Abstract
The ocular lens assembles two separate Intermediate Filament systems sequentially with differentiation. Canonical 8–11 nm IFs composed of Vimentin are assembled in lens epithelial cells and younger fiber cells, while the fiber cell-specific Beaded Filaments are switched on as fiber cell elongation initiates. Some of the key features of both filament systems are reviewed.
Actin filaments and microtubules are essential to the most elemental functions of eukaryotic cells. These filamentous structures are assembled from proteins derived from small, highly conserved gene families. Though tissue specificity exists in the expression of some actin and tubulin family members, they are generally expressed in a ubiquitous manner, and are required for eukaryotic cell survival and replication. In contrast, the family of proteins that comprise the cytoplasmic Intermediate Filaments (IFs) is one of the largest in the human genome with greater than 60 members. IFs are generally not required for cell survival, and are absent from single cell eukaryotes, suggesting a more recent appearance on the evolutionary stage, and a less-essential role in the biology of the cell.
The IF family also differs sharply from actins and tubulins in that there is great variation in both size and sequence among the IF proteins, with sequence identity falling below 30% between more distant members of the human IF family. However, despite the large number of IF proteins available for the construction of IFs, any given cell typically expresses only 1–3 IF proteins, with expression tightly restricted to cell type and state of differentiation. This suggests a considerable degree of cell-specific specialization.
While IF proteins show considerable sequence and size variation, they are unified into a family on the basis of three major features:
-
Predicted domain structure (see figure 1): Algorithms that predict coiled-coil structure show a common predicted domain structure consisting of a) head and tail domains which are quite variable in both size and sequence, and b) a central rod domain where the size (~310 amino acids) and predicted secondary structure is strongly conserved. The rod domain consists of large regions of alpha helical structure (coil domains) interrupted by short non-helical regions (“linkers”) that connect the coil domains. The size, number, and placement of linkers and coils are well-conserved. Moreover, the coil domains exhibit a heptad repeat pattern where the 1, 4 positions in the heptad are dominated by hydrophobic amino acids. Because the 1,4 positions are aligned on one side of the helix, they form a largely hydrophobic “stripe” that runs along one side of the alpha helix. This stripe mediates the dimerization of two matched coil domains. The hydrophobic stripe gently twists around the axis of the helix, giving rise to a supercoiling of two alpha helices, hence the “coiled-coil”.
The requisite hydrophobicity at the 1, 4 positions of the heptad can be conferred by any of several amino acids, thus the central rod domain of IF proteins, while showing conserved secondary structure, also exhibits a generally high degree of sequence variation. The exceptions to this are two short motifs found at either end of the central rod domain, commonly called the Helix Initiation Motif (HIM) and the Helix termination Motif (HTM). At these two sites the primary sequence among IF proteins is well conserved. Not surprisingly, the HIM and HTM motifs are intolerant of mutations, with the majority of known IF diseases arising from point mutations in these sites (http://www.interfil.org).
-
Conserved gene structure: Sequence analysis of IF proteins has allowed clustering of IF proteins into several major classes. Sequence conservation in the rod domain is high within a class (typically greater than 70%) but low between classes. Analysis of the IF genes shows that there is conservation of gene structure as well within the IF family, with the number and placement of introns and exons well conserved, especially in the central rod domain. The degree of gene structure conservation correlates well with the degree of primary sequence conservation, and reinforces the grouping of IF proteins into classes on the basis of primary sequence.
The Type I and II IF classes are called cytokeratins, and these comprise the IFs of epithelia. These begin assembly as an obligate heterodimer of one Type I and one Type II cytokeratin. The Type III IF proteins include vimentin, desmin, GFAP, and peripherin, and these are commonly found in tissues of mesenchymal origin. While Type III IF proteins can heterodimerize, they are more commonly found as homomeric filaments. The Type IV IF proteins are the neurofilament proteins Heavy (NFH), Medium (NFM) and Light (NFL), which assemble collectively into the IFs of neurons.
IF proteins form 8–11 nm diameter IFs. Ultimately, despite the differences in head/tail size and sequence, and variation in the rod domain sequence, all cytoplasmic IF proteins typically assemble into 8–11 nm IFs.
Figure 1.
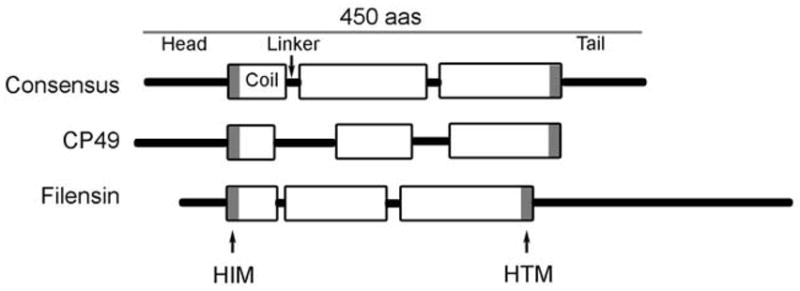
Schematic view of the consensus predicted secondary structure of the IF protein, along with that predicted for CP49 and filensin.
The story of lens IF proteins begins with adherence to the conventions of the IF family. In keeping with its origins from surface ectoderm, the lens placode expresses epithelial cytokeratins(Kasper and Viebahn 1992). However, after severing its connection with the ectoderm, the lens cells switch to the expression of vimentin, a well-conserved Type III IF protein common to cells of mesenchymal origin(Bradley, Ireland et al. 1979; Geisler and Weber 1981; Ellis, Alousi et al. 1984; Bagchi, Caporale et al. 1985; Bloemendal, Willemsen et al. 1985). Figures 2a–b present a localization of both vimentin protein and mRNA, highlighting its abundance in the epithelium and younger elongating fiber cells. This distribution has been highlighted by many investigators, and has been shown to persist in this pattern throughout the life of the lens(Bagchi, Caporale et al. 1985). Both vimentin protein and message appear to be somewhat polarized in their distribution within the epithelial and younger fiber cells. While the protein appears to be dominant in the basal end of the epithelial cell, the message is concentrated in the apical end(Scholz and Rafferty 1988; Blankenship, Hess et al. 2001). In contrast to the epithelial cell which has a significant cytoplasmic labeling, the localization in the fiber cell is predominantly membranous(Bloemendal, Benedetti et al. 1981; Ramaekers, Dunia et al. 1982; Ellis, Alousi et al. 1984; Blankenship, Hess et al. 2001). The functional significance and mechanisms behind the polarized distribution are not known, but IFs have a demonstrated involvement in organelle positioning in polarized cells(Toivola, Tao et al. 2005).
Figure 2.
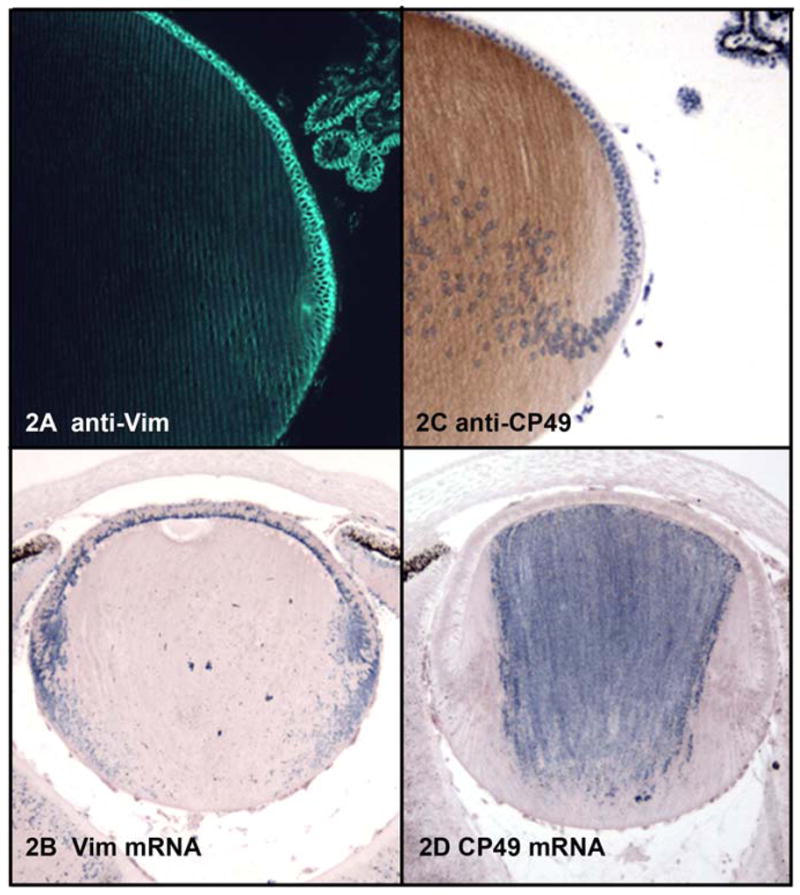
2A: Immunoflurescent localization of vimentin in a paraffin section of mouse lens fixed by freeze substitution in 97% methanol:3% acetic acid. 2B: In situ hybridization localizing vimentin mRNA in paraffin sections of mouse lens. 2C: Immunoperoxidase localization of CP49 in paraffin section of mouse lens, fixed as in 2A. 2D: In situ hybridization demonstrating distribution of CP49 mRNA in mouse lens.
By conventional immunocytochemical approaches, vimentin has been reported to be absent from, or at least greatly reduced in the deeper cortical fiber cells(Bradley, Ireland et al. 1979; Ellis, Alousi et al. 1984; Sandilands, Prescott et al. 1995). However, any retrospective analysis of such data must bear in mind that the immunoreactivity of an antigen in the lens can be dramatically reduced in deeper cortical cells as a result of fixation approach and/or crystallin density-induced artifacts, leading to the incorrect impression that an antigen is absent or sharply reduced in deeper cells(Beebe, Vasiliev et al. 2001; Yoon, Blankenship et al. 2008). Similarly, if vimentin loss is a function of fiber cell age, then the age of the lens in such studies must be considered, as well as the plane of section: off center sections include a higher proportion of younger cells, in contrast to central sections. Thus, any retrospective analysis of such distributions must be critically considered. Electron microscopic examination of ghosted lens fiber cells, an approach that clearly reveals the fiber cell cytoskeleton, also reveals a virtual absence of structurally identifiable cytoplasmic IFs in the organelle-free cells(Alizadeh, Clark et al. 2003), consistent with the majority of immunocytochemical studies(Bradley, Ireland et al. 1979; Ellis, Alousi et al. 1984). However, while cytoplasmic IFs may not be obvious, there can remain a substantial IF presence in the form of filaments adherent to the fiber cell membrane, as seen in tangential sections of fiber cells ghosts (Figure 3).
Figure 3.
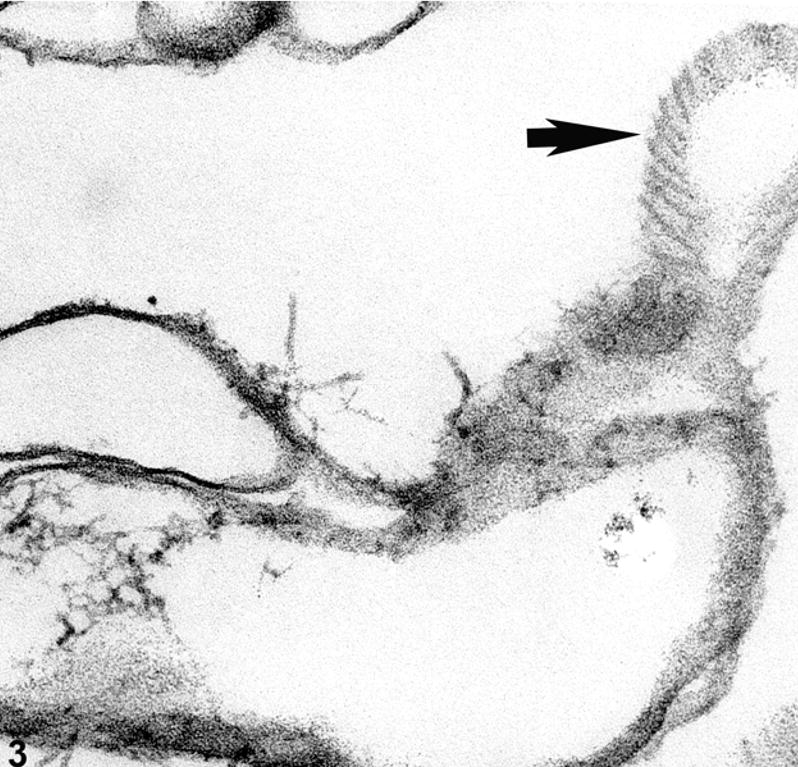
Electron micrograph of ghosted mouse lens, showing a tangential view of a fiber cell membrane (arrow) where IFs are aligned along the surface of the membrane.
While vimentin is clearly the dominant Type III protein in lens cells, the presence of GFAP(Hatfield, Skoff et al. 1984; Hatfield, Skoff et al. 1985), synemin(Granger and Lazarides 1984; Tawk, Titeux et al. 2003), and nestin(Mokry and Nemecek 1998; Yang, Bian et al. 2000) have also been noted. GFAP expression appears to be transient in the course of lens development, and is not universal even among different strains of mice(Achstatter, Moll et al. 1986; Boyer, Maunoury et al. 1990; Boyer, Montagutelli et al. 1991; Bozanic, Bocina et al. 2006)
The function of vimentin in lens has defied identification. Germline knockouts of vimentin were among the first generated(Colucci-Guyon, Portier et al. 1994). However, despite the fact that vimentin is expressed in a variety of tissues, is well-conserved, is often up-regulated in wound repair, and is the “default” IF protein in many de-differentiating cells, no obvious changes in lens phenotype were reported.
In contrast, over expression of vimentin is catastrophic to the lens(Capetanaki, Smith et al. 1989; Bloemendal, Raats et al. 1997). When over expressed, fiber cells proved incapable of dismantling their nuclei, and suffer a generalized problem in phenotypic differentiation. The number of nuclei in the lens epithelium also doubled. The differentiation-dependent disappearance of vimentin in fiber cells correlates well to the loss of organelles, raising the possibility that the loss of vimentin is prerequisite for the successful dismantling of organelles. While the vimentin knockout clearly establishes that nuclear positioning is not dependent on vimentin, is does not preclude a role for vimentin in stabilization of the nucleus, and by inference other organelles(Georgatos and Blobel 1987; Bagchi, Ansari et al. 1997) The capacity of IFs to anchor to membranous organelles(Gao and Sztul 2001) via members of the plakin family, and specifically to the nuclear envelope protein nesparin(Wilhelmsen, Litjens et al. 2005), has been established.
The mechanism by which vimentin is removed as the cell transitions to the organelle-free state is unknown. In cells undergoing mitosis, vimentin IFs are routinely dismantled by phosphorlylation(Inagaki, Nishi et al. 1987), a modification that causes the relatively stable IF polymer break up into smaller subunits, thought to be tetramers. These are subsequently reassembled after cell division is complete. However, vimentin in lens fiber cells appears to removed, and not simply dismantled. IFs are known to be among the first targets of calcium-activated proteases (calpains) in cells that are damaged, and many investigators have demonstrated the calcium-activated degradation of both vimentin and BFs in lens(Yoshida, Murachi et al. 1984; Truscott, Marcantonio et al. 1990; Marcantonio and Duncan 1991; Bettelheim, Qin et al. 1995; Andersson, Sjostrand et al. 1996; Sanderson, Marcantonio et al. 1996; Sanderson, Marcantonio et al. 2000). The dismantling of organelles implies a potential release of calcium from organelles in which it is otherwise routinely sequestered. Whether this release occurs, and whether it alters cytoplasmic calcium levels to a degree that would activate those calpains present in the fiber cell is not known.
Caspases activated in the apoptotic cascade can target conserved sites in IF proteins(Caulin, Salvesen et al. 1997). The elimination of organelles from the fiber cell represents an incomplete apoptotic event, and thus those enzymes responsible for organelle elimination may also represent a viable mechanism for explanation of vimentin’s suggested disappearance(Oshima 2002; Omary, Coulombe et al. 2004).
The loss or reduction of vimentin levels does not leave the mature lens fiber cell devoid of an IF system, however. In a manner that emulates IF switching seen in stratified epithelia, a second generation IF system is switched on in the lens as vimentin is being switched off. It is here where the story of the lens IF system takes the most unusual turn yet described in the IF field.
The initial recognition that the mature lens fiber cells departed from the IF dogma was made when Maisel and co-workers noted the presence of “Beaded Chain Filaments” or Beaded Filaments (BFs) in an electron microscopic analysis of chick lens homogenates(Maisel and Perry 1972; Maisel 1977; Bradley and Maisel 1978; Bradley, Ireland et al. 1979). These studies noted a clearly filamentous structure that was distinct from thin filaments, microtubules, and IFs, which at that time were emerging as the universal cytoskeletal structures common to essentially all vertebrate cells. Speculation emerged that these structures were thin filaments with bound alpha crystallin particles, or nucleosomes on DNA, but these explanations were ruled out experimentally (Bloemendal, Berbers et al. 1984; Ireland and Maisel 1984).
SDS PAGE/western blot analysis of cytoskeletal-enriched fractions from lens revealed the presence of two proteins that failed to label with antibodies that recognized actins, tubulins, and the known IF proteins. Antibodies raised against these two proteins ultimately established that both were components of the BF, but also that neither was expressed in any cell type other than the lens fiber cell(Ireland and Maisel 1984; FitzGerald 1988; FitzGerald and Gottlieb 1989; Ireland and Maisel 1989). (A note on nomenclature which has gotten somewhat confusing: the BF proteins, initially termed CP49 and CP95 by Ireland and Maisel, reflect the fact that they were Cytoskeletal Proteins, of MWr 49 and 95 kDa. Sequencing of the CP95 precipitated a name change to “filensin”(Gounari, Merdes et al. 1993). The initial report of CP49 sequence included the suggestion that CP49 be renamed “phakinin”(Merdes, Gounari et al. 1993). However, the sequence reported in that publication was substantially incorrect. In the first reports of correct partial cDNA sequences of CP49(Hess, Casselman et al. 1993; Orii, Agata et al. 1993), and in the first reported complete sequence of CP49(Hess, Casselman et al. 1996) the name “CP49” was retained, though “phakosin” has also emerged. In recognition of the seminal contributions of Harry Maisel and Mark Ireland to this field, we suggest the retention of their initial name “CP49” in future work (Ireland and Maisel 1984))
Immunocytochemistry of both filensin and CP49, showed that they first emerge in the mouse at embryonic day 12–13, typically at the anterior end of the fiber cell. In more mature lenses, BF proteins are absent from the epithelium, and begin to emerge in the elongating fiber cell, reaching high levels as the cell nears the end of the elongation process. Figures 2c–d present the distribution of CP49 protein and mRNA respectively. BF proteins tend to emerge at the plasma membrane, then extend cytoplasmically as they accumulate(Brunkener and Georgatos 1992; Sandilands, Prescott et al. 1995; Sandilands, Prescott et al. 1995; Blankenship, Hess et al. 2001)
Masaki and Watanabe presented the first sequence data for a BF protein, (filensin(Masaki and Watanabe 1992)), followed closely by Remington and others(Gounari, Merdes et al. 1993; Remington 1993; Hess, Casselman et al. 1998). Sequence data for CP49 appeared subsequently(Hess, Casselman et al. 1993; Orii, Agata et al. 1993).
This sequencing data drew a clear link between the BF proteins and the rapidly expanding IF family. This relationship was surprising, however, as no IF protein had ever been localized to a structure that was not an 8–11 nm IF. This structural difference proved to be one of many where the IF and the where the BF proteins showed a striking divergence from properties otherwise highly conserved among the cytoplasmic IF proteins. IF proteins, despite their divergence in size and primary sequence, were unified by the features described above: predicted secondary structure, conserved HIM/HTM motifs, gene structure, and presence in 8–11 nm IFs. The BF proteins, in contrast, were by far the most unusual members of the family reported, showing the most divergent primary sequences yet reported for IF proteins, notable differences in predicted secondary structure, and extreme divergence in the HIM/HTM motifs, including changes that were known to be disease-causing in every other IF protein(Fuchs 1991; Albers and Fuchs 1992; Fuchs 1994; Fuchs, Coulombe et al. 1994; Pittenger, Hess et al. 2007). Further, while most IF proteins show strong conservation between species, the BF proteins exhibited a relatively high degree of species variation. Bovine CP49, for example, includes a large extension in its tail domain that is absent from mouse, rat, and chick filensins(FitzGerald 1988; Gounari, Merdes et al. 1993). Trout CP49 has a tail domain which is absent from the other species that have been sequenced, and allelic variation in the HIM motif(Binkley, Hess et al. 2002). Chicken shows a variant in gene structure(Wallace, Signer et al. 1998) and at least one alternative splice variant, a property not observed in other species(Sawada, Agata et al. 1995).
Indeed, these exceptional differences initially resulted in legitimate reluctance to consider them members of the IF family. Ultimately however, the demonstration that both BF proteins showed significant conservation of IF gene structure(Hess, Casselman et al. 1996; Gounari, Karagianni et al. 1997; Masaki and Quinlan 1997; Hess, Casselman et al. 1998; Carter, McLean et al. 2000), and that BF proteins could form conventional IFs in vitro(Carter, Hutcheson et al. 1995), confirmed that they were members of the IF family, albeit in their own class of “Orphan Filament Proteins” (Lodish 2000).
The exceptional degree of difference between the BF and IF naturally precipitated the question of why such differences were required to serve the needs of the lens fiber cell: What function of the fiber cell was so unique, so different from all other cells that it required such a divergent IF? Was the formation of a beaded chain structure itself essential to function and the driving force behind its emergence, or was this structure an irrelevant consequence of changes in primary sequence that were necessary for achieving other endpoints?
That BF proteins were likely to be critical to lens clarity was first suggested by linkage analysis studies in two human families with inherited autosomal dominant cataract. These studies pointed to a region of the genome that included CP49(Conley, Erturk et al. 2000; Jakobs, Hess et al. 2000). Sequence analysis of the CP49 gene from these individuals showed two distinct mutations: 1) A three base pair deletion that resulted in the loss of a negatively-charged amino acid from the predicted rod domain. This negative charge was otherwise highly conserved among all human IF proteins. Moreover, the loss of this residue resulted in a frame shift in the heptad repeat pattern of the coiled-coil, a shift that would predict serious consequences for coiled-coil dimer formation; 2) A mis-sense mutation that converted a highly-conserved positive charge to a tryptophan. These mutations resulted in the emergence of cataracts in late childhood/early adulthood. Subsequent studies have implicated filensin as well in inherited human cataract, mediated in one case by a 6kb deletion that results in a shift in the open reading frame(Zhang, Gao et al. 2006; Ramachandran, Perumalsamy et al. 2007)
Thus, as germ line knockouts for each of the two BF proteins were prepared in mice, the expectation was that the absence of the BF proteins, and the BF, would prove catastrophic. However, the change in phenotype produced by the knockout proved surprisingly subtle(Alizadeh, Clark et al. 2002; Alizadeh, Clark et al. 2003; Sandilands, Prescott et al. 2003). Absence of either protein resulted in a total absence of the BF, as judged by electron microscopy, confirming that each was critical to BF assembly. However, the lenses looked clear by routine examination, and there did not appear to be any major perturbation of the elaborate elongation and differentiation of the fiber cells. Careful slit lamp examination(Alizadeh, Clark et al. 2002; Alizadeh, Clark et al. 2003; Alizadeh, Clark et al. 2004; Seeberger, Matsumoto et al. 2004), and laser ray tracing ultimately identified a subtle loss of optical properties: light scatter that grew worse with age, and a decay in the optical quality of the lens(Sandilands, Prescott et al. 2003).
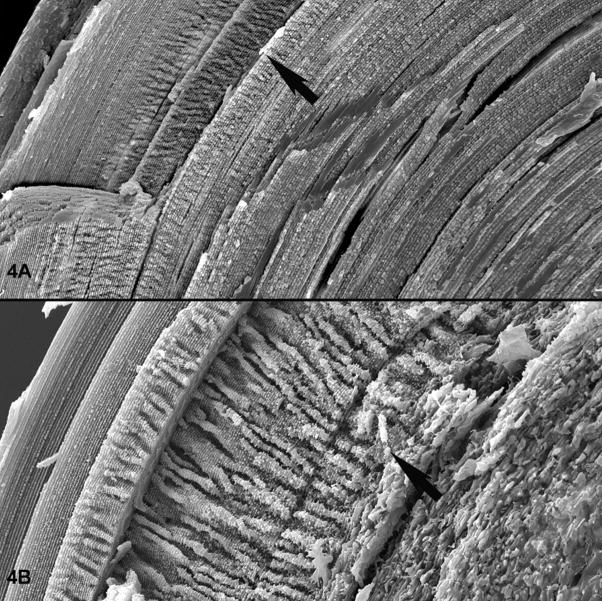
Examination by scanning electron microscopy (SEM) revealed the basis for the light scatter: while fiber cell differentiation proceeded in a manner that was not readily discriminable from the wild type, the fiber cells proved unable to maintain either their uniformly elongated shape, nor the very precise tissue level, long-range order that is a defining characteristic in vertebrate lenses(Sandilands, Prescott et al. 2003; Blankenship, Bradshaw et al. 2007; Yoon, Blankenship et al. 2008). Figure 4A shows an SEM image of a wild type mouse lens. The exceptional degree of alignment is evident, starting with the youngest cells at the surface (upper left), progressing through the emergence then disappearance (arrow) of the elaborate “paddle-like structures (Blankenship, Bradshaw et al. 2007), through the very uniform and regular cells of the inner cortex and nucleus. In contrast, the knockout lens (figure4B) shows normal fiber cell differentiation and maturation through the disappearance of the paddles (arrow), at which point the cells lose their uniform shape and precise alignment, and become much more randomized. This loss of phenotype in older cells suggested that the BF did not contribute to the generation of cellular and tissue level phenotypes in lens, but that it was required to maintain the structural phenotype with age. Such a function in phenotype stabilization is very much in keeping with that determined for several IF proteins, such as the cytokeratins in epidermis and cornea(Fuchs 1994; Coleman, Hannush et al. 1999; Corden, Swensson et al. 2000; Corden, Swensson et al. 2000), and desmin in muscle(Agbulut, Li et al. 1996; Capetanaki and Milner 1998; Balogh, Li et al. 2004). In these examples, structural differentiation of skin, cornea, and muscle appeared to proceed normally, but the cells were incapable of sustaining this phenotype in the face of normal use. Thus despite the divergence of the BF proteins from the rest of their IF kin, there appears to be a conservation of core IF function: stabilization of the differentiated phenotype. This again raises the question of why the BF is the only IF that does not form a typical IF: is there a functional requirement for the BF format that is necessary for its function in lens?
Figure 4.
Scanning electron micrographs of a wild type (A) and BF knockout (B) lens. Figure A is a lower magnification overview, with an arrow highlighting the transition from a region where the paddle-like structures are regressing. Deep to this point, fiber cells retain their unique shape and strict alignment. Figure B is a higher magnification view of a BF knockout lens, with an arrow placed at a similar transition point. The loss of fiber cell shape and alignment at this juncture is evident in the knockout.
The BF knockouts also showed that when one BF protein was eliminated, the level of the other was dramatically reduced(Alizadeh, Clark et al. 2002; Alizadeh, Clark et al. 2003). This suggests that in the absence of an assembly partner, the insoluble BF protein was identified as an unfolded or mis-folded protein and eliminated by the cell’s proteasomal system that recognizes and removes unfolded proteins. A similar phenomenon was established in genetically engineered mice that lack an epidermal cytokeratin. This is probably a critical step in the design of IFs. The IF proteins are insoluble, and in the absence of an assembly partner the aggregated insoluble protein would superimpose an “unfolded protein toxicity” on top of the functional loss due to absence of the filament system, as has been shown experimentally in other cell types(Omary, Coulombe et al. 2004; Watson, Geary-Joo et al. 2007). Indeed, the difference between the overt cataract seen in humans bearing a mutation in CP49, and the much more subtle loss of optical properties seen in mice lacking the BF entirely, might be accounted for by the inability to remove the mutant CP49, and any insoluble complexes it forms with filensin in the early stages of aborted assembly.
It is noteworthy that both labs working on the CP49 knockout encountered a naturally-occurring mutation in the CP49 gene in the 129 strain of mouse(Alizadeh, Clark et al. 2004; Sandilands, Wang et al. 2004). This mutation is a roughly 6kb deletion that results in an absence of CP49, and thus a de facto knockout. The presence of this mutation has been extended to other strains, including the FVB strain which is also used extensively in the preparation of genetically engineered mice(Sandilands, Wang et al. 2004; Simirskii, Lee et al. 2006). Thus, most early knockouts of non-BF lens genes were actually double knockouts, with consequent superimposition of two aberrant phenotypes.
Like many IF proteins, vimentin and the BF proteins undergo post translational changes. For example, Ireland, et. al., demonstrated that both BF and vimentin proteins are phosphorylated in lens(Ireland and Maisel 1984) and that this can be regulated by adrenergic receptors and cAMP. It is well accepted that vimentin phosphorylation is used to induce disassembly of the IF in dividing cells, though, not all vimentin phosphorylation results in disassembly(Ando, Tanabe et al. 1989). While lens epithelial cells are mitotically capable, it is a relatively infrequent event, and unlikely to account for the degree of phosphorylation seen. BFs, in contrast are expressed only in non-dividing cells, thus a role in mitotic disassembly can be ruled out.
BF proteins also undergo a significant reduction in molecular mass with fiber cell maturation, as well as a shift in their immunocytochemically-determined subcellular distribution. Filensin, for example, is cleaved from its parent 95kd to about 51 kd(FitzGerald 1988; Quinlan, Carter et al. 1992; Sandilands, Prescott et al. 1995; Fleschner 1998). This residual 51 kDa fragment appears, on the basis of epitope-specific antibody labeling, to retain the predicted central rod domain, and to remain associated with the plasma membrane(FitzGerald 1988; Brunkener and Georgatos 1992). IFs in general are known to be early targets of calcium-activated proteases, and this class of protease has been shown to be capable of mediating the degradation of BF and IF proteins in the lens, under experimental conditions(Ireland and Maisel 1984; Marcantonio and Duncan 1991; Marcantonio 1992; Marcantonio 1996; Sanderson, Marcantonio et al. 1996; Sanderson, Marcantonio et al. 2000; de Iongh, Lovicu et al. 2001; de Iongh, Wederell et al. 2005). Proteolysis might at first be considered the undesirable fate of a protein destined for life in a cell incapable of protein turn over and renewal. However, the literature on the maturation of fiber cells is showing a surprising number of very specific changes occurring late in the maturation process, even in the organelle-free cell. Many of these changes including the assembly of complex cellular processes(Blankenship, Bradshaw et al. 2007) and shifts in membrane protein localization(Beebe, Vasiliev et al. 2001; Lim, Lorentzen et al. 2006), raise the possibility that such proteolytic events are triggers that may produce an intended change in function late in the differentiation process. Specifically, the fact that the BF proteins show what appears to be a limited susceptibility to proteolysis may be a selective pressure in their favor, leaving a core protein with a specific and different function from the parent function.
Cytoskeletal structures, of course, do not operate as independent entities. For all three major filamentous systems, the same core filament can be functionally tailored to accomplish multiple different tasks by the selective and regulated use of adapter or linker proteins that target the attachment of the filament to specific structures, or to regulate assembly/disassembly and filament stability. In the case of the IF, the number of such linkers (referred to as Intermediate Filament Associated Proteins, or IFAPs) is relatively limited, but they are proving to be very versatile proteins, capable of mediating an unexpectedly large constellation of functions. The plakin family has been at the forefront of studies which demonstrate the adaptation of IFs to multiple functions. The plakins have well documented roles in linking IFs to the plasma membrane and to intercellular junctions such as desmosomes and hemidesmosomes (Bornslaeger, Corcoran et al. 1996; Leung, Green et al. 2002; Groot, Sevilla et al. 2004; Jefferson, Leung et al. 2004; Boczonadi, McInroy et al. 2007; Sonnenberg and Liem 2007). However, IFs have also been shown to attach to organelles such as the golgi(Gao and Sztul 2001; Gao, Vrielink et al. 2002), nucleus(Georgatos and Blobel 1987) and mitochondria, to other cytoskeletal systems, and even to adherens and focal adhesion junctions which had historically been linked to only thin filaments(Windoffer, Woll et al. 2004; Windoffer, Kolsch et al. 2006; Leonard, Chan et al. 2008). Thus, it is clear that IFs are integrated into a variety of non-junctional cellular functions, and the current expectation is that a given IF is likely to accomplish multiple functions, modulated by the IFAPs that are expressed.
Consistent with the emerging role of IFAPs in modulating and adapting IF function is the observation that fiber cell vimentin IFs interact with the N Cadherin-gamma catenin complex(Leonard, Chan et al. 2008), lengsin(Wyatt, Gao et al. 2008), MIP(Lindsey Rose, Gourdie et al. 2006), periplakin(Yoon and FitzGerald 2008), tropomodulin(Fischer, Quinlan et al. 2003) and possibly other complexes which are present in lens(Bagchi, Katar et al. 2002; Straub, Boda et al. 2003; Bagchi, Katar et al. 2004). The number of candidate linker proteins demonstrated in lens leads to the expectation that the BF and IF are likely to accomplish multiple functions, and that these may be modulated as differentiation progresses, and as the fiber cell proteome changes, either by expression or proteolysis.
Similarly, the small heat shock proteins, whose chaperone function appears essential to IF/BF assembly and maintenance, must be considered as critical parts of the biology of IFs in lens. Mutations in the small heat shock proteins have been shown to precipitate a failure in the IF systems in many tissues, and in lens specifically, and to subsequently emulate IF diseases (FitzGerald and Graham 1991; Nicholl and Quinlan 1994; Carter, Hutcheson et al. 1995; Vicart, Caron et al. 1998; Muchowski, Valdez et al. 1999; Perng, Cairns et al. 1999; Perng, Muchowski et al. 1999; Evgrafov, Mersiyanova et al. 2004; Treweek, Rekas et al. 2005; Song, Hanson et al. 2008).
The growing multiplicity of IF interactions underscores the need to expect that failure in “IF function” in the lens can result from failure in a wide spectrum of proteins that affect assembly, phosphorylation, proteolytic modification, stability, removal, or linkage to other cellular structures, and that “IF failure” is likely to show considerable variability in phenotype.
Acknowledgments
I’d like to thank current and former members of my lab, all of whom have contributed extensively to the work included in this manuscript, and who have, and continue to make research flat out fun: John Hess, Ph.D., Jodi Cassleman, Tom Blankenship, Ph.D., Azita Alizadeh, Ph.D., Daryle DePianto, Ph.D., Kyoung-hye Yoon, Ph.D., Josh Pittenger, Ph.D., Atya Aziz, Ph.D., and Brad Shibata. This works was supported by NEI EY08747 to PGF, and by NEI P30 EY12576. Work was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR-12088-01 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achstatter T, Moll R, et al. Expression of glial filament protein (GFP) in nerve sheaths and non-neural cells re-examined using monoclonal antibodies, with special emphasis on the co-expression of GFP and cytokeratins in epithelial cells of human salivary gland and pleomorphic adenomas. Differentiation. 1986;31(3):206–27. doi: 10.1111/j.1432-0436.1986.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Agbulut O, Li Z, et al. Analysis of skeletal and cardiac muscle from desmin knock-out and normal mice by high resolution separation of myosin heavy-chain isoforms. Biol Cell. 1996;88(3):131–5. [PubMed] [Google Scholar]
- Albers K, Fuchs E. The molecular biology of intermediate filament proteins. Int Rev Cytol. 1992;134:243–79. doi: 10.1016/s0074-7696(08)62030-6. [DOI] [PubMed] [Google Scholar]
- Alizadeh A, Clark J, et al. Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin. Invest Ophthalmol Vis Sci. 2003;44(12):5252–8. doi: 10.1167/iovs.03-0224. [DOI] [PubMed] [Google Scholar]
- Alizadeh A, Clark J, et al. Characterization of a mutation in the lens-specific CP49 in the 129 strain of mouse. Invest Ophthalmol Vis Sci. 2004;45(3):884–91. doi: 10.1167/iovs.03-0677. [DOI] [PubMed] [Google Scholar]
- Alizadeh A, Clark JI, et al. Targeted genomic deletion of the lens-specific intermediate filament protein CP49. Invest Ophthalmol Vis Sci. 2002;43(12):3722–7. [PubMed] [Google Scholar]
- Andersson M, Sjostrand J, et al. Calpains in the human lens: relations to membranes and possible role in cataract formation. Ophthalmic Res. 1996;28(Suppl 1):51–4. doi: 10.1159/000267944. [DOI] [PubMed] [Google Scholar]
- Ando S, Tanabe K, et al. Domain- and sequence-specific phosphorylation of vimentin induces disassembly of the filament structure. Biochemistry. 1989;28(7):2974–9. doi: 10.1021/bi00433a035. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Ansari SA, et al. Nonchromatin nuclear proteins of mammalian lens epithelial cells. J Cell Biochem. 1997;64(4):644–50. doi: 10.1002/(sici)1097-4644(19970315)64:4<644::aid-jcb12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Caporale MJ, et al. Vimentin synthesis by ocular lens cells. Exp Eye Res. 1985;40(3):385–92. doi: 10.1016/0014-4835(85)90151-4. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Katar M, et al. Associated proteins of lens adherens junction. J Cell Biochem. 2002;86(4):700–3. doi: 10.1002/jcb.10258. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Katar M, et al. ERM proteins of the lens. J Cell Biochem. 2004;92(3):626–30. doi: 10.1002/jcb.20062. [DOI] [PubMed] [Google Scholar]
- Balogh J, Li Z, et al. Desmin filaments influence myofilament spacing and lateral compliance of slow skeletal muscle fibres. Biophys J. 2004 doi: 10.1529/biophysj.104.042630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Vasiliev O, et al. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest Ophthalmol Vis Sci. 2001;42(3):727–34. [PubMed] [Google Scholar]
- Bettelheim FA, Qin C, et al. Calcium cataract: a model for optical anisotropy fluctuations. Exp Eye Res. 1995;60(2):153–7. doi: 10.1016/s0014-4835(95)80005-0. [DOI] [PubMed] [Google Scholar]
- Binkley PA, Hess J, et al. Unexpected variation in unique features of the lens-specific type I cytokeratin CP49. Invest Ophthalmol Vis Sci. 2002;43(1):225–35. [PubMed] [Google Scholar]
- Blankenship T, Bradshaw L, et al. Structural specializations emerging late in mouse lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2007;48(7):3269–76. doi: 10.1167/iovs.07-0109. [DOI] [PubMed] [Google Scholar]
- Blankenship TN, Hess JF, et al. Development- and differentiation-dependent reorganization of intermediate filaments in fiber cells. Invest Ophthalmol Vis Sci. 2001;42(3):735–42. [PubMed] [Google Scholar]
- Bloemendal H, Benedetti EL, et al. The lens cytoskeleton. Intermediate-sized filaments, their biosynthesis and association with plasma membranes. Mol Biol Rep. 1981;7(13):167–8. doi: 10.1007/BF00778749. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, Berbers GA, et al. Interaction of crystallins with the cytoskeletal-plasma membrane complex of the bovine lens. Ciba Found Symp. 1984;106:177–90. doi: 10.1002/9780470720875.ch10. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, Raats JM, et al. Transgenic mice carrying chimeric or mutated type III intermediate filament (IF) genes. Cell Mol Life Sci. 1997;53(1):1–12. doi: 10.1007/PL00000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H, Willemsen M, et al. Isolation of the intermediate filament protein vimentin by chromatofocusing. FEBS Lett. 1985;180(2):181–4. doi: 10.1016/0014-5793(85)81067-x. [DOI] [PubMed] [Google Scholar]
- Boczonadi V, McInroy L, et al. Cytolinker cross-talk: periplakin N-terminus interacts with plectin to regulate keratin organisation and epithelial migration. Exp Cell Res. 2007;313(16):3579–91. doi: 10.1016/j.yexcr.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Bornslaeger EA, Corcoran CM, et al. Breaking the connection: displacement of the desmosomal plaque protein desmoplakin from cell-cell interfaces disrupts anchorage of intermediate filament bundles and alters intercellular junction assembly. J Cell Biol. 1996;134(4):985–1001. doi: 10.1083/jcb.134.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S, Maunoury R, et al. Expression of glial fibrillary acidic protein and vimentin in mouse lens epithelial cells during development in vivo and during proliferation and differentiation in vitro: comparison with the developmental appearance of GFAP in the mouse central nervous system. J Neurosci Res. 1990;27(1):55–64. doi: 10.1002/jnr.490270109. [DOI] [PubMed] [Google Scholar]
- Boyer S, Montagutelli X, et al. Recent evolutionary origin of the expression of the glial fibrillary acidic protein (GFAP) in lens epithelial cells. A molecular and genetic analysis of various mouse species. Brain Res Mol Brain Res. 1991;10(2):159–66. doi: 10.1016/0169-328x(91)90106-8. [DOI] [PubMed] [Google Scholar]
- Bozanic D, Bocina I, et al. Involvement of cytoskeletal proteins and growth factor receptors during development of the human eye. Anat Embryol (Berl) 2006;211(5):367–77. doi: 10.1007/s00429-006-0087-z. [DOI] [PubMed] [Google Scholar]
- Bradley R, Maisel H. Chain-proteins of the vertebrate lens. Experientia. 1978;34(4):470–2. doi: 10.1007/BF01935933. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Ireland ME, et al. Age changes in the skeleton of the human lens. Acta Ophthalmol (Copenh) 1979;57(3):461–9. doi: 10.1111/j.1755-3768.1979.tb01830.x. [DOI] [PubMed] [Google Scholar]
- Brunkener M, Georgatos SD. Membrane-binding properties of filensin, a cytoskeletal protein of the lens fiber cells. J Cell Sci. 1992;103(Pt 3):709–18. doi: 10.1242/jcs.103.3.709. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y, Milner DJ. Desmin cytoskeleton in muscle integrity and function. In: Herrmann H, Harris JR, editors. Intermediate Filaments. Vol. 31. New York and London: Plenum Press; 1998. pp. 463–495. [PubMed] [Google Scholar]
- Capetanaki Y, Smith S, et al. Overexpression of the vimentin gene in transgenic mice inhibits normal lens cell differentiation. J Cell Biol. 1989;109(4 Pt 1):1653–64. doi: 10.1083/jcb.109.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JM, Hutcheson AM, et al. In vitro studies on the assembly properties of the lens proteins CP49, CP115: coassembly with alpha-crystallin but not with vimentin. Exp Eye Res. 1995;60(2):181–92. doi: 10.1016/s0014-4835(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Carter JM, McLean WH, et al. Mapping of the human CP49 gene and identification of an intragenic polymorphic marker to allow genetic linkage analysis in autosomal dominant congenital cataract. Biochem Biophys Res Commun. 2000;270(2):432–6. doi: 10.1006/bbrc.2000.2442. [DOI] [PubMed] [Google Scholar]
- Caulin C, Salvesen GS, et al. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138(6):1379–94. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CM, Hannush S, et al. A novel mutation in the helix termination motif of keratin K12 in a US family with Meesmann corneal dystrophy. Am J Ophthalmol. 1999;128(6):687–91. doi: 10.1016/s0002-9394(99)00317-7. [DOI] [PubMed] [Google Scholar]
- Colucci-Guyon E, Portier MM, et al. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79(4):679–94. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Conley YP, Erturk D, et al. A juvenile-onset, progressive cataract locus on chromosome 3q21–q22 is associated with a missense mutation in the beaded filament structural protein-2. Am J Hum Genet. 2000;66(4):1426–31. doi: 10.1086/302871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden LD, Swensson O, et al. A novel keratin 12 mutation in a German kindred with Meesmann’s corneal dystrophy. Br J Ophthalmol. 2000;84(5):527–30. doi: 10.1136/bjo.84.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden LD, Swensson O, et al. Molecular genetics of Meesmann’s corneal dystrophy: ancestral and novel mutations in keratin 12 (K12) and complete sequence of the human KRT12 gene. Exp Eye Res. 2000;70(1):41–9. doi: 10.1006/exer.1999.0769. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Lovicu FJ, et al. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development. 2001;128(20):3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Wederell E, et al. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179(1–2):43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- Ellis M, Alousi S, et al. Studies on lens vimentin. Exp Eye Res. 1984;38(2):195–202. doi: 10.1016/0014-4835(84)90103-9. [DOI] [PubMed] [Google Scholar]
- Evgrafov OV, Mersiyanova I, et al. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36(6):602–6. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- Fischer RS, Quinlan RA, et al. Tropomodulin binds to filensin intermediate filaments. FEBS Lett. 2003;547(1–3):228–32. doi: 10.1016/s0014-5793(03)00711-7. [DOI] [PubMed] [Google Scholar]
- FitzGerald PG. Age-related changes in a fiber cell-specific extrinsic membrane protein. Curr Eye Res. 1988;7(12):1255–62. doi: 10.3109/02713688809033229. [DOI] [PubMed] [Google Scholar]
- FitzGerald PG. Immunochemical characterization of a Mr 115 lens fiber cell-specific extrinsic membrane protein. Curr Eye Res. 1988;7(12):1243–53. doi: 10.3109/02713688809033228. [DOI] [PubMed] [Google Scholar]
- FitzGerald PG, Gottlieb W. The Mr 115 kd fiber cell-specific protein is a component of the lens cytoskeleton. Curr Eye Res. 1989;8(8):801–11. doi: 10.3109/02713688909000870. [DOI] [PubMed] [Google Scholar]
- FitzGerald PG, Graham D. Ultrastructural localization of alpha A-crystallin to the bovine lens fiber cell cytoskeleton. Curr Eye Res. 1991;10(5):417–36. doi: 10.3109/02713689109001750. [DOI] [PubMed] [Google Scholar]
- Fleschner CR. Intermediate filament cytoskeletal proteins associated with bovine lens native membrane fractions. Curr Eye Res. 1998;17(4):409–18. doi: 10.1080/02713689808951222. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Keratin genes, epidermal differentiation and animal models for the study of human skin diseases. Biochem Soc Trans. 1991;19(4):1112–5. doi: 10.1042/bst0191112. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Intermediate filaments and disease: mutations that cripple cell strength. J Cell Biol. 1994;125(3):511–6. doi: 10.1083/jcb.125.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Coulombe P, et al. Genetic bases of epidermolysis bullosa simplex and epidermolytic hyperkeratosis. J Invest Dermatol. 1994;103(5 Suppl):25S–30S. doi: 10.1111/1523-1747.ep12398924. [DOI] [PubMed] [Google Scholar]
- Gao Y, Sztul E. A novel interaction of the Golgi complex with the vimentin intermediate filament cytoskeleton. J Cell Biol. 2001;152(5):877–94. doi: 10.1083/jcb.152.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YS, Vrielink A, et al. A novel type of regulation of the vimentin intermediate filament cytoskeleton by a Golgi protein. Eur J Cell Biol. 2002;81(7):391–401. doi: 10.1078/0171-9335-00260. [DOI] [PubMed] [Google Scholar]
- Geisler N, Weber K. Isolation of polymerization-competent vimentin from porcine eye lens tissue. FEBS Lett. 1981;125(2):253–6. doi: 10.1016/0014-5793(81)80732-6. [DOI] [PubMed] [Google Scholar]
- Georgatos SD, Blobel G. Two distinct attachment sites for vimentin along the plasma membrane and the nuclear envelope in avian erythrocytes: a basis for a vectorial assembly of intermediate filaments. J Cell Biol. 1987;105(1):105–15. doi: 10.1083/jcb.105.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounari F, Karagianni N, et al. The mouse filensin gene: structure and evolutionary relation to other intermediate filament genes. FEBS Lett. 1997;413(2):371–8. doi: 10.1016/s0014-5793(97)00937-x. [DOI] [PubMed] [Google Scholar]
- Gounari F, Merdes A, et al. Bovine filensin possesses primary and secondary structure similarity to intermediate filament proteins. J Cell Biol. 1993;121(4):847–53. doi: 10.1083/jcb.121.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger BL, Lazarides E. Expression of the intermediate-filament-associated protein synemin in chicken lens cells. Mol Cell Biol. 1984;4(10):1943–50. doi: 10.1128/mcb.4.10.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot KR, Sevilla LM, et al. Kazrin, a novel periplakin-interacting protein associated with desmosomes and the keratinocyte plasma membrane. J Cell Biol. 2004;166(5):653–9. doi: 10.1083/jcb.200312123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield JS, Skoff RP, et al. Glial fibrillary acidic protein is localized in the lens epithelium. J Cell Biol. 1984;98(5):1895–8. doi: 10.1083/jcb.98.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield JS, Skoff RP, et al. The lens epithelium contains glial fibrillary acidic protein (GFAP) J Neuroimmunol. 1985;8(4–6):347–57. doi: 10.1016/s0165-5728(85)80072-2. [DOI] [PubMed] [Google Scholar]
- Hess JF, Casselman JT, et al. cDNA analysis of the 49 kDa lens fiber cell cytoskeletal protein: a new, lens-specific member of the intermediate filament family? Curr Eye Res. 1993;12(1):77–88. doi: 10.3109/02713689308999499. [DOI] [PubMed] [Google Scholar]
- Hess JF, Casselman JT, et al. Gene structure and cDNA sequence identify the beaded filament protein CP49 as a highly divergent type I intermediate filament protein. J Biol Chem. 1996;271(12):6729–35. doi: 10.1074/jbc.271.12.6729. [DOI] [PubMed] [Google Scholar]
- Hess JF, Casselman JT, et al. Primary sequence, secondary structure, gene structure, and assembly properties suggests that the lens-specific cytoskeletal protein filensin represents a novel class of intermediate filament protein. Exp Eye Res. 1998;66(5):625–44. doi: 10.1006/exer.1998.0478. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Nishi Y, et al. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987;328(6131):649–52. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- Ireland M, Maisel H. A cytoskeletal protein unique to lens fiber cell differentiation. Exp Eye Res. 1984;38(6):637–45. doi: 10.1016/0014-4835(84)90182-9. [DOI] [PubMed] [Google Scholar]
- Ireland M, Maisel H. Phosphorylation of chick lens proteins. Curr Eye Res. 1984;3(7):961–8. doi: 10.3109/02713688409167214. [DOI] [PubMed] [Google Scholar]
- Ireland M, Maisel H. A family of lens fiber cell specific proteins. Lens Eye Toxic Res. 1989;6(4):623–38. [PubMed] [Google Scholar]
- Jakobs PM, Hess JF, et al. Autosomal-dominant congenital cataract associated with a deletion mutation in the human beaded filament protein gene BFSP2. Am J Hum Genet. 2000;66(4):1432–6. doi: 10.1086/302872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson JJ, Leung CL, et al. Plakins: goliaths that link cell junctions and the cytoskeleton. Nat Rev Mol Cell Biol. 2004;5(7):542–53. doi: 10.1038/nrm1425. [DOI] [PubMed] [Google Scholar]
- Kasper M, Viebahn C. Cytokeratin expression and early lens development. Anat Embryol (Berl) 1992;186(3):285–90. doi: 10.1007/BF00174151. [DOI] [PubMed] [Google Scholar]
- Leonard M, Chan Y, et al. Identification of a novel intermediate filament-linked N-cadherin/gamma-catenin complex involved in the establishment of the cytoarchitecture of differentiated lens fiber cells. Dev Biol. 2008;319(2):298–308. doi: 10.1016/j.ydbio.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CL, Green KJ, et al. Plakins: a family of versatile cytolinker proteins. Trends Cell Biol. 2002;12(1):37–45. doi: 10.1016/s0962-8924(01)02180-8. [DOI] [PubMed] [Google Scholar]
- Lim J, Lorentzen KA, et al. Molecular identification and characterisation of the glycine transporter (GLYT1) and the glutamine/glutamate transporter (ASCT2) in the rat lens. Exp Eye Res. 2006;83(2):447–55. doi: 10.1016/j.exer.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Lindsey Rose KM, Gourdie RG, et al. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest Ophthalmol Vis Sci. 2006;47(4):1562–70. doi: 10.1167/iovs.05-1313. [DOI] [PubMed] [Google Scholar]
- Lodish HMP, Kaiser c. Molecular Cell Biology. New York: Freeman; 2000. [Google Scholar]
- Maisel H. Filaments of the vertebrate lens. Experientia. 1977;33(4):525. doi: 10.1007/BF01922252. [DOI] [PubMed] [Google Scholar]
- Maisel H, Perry MM. Electron microscope observations on some structural proteins of the chick lens. Exp Eye Res. 1972;14(1):7–12. doi: 10.1016/0014-4835(72)90136-4. [DOI] [PubMed] [Google Scholar]
- Marcantonio JM. Susceptibility of the bovine lens 115kDa beaded filament protein to degradation by calcium and calpain. Curr Eye Res. 1992;11(1):103–8. doi: 10.3109/02713689209069172. [DOI] [PubMed] [Google Scholar]
- Marcantonio JM. Calcium-induced disruption of the lens cytoskeleton. Ophthalmic Res. 1996;28(Suppl 1):48–50. doi: 10.1159/000267943. [DOI] [PubMed] [Google Scholar]
- Marcantonio JM, Duncan G. Calcium-induced degradation of the lens cytoskeleton. Biochem Soc Trans. 1991;19(4):1148–50. doi: 10.1042/bst0191148. [DOI] [PubMed] [Google Scholar]
- Masaki S, Quinlan RA. Gene structure and sequence comparisons of the eye lens specific protein, filensin, from rat and mouse: implications for protein classification and assembly. Gene. 1997;201(1–2):11–20. doi: 10.1016/s0378-1119(97)00419-8. [DOI] [PubMed] [Google Scholar]
- Masaki S, Watanabe T. cDNA sequence analysis of CP94: rat lens fiber cell beaded-filament structural protein shows homology to cytokeratins. Biochem Biophys Res Commun. 1992;186(1):190–8. doi: 10.1016/s0006-291x(05)80792-2. [DOI] [PubMed] [Google Scholar]
- Merdes A, Gounari F, et al. The 47-kD lens-specific protein phakinin is a tailless intermediate filament protein and an assembly partner of filensin. J Cell Biol. 1993;123(6 Pt 1):1507–16. doi: 10.1083/jcb.123.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokry J, Nemecek S. Immunohistochemical detection of intermediate filament nestin. Acta Medica (Hradec Kralove) 1998;41(2):73–80. [PubMed] [Google Scholar]
- Muchowski PJ, Valdez MM, et al. AlphaB-crystallin selectively targets intermediate filament proteins during thermal stress. Invest Ophthalmol Vis Sci. 1999;40(5):951–8. [PubMed] [Google Scholar]
- Nicholl ID, Quinlan RA. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. EMBO J. 1994;13(4):945–53. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary MB, Coulombe PA, et al. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351(20):2087–100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- Orii H, Agata K, et al. Evidence that the chick lens cytoskeletal protein CP 49 belongs to the family of intermediate filament proteins. Curr Eye Res. 1993;12(6):583–8. doi: 10.3109/02713689309001836. [DOI] [PubMed] [Google Scholar]
- Oshima RG. Apoptosis and keratin intermediate filaments. Cell Death Differ. 2002;9(5):486–92. doi: 10.1038/sj.cdd.4400988. [DOI] [PubMed] [Google Scholar]
- Perng MD, Cairns L, et al. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci. 1999;112( Pt 13):2099–112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Perng MD, Muchowski PJ, et al. The cardiomyopathy and lens cataract mutation in alphaB-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J Biol Chem. 1999;274(47):33235–43. doi: 10.1074/jbc.274.47.33235. [DOI] [PubMed] [Google Scholar]
- Pittenger JT, Hess JF, et al. Identifying the role of specific motifs in the lens fiber cell specific intermediate filament phakosin. Invest Ophthalmol Vis Sci. 2007;48(11):5132–41. doi: 10.1167/iovs.07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RA, Carter JM, et al. The 53kDa polypeptide component of the bovine fibre cell cytoskeleton is derived from the 115kDa beaded filament protein: evidence for a fibre cell specific intermediate filament protein. Curr Eye Res. 1992;11(9):909–21. doi: 10.3109/02713689209033488. [DOI] [PubMed] [Google Scholar]
- Ramachandran RD, Perumalsamy V, et al. Autosomal recessive juvenile onset cataract associated with mutation in BFSP1. Hum Genet. 2007;121(3–4):475–82. doi: 10.1007/s00439-006-0319-6. [DOI] [PubMed] [Google Scholar]
- Ramaekers FC, Dunia I, et al. Lenticular intermediate-sized filaments: biosynthesis and interaction with plasma membrane. Proc Natl Acad Sci U S A. 1982;79(10):3208–12. doi: 10.1073/pnas.79.10.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington SG. Chicken filensin: a lens fiber cell protein that exhibits sequence similarity to intermediate filament proteins. J Cell Sci. 1993;105( Pt 4):1057–68. doi: 10.1242/jcs.105.4.1057. [DOI] [PubMed] [Google Scholar]
- Sanderson J, Marcantonio JM, et al. Calcium ionophore induced proteolysis and cataract: inhibition by cell permeable calpain antagonists. Biochem Biophys Res Commun. 1996;218(3):893–901. doi: 10.1006/bbrc.1996.0159. [DOI] [PubMed] [Google Scholar]
- Sanderson J, Marcantonio JM, et al. A human lens model of cortical cataract: Ca2+-induced protein loss, vimentin cleavage and opacification. Invest Ophthalmol Vis Sci. 2000;41(8):2255–61. [PubMed] [Google Scholar]
- Sandilands A, Prescott AR, et al. Vimentin and CP49/filensin form distinct networks in the lens which are independently modulated during lens fibre cell differentiation. J Cell Sci. 1995;108(Pt 4):1397–406. doi: 10.1242/jcs.108.4.1397. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Prescott AR, et al. Filensin is proteolytically processed during lens fiber cell differentiation by multiple independent pathways. Eur J Cell Biol. 1995;67(3):238–53. [PubMed] [Google Scholar]
- Sandilands A, Prescott AR, et al. Knockout of the intermediate filament protein CP49 destabilises the lens fibre cell cytoskeleton and decreases lens optical quality, but does not induce cataract. Exp Eye Res. 2003;76(3):385–91. doi: 10.1016/s0014-4835(02)00330-5. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Wang X, et al. Bfsp2 mutation found in mouse 129 strains causes the loss of CP49’ and induces vimentin-dependent changes in the lens fibre cell cytoskeleton. Exp Eye Res. 2004;78(4):875–89. doi: 10.1016/j.exer.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Sawada K, Agata J, et al. The predicted structure of chick lens CP49 and a variant thereof, CP49ins, the first vertebrate cytoplasmic intermediate filament protein with a lamin-like insertion in helix 1B. Curr Eye Res. 1995;14(7):545–53. doi: 10.3109/02713689508998401. [DOI] [PubMed] [Google Scholar]
- Scholz DL, Rafferty NS. Immunogold-EM localization of actin and vimentin filaments in relation to polygonal arrays in lens epithelium in situ. Curr Eye Res. 1988;7(7):705–19. doi: 10.3109/02713688809033200. [DOI] [PubMed] [Google Scholar]
- Seeberger TM, Matsumoto Y, et al. Digital image capture and quantification of subtle lens opacities in rodents. J Biomed Opt. 2004;9(1):116–20. doi: 10.1117/1.1630034. [DOI] [PubMed] [Google Scholar]
- Simirskii VN, Lee RS, et al. Inbred FVB/N mice are mutant at the cp49/Bfsp2 locus and lack beaded filament proteins in the lens. Invest Ophthalmol Vis Sci. 2006;47(11):4931–4. doi: 10.1167/iovs.06-0423. [DOI] [PubMed] [Google Scholar]
- Song S, Hanson MJ, et al. Protein-protein interactions between lens vimentin and alphaB-crystallin using FRET acceptor photobleaching. Mol Vis. 2008;14:1282–7. [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313(10):2189–203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- Straub BK, Boda J, et al. A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. J Cell Sci. 2003;116(Pt 24):4985–95. doi: 10.1242/jcs.00815. [DOI] [PubMed] [Google Scholar]
- Tawk M, Titeux M, et al. Synemin expression in developing normal and pathological human retina and lens. Exp Neurol. 2003;183(2):499–507. doi: 10.1016/s0014-4886(03)00240-1. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Tao GZ, et al. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 2005;15(11):608–17. doi: 10.1016/j.tcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Treweek TM, Rekas A, et al. R120G alphaB-crystallin promotes the unfolding of reduced alpha-lactalbumin and is inherently unstable. FEBS J. 2005;272(3):711–24. doi: 10.1111/j.1742-4658.2004.04507.x. [DOI] [PubMed] [Google Scholar]
- Truscott RJ, Marcantonio JM, et al. Calcium-induced opacification and proteolysis in the intact rat lens. Invest Ophthalmol Vis Sci. 1990;31(11):2405–11. [PubMed] [Google Scholar]
- Vicart P, Caron A, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20(1):92–5. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- Wallace P, Signer E, et al. The chicken CP49 gene contains an extra exon compared to the human CP49 gene which identifies an important step in the evolution of the eye lens intermediate filament proteins. Gene. 1998;211(1):19–27. doi: 10.1016/s0378-1119(98)00117-6. [DOI] [PubMed] [Google Scholar]
- Watson ED, Geary-Joo C, et al. The Mrj co-chaperone mediates keratin turnover and prevents the formation of toxic inclusion bodies in trophoblast cells of the placenta. Development. 2007;134(9):1809–17. doi: 10.1242/dev.02843. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K, Litjens SH, et al. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171(5):799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windoffer R, Kolsch A, et al. Focal adhesions are hotspots for keratin filament precursor formation. J Cell Biol. 2006;173(3):341–8. doi: 10.1083/jcb.200511124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windoffer R, Woll S, et al. Identification of novel principles of keratin filament network turnover in living cells. Mol Biol Cell. 2004;15(5):2436–48. doi: 10.1091/mbc.E03-09-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt K, Gao C, et al. A role for lengsin, a recruited enzyme, in terminal differentiation in the vertebrate lens. J Biol Chem. 2008;283(10):6607–15. doi: 10.1074/jbc.M709144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bian W, et al. Nestin expression during mouse eye and lens development. Mech Dev. 2000;94(1–2):287–91. doi: 10.1016/s0925-4773(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Yoon KH, Blankenship T, et al. Resisting the effects of aging: a function for the fiber cell beaded filament. Invest Ophthalmol Vis Sci. 2008;49(3):1030–6. doi: 10.1167/iovs.07-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KH, FitzGerald P. Periplakin Serves As A Linker For Both Vimentin And The Highly Divergent Beaded Filament Proteins In The Ocular Lens. IOVS. 2008 In Press. [Google Scholar]
- Yoshida H, Murachi T, et al. Degradation of actin and vimentin by calpain II, a Ca2+-dependent cysteine proteinase, in bovine lens. FEBS Lett. 1984;170(2):259–62. doi: 10.1016/0014-5793(84)81324-1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gao L, et al. Progressive sutural cataract associated with a BFSP2 mutation in a Chinese family. Mol Vis. 2006;12:1626–31. [PubMed] [Google Scholar]