Abstract
Prenatal exposure to opiates, which is invariably followed by postnatal withdrawal, can affect cognitive performance. To further characterize these effects, we examined radial 8-arm maze performance and expression of brain derived neurotrophic factor (BDNF) in male rats prenatally exposed to the opiate l-α-acetylmethadol (LAAM). Female rats received 1.0 mg/kg/day LAAM or water via daily oral gavage for 28 days prior to breeding, during breeding, and throughout pregnancy. Pups were fostered to non-treated lactating dams at birth and underwent neonatal opiate withdrawal. At 5–6 months, prenatal water- and LAAM-exposed males (n=6 each; non-littermates) received radial arm maze training consisting of ten trials a day for five days and three retention trials on day six. Rats prenatally exposed to LAAM had poorer maze performance, decreased percent correct responding and more reference and working memory errors than prenatal water-treated controls. However, they were able to acquire the task by the end of training. There were no differences between the groups on retention 24 hr after testing. Following retention testing, hippocampi were removed and protein extracted from cytosol and synaptic fractions. Western blots were used to measure levels of mature and precursor BDNF protein, as well as the BDNF receptor TrkB. BDNF precursor protein was significantly decreased in the synaptic fraction of trained prenatal LAAM-treated rats compared to prenatal water-treated trained controls. No effects were found for the full-length or truncated TrkB receptor. In untrained rats, prenatal treatment did not affect any of the measures. These data suggest that prenatal opiate exposure and/or postnatal withdrawal compromise expression of proteins involved in the neural plasticity underlying learning.
Keywords: Drug abuse, pro-BDNF, Radial Arm Maze, Spatial Learning, LAAM Opioids
1. Introduction
Methadone-maintenance remains the only recommended treatment option for opiate-dependent pregnant women (Jarvis and Schnoll, 1994; SAMSHA, 2003). In addition to the heroin and methadone-exposed population, there is growing concern about prenatal opiate exposure in offspring of women dependent on prescriptive opiates. Studies from animal models suggest that prenatal exposure to opiates may compromise aspects of cognitive function and synaptic plasticity.
Morphine administered in mid to late gestation increased the latency to a complete a non-delay version of the radial arm maze (Slamberová et al., 2001). Sex specific changes in the hippocampus in mu-opioid receptors and endogenous opioids were suggested as mechanisms for the poorer learning (Schindler et al., 2004; Slamberová et al., 2003). Other groups have demonstrated poorer performance in the Morris water maze and lower levels of long-term depression in juvenile rats exposed prenatally and postnatally to morphine (Yang et al., 2003; 2006). In these studies morphine was administered throughout pregnancy and for the first 30 days post-partum. The rats were assessed while on morphine exposure or shortly after treatment ended. The detrimental effects of morphine could be attenuated by coadministration of dextromethorphan during pregnancy, suggesting that NMDA receptor changes were involved in the effect (Tao et al., 2001; Yang et al., 2006). The effects of prenatal heroin exposure on neural plasticity have also been noted. Rats exposed to heroin during mid to late gestation (days 9–18) had impaired radial arm maze performance and both an increase in the number of cholinergic transporter binding sites and a different pattern of transporter localization in the hippocampus (Steingart et al., 2000; Vatury et al., 2004).
The aim of the present study was two-fold, to replicate the prior studies using an opiate with pharmacological properties that differ from those of morphine and heroin and to extend the previous research by examining the role of neurotrophins in the cognitive deficits. We exposed rats in utero to the long-acting opiate l-alpha-acetylmethadol (LAAM) and allowed the pups to undergo spontaneous withdrawal after birth. LAAM is a full mu opioid receptor agonist, but differs from other commonly studied opiates in its long duration of action. Morphine has a short half-life in the rat, such that even with twice-daily injections withdrawal signs are present (Gellert and Sparber, 1979). The half-life of heroin is even shorter (Cohn et al., 1973). Drugs used in opiate substitution therapy are generally longer acting; however, methadone has a half-life of less than 3 hr in the rat (Misra et al., 1973). This means that in rat studies, with daily administration of heroin, morphine, or methadone cycles of exposure and withdrawal are likely to occur. This cycling may have greater detrimental effects than opiate exposure alone. The presence of active metabolites lengthens the duration of LAAM’s action, such that with daily administration, withdrawal signs are not present in rats, unless antagonist-challenged (Henderson et al., 1977; Lichtblau and Sparber, 1982; 1983; York et al., 2002). After birth, opiate withdrawal emerges in the pups as the remaining drug is metabolized (e.g., Lichtblau et al., 1982) and this is manifest as poorer body weight gain (e.g., Hamilton et al., 2005; Lichtblau and Sparber, 1981), behavioral activation and increased concentrations of stress hormones such as corticosterone (unpublished data). Thus, we examined whether continuous exposure to the opiate LAAM during gestation followed by postnatal withdrawal affected acquisition or retention of a complex learning task, the eight-arm radial maze.
A second goal of the study was to examine potential mechanisms for changes obtained in the acquisition of the task. We investigated the role of BDNF because it has been implicated in the development of synaptic architecture throughout development (i.e., Chan et al., 2006; Bao et al., 1999; Liou et al; 1997; Martinez et al., 1998; Wang et al., 1995) and thus has the potential to be affected by drug insults. In addition, in the adult animal, BDNF has been implicated in changes in plasticity associated with drug exposure, escalation, and abuse (reviewed by Bolanos and Nestler, 2005; see also Berglind et al., 2007; Cheng et al., 2005; Tsai et al, 2007). Finally, there is a substantial literature documenting the role of BDNF in maintaining synaptic plasticity and modulating learning and memory in adult rats (i.e., Bekinschtein et al., 2007; Hall et al., 2000; Linnarsson et al., 1997; Ma et al., 1998; Mu et al., 1999). The convergence of these factors makes BDNF an attractive target for changes in learning and memory that follow prenatal drug exposure.
While most studies that have implicated BDNF in the above areas have examined BDNF mRNA or the mature or secreted form of BDNF, there is growing interest in the precursor form of the protein. BDNF mRNA is translated into a pre-proprotein that is packaged in secretory vesicles before cleavage to the mature, secreted protein (Mowla et al., 2001). The precursor form of BDNF may actually play a different role in the brain than the mature form (Teng et al., 2005), including affecting learning and memory and drug abuse (Cheng et al., 2005; Hariri et al., 2003; Silhol et al., 2007). We also examined changes in BDNF’s high affinity receptor, TrkB. There are two forms of this receptor, a full-length signal transducing receptor with a tyrosine kinase domain and a truncated, non-signal transducing receptor that shares the extracellular (ligand-binding) domain, but has no kinase domain (Klein et al., 1990; Middlemas et al., 1991). The two forms have different age and cellular localizations. The full-length receptor is expressed both prenatally and postnatally, primarily on neurons. In contrast, the truncated receptor appears postnatally, and it is primarily found on glia and ependymal cells (Alderson et al., 2000; Fryer et al., 1997; Rubio, 1997). Thus, in the present study, we not only examined changes in the mature form of BDNF and the precursor form of the protein, but we also assessed the level of the full length and truncated forms of the TrkB receptor in the hippocampus of rats that were prenatally-treated with LAAM or water and then trained in the radial arm maze.
2. Results
2.1 Pregnancy and Neonatal Measures
We have previously published that the LAAM treatment was well tolerated during pregnancy and that exposed pups undergo a transient opiate withdrawal postnatally (Hamilton et al., 2005). Briefly, dams gained a similar amount of weight throughout gestation (approximately 150–160% of pre-breeding weight), regardless of treatment. There were no differences in the pups’ body weights across the treatments at birth. Changes in body weight during the first postnatal week were used to index post-parturition opiate withdrawal. Rat pups treated prenatally with LAAM displayed reduced weight gain during the first five days of life, indicative of a transient postnatal withdrawal. By postnatal day (PND) 5, weight gain normalized, and through PND 21, there were no differences in body weight between prenatal LAAM and prenatal water-treated rats (Hamilton et al., 2005). There was also no difference in the body weights between the groups at the time of testing.
2.2 Radial Arm Maze Acquisition
Rats prenatally exposed to LAAM had poorer radial arm maze acquisition compared to rats prenatally treated with water. Repeated measures ANOVA revealed an overall Prenatal Treatment effect (F1,10 = 13.66, p <0.005), but not a Treatment × Trial Block interaction (p = 0.36) for percent correct responding. Despite this poorer performance, both groups showed acquisition of the task, with increased percent correct responding across the ten trial blocks (Figure 1; Trial Block effect for Prenatal Water group - F5,9 = 19.54, p <0.0001; Prenatal LAAM group - F5,9 = 17.93, p <0.0001).
Figure 1.
Prenatal LAAM-treated male rats (dark squares) had reduced performance in the radial arm maze compared with prenatal water-treated rats (open circles) as assessed by the percent of correct responses. However by the final trial blocks, there was no difference between the groups. Data depict mean ± SEM for trial blocks of 5 trials. (N = 6 per group).
Not surprisingly, the total number of errors (data not shown; F1,10 = 18.48, p <0.002) also differed between the prenatal treatment groups. When the types of errors committed was examined, it was found that prenatal LAAM-treated rats made more reference memory errors (Figure 2; F1,10 = 29.75, p <0.0003), as well as working memory errors (Figure 3; F1,10 = 11.52, p <0.007). Reference memory errors were scored when a rat visited an arm that was never baited with food. Working memory errors were scored when a rat revisited an arm within a trial that it had already obtained the food reward from. However, both groups reduced the numbers of errors across the training days, with no differences between groups on the final 10 trials (last two trial blocks) on either measure.
Figure 2.
Prenatal LAAM-treated male rats (dark squares) made more reference memory errors in the radial arm maze compared with prenatal water-treated rats (open circles). Reference memory errors were scored when a rat visited an arm that was never baited with food. Both groups decreased the number of errors to almost zero by the end of the training. Data depict mean ± SEM for trial blocks of 5 trials. (N = 6 per group).
Figure 3.
Prenatal LAAM-treated male rats (dark squares) made more working memory errors in the radial arm maze compared with prenatal water-treated rats (open circles). Working memory errors were scored when a rat revisited an arm within a trial that it had already obtained the food reward from. Both groups decreased the number of errors to less than one error by the end of the training and there were no differences between the groups on the final 10 trials. Data depict mean ± SEM for trial blocks of 5 trials. (N = 6 per group).
2.3 Retention of the Radial Arm Maze Task
Since the groups did not have different performance on the final 5 trials, we examined short-term retention in this task 24 hr after the final trials. Three retention were trials were given and the average score was analyzed. As can be seen in Figures 4a – c, there was no difference between the prenatal treatment groups for % correct responding (p = 0.84), reference memory errors (p = 0.84), or working memory errors (p = 0.51). Retention in both groups was very good, with values similar to their final training trials.
Figure 4.
Prenatal LAAM-treated male rats (dark bars) did not differ from prenatal water-treated rats (open bars) in the 24-hr retention of the radial arm maze. Panel A depicts the % of correct responses, Panel B depicts the number of reference memory errors and Panel C depicts the number of working memory errors. Data depict mean ±SEM for the average of the three retention trials. (N = 6 per group).
2.4 Hippocampal BDNF and TrkB Protein–Trained Subjects
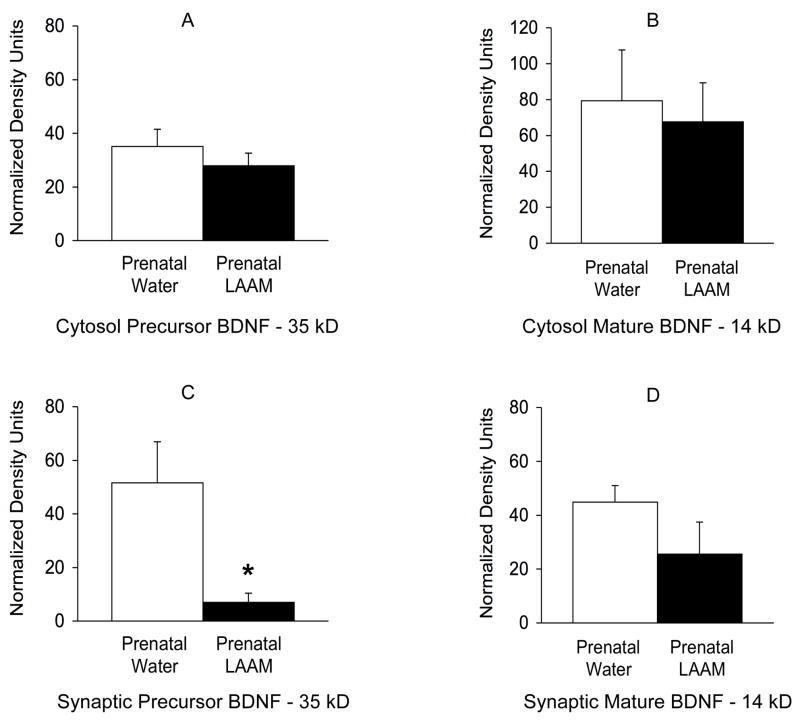
Following retention testing, hippocampi were removed and protein extracted from cytosol and synaptic fractions. Western blots were used to measure levels of mature and precursor BDNF protein, as well as the BDNF receptor TrkB. See Figure 5 for a representative Western. No effects of prenatal treatment were found for the cytosol fraction (Figures 6a and b; p = 0.40 and 0.76 respectively). However, BDNF precursor protein was significantly decreased in the synaptic fraction of prenatal LAAM-treated rats compared to controls (Figure 6c, t8 = 2.84, p <0.03). The protein levels in the prenatal LAAM-treated rats were reduced by more than 7-fold. There was a non-significant trend for a similar effect for the mature, secreted protein in the synaptic fraction (p = 0.18; Figure 6d).
Figure 5.
Representative Western blot for the synaptic fraction of the hippocampus from two prenatal water- and two prenatal LAAM-treated rats trained in the radial arm maze. The blot depicts the mature and precursor forms of BDNF and the tubulin loading control.
Figure 6.
BDNF levels in the cytosolic fraction (Panels A and B) and synaptic fraction (Panels C and D) of hippocampi from prenatal water and LAAM-treated rats after retention testing. Data depict mean ± SEM optical density in normalized optical density units of the quantified Western blots. No significant differences were found for the precursor protein (Panel A) and the mature form of the protein (Panel B) in the cytosolic fraction. In the synaptic fraction, there was a significant decrease in BDNF precursor protein (Panel C) in prenatal LAAM-treated rats, and a similar, but non-significant trend for the mature protein (Panel D). * p<0.03 vs. Prenatal Water. N=6 per group.
Interestingly, there were no effects of prenatal treatment for either the full-length (p = 0.75) or truncated TrkB receptor (p = 0.24) as assessed in the synaptic fraction. The values (means ± SEM) were as follows: Full length TrkB (normalized optical density units) prenatal water = 21.59 ± 5.18 and prenatal LAAM = 25.35 ± 10.66; Truncated TrkB (normalized optical density units) prenatal water = 20.87 ± 8.39 and prenatal LAAM = 44.48 ± 17.80.
2.5 Hippocampal BDNF and TrkB Protein–Untrained Subjects
To determine if the differences in BDNF were solely a consequence of prenatal treatment or whether they emerged due to differences in the rate of acquisition, hippocampi were analyzed from like prenatal-treated rats that were otherwise unmanipulated as adults. There were no effects of prenatal treatment in the cytosolic (p = 0.83 and 0.57) or synaptic fractions (p = 0.51 and 0.38) for BDNF in rats that had not undergone radial arm maze training, indicating that training is necessary for effects to emerge. Likewise, there was no difference in the TrkB receptor levels (p = 0.23 and 0.22). See Table 1 for data. We were not able directly compare the trained and untrained subjects because the samples were processed at different times and there was insufficient sample to rerun them on a common assay. However, in a separate group of untreated rats we examined changes in BDNF as a consequence of radial arm maze training. We found that 60 training trials led to an approximately 2-fold increase in the precursor and mature forms of BDNF in the synaptic fraction compared to untrained control rats (Supplementary Figure 1).
Table 1. BDNF and TrkB levels in untrained prenatal water- and LAAM treated rats.
Table 1: Quantification of Western blot data for untrained prenatal water- and LAAM treated rats in arbitrary units. Data depict the mean ± SEM for the mature and precursor forms of BDNF and the full-length and truncated TrkB receptors in the synaptic and/or cytosol fractions of hippocampi. There were no differences between the prenatal treatment groups for any measure. (N = 6 per group).
| Untrained Controls Prenatal Water | Untrained Controls Prenatal LAAM | |
|---|---|---|
| BDNF Precursor Protein (Cytosolic Fraction) | 42.17 ± 15.98 | 55.95 ± 16.94 |
| BDNF Mature Protein (Cytosolic Fraction) | 84.21 ± 20.15 | 77.18 ± 24.75 |
| BDNF Precursor Protein (Synaptic Fraction) | 26.76 ± 6.59 | 45.73 ± 12.77 |
| BDNF Mature Protein (Synaptic Fraction) | 102.20 ± 20.74 | 110.28 ± 32.80 |
| Full length TrkB Receptor (Synaptic Fraction) | 1.82 ± 0.78 | 4.49 ± 1.95 |
| Truncated TrkB receptor (Synaptic Fraction) | 8.94 ± 4.10 | 14.45 ± 1.01 |
3. Discussion
The present experiments demonstrate that prenatal exposure to the long acting opiate LAAM compromises the acquisition of a spatial and working memory task, the eight-arm radial maze. Prenatal LAAM-treated rats had fewer percent correct responses across the first eight testing sessions, but did reach the same level of performance by the last two trial blocks. When the types of errors that the prenatal LAAM-treated rats were making were analyzed, increased numbers of both reference memory and working memory errors were found. This suggests compromised function in the hippocampus and possibly the prefrontal cortex. While we did not find any differences in the number of food rewards obtained during the adaptation phase of the task where no learning was involved, we can not completely rule out differences between the prenatal treatments with respect to the rewarding value of the sweet cereal that was used to bait the radial arm maze.
There was no difference in their short-term retention (24 hr) of the correct pattern of responses, as assessed by percent correct responses, number of reference memory errors or working memory errors. It is important to note that retention test was essentially an additional day of testing with a reduced number of trials. Since the prenatal treatment groups did not display differences on the final day of testing, the lack of a retention deficit was not surprising. It remains to be determined if longer-term retention would be compromised by the prenatal opiate treatment or if a larger number of subjects would reveal more subtle deficits.
Hippocampal samples from the rats were analyzed for changes in the neurotrophin BDNF and its high affinity receptor TrkB. Interestingly, prenatal LAAM-treated rats that were trained in the radial arm maze had dramatically lower amounts of the BDNF precursor protein in the synaptic fractions of their hippocampal samples. A non-significant trend toward decreased mature or secreted BDNF in the synaptic fraction was also noted. The effects were specific for the synaptic fraction, with no differences found in the cytosol. No changes in the levels of the full length or truncated TrkB receptor were noted. The lower levels of BDNF in synaptic fraction of prenatal LAAM-treated rats was specific to the training condition, as there were no differences as a consequence of prenatal treatment in animals that had not received radial arm training.
We were not able to directly compare the levels of BDNF in trained and untrained prenatal treated rats in the present study. However, data presented as supplementary figures showed that in normal (untreated rats) an increase in both precursor and mature BDNF protein following 60 trials in the radial arm maze as compared to untrained rats. Thus, we can infer that the prenatal LAAM-treated rats were failing to upregulate or mobilize BDNF to their synaptic regions to the same extent as the prenatal water-treated rats. Whether this is a consequence of the slower acquisition or the slower acquisition resulted from the lower synaptic BDNF levels cannot be ascertained from the present study. However, a recent report by Bekinschtein et al. (2007) suggests that BDNF synthesis in the hippocampus may be necessary for long-term storage of new information. The radial arm maze protocol requires that the rats remember the spatial location of the baited arms (reference memory) across the five days of the task and new BDNF synthesis may be required for this between day retention. In the present study, the protein differences between the groups might have been greater (especially for the mature form of BDNF) if we had sampled at an earlier stage of testing. Tissue samples were taken after 53 trials and there might have been some “catch-up” in BDNF levels by that time.
It is interesting to note that the most dramatic change in BDNF occurred in the precursor form of the protein. There has been growing interest in the distinct role that the BDNF precursor plays as compared to the better-studied secreted form of BDNF. BDNF precursor can be localized to synaptic regions where it may serve to stabilize synapses or it can be secreted (Agassandian, 2006; Mowla et al., 2001; Zhou et al., 2004). BDNF precursor can be transported in the axons and has been found to be both an anterograde and retrograde signaling molecule (Wang et al., 2006). A recent report found that the BDNF precursor can be secreted by cultured neurons and activate apoptosis pathways (Teng et al., 2005) suggesting a role in pruning. Most relevant to the present study, Silhol and colleagues (2007) reported that precursor BDNF was increased in the hippocampus following water maze training in both young (2 month) and older rats (24 month), while the mature form was only increased after training in the younger animals. Since the rats tested in the present study were approximately 5–6 months of age, the smaller difference between the prenatal treated groups with respect to the mature form of BDNF may be age-related. It is also important to note that developmental insults have been found to differentially affect BDNF precursor vs. the mature form. Adult rats that underwent maternal separation during the neonatal period had increased precursor BDNF in the ventral tegmental area, but decreased mature form in the hippocampus and striatum (Lippmann et al., 2007). Thus, the data in the present study add to the growing body of literature implicating the BDNF precursor as a mediator of synaptic plasticity, and suggest that it may be especially relevant after developmental insults.
In conclusion, the brain and subsequent behavioral responses can be dramatically shaped by insults during pregnancy. One such insult is exposure to drugs of abuse. This can result in altered behavior in the offspring that ranges from changes in developmental parameters, affective states, emotional responsivity, response to drug challenge and cognition. Since methadone-maintenance remains the recommended treatment option for opiate-dependent pregnant women and increasing numbers of young women are abusing prescription opiates, understanding the long-term consequences of prenatal opiate exposure on cognitive function takes on added importance.
4. Experimental Procedure
4.1 Subjects
The subjects in the present study were the adult male offspring of females that had been treated for one month prior to and then throughout breeding and gestation with water or 1 mg/kg/day of the long-acting opiate LAAM. Details of the prenatal treatment and developmental outcome measures have been previously reported (Hamilton et al., 2005). The rats tested in the present study were not used for any other dependent measures.
Briefly, nulliparous female Sprague-Dawley rats (7–8 wks of age; Harlan, Madison, WI) were adapted to an oral gavage procedure using water. The females were group-housed and maintained on a 12:12 light:dark schedule, with lights on at 0700. Food and water were provided ad libitum throughout the treatment phase. Treatment consisted of an oral gavage of LAAM HCl (kindly provided by the National Institute on Drug Abuse through the Research Triangle Institute, Research Triangle Park, NC) or the water vehicle. The 1.0-mg/kg dose was chosen because previous studies found that higher doses (e.g., 2.0 mg/kg) increased neonatal morbidity (Licthblau and Sparber, 1982, York et al., 2002). An 18-gauge, 7.6 cm long gavage tube (Popper and Sons, New Hyde Park, New York) was used for drug administration. Treatment was initiated on Day 0 and the rats were gradually made chronically opiate dependent via treatments on days 3, 6, and 8, followed by daily treatments from days 10 to 28. Females were harem-bred, with 1 male placed in each cage of 3 females, after 28 days of treatment. Pregnancy was confirmed by a sperm-positive vaginal smear and/or appropriate weight gain. Treatment continued through the 10 days of breeding and during pregnancy until parturition. Throughout pregnancy the females were weighed daily and monitored for signs of opiate withdrawal. On approximately gestation day (GD) 14, pregnant females were placed in individual nesting cages. From GD 20–23 the pregnant dams were checked for births twice a day. Litters were handled only after pups had been cleaned and fed and the day of birth was designated as postnatal day (PND 0). At this time, the pups were sexed and weighed. Within 12 to 36 hr of parturition, pups from both water-and LAAM-treated dams were fostered to non-treated dams. Pups were culled to 12 per litter at fostering, balancing by sex when possible. The pups were weaned at PND 21, with 3–5 same-sex littermates housed per cage. The offspring were weighed weekly until approximately 24 weeks of age, when non-littermate subjects from each treatment were randomly selected for testing.
4.2 Radial Arm Maze Equipment
The radial arm maze was constructed of black painted wood finished with a polyurethane coating. The center platform was 60 cm in diameter with 8 arms extending from the center. The arms were 80 cm long and 15 cm wide. The distal 13 cm of each arm were 5 cm wider and a 3 cm diameter hole was in the center of the arm. A plastic disposable cup was placed in this hole to contain the food reinforcement and all arms were baited with either a food cup or an empty cup. The food reinforcer was a wet mash of Maypo (Parsippany, NJ), a sweetened maple-flavored oatmeal cereal. A 0.64cm lip surrounded the arms and center platform. The entire radial arm maze was elevated 1 m off the floor. Visual cues were located throughout the room to provide spatial cues. Before use, and between each trial, the maze was wiped down with 95% ethanol to prevent the use of odor cues.
4.3 Radial Arm Maze Procedure
Twelve male rats (n=6 prenatal water and n=6 prenatal LAAM) rats were food restricted to 85–90% of free-feeding weight and maintained at this weight for the duration of training and testing. The radial arm maze task was divided into three phases: adaptation, training, and retention testing. The rats were adapted to the radial arm maze apparatus before training by three 5-min trials conducted after the rats reached 85–90% of their baseline weight. During adaptation, all of the arms of the maze were baited with the Maypo mash so that the rats received experience in gaining a food reinforcement by completely traversing an arm and reaching into the food cup. The number of food rewards eaten during the adaptation phase was recorded and there were no differences between the groups.
During the training trials, four arms were baited. The baited arms contained a small drop of Maypo mash. The same arms remained baited for all training and retention trials. Four patterns of baited arms were used and the patterns were chosen to reflect equal difficulty and minimize non-spatial search strategies (i.e., visiting more than two consecutive unbaited arms). There were ten massed trials for each subject, each of which was preceded by a one-minute confinement in the center platform. The trial was terminated when the rat has entered and eaten from all our baited arms or following a maximum latency of 180 seconds. At the end of the ten trials, the rat was returned to its home cage. This procedure was repeated for five training days (50 trials total). Retention testing was conducted 24 hr after the final training trials. They received three trials as described under the training procedure. The data were analyzed for the following measures: % correct, number of reference memory errors, and number of working memory errors. Reference memory errors were scored when a rat visited an arm that was never baited with food. Working memory errors were scored when a rat revisited an arm within a trial that it had already obtained the food reward from.
4.4 Hippocampal Protein Isolation
After the final training trial, the rats were euthanized by carbon dioxide. The brain was removed, and the hippocampus rapidly dissected on dry ice. Along with rats that had received training, tissue was removed from food-restricted control rats that were not tested on the maze (n = 6 prenatal water and n=6 prenatal LAAM). The brain samples were stored at −80 °C until protein isolation.
A subcellular fractionation procedure was used to separate protein from the cytosolic and synaptic membrane compartments because there can also be local synthesis of BDNF in dendrites (Hartmann et al., 2001; Kovalchuk et al., 2002), where it can directly affect synaptic plasticity. Tissue was homogenized in a Tris buffer containing a cocktail of protease inhibitors (Sacktor et al., 1993). After low speed centrifugation to remove particulates (5 min at 3,000 g), the supernatant underwent ultracentrifugation (30 min at 100,000 g) and this resulting supernatant reflected the cytosol. The pellet from this spin was lysed in a Tris-buffer containing 0.05% Triton-X for 1 h at 4°C before a second ultracentrifugation (100,000 g for 60 min). The pellet from this second spin was resuspended in Tris buffer, and reflected the synaptic fraction. Total protein was quantified using a bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Following quantification, the samples were diluted with 5x Laemmli sample buffer (BioRad, Hercules, CA) containing β-mercaptoethanol and boiled to denature the proteins. Aliquotted samples were stored at −80°C until analysis. To confirm identity of the synaptic fraction, Western blot analysis (described below) was performed using antibodies to proteins known to localize to this fraction, including post-synaptic density-95 (PSD-95; Chemicon, Temecula, CA), synaptophysin (Chemicon, Temecula, CA), and Cam Kinase II-a (gift of Dr. Y. Yamagata, Okazaki, Japan). There was insufficient protein from the synaptic fraction of two samples from trained rats (one prenatal water and one prenatal LAAM) to analyze by Western blot. Thus, n = 5 prenatal water and n = 5 prenatal LAAM for the synaptic fraction analyses.
4.5 Western Blot Analysis
Levels of BNDF and its receptor TrkB were analyzed in the isolated cytosolic and synaptic fractions. A 4–20% Tris-glycine gradient gel (BioRad, Hercules, CA) was used and this allowed for resolution of bands for both BDNF (14–35 kD) and the TrkB receptor (95–145 kD). On each gel, protein samples (20 μg/cm well) from both prenatal LAAM and prenatal water-exposed rats were run. The gel was run at a constant 200 V for 47 min with appropriate buffers using the Criterion system (BioRad, Hercules, CA). The proteins were transferred from the gel (30 min at 100 V) onto a 0.5-μm nitrocellulose membrane using the Criterion system. The membrane was stained was Ponceau S to ensure adequate transfer of proteins.
The membrane was incubated with the antibodies of interest. The BDNF antibody was at a dilution of 1:500 and TrkB antibody at a dilution of 1:200 (both antibodies from Santa Cruz Biotechnology, Santa Cruz, CA). Tubulin served as the loading control (antibody at 1:1000 dilution; Promega, Madison, WI). The BDNF polyclonal antibody recognizes the precursor (approximately 35–40 kD) and the mature form of the protein (13–14 kD). The polyclonal TrkB receptor antibody recognizes the truncated non-signal transducing receptor (95kD) and the full-length signal transducing receptor (145 kD). Following washes, the membrane was incubated with alkaline-phosphatase conjugated secondary antibody. The secondary antibody for BDNF and TrkB was goat anti-rabbit IgG (H+L) (1:1000 dilution; Pierce, Rockford, IL) and for tubulin was anti-mouse IgG (H+L) (1:1000 dilution; Promega, Madison, WI). BCIP (5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt) and NBT (nitro-blue tetrazolium chloride) colorometric detection (MP Biomedical, Irvine, CA) was used to detect the reaction product. After detection and drying, the membrane was scanned and band density measured using NIH Image (Bethesda, MD).
4.6 Data Analysis
The radial arm maze data (% correct, latency, and errors) during the 50 training trials were collapsed into trial blocks of five or ten trials for analysis, by taking the mean across the trials for each measure. There was no difference in the pattern of results based across the two methods. We chose to depict the data across trial blocks of five so that both between-session (e.g., Trials 6–10 vs. 11 –15) and within-session learning (Trials 1–5 vs. 6–10) would be observable. The data were analyzed by two-way mixed factorial analysis of variance (ANOVA) with Prenatal Treatment as the between subjects factor and Trial Block as the within subjects factor. The retention data for the radial arm maze was collapsed across the three trials and analyzed by an unpaired t-test with Prenatal Treatment as the between subjects factor. Western blot data were standardized to the tubulin loading control and an unpaired t-test ANOVA compared protein levels between rats of the two prenatal treatment groups.
Supplementary Material
Acknowledgments
This research was supported in part by USPHS DA018181 and DA00362 (LMS). The authors wish to thank Drs. Kathryn Hamilton and Sheldon Sparber for their assistance in the preliminary studies and the developmental portion of the present study.
Abbreviations
- BCA
bicinchoninic acid
- BCIP
5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt
- BDNF
Brain derived neurotrophic factor
- LAAM
l-α-acetylmethadol
- PND
postnatal day
- TrkB
tyrosine kinase receptor B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agassandian K, Gedney M, Cassell MD. Neurotrophic factors in the central nucleus of amygdala may be organized to provide substrates for associative learning. Brain Res. 2006;1076:78–86. doi: 10.1016/j.brainres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Alderson RF, Curtis R, Alterman AL, Lindsay RM, DiStefano PS. Truncated TrkB mediates the endocytosis and release of BDNF and neurotrophin – 4/5 by rat astrocytes and Schwann cells in vitro. Brain Res. 2000;871:210–222. doi: 10.1016/s0006-8993(00)02428-8. [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Qiao X, Thompson RF. Transgenic brain-derived neurotrophic factor modulates a developing cerebellar cortex. Learn Mem. 1999;6:276–283. [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis-and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–77. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–66. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. NeuroMolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting Bdnf in fetal or postnatal brains of mice. Neuroscience. 2006;142:49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Hong CJ, Yu YW, Chen TJ, Wu HC, Tsai SJ. Brain-derived neurotrophic factor (Val66Met) genetic polymorphism is associated with substance abuse in males. Molec Brain Res. 2005;140:86–90, 2005. doi: 10.1016/j.molbrainres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Cohn GL, Cramer JA, Kleber HD. Heroin metabolism in the rat. Proc Soc Exp Biol Med. 1973;144:351–5, 1973. doi: 10.3181/00379727-144-37589. [DOI] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Kromer LF. Truncated TrkB receptors on nonneuronal cells inhibit BDNF-induced neurite outgrowth in vitro. Exp Neurol. 1997;148:616–627. doi: 10.1006/exnr.1997.6699. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Sparber SB. Effects of morphine withdrawal on food competition hierarchies and fighting behavior in rats. Psychopharmacology. 1979;60:165–172. doi: 10.1007/BF00432288. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Harris AC, Sparber SB, Gewirtz JC, Schrott LM. HPA axis dysregulation following prenatal opiate exposure and postnatal withdrawal. Neurotoxicol Teratol. 2005;27:95–103. doi: 10.1016/j.ntt.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–4. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GL, North-Root H, Kuttab SH. Metabolism and disposition of l-alpha-acetylmethadol in the rat. Drug Metab Dispos. 1977;5:321–328. [PubMed] [Google Scholar]
- Jarvis MAE, Schnoll SH. Methadone treatment during pregnancy. J Psychoact Drugs. 1994;26:155–161. doi: 10.1080/02791072.1994.10472263. [DOI] [PubMed] [Google Scholar]
- Klein R, Martin-Zanca D, Barbacid M, Parada LF. Expression of the tyrosine kinase receptor gene TrkB is confined to the murine embryonic and adult nervous system. Development. 1990;109:845–850. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Lichtblau L, Finkle BS, Sparber SB. Cumulation of active metabolites of levo-alpha-acetylmethadol in the rat fetus. Life Sci. 1982;30:307–312. doi: 10.1016/0024-3205(82)90513-6. [DOI] [PubMed] [Google Scholar]
- Lichtblau L, Sparber SB. Outcome of pregnancy in rats chronically exposed to l-α-acetylmethadol (LAAM) J Pharmacol Exp Therap. 1981;218:303–308. [PubMed] [Google Scholar]
- Lichtblau L, Sparber SB. Congenital behavioral effects in mature rats prenatally exposed to levo-alpha-acetylmethadol (LAAM) Neurobehav Toxicol Teratol. 1982;4:557–565. [PubMed] [Google Scholar]
- Lichtblau L, Sparber SB. Prenatal levo-alpha-acetylmethadol (LAAM) and/or naloxone: Effects on brain chemistry and postweanling behavior. Neurobehav Toxicol Teratol. 1983;5:479–486. [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Liou JC, Yang RS, Fu WM. Regulation of quantal secretion by neurotrophic factors at developing motoneurons in Xenopus cell cultures. J Physiol. 1997;503:129–139. doi: 10.1111/j.1469-7793.1997.129bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–8. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–67. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- Martinez A, Alcantara S, Borrell V, Del Rio JA, Blasi J, Otal R, Campos N, Boronat A, Barbacid M, Silso-Santiago I, Soriano E. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J Neurosci. 1998;18:7336–7450. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemas DS, Lindberg RA, Hunter T. TrkB, a neural receptor protein-tyrosine kinase evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra AL, Mule SJ, Bloch R, Vadlamani NL. Physiological disposition and metabolism of levo-methadone-1-3H in nontolerant and tolerant rats. J Pharmacol Exp Therap. 1973;185:287–299. [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–6. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–65. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Rubio N. Mouse astrocytes store and deliver brain-derived neurotrophic factor using the non-catalytic gp95 trkB receptor. Eur J Neurosci. 1997;9:1847–1853. doi: 10.1111/j.1460-9568.1997.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA. 1993;90:8342–6. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Opioid drugs in maintenance and detoxification treatment of opiate addiction; Addition of buprenorphine and buprenorphine combination to list of approved opioid treatment medications. Fed Regist. 2003;68:27937–27939. [PubMed] [Google Scholar]
- Schindler CJ, Slamberová R, Rimanoczy A, Hnactzuk OC, Riley MA, Vathy I. Field-specific changes in hippocampal opioid mRNA, peptides, and receptors due to prenatal morphine exposure in adult male rats. Neuroscience. 2004;126:355–64. doi: 10.1016/j.neuroscience.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Silhol M, Arancibia S, Maurice T, Tapia-Arancibia L. Spatial memory training modifies the expression of brain-derived neurotrophic factor tyrosine kinase receptors in young and aged rats. Neuroscience. 2007;146:962–73. doi: 10.1016/j.neuroscience.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Slamberová R, Rimanoczy A, Bar N, Schindler CJ, Vathy I. Density of mu-opioid receptors in the hippocampus of adult male and female rats is altered by prenatal morphine exposure and gonadal hormone treatment. Hippocampus. 2003;13:461–71. doi: 10.1002/hipo.10076. [DOI] [PubMed] [Google Scholar]
- Slamberová R, Schindler CJ, Pometlova M, Urkuti C, Purow-Sokol JA, Vathy I. Prenatal morphine exposure differentially alters learning and memory in male and female rats. Physiol Behav. 2001;73:93–103. doi: 10.1016/s0031-9384(01)00469-3. [DOI] [PubMed] [Google Scholar]
- Steingart RA, Silverman WF, Barron S, Slotkin TA, Awad Y, Yanai J. Neural grafting reverses prenatal drug-induced alterations in hippocampal PKC and related behavioral deficits. Develop Brain Res. 2000;125:9–19. doi: 10.1016/s0165-3806(00)00123-1. [DOI] [PubMed] [Google Scholar]
- Tao PL, Yeh GC, Su CH, Wu YH. Co-administration of dextromethorphan during pregnancy and throughout lactation significantly decreases the adverse effects associated with chronic morphine administration in rat offspring. Life Sci. 2001;69:2439–50. doi: 10.1016/s0024-3205(01)01316-9. [DOI] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–63. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ. Increased central brain-derived neurotrophic factor activity could be a risk factor for substance abuse: Implications for treatment. Med Hypoth. 2007;68:410–4. doi: 10.1016/j.mehy.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Vatury O, Barg J, Slotkin TA, Yanai J. Altered localization of choline transporter sites in the mouse hippocampus after prenatal heroin exposure. Brain Res Bull. 2004;63:25–32. doi: 10.1016/j.brainresbull.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Wang H, Wu LL, Song XY, Luo XG, Zhong JH, Rush RA, Zhou XF. Axonal transport of BDNF precursor in primary sensory neurons. Eur J Neurosci. 2006;24:2444–52. doi: 10.1111/j.1460-9568.2006.05138.x. [DOI] [PubMed] [Google Scholar]
- Wang T, Xie KW, Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Huang LT, Wang CL, Chen WF, Yang CH, Lin SZ, Lai MC, Chen SJ, Tao PL. Prenatal administration of morphine decreases CREBSerine-133 phosphorylation and synaptic plasticity range mediated by glutamatergic transmission in the hippocampal CA1 area of cognitive-deficient rat offspring. Hippocampus. 2003;13:915–21. doi: 10.1002/hipo.10137. [DOI] [PubMed] [Google Scholar]
- Yang SN, Liu CA, Chung MY, Huang HC, Yeh GC, Wong CS, Lin WWYang CH, Tao PL. Alterations of postsynaptic density proteins in the hippocampus of rat offspring from the morphine-addicted mother: Beneficial effect of dextromethorphan. Hippocampus. 2006;16:521–30. doi: 10.1002/hipo.20179. [DOI] [PubMed] [Google Scholar]
- York RG, Denny KH, Moody DE, Alburges ME. Developmental toxicity of levo-alpha-acetylmethadol (LAAM) in tolerant rats. Int J Toxicol. 2002;21:147–159. doi: 10.1080/10915810252866105. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Song XY, Zhong JH, Barati S, Zhou FH, Johnson SM. Distribution and localization of pro-brain-derived neurotrophic factor-like immunoreactivity in the peripheral and central nervous system of the adult rat. J Neurochem. 2004;91:704–15. doi: 10.1111/j.1471-4159.2004.02775.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.