Abstract
The neurotransmitter acetylcholine is an important modulator of cognitive functions including attention, learning, and memory. The actions of acetylcholine are mediated by five distinct muscarinic acetylcholine receptor subtypes (M1-M5). The lack of drugs with a high degree of selectivity for these subtypes has impeded the determination of which subtypes mediate which components of cholinergic neurotransmission relevant to cognitive abilities. The present study examined the behavioral functions of the M2 muscarinic receptor subtype by utilizing congenic C57BL/6 mice possessing a null-mutation in the M2 muscarinic receptor gene (M2−/− mice). Comprehensive assessment of general health and neurological function found no major differences between M2−/− and wild-type (M2+/+) mice. In tests of learning and memory, M2−/− mice were impaired in the acquisition (trials to criterion), but not the retention (72 hr) of a passive avoidance task. In a novel open field, M2−/− mice were impaired in between-sessions, but not within-session habituation. In a holeboard test of spatial memory, M2−/− mice committed more errors in working memory than M2+/+ mice. Reference memory did not differ between the genotypes. M2−/− mice showed no impairments in either cued or contextual fear conditioning. These findings replicate and extend earlier findings in a hybrid strain and solidify the interpretation that the M2 receptor plays a critical role in specific components of cognitive abilities.
Keywords: acetylcholine, muscarinic, memory, knockout, M2, passive avoidance, habituation, holeboard
Introduction
Many clinically important functions of the neurotransmitter acetylcholine (ACh) are mediated by muscarinic acetylcholine receptors [8,25,49]. Muscarinic receptor subtypes have been implicated in components of attentional processes, learning, and memory [26]. The role of muscarinic receptors in learning and memory is of particular interest because cholinergic dysfunction plays a major role in neurodegenerative disorders such as Alzheimer’s disease [4,10,51].
There are five molecularly distinct muscarinic receptor subtypes (M1-M5) [9], but little is known about the specific physiologic roles of each subtype because of a lack of subtype-selective ligands. Furthermore, most organs, tissues, and cells express two or more muscarinic receptor subtypes, making it difficult to determine the role of each individual subtype [30,47,52]. In mice, studies using the null mutation strategy (gene knockout) to study individual receptor subtypes provide a way to address these issues [48–50].
Previous studies using the null mutation strategy have demonstrated that the M2 muscarinic receptor subtype facilitates learning and memory. Mice homozygous for a null mutation in the M2 receptor gene (M2−/− mice) showed impaired performance in a passive avoidance task relative to their wild-type littermates (M2+/+ mice) indicating a learning and memory deficit [45]. M2−/− mice were also impaired in a simple form of learning, between-sessions habituation to the open field [14]. In another study, using the Barnes circular maze and the T maze delayed alternation task, M2−/− mice were impaired in spatial learning and behavioral flexibility [41].
These behavioral findings were obtained in mice with a hybrid genetic background (129J1/CF1;50/50%) leaving open the questions of whether the observed phenotypes depend on genetic background and whether flanking genes from the embryonic stem cell donor strain contribute to the phenotypes [5,12,18,53]. In the present study we addressed these questions by generating a congenic strain of M2−/− mice through the backcrossing of heterozygotes (M2+/− mice) into the C57BL/6NTac strain for 10 successive generations. M2−/− mice and M2+/+ mice (littermate controls) were then compared in a battery of learning and memory tasks. The learning and memory tests included passive avoidance to criterion, within session habituation to the open field, between session habituation to the open field, cued and contextual fear conditioning, and spatial memory in the holeboard task. These studies were undertaken because knowledge of the precise functions of muscarinic receptors is essential for the development of new therapeutic approaches to diseases involving memory and cognition.
Materials and Methods
Mice
M2−/− mice were generated on a mixed genetic background as previously described [19]. Heterozygous (M2+/−) mice were backcrossed for 10 generations into the C57BL/6NTac background (Taconic Farms, Germantown, NY). Two batches of mice were produced by breeding heterozygous (M2+/−) mice at the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD). Mouse genotyping was performed by PCR analysis of mouse tail DNA. Batch 1 (males and females) was used to assess whether the null mutation affected the general health and neurological function of the mice. Batch 2 (males only) was shipped at 10 months of age from the National Institute of Diabetes and Digestive and Kidney Disease to the Drake University College of Pharmacy and Health Sciences where behavioral assessment was conducted at 12–17 months of age. The test order for Batch 2 is indicated by the order of description in the methods. The time interval between behavioral tests is provided at the beginning of the description of each test. In all procedures measures were taken to minimize pain and discomfort of the mice, and all procedures were approved by the Institutional Animal Care and Use Committee at Drake University.
Assessment of General Health and Neurological Function
Measures of general health and neurological function were performed as previously described [11]. Briefly, general health was determined by assessment of fur condition, whisker condition, body and limb tone, and observation of home cage behavior. Neurological reflexes assessed were trunk curl, forepaw reaching, eye-blink, pinna twitch, vibrissae response, toe pinch response, and the righting reflex. Behavioral reactivity was assessed by attempted escape during petting, struggling or vocalization during handling, and biting of a wooden dowel. Empty cage behavior was assessed by placing each subject in a clean, empty cage for 3 min, and noting the occurrence of freezing, wild running, stereotypies, exploration, and grooming. Olfactory ability was assessed by measuring the latency to find a buried peanut, as previously described. All subjective assessments were done by an experimenter who was uninformed of the subjects’ genotypes.
Open Field Activity and Within Session Habituation
An automated open field apparatus was used to assess spontaneous motor activity and within session habituation to a novel environment. The open field was a square arena (40 × 40 × 35 cm; Med Associates, St. Albans, VT) with clear Plexiglas walls and floor, evenly illuminated by white light (~ 700 lux). Each subject (males, M2+/+, n = 23; M2−/−, n = 21) was placed in the center of the open field and allowed to explore for 60 min. The arena was traversed by 32 infrared beams emitted from each of two sides of the arena (16 per side) such that the beams formed a grid of equally sized squares. As the mouse moved about in the arena, the automated computer software (Activity Monitor, version 5.8, Med Associates, St. Albans, VT) counted beam breaks, termed ambulatory counts, as a measure of horizontal activity. Beam breaks were counted as ambulatory counts whenever 3 beams were broken and the interval between breaks was less than 500 msec. Within-session habituation was measured as the decrease in horizontal activity across the duration of the test. A second array of infrared beams, situated above those that assessed horizontal activity, detected the vertical activity (rearing) of the mouse. The apparatus was cleaned with 70% ethanol between subjects.
Passive Avoidance
Two to four days after open field testing, passive avoidance learning was assessed in male M2+/+ mice (n = 23) and M2−/− mice (n = 20) in a computer-controlled Gemini shuttle-box (San Diego Instruments, San Diego, CA) that was divided into two equal chambers by a guillotine door. Training began by placing the subject into the chamber on the right and allowing free exploration for 1 min. At the end of this exploration period, the chamber was illuminated (6,340 lux) and the guillotine door was opened. Latency to pass through the doorway and to enter the other chamber, which remained darkened (3 lux), was recorded by the computer. Upon entering the darkened chamber, the guillotine door closed and a mild footshock (2 sec, 0.75 mA) was delivered. The mouse was then removed from the darkened chamber after 10 sec and placed back into the illuminated chamber. This sequence was repeated until the mouse remained in the illuminated chamber for 300 sec. The number of trials needed to reach this criterion was taken as the measure of task acquisition. Approximately 72 hrs after task acquisition, retention of passive avoidance was assessed by placing each mouse in the illuminated chamber as before. Latency to enter the darkened chamber was taken as the measure of task retention.
Between Sessions Habituation to the Open Field
Approximately 2 months after passive avoidance training, between sessions habituation to a novel environment was assessed. Male mice (M2+/+, n = 20; M2−/−, n = 20) were placed in the automated open field described above for a duration of 15 min. After an intersession interval of 4 hrs, the mice were placed in the apparatus again for 15 min. During both trials, horizontal activity was recorded as before. To create a novel environment from the previous exposure to the open field box, the top half of the box was “wall papered” with white bench paper and was cleaned with orange-scented cleaner (Fantastik Orange Action, S.C, Johnson, Racine, WI) between subjects rather than ethanol. The percent reduction in horizontal activity during the entire second exposure to the apparatus relative to the entire first exposure served as a measure of between sessions habituation.
Cued and Contextual Fear Conditioning
Approximately 3 weeks after assessment of between-sessions habituation, cued and contextual fear conditioning was conducted in an automated conditioning chamber (32 cm × 25 cm × 23 cm, Med Associates, St. Albans, VT) equipped with a digital video camera interfaced to a PC installed with commercially available software (Video Freeze, version 1.12.0.0, Med Associates, St. Albans, VT) that uses a pixel-based method for determining the occurrence of freezing behavior, the species-specific conditioned response produced by fear conditioning protocols. The conditioning chamber was housed in a wooden, sound attenuating cubicle (64 cm × 76 cm × 42 cm). The chamber was illuminated by a fluorescent light mounted on the back wall of the sound-attenuating cubicle (1,800 lux). The user-determined settings for the determination of freezing behavior were: observation interval of 10 sec, observation duration of 0.5 sec, and a motion threshold of 20 arbitrary units. These settings were found in pilot studies in our laboratory to reliably correlate with human observation of freezing behavior (r = 0.944).
For fear conditioning training, male subjects (M2+/+, n = 23; M2−/−, n = 21) were placed in the chamber and presented with two white noise (conditioned stimulus (CS), 90 dB) and footshock (unconditioned stimulus (US), 0.75 mA) pairings. The footshock was delivered through the floor which was in the form of parallel bars with a diameter of 3.2 mm.. Each pairing was preceded and followed by a 2 min exploration period. The CS-US pairings were comprised of 30 sec of white noise and 1 sec of footshock that overlapped and co-terminated with the CS.
Twenty-four and 48 hrs after training, the mice were tested for contextual and cued fear conditioning, respectively. For contextual conditioning, the mice were individually removed from their home cages and placed in the conditioning chamber under environmental conditions identical to those of the training day. No stimuli were presented to the mice during the contextual conditioning test. Freezing behavior was recorded by the computer software for 5 min and served as the measure of the strength of association between the shock and the training environment.
In the cued fear conditioning test, mice were individually removed from their home cages and placed in the conditioning chambers. To isolate cued conditioning from contextual conditioning, the environmental context was altered by replacing the parallel bar floor with a grid-mesh floor made of smaller bars and a white, rounded plastic insert was placed along the walls in order to change the overall appearance of the chamber. Furthermore, the chambers were cleaned with an orange-scented cleaning solution rather than the usual 70% ethanol between subjects in order to alter the olfactory stimuli in the environment. Each mouse was placed in the altered-context environment for 6 min. The first 3 min established the baseline exploration in the absence of any stimuli. During the last 3 min, the CS white noise was presented. In order to analyze the effect of cue onset freezing behavior during the last minute of cue offset and the first minute of cue onset were compared. The presence or absence of freezing behavior was recorded by computer software and served as the measure of the strength of the learned association between the auditory cue and the footshock.
Holeboard Task of Spatial Memory
Approximately two months after fear conditioning, a food-motivated holeboard task was used to assess spatial learning and memory [27]. The apparatus consisted of an open field arena (40 × 40 × 35 cm; Med Associates, St. Albans, VT) with a floor insert that consisted of 16 evenly spaced holes (diameter of 1.3 cm, depth of 9 cm). An array of infrared beams was used to detect nosepokes (entries) into the holes. Extra-maze spatial cues consisted of cables, hardware, and the experimenter who always sat in the same location. The apparatus was illuminated at a level of approximately 700 lux.
Prior to being trained in this task, male mice (M2+/+, n = 16; M2−/−, n = 15) were singly housed and placed on a food restriction schedule in which their body weights were maintained at 80–85% of their free-feeding body weights. The first three days of the task consisted of acclimation and shaping sessions in which all the holes were baited with a food pellet and the mouse was allowed to freely explore the arena. The habituation session ended when the mouse had obtained all of the food rewards or 15 min had expired. Training of the mice in the task began on the fourth day. In the training trials, 4 randomly chosen holes (holes number 1, 8, 10, and 15 with the top left hole being #1 and bottom right being #16) were baited with a 20 mg pellet (Bio-Serve, Frenchtown, NJ). The 12 unbaited holes and 4 baited holes also contained a 45-mg pellet (changed daily) that was located beneath a wire grid and thus inaccessible to the mice. By setting up the baited and unbaited holes in this manner, the mice could not use olfaction to locate the baited holes and were required to learn their location relative to spatial cues. Training days consisted of 3 trials per day with each trial being separated by an interval of approximately 2.5 min. Trials continued until all 4 baits were obtained or 3 min had expired. The apparatus was cleaned with 70% ethanol between subjects. Mice were run in the task for 19 days, but only data from the first six days is shown because performance did not significantly improve after that time (asymptotic performance).
The performance measures in the holeboard task were working memory errors and reference memory errors. Working memory errors were defined as any re-entry into a baited or unbaited hole. Reference memory errors were defined as any first-time entry into an unbaited hole.
Statistical Analysis
All passive avoidance measurements were analyzed by t-test. Ambulatory counts in the open field were analyzed by two-way repeated measures analysis of variance (ANOVA) using 5-min time bin and genotype as the factors. For between-sessions habituation to the open field, percent reduction in ambulatory activity during the entire second session relative to the entire first session was analyzed by t-test. Freezing behavior during training and during the test of cued conditioning was analyzed by two-way repeated measures ANOVA using genotype and stage of session (pre CS-US pairing or post CS-US pairing) as the factors. Freezing behavior during the contextual conditioning test was analyzed by t-test. For cued conditioning, freezing behavior during the last minute of the cue off condition and first minute of cue on condition were analyzed by two-way repeated measures ANOVA using genotype and cue state as the factors. Working and reference memory errors in the holeboard task were analyzed by two-way repeated measures ANOVA using genotype and day as the factors. The threshold for significance for all tests was p < 0.05, and all analyses were conducting using SigmaStat (version 3.10).
Results
General Health and Neurological Function
Table 1 summarizes the results of the assessment of general health and neurological function. M2−/− mice were generally indistinguishable from M2+/+ mice on all measures indicating that the null mutation did not preclude testing of performance in more sophisticated behavioral domains such as learning and memory. Although the mice were older than those used in previous behavioral studies of M2 knockout mice (12–17 months vs. 3–6 months), we found no grossly apparent, age-related characteristics that would limit our studies.
Table 1.
Assessment of general health and neurological function in Batch 1
| M2+/+, male (n=9) | M2−/−, male (n=11) | M2+/+, female (n=12) | M2−/−, female (n=15) | |
|---|---|---|---|---|
| General health | ||||
| Fur condition (3 point scale) | 2.5 | 3.0 | 3.0 | 3.0 |
| Bald patches (%) | 0 | 0 | 0 | 0 |
| Piloerection (%) | 0 | 0 | 0 | 0 |
| Body tone (3 point scale) | 2.0 | 2.0 | 2.0 | 2.0 |
| Limb tone (3 point scale) | 2.0 | 2.0 | 2.0 | 2.0 |
| Motoric abilities | ||||
| Positional passivity (%) | 0 | 0 | 0 | 0 |
| Trunk curl (%) | 100 | 100 | 100 | 100 |
| Reflexes | ||||
| Forepaw reaching (%) | 100 | 100 | 100 | 100 |
| Righting reflex (%) | 100 | 100 | 100 | 100 |
| Corneal (%) | 100 | 100 | 100 | 100 |
| Pinna (%) | 100 | 100 | 100 | 100 |
| Vibrissae (%) | 100 | 100 | 100 | 100 |
| Toe pinch (%) | 100 | 46 | 92 | 60 |
| Reactivity | ||||
| Petting escape (%) | 100 | 100 | 100 | 100 |
| Dowel biting (3point scale) | 1.2 | 1.0 | 1.5 | 1.1 |
| Empty cage behavior | ||||
| Transfer freezing (%) | 0 | 0 | 0 | 0 |
| Wild running (%) | 0 | 0 | 0 | 0 |
| Stereotypies (%) | 0 | 0 | 0 | 0 |
| Exploration (3 point scale) | 2.0 | 2.0 | 1.8 | 2.0 |
| Grooming (3 point scale) | 1.0 | 1.0 | 1.2 | 1.3 |
Open Field Motor Activity
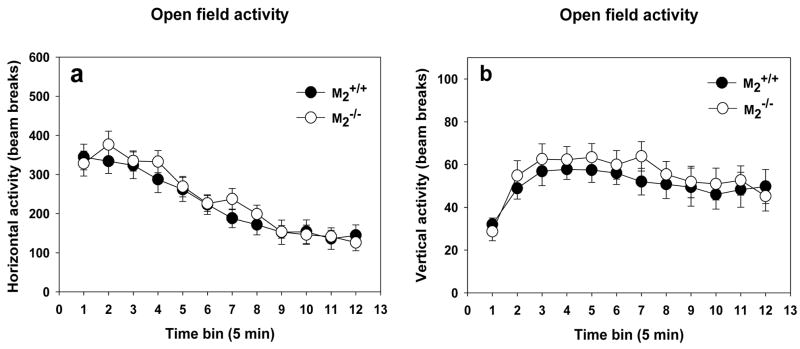
M2−/− mice and M2+/+ mice did not differ in their motor activity levels in an open field nor in their habituation to the open field within the test session, as indicated by significantly decreased motor activity across a 60 min test session (Figure 1a; two-way repeated measures ANOVA, main effect of time bin, F(11, 442) = 44.69, p < 0.001). The genotypes also did not differ in vertical activity (rearing; Figure 1b).
Figure 1.
Spontaneous Motor Activity and Within Session Habituation. (a) There was no difference between M2+/+ (n = 23) and M2−/− (n = 21) mice in spontaneous motor activity in a novel environment. The genotypes also did not differ in their habituation to the novel environment as indicated by a decrease in motor activity with time. (b) There was no difference between M2+/+ and M2−/− mice in vertical activity (rearing) in a novel environment.
Passive Avoidance
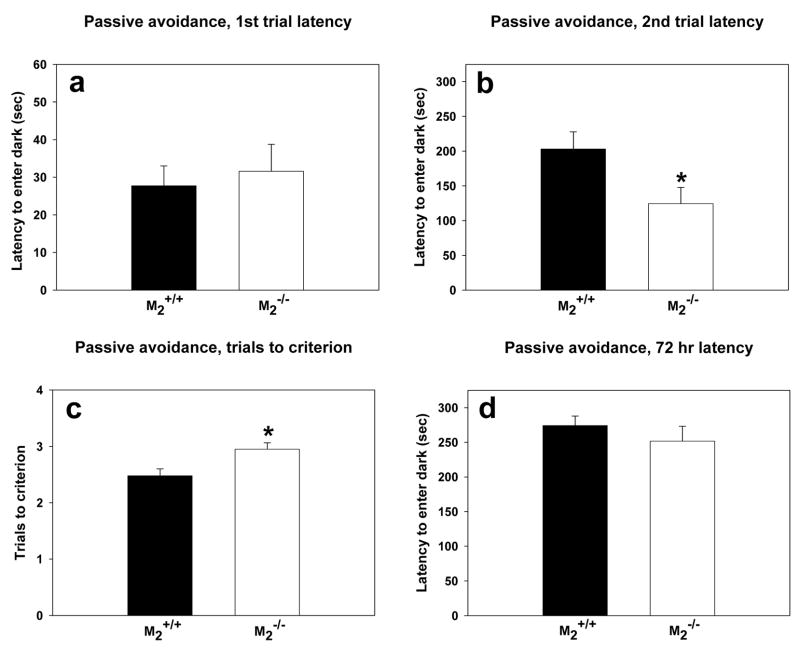
In the first trial of passive avoidance training to criterion, M2+/+ mice and M2−/− mice entered the darkened chamber with similar latencies (Figure 2a) indicating that there was no confounding effect of genotype on motor activity in this task. M2−/− mice were impaired in their ability to learn the passive avoidance as indicated by significantly lower latencies (Figure 2b; t = 2.29, df = 41, p = 0.03) than M2+/+ mice to enter the dark on the second trial. Furthermore, an acquisition deficit was indicated by the M2−/− requiring significantly more training trials than M2+/+ mice to obtain the criterion of remaining in the illuminated chamber for 300 sec (Figure 2c; t = −2.77, df = 41, p =0.008). The passive avoidance deficit in M2−/− mice was limited to acquisition as shown by the 72-hr retention test (Figure 2d) in which there was no effect of genotype.
Figure 2.
Passive Avoidance Trials to Criterion. (a) There was no difference between the genotypes in latency to enter the dark chamber on the first training trial. (b) As indicated by the asterisk, on the second training trial, M2−/− mice (n = 20) had significantly lower latencies (t = 2.29, df = 41, p = 0.03) than M2+/+ mice (n = 23) to enter the dark chamber. (c) As indicated by the asterisk, M2−/− mice required significantly more trials (t = −2.77, df = 41, p =0.008) than M2+/+ mice to reach the criterion of remaining in the lit chamber for 300 sec. (d) There was no effect of genotype on latency to enter the dark chamber in the 72 hr retention trial.
Between Session Habituation to Open Field
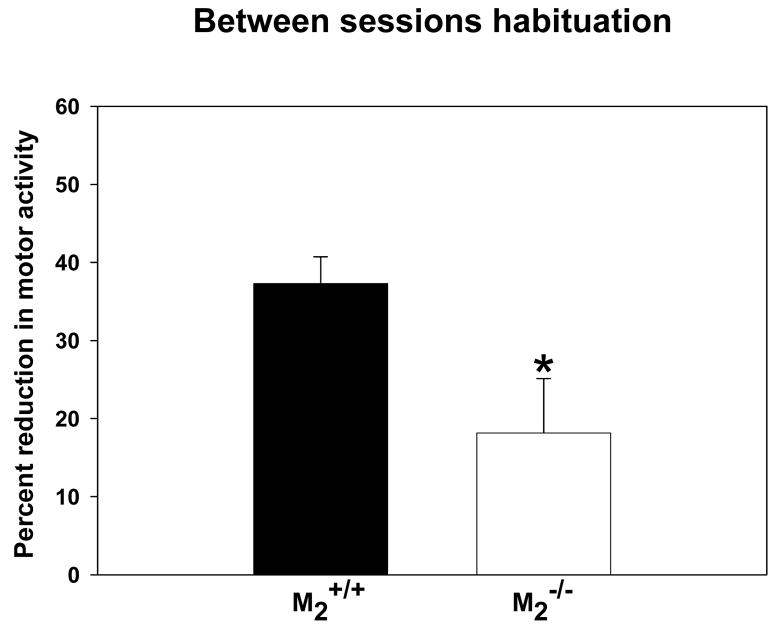
In the test of between session habituation to a novel open field, there was no effect of genotype on ambulatory counts during either the first or second 15-min exposure to the open field (M2+/+ first exposure mean = 720.10 ± 92.08; M2+/+ second exposure mean = 463.80 ± 71.41; M2−/− first exposure mean = 602.65 ± 65.49; M2−/− second exposure mean = 468.65 ± 56.21), replicating the finding described above. However, during the second exposure (Figure 3), M2−/− mice decreased their motor activity significantly less (approx. 20%) than M2+/+ mice (approx. 40%; t = 2.46, df = 38, p = 0.02). These data indicate decreased between session habituation to the novel environment in M2−/− mice suggesting an impairment in their recognition memory of the novel environment.
Figure 3.
Between Sessions Habituation. As indicated by the asterisk, M2−/− (n = 20) mice exhibited a significantly smaller (t = 2.46, df = 38, p = 0.02) degree of habituation (as measured by a decrease in motor activity) than M2+/+ (n = 20) mice during the second exposure (4 hr interval) to a novel environment.
Cued and Contextual Fear Conditioning
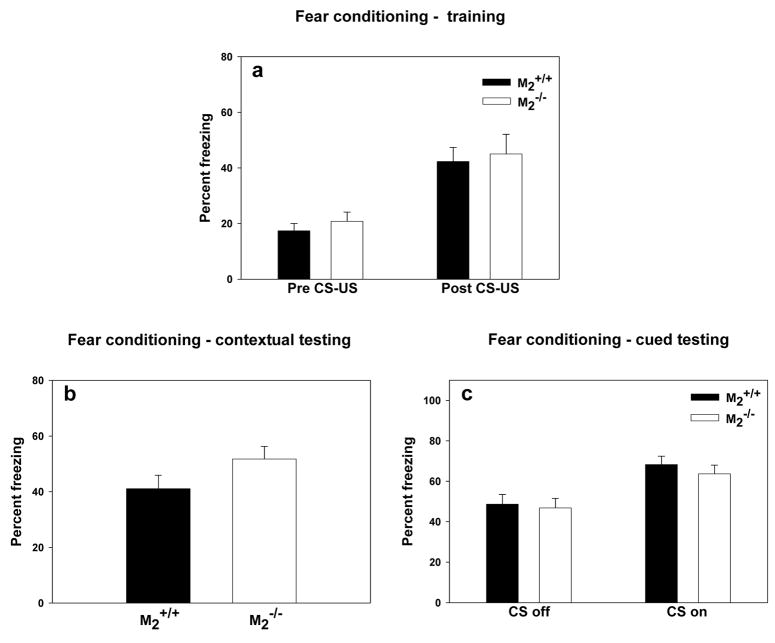
During fear conditioning training (Figure 4a), both genotypes increased freezing subsequent to the CS-US pairings (two-way repeated measures ANOVA, F(1, 42) = 30.81, p < 0.001), but the genotypes did not differ in their degree of freezing either prior or subsequent to the CS-US pairings. There was not a significant interaction between genotype and phase of training. During testing of contextual conditioning, the genotypes did not differ in the amount of freezing exhibited in the training context (Figure 4b). During testing of cued conditioning, both genotypes increased their freezing behavior during the first minute of cue onset relative to the last minute of the cue off condition (Figure 4c; two-way repeated measures ANOVA, F(1, 42) = 24.91, p < 0.01) indicating that the mice associated the auditory cue with footshock. There was no effect of genotype on freezing either during the cue off or cue on phases of the experiment, and there was no significant interaction between genotype and state of the cue.
Figure 4.
Cued and Contextual Fear Conditioning. (a) During training freezing behavior did not differ by genotype (M2+/+, n = 23; M2−/−, n = 21) prior or subsequent to cue-shock pairings. (b) During testing for contextual conditioning, there was no effect of genotype on freezing. (c) During testing for cued conditioning, freezing was significantly greater during the first minute of “cue on” than during the last minute of “cue off” (p < 0.01), but there was no effect of genotype on freezing.
Holeboard Task of Spatial Memory
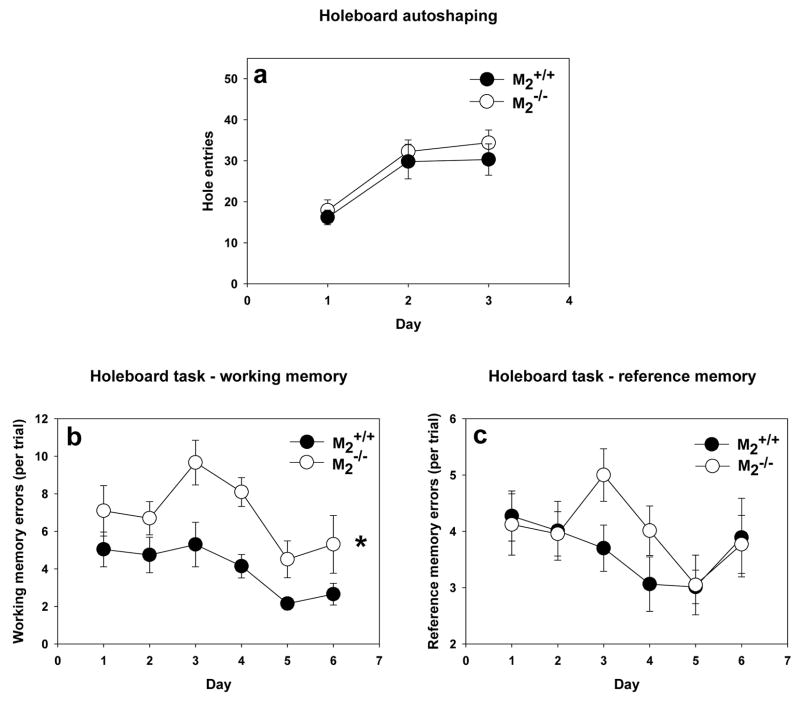
In the autoshaping stage of the holeboard task, M2−/− mice and M2+/+ mice showed equivalent propensity to explore the holes (Figure 5a) and consume the pellets. When spatial memory was taxed by baiting only 4 of the 16 holes, M2−/− made significantly more working memory errors (two-way repeated measures ANOVA, F(1, 29) = 8.04, p =0.008) than M2+/+ mice (Figure 5b). Both genotypes decreased their working memory errors with training (F(5, 130) = 6.89, p < 0.001), and there was not a significant interaction between genotype and day of training. The genotypes did not differ in terms of reference memory errors (Figure 5c) with both genotypes decreasing their reference memory errors with training (F(5, 130) = 3.10, p = 0.01). There was not a significant interaction between genotype and day.
Figure 5.
Holeboard Task of Spatial Memory. (a) During autoshaping trials, the M2−/− mice (n = 15) and M2+/+ mice (n = 16) did not differ in their propensity to explore the holes. (b) As indicated by the asterisk, during training in the holeboard task, M2−/− mice made a significantly greater number of working memory errors than M2+/+ mice (F(1, 29) = 8.04, p =0.008). (c) During training in the holeboard task, M2−/− and M2+/+ made a similar number of reference memory errors.
Discussion
The principal finding of the current study is that congenic C57BL/6TacN mice possessing a null mutation in the gene for the M2 receptor have impaired performance in several learning and memory tests. These impairments included passive avoidance acquisition, recognition of an open field, and spatial working memory. However, these mice were not impaired in cued or contextual fear conditioning. M2−/− mice were not different from M2+/+ mice in general health and neurological function, a finding that minimizes the likelihood that the differential behavioral phenotype is a false positive for mnemonic dysfunction.
In our passive avoidance experiment hyperactivity is one potential genotype effect that could lead to a false positive finding for memory impairment. Several of our measurements dismiss this interpretation. Within the passive avoidance experiment itself, there was no genotype effect on either first trial or 72 hr latency which argues against general hyperactivity. Further, spontaneous motor activity in the open field did not differ between the genotypes.
Genotype effects on exploratory activity can also confound the holeboard task. The measures of re-entries for working memory and entries into unbaited holes for reference memory assume that experimental and control mice do not differ in their general propensity to explore the holes in the floor. We dealt with this issue by confirming that during the pre-training days (autoshaping), M2−/− mice and M2+/+ mice made the same number of hole entries. The difference between the genotypes arose only when working memory was taxed by baiting only four holes. Moreover, the lack of a genotype effect on reference memory argues against a confound caused by a general increase in exploratory behavior.
Despite observing a genotype effect on passive avoidance acquisition, no effect was observed on contextual or cued fear conditioning. We favor a cautious interpretation of our fear conditioning data because the same subjects were used in both the passive avoidance and fear conditioning experiments. Interestingly, the level of pre-cue freezing in the test of cued fear was relatively elevated in both genotypes. These data suggest that experience in passive avoidance may have sensitized the mice to exhibit generalized fear in subsequent shock-motivated paradigms. We are not aware of any controlled studies suggesting that passive avoidance testing influences subsequent testing in fear conditioning, but the possibility of an interaction between the paradigms should be considered when interpreting the present fear conditioning data.
In light of the fact that genetic deletions of muscarinic receptor subtypes other than M2 can impact behavior [2,33,43], one must consider the possibility that the cognitive impairments reported here are not due to the absence of M2 directly, but may be due to changes in the expression of other muscarinic receptors. We do not favor this interpretation of the present data because knockout mice of the various individual muscarinic receptor subtypes have not shown changes in protein expression levels of the other receptors in hippocampus, cortex, striatum, and other brain regions [19,20,33,54]. Although changes in expression of other muscarinic receptors seems to be an unlikely cause of the present findings, it remains a possibility that alterations in signal transduction pathways of other muscarinic receptors may be playing a role. Further, as is the case for all life-long gene knockouts, undetected developmental effects of the loss of M2 may contribute to the phenotype.
The learning and memory deficits in our C57BL/6NTac congenic line replicate the findings of previous studies of a non-backcrossed, hybrid background line of M2−/− mice. Similar to the spatial working memory impairment reported in the present study, Seeger et al. [41] showed that M2−/− mice with a genetic background of 50% 129/J1 and 50% CF1 had spatial working memory deficits in the T-maze delayed alternation test. This same hybrid line was also shown to have impaired passive avoidance learning [45] and diminished between sessions habituation to an open field [14]. All of these findings are similar to the phenotypes in the present report.
The replication of the behavioral phenotype of M2−/− mice in our backcrossed line is important for two reasons. Firstly, it is now well-understood that the phenotype produced by a genetic manipulation can vary depending on the genetic background in which the mutation is expressed [23,38,42,44]. Based on the two background strains studied so far, there is no strain-dependent variation in penetrance for the genetic deletion of M2 receptors. Secondly, a differential phenotype can conceivably result from “flanking” genes that are located near the null mutation in the genome and are polymorphic between the embryonic stem cell donor and background strains [5,12,18,53]. This situation can cause a false positive finding in which the phenotypic difference between mutants and wild-types is erroneously attributed to the null mutation. The use of a congenic background strain produced by repeated backcrossing, as was done in the present study, has been suggested as a means of addressing this issue [3]. These backcrosses serve to reduce the size of the flanking region through crossing-over events and thus reduce the number of genes that differ between the mutants and controls. Nevertheless, it must be kept in mind that the size of the flanking region is reduced asymptotically with each successive backcross such that even after 12 backcrosses, the flanking region can represent 1% of the genome [12,18] - an amount that would be 300 genes in a 30,000 gene genome. Hence, in the present study we have only reduced, but not eliminated [40] the likelihood of the behavioral phenotype of M2−/− mice being due to flanking genes.
The role of M2 receptors in learning and memory was first examined pharmacologically using M2 receptor-preferring antagonists. The rationale for these studies was based on the premise the M2 receptor functions as an inhibitory autoreceptor on cholinergic terminals of the hippocampus and neocortex [55]. Thus, in situations such as Alzheimer’s disease in which cholinergic deficiency is associated with cognitive impairment, M2 receptor antagonists may prove therapeutically useful by increasing ACh release in the hippocampus and neocortex. Pharmacological studies stemming from this line of reasoning have shown that in aged animals that have impaired cognition and impaired cholinergic function, M2 receptor-preferring antagonists increase ACh release and improve cognitive performance [36,37,46]. Similarly, M2 receptor-preferring antagonists reverse scopolamine-induced cognitive deficits in young animals [7,28,36,46]. Based on these data, it appears that blockade of M2 receptors offers a way of fine-tuning a disrupted cholinergic system by restoring cholinergic activity to some optimal level for cognitive function. Nevertheless, all of these pharmacological studies are limited by the fact that the available M2 receptor antagonists have limited subtype selectivity [6,15,16,22,28,29,32].
Similar to the pharmacological studies, data from M2−/− mice have provided evidence that the M2 receptor functions as an inhibitory autoreceptor that regulates evoked acetylcholine release. Tzavera et al. [45] showed that stimulus-evoked release of ACh is increased in the hippocampus of M2−/− mice while the scopolamine-evoked increase in ACh release is diminished. However, counter to what might be expected based on the behavioral pharmacological studies, the increase in ACh levels was associated with impaired passive avoidance learning [45]. Furthermore, M2−/− mice are impaired in a number of other rodent memory tasks. In addition, despite the fact that pharmacological blockade of M2 receptors and genetic deletion of M2 receptors seem to have a similar effect on ACh release, their respective impacts on learning and memory are in opposition.
The pattern of contradictory behavioral results from pharmacological and genetic studies of M2 receptors function suggests that there is an optimal level of cholinergic activity for cognition. Thus, elevating ACh release through pharmacological antagonism of M2 receptors is beneficial because it restores ACh release to this optimal level after cholinergic signaling has been decreased by aging [36,37,46], drugs (e.g. scopolamine) [7,28,36,46], or disease (e.g. AD). However, when cholinergic function has not been lowered by some deleterious process, the loss of M2 receptors by genetic deletion leads to a dysregulated ACh release that is in excess of the optimum and is detrimental to cognitive function. Dysregulation of ACh release would be expected to be deleterious to cognition because ACh normally has a strong temporal association with the detection of novel or behaviorally-significant stimuli [1,13,24,34,35]. This stimulus-bound character of ACh release is thought to be critical to its role in detecting and processing stimuli [39]. Thus, when ACh release is excessive and not reliably associated with behaviorally relevant stimuli, one would expect to find, as reported here, impairments in tasks such as between-sessions habituation that require learning about novel stimuli.
Complicating the interpretation of pharmacological and genetic studies of M2 function is the fact that M2 is not exclusively an inhibitory autoreceptor, but is also found on GABAergic neurons of the basal forebrain [31], and on GABAergic interneurons of Ammon’s horn [17,21]. Expression of M2 by hippocampal interneurons places this receptor in a location where it may be involved in the plasticity of Schaffer-CA1 synapses. Indeed, Seeger et al. [41] showed that short-term potentiation was abolished and long-term potentiation was diminished in Schaffer-CA1 synapses in M2−/− mice. Given the role of CA1 pyramidal cells in spatial memory, this disruption in plasticity likely underlies the impaired spatial working memory of M2−/− mice reported here and elsewhere [41].
In summary, mice possessing a null mutation in the gene encoding the M2 receptor are impaired in the acquisition of passive avoidance, between-sessions habituation to a novel open field, and spatial working memory in a holeboard task. These deficits were demonstrated in a C57BL/6NTac congenic strain. This replication and extension of previous findings from a non-backcrossed hybrid line shows that the behavioral phenotype of M2−/− mice does not vary between two different genetic backgrounds. These findings are in contrast with a number of behavioral pharmacological studies, underscoring the complexity of M2 receptor function. Further studies that address this complexity by using inducible and anatomically-restricted null mutations will be necessary to inform the development of therapeutics that target cholinergic dysfunction in diseases such as Alzheimer’s disease.
Acknowledgments
This work was supported by Drake University, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Mental Health. The authors thank Ms. Stephanie Swain for pilot work with the passive avoidance and holeboard tasks. The authors thank Ms. Elizabeth Stucker and Ms. Erika Kilker for care of the mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acquas E, Wilson C, Fibiger HC. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: Effects of novelty, habituation, and fear. J Neurosci. 1996;16:3089–3096. doi: 10.1523/JNEUROSCI.16-09-03089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine m-1 muscarinic receptor mutant mice. Nature Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- 3.Banbury CoGBiM. Mutant mice and neuroscience: Recommendations concerning genetic background. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- 4.Bartus R, Dean R, 3rd, Beer B, Lippa A. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 5.Bolivar VJ, Cook MN, Flaherty L. Mapping of quantitative trait loci with knockout/congenic strains. Genome Res. 2001;11:1549–1552. doi: 10.1101/gr.194001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke R. Gallamine binding to muscarinic m1 and m2 receptors, studied by inhibition of [3H]pirenzepine and [3H]quinuclidinylbenzilate binding to rat brain membranes. Mol Pharmacol. 1986;30:58–68. [PubMed] [Google Scholar]
- 7.Carey GJ, Billard W, Binch I, Herbert Cohen-Williams M, Crosby G, Grzelak M, Guzik H, Kozlowski JA, Lowe DB. SCH 57790, a selective muscarinic m2 receptor antagonist, releases acetylcholine and produces cognitive enhancement in laboratory animals. Eur J Pharmacol. 2001;431:189–200. doi: 10.1016/s0014-2999(01)01440-6. [DOI] [PubMed] [Google Scholar]
- 8.Caulfield MP. Muscarinic receptors--characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 9.Caulfield MP, Birdsall NJM. International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 10.Coyle J, Price D, DeLong M. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 11.Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- 12.Crusio WE. Flanking gene and genetic background problems in genetically manipulated mice. Biol Psychiatry. 2004;56:381–385. doi: 10.1016/j.biopsych.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Day J, Damsma G, Fibiger HC. Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: An in vivo microdialysis study. Pharmacol Biochem Behav. 1991;38:723–729. doi: 10.1016/0091-3057(91)90233-r. [DOI] [PubMed] [Google Scholar]
- 14.Degroot A, Salhoff C, Davies RJ, Nomikos GG. Genetic deletion of CB1 receptors improves non-associative learning. Behav Brain Res. 2005;162:161–164. doi: 10.1016/j.bbr.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Doods H, Entzeroth M, Ziegler H, Schiavi G, Engel W, Mihm G, Rudolf K, Eberlein W. Characterization of BIBN 99: A lipophilic and selective muscarinic m2 receptor antagonist. Euro J Pharmacol. 1993;242:23–30. doi: 10.1016/0014-2999(93)90005-3. [DOI] [PubMed] [Google Scholar]
- 16.Dorje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann M. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- 17.Freund TF, Gulyas AI. Inhibitory control of GABAergic interneurons in the hippocampus. Can J Physiol Pharmacol. 1997;75:479–487. [PubMed] [Google Scholar]
- 18.Gerlai R. Gene-targeting studies of mammalian behavior: Is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 19.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in m2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C-x, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajos N, Papp EC, Acsady L, Levey AI, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1997;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- 22.Hammer R, Giraldo E, Schiavi GB, Monferini E, Ladinsky H. Binding profile of a novel cardioselective muscarine receptor antagonist, AF-DX 116, to membranes of peripheral tissues and brain in the rat. Life Sci. 1986;38:1653–1662. doi: 10.1016/0024-3205(86)90409-1. [DOI] [PubMed] [Google Scholar]
- 23.Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: Parallels with human anxiety and depression. Biol Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Inglis FM, Fibiger HC. Increases in hippocampal and frontal cortical acetylcholine release associated with presentation of sensory stimuli. Neuroscience. 1995;66:81–86. doi: 10.1016/0306-4522(94)00578-s. [DOI] [PubMed] [Google Scholar]
- 25.Ishii M, Kurachi Y. Muscarinic acetylcholine receptors. Curr Pharm Des. 2006;12:3573–3581. doi: 10.2174/138161206778522056. [DOI] [PubMed] [Google Scholar]
- 26.Iversen SD. Behavioural evaluation of cholinergic drugs. Life Sci. 1997;60:1145–1152. doi: 10.1016/s0024-3205(97)00059-3. [DOI] [PubMed] [Google Scholar]
- 27.Kuc KA, Gregersen BM, Gannon KS, Dodart JC. Holeboard discrimination learning in mice. Genes Brain Behav. 2006;5:355–363. doi: 10.1111/j.1601-183X.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- 28.Lachowicz JE, Duffy RA, Ruperto V, Kozlowski J, Zhou GW, Clader J, Billard W, Binch H, Crosby G, Cohen-Williams M, Strader CD, Coffin V. Facilitation of acetylcholine release and improvement in cognition by a selective M-2 muscarinic antagonist, SCH 72788. Life Sci. 2001;68:2585–2592. doi: 10.1016/s0024-3205(01)01056-6. [DOI] [PubMed] [Google Scholar]
- 29.Lachowicz JE, Lowe D, Duffy RA, Ruperto V, Taylor LA, Guzik H, Brown J, Berger JG, Tice M, McQuade R. SCH 57790: A novel m2 receptor selective antagonist. Life Sci. 1999;64:535–539. doi: 10.1016/s0024-3205(98)00598-0. [DOI] [PubMed] [Google Scholar]
- 30.Levey AI. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 31.Levey AI, Edmunds SM, Hersch SM, Wiley RG, Heilman CJ. Light and electronic microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol. 1995;351:339–356. doi: 10.1002/cne.903510303. [DOI] [PubMed] [Google Scholar]
- 32.Michel AD, Whiting RL. Methoctramine, a polymethylene tetraamine, differentiates three subtypes of muscarinic receptor in direct binding studies. Eur J Pharmacol. 1988;145:61–66. doi: 10.1016/0014-2999(88)90349-4. [DOI] [PubMed] [Google Scholar]
- 33.Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the m-1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore H, Sarter M, Bruno JP. Age-dependent modulation of in vivo cortical acetylcholine release by benzodiazepine receptor ligands. Brain Res. 1992;596:17–29. doi: 10.1016/0006-8993(92)91527-l. [DOI] [PubMed] [Google Scholar]
- 35.Moore H, Sarter M, Bruno JP. Bidirectional modulation of stimulated cortical acetylcholine release by benzodiazepine receptor ligands. Brain Res. 1993;627:267–274. doi: 10.1016/0006-8993(93)90330-p. [DOI] [PubMed] [Google Scholar]
- 36.Quirion R, Wilson A, Rowe W, Aubert I, Richard J, Doods H, Parent A, White N, Meaney M. Facilitation of acetylcholine release and cognitive performance by an m(2)-muscarinic receptor antagonist in aged memory-impaired. J Neurosci. 1995;15:1455–1462. doi: 10.1523/JNEUROSCI.15-02-01455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowe WB, O’Donnell JP, Pearson D, Rose GM, Meaney MJ, Quirion R. Long-term effects of BIBN-99, a selective muscarinic m2 receptor antagonist, on improving spatial memory performance in aged cognitively impaired rats. Behav Brain Res. 2003;145:171–178. doi: 10.1016/s0166-4328(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 38.Roy IL, Pothion S, Mortaud S, Chabert C, Nicolas L, Cherfouh A, Roubertoux PL. Loss of aggression, after transfer onto a C57BL/6J background, in mice carrying a targeted disruption on the neuronal nitric oxide synthase gene. Behav Genet. 2000;30:367–373. doi: 10.1023/a:1002796404278. [DOI] [PubMed] [Google Scholar]
- 39.Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 40.Schalkwyk LC, Fernandes C, Nash MW, Kurrikoff K, Vasar E, Koks S. Interpretation of knockout experiments: The congenic footprint. Genes Brain Behav. 2007;6:299–303. doi: 10.1111/j.1601-183X.2007.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci. 2004;24:10117–10127. doi: 10.1523/JNEUROSCI.3581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibilia M, Wagner E. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 43.Thomsen M, Woldbye DPD, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic m5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25:8141–8149. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Threadgill D, Dlugosz A, Hansen L, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris R, et al. Targeted disruption of mouse EGF receptor: Effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 45.Tzavara ET, Bymaster FP, Felder CC, Wade M, Gomeza J, Wess J, McKinzie DL, Nomikos GG. Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in m2, m4 and m2/m4 muscarinic receptor knockout mice. Mol Psychiatry. 2003;8:673–679. doi: 10.1038/sj.mp.4001270. [DOI] [PubMed] [Google Scholar]
- 46.Vannucchi MG, Scali C, Kopf SR, Pepeu G, Casamenti F. Selective muscarinic antagonists differentially affect in vivo acetylcholine release and memory performances of young and aged rats. Neuroscience. 1997;79:837–846. doi: 10.1016/s0306-4522(97)00091-2. [DOI] [PubMed] [Google Scholar]
- 47.Vilaro MT, Mengod G, Palacios JM. Advances and limitations of the molecular neuroanatomy of cholinergic receptors: The example of multiple muscarinic receptors. Prog Brain Res. 1993;98:95–101. doi: 10.1016/s0079-6123(08)62385-7. [DOI] [PubMed] [Google Scholar]
- 48.Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol Sci. 2003;24:414–420. doi: 10.1016/S0165-6147(03)00195-0. [DOI] [PubMed] [Google Scholar]
- 49.Wess J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annual Rev Pharmacol Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- 50.Wess J, Duttaroy A, Zhang W, Gomeza J, Cui Y, Miyakawa T, Bymaster FP, McKinzie L, Felder CC, Lamping KG, Faraci FM, Deng C, Yamada M. M-1-M-5 muscarinic receptor knockout mice as novel tools to study the physiological roles of the muscarinic cholinergic system. Receptors Channels. 2003;9:279–290. [PubMed] [Google Scholar]
- 51.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delong MR. Alzheimers-disease and senile dementia - loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 52.Wolfe BB, Yasuda RP. Development of selective antisera for muscarinic cholinergic receptor subtypes. Ann N Y Acad Sci. 1995;757:186–193. doi: 10.1111/j.1749-6632.1995.tb17474.x. [DOI] [PubMed] [Google Scholar]
- 53.Wolfer DP, Crusio WE, Lipp HP. Knockout mice: Simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25:336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- 54.Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the m3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 55.Zhang WL, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci. 2002;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]