow rate was 15 ml/min.
ES-TOF Mass Spectroscopic Analysis (Figure S1): The purity of all compounds was measured using liquid chromatography-mass spectrometry (LCMS) on a Waters system consisting of a 1525 binary HPLC pump with a manual 7725i Rheodyme injector, a 2487 dual wavelength absorbance detector, and a 2475 multi wavelength fluorescence detector (Waters). The column eluate was divided in two using a flow splitter (Upchurch Scientific). A portion of the eluate flowed into an ELSD (Richards Scientific) while the rest flowed into a Micromass LCT TOF-ES spectrometer (Waters) equipped with a Symmetry®C18 (4.6 × 150 mm, 5 μm) reverse-phase HPLC column. For mass spectrometry mobile phase was solvent A = TEAA, solvent B = Methanol, flow rate was 0.5 ml/min, capillary voltage was −3317V, and sample cone voltage was −50V.
Spectral Measurements: Absorbance spectroscopy (Figure S1) was performed in a 1 cm path length quartz cuvette (Starna, Atascadero, CA, USA), mounted in a CUV-ALL-UV four-way cuvette holder (Ocean Optics, Dunedin, FL, USA), and excited with a balanced deuterium-tungsten light source (Ocean Optics) spectrometer with a 1.3 nm resolution from 200 to 850 nm, using 2 μM of NIR fluorophore in the indicated buffer. Fluorescence spectrometry was performed in a three-sided quartz cuvette (Starna) excited with a 5 mW 770 nm laser diode coupled through a 300 μm core diameter, NA 0.22 fiber (Fiberguide Industries, Stirling, NJ, USA). Fluorescence measurements (Figure S1) were made on an HR2000 (Ocean Optics) spectrometer with a 7.6 nm resolution from 350 to 1000 nm, using 2 μM NIR fluorophore in the indicated buffer. QYs of Pam-Re-800 6b in PBS, pH 7.4 or 100% fetal bovine serum were calculated using ICG in DMSO (QY 13%2) as calibration standard, under conditions of matched fluorophore absorbance at the 770 nm laser line.
Figure S1. Analytical and spectral characterization. (A) HPLC of Pam-Tc-800 6a: Fluorescence (top; 770 nm excitation) and gamma emission (bottom). (B) MALDI-TOF m/z of 6b: Calculated C66H85N8O30P2ReS53−: 1878.30, found 1875.02 (C) Absorbance/fluorescence spectra of Pam-Re-800 6b.
Intraoperative NIR Fluorescence Imaging System: The principles of the optical path were published in detail previously.3 Briefly, a zoom lens with a working distance of 18” directs light to a dichroic mirror, where the visible light (400−700 nm) is deflected to a color video camera (Imitech IMC-80F, IMI Technology Co., Seoul, Korea), and the NIR fluorescence light (> 785 nm) is filtered by an emission filter (> 795 nm longpass filter) prior to reaching an NIR sensitive camera (Orca-AG, Hamamatsu, Bridgewater, NJ, USA). The entire optical system is mounted on a shock-resistant aluminum frame, enclosed by a sealed cover measuring 12”W × 12”H × 9”D, and mounted on an adjustable arm that swings out over the surgical field.
NIR excitation light is provided by an array of 10 high-powered (1W) light emitting diodes (LEDs; L-760−66−60, Marubeni Epitex, New York, NY, USA), which are filtered (HHQ750/50x, Chroma Technology, Brattleboro, VT, USA) and focused on the surgical field. White light is provided by an array of four Luxeon III white light diodes (Lumileds, San Jose, CA, USA) from which all NIR wavelengths are filtered out (Filter E680SP, Chroma). The generated fluence rates for NIR excitation and white light range from 0−5 mW/cm2 and 0−1 mW/cm2, respectively.
Spatial resolution at a field-of-view of 20 × 15 cm is 625 μm, and at a field-of-view of 4 × 3 cm is 125 μm. After computer-controlled (LabVIEW) camera acquisition via custom LabVIEW (National Instruments, Austin, TX, USA) software, anatomic (white light) and functional (NIR fluorescence light) images can be displayed separately and merged. To create a single image that displays both anatomy (color video) and function (NIR fluorescence), the NIR fluorescence image is pseudo-colored in lime green and overlaid images are refreshed up to 15 times per second. The entire apparatus is suspended on an articulated arm over the surgical field, thus permitting noninvasive and non-intrusive imaging. Hands-free operation utilizing motorized zoom and focus lenses, a four-pedal footswitch, and a multifunction data acquisition board were described in detail previously.4
Gamma scintigraphy and SPECT/CT: Animals were monitored by gamma radioscintigraphy performed with a Research Digital Camera (Isochem Technologies, Castana, IA, USA) equipped with a 1/2” NaI crystal, 86 photomultiplier tubes, and high-resolution, low-energy lead collimator. Sequential anterior images were collected at specified time 4 h using 512 × 512 image matrix while the animal remained under anesthesia (data not shown).
SPECT/CT (single photon emission computed tomography/computed tomography) scans and image analysis were performed using a rodent scanner (NanoSPECT/CT; Philips). Five-minute static scans were acquired at 4 h after injection. The images were reconstructed by a 2-dimensional ordered-subsets expectation maximum algorithm, and no correction was applied for attenuation or scatter.
Detailed Procedures
Synthesis of Lys(t.-Boc)-800CW-carboxylic acid 2: NHS ester of the NIR fluorophore IRDye800CW (1 mg, 0.91 μmol) and N,N-diisopropylethylamine (0.15 μL, 0.91 μmol) were added at 0°C under nitrogen atmosphere to the N-ε-t.-Boc-L-lysine 1 (0.23 mg, 0.91 μmol) taken in 1 mL anhydrous DMSO. The vortexing continued for 2 h at RT in the dark. The reaction mixture was poured over 4 mL ice-cold water and purified by HPLC. After concentration on an Oasis HLB desalting cartridge and subsequent lyophilization a bright green solid reaction component, Lys(t.-Boc)-800CW-carboxylic acid 2 was obtained (∼1 mg, 89%).
Synthesis of Lys(t.-Boc)-800CW-Pam-Me 3: Me-Pam5 (0.23 mg, 0.81 μmol), HCTU (0.33 mg, 0.81 μmol) and n-methylmorpholine (0.11 μL, 0.98 μmol) were added at RT under N2 atmosphere to Lys(t.-Boc)-800CW-carboxylic acid 2 (1 mg, 0.81 μmol) taken in 0.5 mL anhydrous DMSO. The stirring continued for 0.5 h at RT, the reaction mixture was poured over 2 mL ice-cold water and purified by HPLC. After concentration on an Oasis HLB desalting cartridge and subsequent lyophilization a bright green solid reaction component, Lys(t.-Boc)-800CW-Pam-Me 3 was obtained (0.95 mg, 78%).
Synthesis of Lys-800CW-Pam-Me 4: Lys(t.-Boc)-800CW-Pam-Me 3 (0.95 mg, 0.6 μmol) was taken in 95% trifluoroacetic acid (0.4 mL). The solution was stirred at RT for 2.5 h then removed the acid by N2 stream. After lyophilization, the compound 4 was obtained without further purification as a green powder (0.86 mg, 97 %).
Lys-800CW-Pam 5: Trimethylsilyl bromide (0.39 μL, 3.05 μmol) was added slowly to a solution of Lys-800CW-Pam-Me 4 (0.86 mg, 0.61 μmol) in dry DMF (0.2 mL) at 0°C under nitrogen atmosphere. The reaction mixture was vortexed at RT for 12 h in the dark and solvent was evaporated off to dryness. 0.5 mL of CH3OH-H2O (4:1) was added to yield a bright green solution, which was vortexed for 30 min at RT and purified by HPLC. After concentration on an Oasis HLB desalting cartridge and subsequent lyophilization, a bright green solid reaction component termed Lys-800CW-Pam 5 was obtained (0.78 mg, 95%).
Synthesis of Pam-Tc-800 6a: The N-hydroxysuccinimide (NHS) of the 99mTc-MAS3 was prepared with high radiochemical purity (>99%) and high specific activity in DMSO as described previously.6 For radiolabeling, Lys-800CW-Pam 5 (0.75 nmol) in 20 μL DMSO was conjugated to 500 μL of [99mTcMAS3]-NHS (4 mCi, 0.36 nmol)) in DMSO in presence of triethylamine (0.75 nmol). Constant vortexing at RT was maintained for 1 h. The radiolabeled compound was purified by reverse phase HPLC.
Synthesis of Pam-Re-800 6b: Lys-800CW-Pam 5 (0.60 mg, 0.53 μmol) was taken in 0.2 mL dry DMSO and reacted with 1 equivalent Re-MAS3- NHS in presence of 1.5 equivalent DIEA at RT for 1 h in dark under anhydrous conditions. The reaction mixture was poured over 2 mL ice-cold water and purified by HPLC. After concentration on an Oasis HLB desalting cartridge and subsequent lyophilization a bright green solid reaction component, Pam-Re-800 6b was obtained (0.70 mg, 83%).
Table S1: Preparative HPLC purification and LCMS characterization of compounds.
In Vitro Calcium Salt Specificity Experiments: In a 96-well plate, 5 mg/mL of hydroxyapatite (HA) or the phosphate, oxalate, carbonate, and pyrophosphate salts of calcium were separately incubated with Pam-Tc/Re-800 containing 200 nCi (99mTc) and 100 nM (Re) in 100 μl PBS for 30 min with continuous vortexing at RT. Crystals were washed 4 times with a 100-fold excess of PBS, centrifuged and visualized using the previously described NIR fluorescence imaging system at a fluence rate of 5 mW/cm2. All NIR fluorescence images had identical exposure times and normalizations.
The same crystals were also mounted on SPECT/CT and 5 minute static scans were acquired. The images were reconstructed by a 2-dimensional ordered-subsets expectation maximum algorithm, and no correction was applied for attenuation or scatter.
In control experiments, there was no detectable binding of IRDye800CW carboxylic acid or 99mTc-MAS3 to any of the calcium salts. Also, the possibility of fluorescence quenching of Pam-Tc/Re-800 leading to a false-negative result with non-HA salts was ruled out because absorbance measurements of the supernatant confirmed complete recovery of unbound dye.
In vivo SPECT/CT and NIR fluorescence imaging study: Rats with breast cancer microcalcification tumors were intravenously administered Pam-Tc/Re-800 with a dose of 500 μCi (99mTc) and 25 nmol (Re) per rat in 200 μl saline. Four hours post-injection, rats were imaged with a custom intraoperative NIR fluorescence imaging system.7 The same rats were imaged using nanoSPECT/CT.
For biodistribution, 500 μCi of Pam-Tc-800 in 200 μL of saline was administered intravenously. Blood was collected at 0, 1, 2, 5, 10, 15, 30, 60, 90, 120, 180, and 240 min post-injection from the tail vein using micro-capillary tubes, weighed, and counted on a model 1470 Wallac Wizard (Perkin Elmer, Wellesley, MA, USA) 10-detector gamma counter. Curve fitting was performed using Prism version 4.0a (GraphPad, San Diego, CA, USA) software. For measurement of total body retention and clearance, at 4-h post-injection, we ligated the ureters and urethra with silk sutures, removed the bladder en masse, and combined it with excreted urine and feces before measurement of radioactivity in a dose calibrator.8 The remaining carcass was also measured in a dose calibrator, then the heart, lungs, spleen, liver, kidneys, stomach, intestine, tumor, and brain were resected, washed twice in PBS, weighed, and counted as described above. An additional set of rats with breast tumors were injected with clinically available ∼ 500μCi 99mTc MDP to measure the tumor uptake. Statistical analysis was performed with an unpaired t-test using Prism.
References
1. Liu, F.; Bloch, N.; Bhushan, K. R.; De Grand, A. M.; Tanaka, E.; Solazzo, S.; Mertyna, P. M.; Goldberg, S. N.; Frangioni, J. V.; Lenkinski, R. E. Mol. Imaging 2008, In Press.
2. Benson, C.; Kues, H. A. J. Chem. Eng. Data 1977, 22, 379−383.
3. De Grand, A. M.; Frangioni, J. V. Technol. Cancer Res. Treat. 2003, 2, 553−62.
4. Gioux, S.; De Grand, A. M.; Lee, D. S.; Yazdanfar, S.; Idoine, J. D.; Lomnes, S. J.; Frangioni, J. V. SPIE Proceedings 2005, 6009, 39−48.
5. Bhushan, K. R.; Tanaka, E.; Frangioni, J. V. Angew. Chem. Int. Ed. Engl. 2007, 46, 7969−71.
6. Misra, P.; Humblet, V.; Pannier, N.; Maison, W.; Frangioni, J. V. J. Nucl. Med. 2007, 48, 1379−89.
7. Tanaka, E.; Choi, H. S.; Fujii, H.; Bawendi, M. G.; Frangioni, J. V. Ann. Surg. Oncol. 2006, 13, 1671−81.
8. Misra, P.; Lebeche, D.; Ly, H.; Schwarzkopf, M.; Diaz, G.; Hajjar, R. J.; Schecter, A. D.; Frangioni, J. V. J. Nucl. Med. 2008, 49, 963−9.