Abstract
Myocardial perfusion imaging can predict outcomes in cardiac patients. However, limited data exist regarding its prediction of cardiovascular outcomes in cancer patients. We sought to determine whether myocardial perfusion imaging predicts long-term cardiovascular outcomes in cancer patients.
We performed a retrospective review of 787 consecutive patients at our institution who underwent myocardial perfusion imaging from January 2001 through March 2003. The Cox proportional hazard model was applied, and total cardiac events, cardiac death, and all-cause death were determined for 3 years. We considered P <0.05 to be statistically significant.
Patients with abnormal myocardial perfusion imaging results were more likely to be male and older, with heart disease, more vascular risk factors, and lower left ventricular ejection fraction (0.52 ± 0.14 vs 0.63 ± 0.11; P <0.001) than patients with normal myocardial perfusion imaging results. Multivariate predictors of total cardiac events included age (P = 0.023), hyperlipidemia (P = 0.0021), pharmacologic myocardial perfusion imaging (P <0.01), left ventricular ejection fraction (P <0.001), and abnormal myocardial perfusion imaging (P = 0.012). Multivariate predictors of cardiac death included age (P = 0.026) and left ventricular ejection fraction (P = 0.0001). Multivariate predictors of all-cause death were age (P = 0.0001), atrial fibrillation (P = 0.0012), and smoking (P <0.001). Overall survival was improved when patients took aspirin (P = 0.0002) and upon each unit increase in left ventricular ejection fraction (P <0.001).
Myocardial perfusion imaging in cancer patients can predict 3-year cardiac outcomes. Increasing age, atrial fibrillation, and smoking were associated with worse outcomes, whereas higher left ventricular ejection fraction and the taking of aspirin were protective.
Key words: Aspirin/therapeutic use; comorbidity; coronary disease/epidemiology/radionuclide imaging; death, sudden, cardiac/prevention & control; electrocardiography; exercise test/methods/statistics & numerical data; heart/radionuclide imaging; multivariate analysis; neoplasms/epidemiology/mortality; predictive value of tests; proportional hazards model; retrospective studies; risk assessment/methods; ventricular function, left
The use of myocardial perfusion imaging (MPI) for estimating cardiac risk and for guiding the management of heart disease has been validated in multiple studies.1–6 Most of these studies were of patients with known or suspected coronary artery disease (CAD),1–3,7 but without substantial comorbidities, such as cancer. Some authors have evaluated the role of other comorbidities, such as diabetes mellitus6 and advanced age,4,5 in conjunction with CAD, and MPI is useful for risk stratification in these patients. However, there exists little information on the usefulness of MPI in cancer patients with coexistent CAD.7 This is a relevant concern, primarily due to substantially increased survival of patients with all types of cancer over the past 2 decades,8 which has resulted in cancer's being thought of as a chronic disease. One of the most important factors that has been identified to predict outcomes in cancer patients is comorbidity, such as heart disease.9 The management of cardiac disease in cancer patients is emerging as a crucial approach for the improvement of outcomes. A 1999 study of more than 34,000 newly diagnosed cancer patients found that the prevalence of cardiovascular disease and hypertension was as high as 30% in patients who were older than 75 years and who had certain forms of cancer.10 Cardiovascular disease and cancer are the 2 leading causes of death worldwide, accounting for more than 70% of deaths.11 In addition, cancer occurs as a comorbidity in cardiac patients more often than may be acknowledged. In fact, a recent community heart-failure study indicated that existing cancer occurred nearly as often as did peripheral arterial disease in a population of patients who had heart disease.12 There is no doubt that cancer would have an important influence on morbidity and death in any population, especially in patients who have heart disease. Furthermore, the interaction between these 2 major diseases is currently unknown, because clinical trials involving cardiovascular disease generally exclude patients who have cancer, and, similarly, cancer-treatment trials exclude patients who have heart disease.
We sought to define the outcomes of cancer patients who underwent MPI, in an effort to understand the clinical importance of such findings. To date, there are few data surrounding the performance of MPI in cancer patients, and previous studies have been limited to preoperative evaluations with short-term follow-up of 30 days.7,13 We therefore analyzed the role of MPI in predicting 3-year outcomes in cancer patients.
Patients and Methods
Selection of Patients and Collection of Data
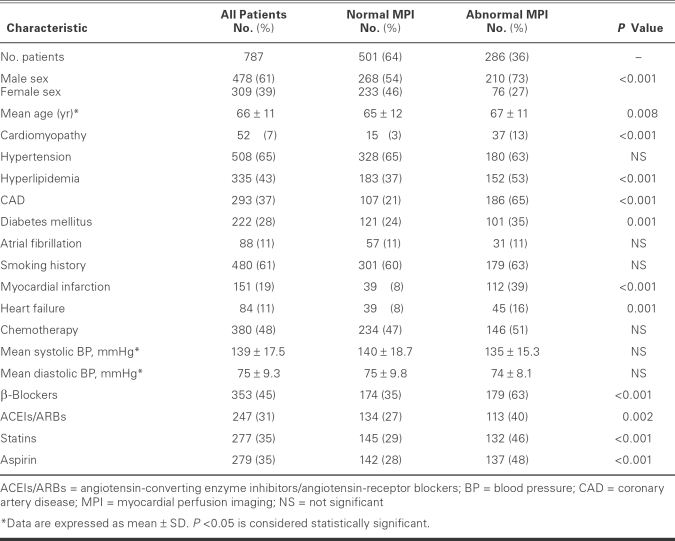
Approval for this outcomes analysis was obtained from the institutional review board at the University of Texas M.D. Anderson Cancer Center, Houston, Texas. A total of 787 consecutive patients who had undergone MPI from January 2001 through March 2003 were identified from the Stress Laboratory database and were included in the retrospective study. Data were collected on the patients' age and sex, vascular risk factors, medical histories, cardiac medications, recent chemotherapy, and MPI results, which involved left ventricular ejection fraction (LVEF) and the extent of vascular defects. Cardiac endpoints and vital status were also confirmed in all patients. The outcomes of all patients were documented for a median follow-up period of 1.8 years, and maximum follow-up was 3.6 years (25th quartile, 0.8 yr; 75th quartile, 2.2 yr). The baseline data of the 787 patients are shown in Table I.
TABLE I. Characteristics of 787 Cancer Patients Who Underwent Myocardial Perfusion Imaging
Endpoint Definitions
Endpoints included total cardiac events, cardiac death, and all-cause death. Total cardiac events were defined as cardiac death, nonfatal myocardial infarction (MI), coronary revascularization, or symptomatic CAD confirmed by angiography (≥70% stenosis). A diagnosis of acute MI was made in accordance with the universal definition of MI.14 Coronary revascularization was defined as percutaneous coronary angioplasty, stenting, or bypass surgery. For any patient who died during the study period, the cause of death was determined from the medical records and was classified as cardiac or noncardiac. Cardiac death was defined as death that was caused by acute MI, arrhythmias, heart failure, or any unexplained sudden death. Noncardiac death was the term assigned to the death of any patient who experienced a progression of cancer and in whom the proximate cause was not cardiac-related.
Myocardial Perfusion Imaging
All patients who were able to exercise underwent standard Bruce protocol exercise stress testing, while those unable to exercise underwent chemical stress testing with either intravenous dobutamine (infusion rate, 5– 40 μg/[kg·min]) or adenosine (infusion rate, 140 μg/[kg·min] for 6 min).15 The electrocardiographic (ECG) portion of the test was interpreted as positive, negative, or inconclusive. The ECG result was considered positive when there was at least a 1-mm horizontal or down-sloping ST-segment depression that was measured at 80 ms after the J-point.16
Myocardial perfusion imaging was performed with use of the dual-isotope technique. Images with the patients at rest were acquired via intravenous thallium-201 chloride (3 mCi), and stress images via 30 mCi of technetium-99m tetrofosmin.17 All images were acquired on a large-field-of-view dual-head camera via low-energy, high-resolution collimeters with rod-source-attenuation correction. Imaging was performed over a 180° semicircular orbit from the right anterior oblique position to the left posterior oblique position. Acquired data were organized into a 64 × 64 matrix for 64 projections of technetium-99m and 32 projections of thallium-201. Image processing was performed by use of a rampback projection filter. Image sets (horizontal and vertical long- and short-axis planes) were normalized to maximal myocardial activity.15,17
All images were reviewed by independent readers and were interpreted as normal or abnormal. Patients with abnormal scans were further classified into 3 groups on the basis of the type of vascular perfusion defect: scar only (fixed defects), ischemia only (reversible defects), and both scar and ischemia (fixed and reversible defects).15 Figure 1 shows an example of the imaging that was performed.
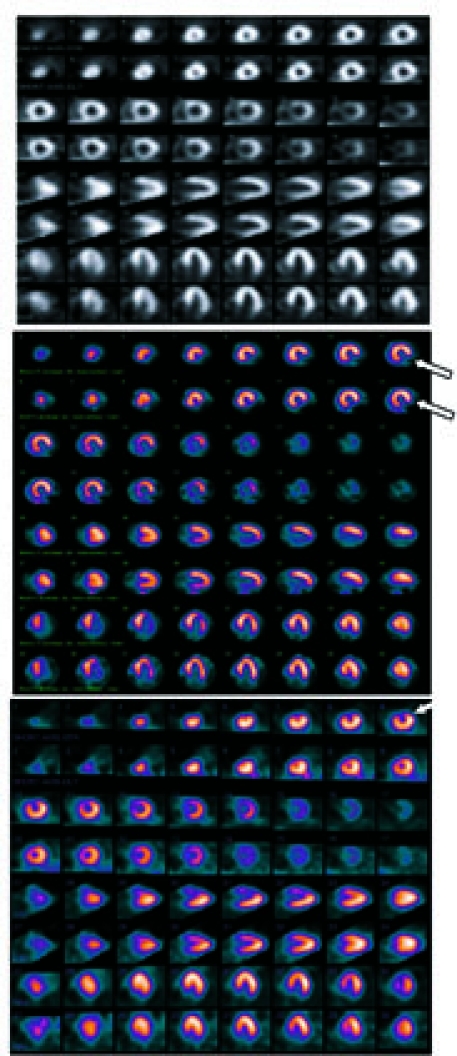
Fig. 1 Examples of myocardial perfusion images in a cancer population. The top panel (normal) shows a normal perfusion image, the middle panel (scar) indicates a fixed defect (double arrows), and the bottom panel (ischemia) indicates a reversible defect (single arrow).
Statistical Analysis
Values were summarized as mean ± SD for continuous variables and as frequency and percentage for categorical variables. The Kaplan-Meier method was used to estimate event-free survival and the distribution of time from initiation of follow-up to 1st cardiovascular event. Nine patients who underwent coronary revascularization or cardiac catheterization within 60 days of an abnormal MPI scan result were excluded from event-free survival analysis, because of the observation in previous studies1,3,4,6 that decisions to revascularize patients within 60 days of abnormal MPI were influenced by abnormal MPI and not entirely by symptoms. Any coronary revascularization after the initial 60 days was considered an event. Patients with follow-up times of longer than 3 years were evaluated at the 3-year time point. Comparisons of survival curves by categorical cardiac risk factors were made by use of the log-rank test. The Cox proportional hazard model was used to estimate the prognostic impact of multiple risk factors. Differences in risk were expressed as hazard ratio (HR) with the corresponding 95% confidence interval (CI). Analysis was performed on all of the patients who were included in the study, and results were compared on the basis of the MPI scan results and the cardiac risk factors. All tests were 2-sided, and P <0.05 was considered statistically significant. Statistical analyses were performed with the use of SAS Release 9.1 (SAS Institute; Cary, NC), and graphs were produced by use of S-PLUS 7.0 (Insightful Co.; Seattle, Wash).
Results
Characteristics of the Patients
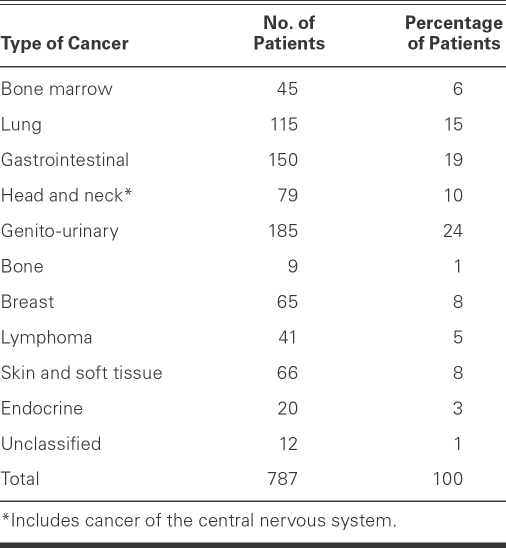
The mean age of the 787 patients was 66 ± 11 years; 61% were men. Of the MPI scans that were performed, 286 (36%) produced abnormal results (Table I). Most of the perfusion studies (71%) were part of preoperative evaluation before cancer therapy or surgery, and the others were performed in order to evaluate suspected coronary disease on the basis of symptoms or other clinical indicators. There were significant differences in risk factors between the normal-MPI and abnormal-MPI groups. In the abnormal-MPI group, the patients were slightly older (mean age, 67 ± 11 vs 65 ± 11.5 yr; P <0.008) with greater male predominance (73%). The abnormal-MPI group had a higher prevalence of cardiomyopathy, hyperlipidemia, CAD, diabetes mellitus, MI, and heart failure, and a higher percentage of patients treated with β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, and aspirin (all P <0.05) (Table I). There was no difference between groups with regard to the number of patients who had received chemotherapy (P = 0.24). The various types of cancer in the study population are shown in Table II.
TABLE II. Types of Cancer in the Study Population
Myocardial Perfusion Imaging Testing Characteristics
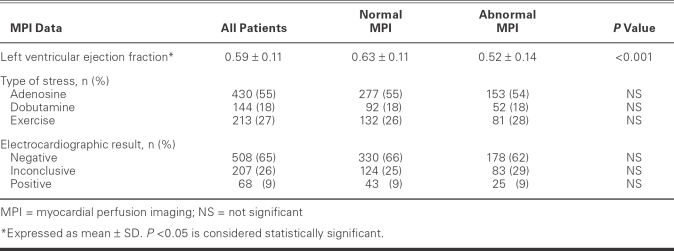
Test characteristics were similar in both MPI groups (Table III). Most patients had undergone an adenosine stress study (55%); fewer had undergone exercise and dobutamine stress studies (27% and 18%, respectively). The mean LVEF was 0.59 ± 0.11 for the total population and 0.63 ± 0.11 for the normal-MPI group, but lower—0.52 ± 0.14—for the abnormal-MPI group (P <0.0001). The stress-test ECG results were similar between the normal-MPI and abnormal-MPI groups: negative readings, 66% vs 62%; inconclusive readings, 25% vs 29%; and positive readings, 9% vs 9% (Table III).
TABLE III. Stress Myocardial Perfusion Scan Results
Survival Data
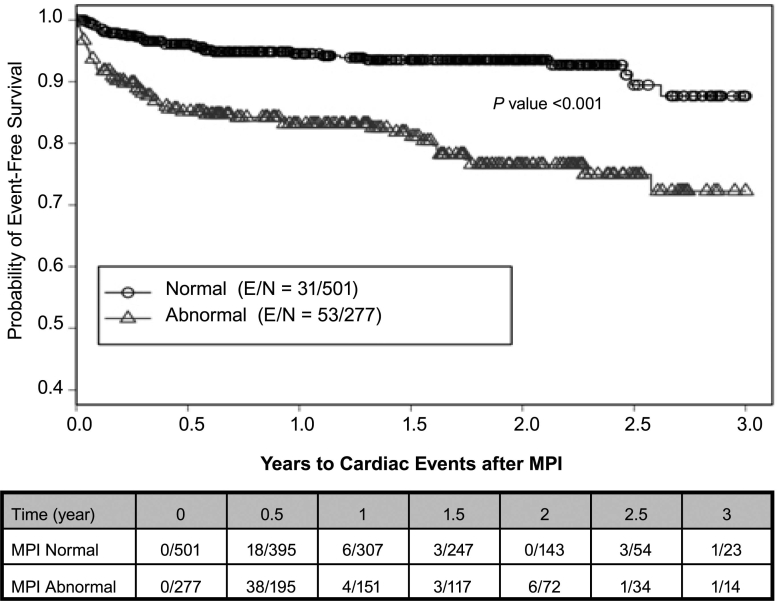
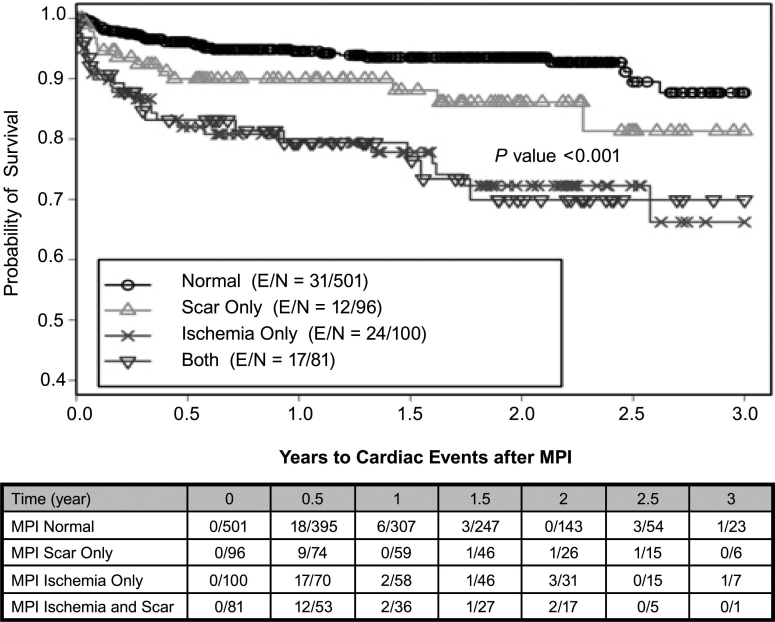
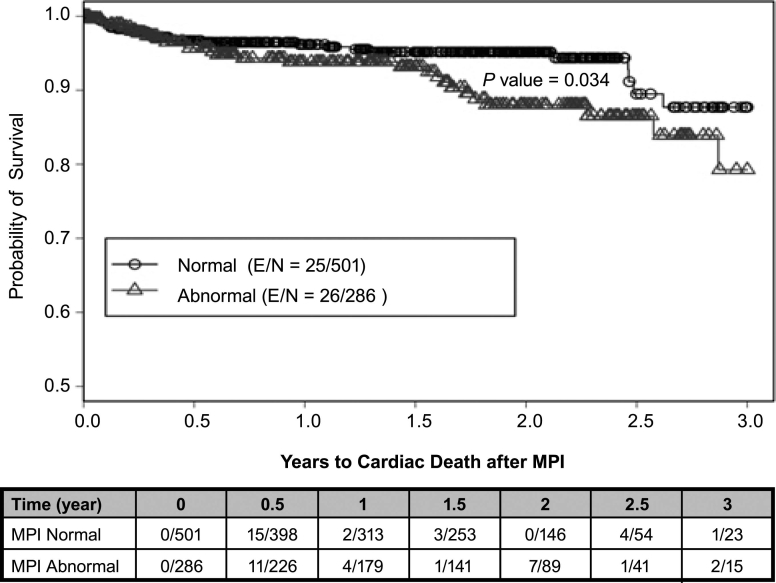
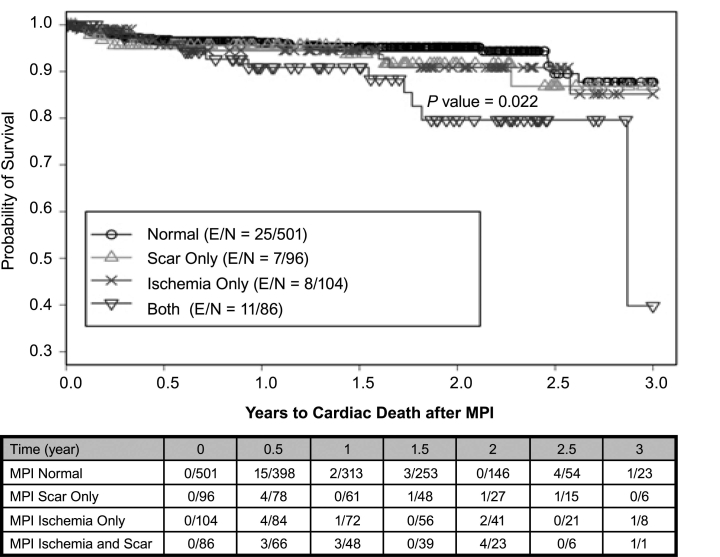
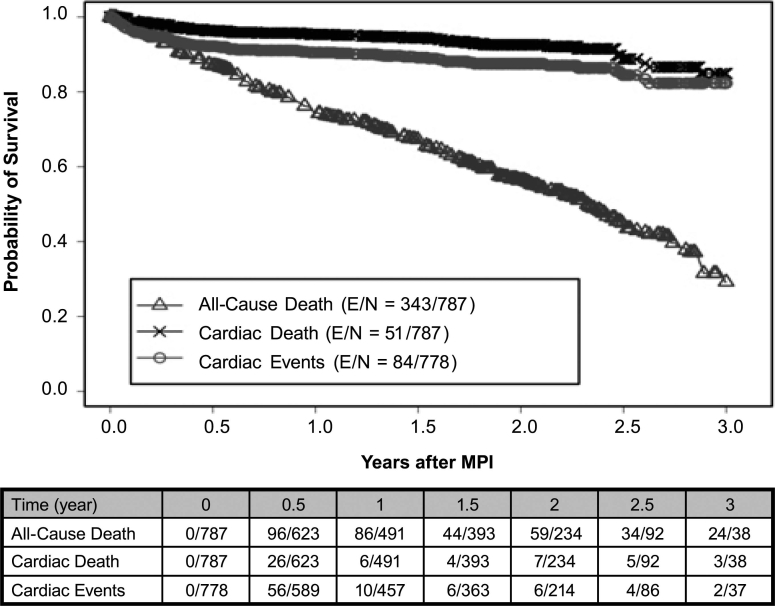
There were 84 total cardiac events, 51 cardiac deaths, and 343 all-cause deaths during the study. Using a Kaplan-Meier survival analysis, we found that the probability of 3-year total cardiac-event-free survival was 0.82 (0.78–0.87) for the entire study population. In the abnormal-MPI group, the probability of 3-year total cardiac-event-free survival was 0.72 (0.64–0.81) versus 0.88 (0.82–0.94) in the normal-MPI group (P <0.001) (Fig. 2). Of all abnormal scans (n = 277), the probability of 3-year total cardiac-event-free survival on the basis of the type of perfusion defects varied: 0.81 (0.7–0.94) for scarring only, 0.66 (0.53–0.83) for ischemia only, and 0.70 (0.58–0.85) for coexistent scarring and ischemia (P <0.001) (Fig. 3). The probability of 3-year cardiac-death-free survival was 0.85 (0.79–0.91) overall, 0.79 (0.69–0.91) for the abnormal-MPI group, and 0.88 (0.81–0.95) for the normal-MPI group (P = 0.034) (Fig. 4). Furthermore, the probability of 3-year cardiac-death-free survival in patients with both scarring and ischemic defects was 0.4 (0.1–1.0), which was much worse than those with scarring alone (0.87 [0.76–0.99]) or ischemia alone (0.85 [0.74–0.99]) (P = 0.022) (Fig. 5). All-cause death in the study population was extremely high: the probability of overall survival, which was 0.74 (0.71–0.78) at the end of 1 year, decreased to 0.29 (0.24–0.36) by the end of 3 years (Fig. 6). There was no significant difference in the probability of overall survival between those with normal MPI versus those with abnormal MPI results.
Fig. 2 Total cardiac events associated with MPI results.
E = patients who experienced an event; MPI = myocardial perfusion imaging; N = total number of patients
Fig. 3 Total cardiac events associated with scarring, ischemia, or both, as seen on MPI.
E = patients who experienced an event; MPI = myocardial perfusion imaging; N = total number of patients
Fig. 4 Cardiac death associated with MPI results.
E = patients who experienced an event; MPI = myocardial perfusion imaging; N = total number of patients
Fig. 5 Cardiac death associated with scarring, ischemia, or both, as seen on MPI.
E = patients who experienced an event; MPI = myocardial perfusion imaging; N = total number of patients
Fig. 6 Survival curves on the basis of defined endpoints (total cardiac events, cardiac death, and all-cause death) in the cancer population.
E = patients who experienced an event; MPI = myocardial perfusion imaging; N = total number of patients
The effect of surgery on overall survival, cardiac-specific survival, and total cardiac-event-free survival was evaluated. Surgery status was modeled as a time-dependent covariate in a univariate Cox proportional hazard model for each of the survival endpoints. No significant association was detected between surgery status and any of the 3 survival-based endpoints; the data are not shown.
Univariate Analysis
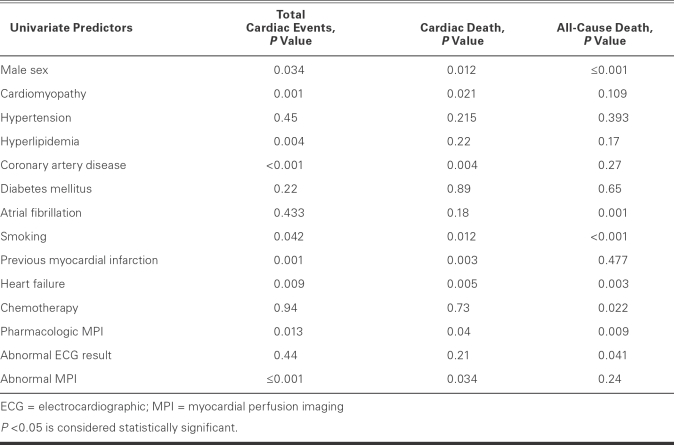
Univariate predictors of total cardiac events included male sex (P = 0.034), cardiomyopathy (P = 0.001), hyperlipidemia (P = 0.004), CAD (P <0.001), history of smoking (P = 0.042), previous MI (P <0.001), heart failure (P = 0.009), pharmacologic MPI (P = 0.013), and abnormal MPI (P <0.001) (Table IV). Similarly, the univariate predictors of cardiac death included male sex (P = 0.01), cardiomyopathy (P = 0.021), CAD (P = 0.004), history of smoking (P = 0.012), previous MI (P = 0.003), heart failure (P = 0.005), pharmacologic MPI (P = 0.04), and abnormal MPI results (P = 0.0034). All-cause death was predicted by male sex (P <0.001), atrial fibrillation (P = 0.001), history of smoking (P <0.001), heart failure (P = 0.003), chemotherapy (P = 0.022), pharmacologic MPI (P = 0.009), and abnormal ECG on stress testing (P = 0.041).
TABLE IV. Univariate Predictors of Cardiac Outcomes and All-Cause Death
Multivariate Analysis
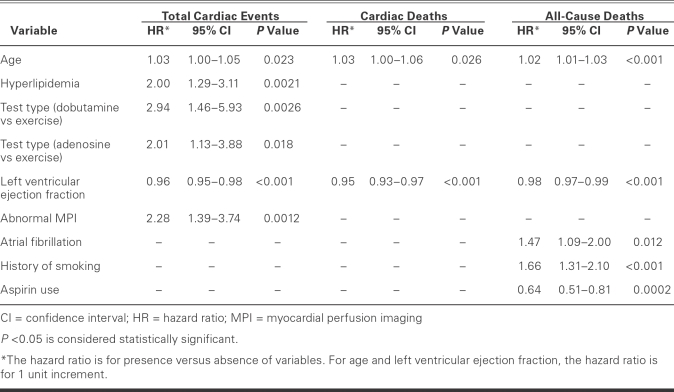
Upon multivariate analysis for total cardiac events (Table V), significant multivariate predictors of worse outcomes were age (HR, 1.03; CI, 1.0–1.05; P = 0.023), hyperlipidemia (HR, 2.00; CI, 1.29–3.11; P = 0.0021), dobutamine MPI (HR, 2.94; CI, 1.46–5.93; P = 0.0026), adenosine MPI (HR, 2.01; CI, 1.13–3.8; P = 0.018), and abnormal MPI (HR, 2.28; CI, 1.39–3.74; P = 0.0012). Each percentage point of increase in LVEF (HR, 0.96; CI, 0.95–0.98; P <0.001) had a protective effect on total cardiac-event-free survival. In the multivariate model for predictors of cardiac death, each year of increment in age (HR, 1.03; CI, 1.0–1.06; P = 0.026) was a predictor of cardiac death, while each unit-increment increase in LVEF (HR, 0.95; CI, 0.93–0.97; P <0.001) was associated with a lower incidence of cardiac death. The multivariate model for all-cause death revealed worse outcomes with increasing age (HR, 1.02; CI, 1.01–1.03; P <0.001), atrial fibrillation (HR, 1.47; CI, 1.09–2.0; P = 0.012), and history of smoking (HR, 1.66; CI, 1.31–2.10; P <0.001). Overall survival was better with the taking of aspirin (HR, 0.64; CI, 0.51–0.81; P = 0.0002) and with each percentage point of increment in LVEF (HR, 0.98; CI, 0.97–0.99; P <0.001).
TABLE V. Multivariate Predictors of Cardiac Events, Cardiac Deaths, and All-Cause Deaths
Discussion
This study of 787 cancer patients is the 1st extensive evaluation of the role of MPI in predicting long-term cardiovascular outcomes in cancer patients. The study suggests that the findings on MPI provide incremental information, in addition to identifying clinical risk factors. We found that abnormal MPI is highly predictive of future cardiac events and cardiac death in this cancer population. In univariate and Kaplan-Meier analyses, the probabilities of a cardiac event and of cardiac death were significantly higher in patients who had abnormal MPI scans compared with those who had normal scans. Upon multivariate analysis, MPI retained its incremental value in predicting total cardiac events (cardiac death, acute MI, revascularizations, and symptomatic CAD), but not in predicting all-cause death alone. This finding can be explained by the high mortality rate in the normal-MPI group, compared with previous studies.1–3,5,6,18 This high probability of death in the normal-MPI group might have mitigated the discriminating power of this test. Furthermore, all patients in this study appeared to be at higher risk of CAD than the general population. In the normal-MPI group, more than 40% of the patients had 3 or more risk factors for obstructive CAD, and more than 60% had 2 or more. In addition, more than 75% of patients in the normal-MPI group were referred for a pharmacologic stress test, either due to baseline ECG abnormality or low functional capacity. Therefore, the increased probability of death in the normal-MPI group might reflect the combination of multiple risk factors and low functional capacity in cancer patients. These data emphasize the need to investigate and treat vascular risk factors in a cancer population in order to optimally affect patient outcomes.
The results of our study in a cancer population include several important findings. In terms of predicting cardiac events and cardiac death, typical vascular risk factors (hyperlipidemia, CAD, and previous MI) are powerful predictors of such outcomes; however, all-cause death is best predicted by atrial fibrillation and smoking. A higher LVEF is a protective clinical value that predicts a reduction of all events, including all-cause death, and, not surprisingly, older age is predictive of worse outcomes in an incremental fashion. Of note, the taking of aspirin is strongly associated with reduced all-cause death and has as great an influence as any other factor. This emphasizes the benefit of aspirin as a treatment for cardiac disease, especially in a cancer population.19 In addition, other methods to protect cardiac function during cancer therapy are crucial.20
The type of stress chosen during MPI—exercise versus pharmacologic—may also have some effect on outcomes, although this is likely a reflection of the perceived functional status in this patient population. Even though exercise testing by itself has low discriminatory power, exercise MPI discloses important functional information.1,3,6,21,22 Our study showed that patients who underwent exercise MPI had significantly better survival compared with those who underwent pharmacologic MPI, and that this was a strong predictor of cardiac death and total cardiac events. Even after controlling for cardiac risk factors in a multivariate analysis, we found that the undergoing of pharmacologic MPI was an important and independent predictor of total cardiac events. A previous study of elderly patients5 also indicated that the event rate was significantly higher in patients who had undergone pharmacologic MPI. Again, this difference is largely due to the decreased functional status of individual patients, yet it seems to emphasize that functional status is an important predictor of outcome.
In the current study, patients could be stratified into different risk groups on the basis of the types of perfusion defects that were seen on MPI. Fixed or reversible perfusion defects are strong predictors of cardiac death, as previous studies have shown,1–7,18,23 and the present study is of similar usefulness regarding the detailed findings. In our population, combination fixed and reversible defects correlated strongly with cardiac death, as suggested by previous data,6,18 indicating that combined defects usually involve myocardial tissue more extensively. Furthermore, patients with extensive reversible defects usually undergo early revascularization, and such patients were excluded from cardiac-event analysis in our study. This exclusion may attenuate the prognostic value of reversible defects and could be a plausible explanation for the lack of association of cardiac death and reversible defects. Our results are in accordance with those of other studies and support the concept that death correlates more strongly with conditions that affect left ventricular dysfunction, such as scar tissue and not only ischemia, on stress testing.
Our study shows a lack of association between MPI abnormality and all-cause death. This is partially explained by the different types and stages of cancer that may have radically different prognoses. Furthermore, improved medical therapy in the abnormal-MPI group may have averted the death of some patients. This is evident from the statistically higher taking of aspirin, β-blockers, ACE inhibitors, and statins in the abnormal-MPI group (Table I). Also, all-cause death is directly related to concomitant comorbidities, even in a cardiac-disease population. Other studies have shown that, in the presence of other comorbidities, MPI does not predict the endpoint of death from all causes.24 In the current study, noncardiac-related death was substantial (Fig. 6), which suggests that other comorbidities (such as cancer) have a great effect on outcomes.
Study Limitations
The population in this study comprised patients who were referred to a tertiary-care cancer center with a diagnosis of cancer, and there is the potential of referral bias; accordingly, these results may not be applicable to an unselected population of cancer patients. This study has limitations that are inherent to all retrospective trials; however, the data were meticulously collected, and long-term outcomes were all confirmed. In addition, we were unable to reliably classify the staging of the malignancies, which may have affected the mortality data.
Conclusion
Abnormal MPI test results in cancer patients predict increased cardiovascular morbidity and death over a 3-year period. All-cause death in this population is directly related to increasing age, the presence of atrial fibrillation, smoking history, and reduced LVEF. Upon performing multivariate analysis, we found that the taking of aspirin profoundly reduces death in cancer patients.
Acknowledgment
The authors greatly appreciate the expert preparation of this manuscript by Amy Chiu, MBA.
Footnotes
Address for reprints: Daniel J. Lenihan, MD, Department of Cardiology – 449, 1515 Holcombe Blvd., Houston, TX 77030. E-mail: dlenihan@mdanderson.org
References
- 1.Berman DS, Hachamovitch R, Kiat H, Cohen I, Cabico JA, Wang FP, et al. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: a basis for optimal utilization of exercise technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography [published erratum appears in J Am Coll Cardiol 1996;27(3):756]. J Am Coll Cardiol 1995;26(3):639–47. [DOI] [PubMed]
- 2.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction [published erratum appears in Circulation 1998;98(2):190]. Circulation 1998;97(6):535–43. [DOI] [PubMed]
- 3.Galassi AR, Azzarelli S, Tomaselli A, Giosofatto R, Ragusa A, Musumeci S, et al. Incremental prognostic value of technetium-99m-tetrofosmin exercise myocardial perfusion imaging for predicting outcomes in patients with suspected or known coronary artery disease. Am J Cardiol 2001;88(2):101–6. [DOI] [PubMed]
- 4.Schinkel AF, Elhendy A, Biagini E, van Domburg RT, Valkema R, Rizello V, et al. Prognostic stratification using dobutamine stress 99mTc-tetrofosmin myocardial perfusion SPECT in elderly patients unable to perform exercise testing. J Nucl Med 2005;46(1):12–8. [PubMed]
- 5.Lima RS, De Lorenzo A, Pantoja MR, Siqueira A. Incremental prognostic value of myocardial perfusion 99m-technetium-sestamibi SPECT in the elderly. Int J Cardiol 2004;93(2–3): 137–43. [DOI] [PubMed]
- 6.De Lorenzo A, Lima RS, Siqueira-Filho AG, Pantoja MR. Prevalence and prognostic value of perfusion defects detected by stress technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography in asymptomatic patients with diabetes mellitus and no known coronary artery disease. Am J Cardiol 2002;90(8):827–32. [DOI] [PubMed]
- 7.Pryma DA, Ravizzini G, Amar D, Richards VL, Patel JB, Strauss HW. Cardiovascular risk assessment in cancer patients undergoing major surgery. J Nucl Cardiol 2005;12(2):151–7. [DOI] [PubMed]
- 8.ASCO.org [homepage on the Internet]. American Society of Clinical Oncology; Alexandria, VA: c2005-9 [cited 2009 Feb 17]. Available from: http://www.asco.org/portal/site/ASCO.
- 9.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291(20):2441–7. [DOI] [PubMed]
- 10.Coebergh JW, Janssen-Heijnen ML, Post PN, Razenberg PP. Serious co-morbidity among unselected cancer patients newly diagnosed in the southeastern part of The Netherlands in 1993-1996. J Clin Epidemiol 1999;52(12):1131–6. [DOI] [PubMed]
- 11.Sanz J, Moreno PR, Fuster V. The year in atherothrombosis. J Am Coll Cardiol 2007;49(16):1740–9. [DOI] [PubMed]
- 12.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 2003;290(19):2581–7. [DOI] [PubMed]
- 13.Chang K, Sarkiss M, Won KS, Swafford J, Broemeling L, Gayed I. Preoperative risk stratification using gated myocardial perfusion studies in patients with cancer. J Nucl Med 2007;48(3):344–8. [PubMed]
- 14.Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50(22):2173–95. [DOI] [PubMed]
- 15.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol 2003;42(7):1318–33. [DOI] [PubMed]
- 16.Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina) [published erratum appears in J Am Coll 1999;34(1):314]. J Am Coll Cardiol 1999;33(7):2092–197. [DOI] [PubMed]
- 17.Gibbons RJ. Myocardial perfusion imaging. Heart 2000; 83(3):355–60. [DOI] [PMC free article] [PubMed]
- 18.Shaw LJ, Hachamovitch R, Heller GV, Marwick TH, Travin MI, Iskandrian AE, et al. Noninvasive strategies for the estimation of cardiac risk in stable chest pain patients. The Economics of Noninvasive Diagnosis (END) Study Group. Am J Cardiol 2000;86(1):1–7. [DOI] [PubMed]
- 19.Sarkiss MG, Yusuf SW, Warneke CL, Botz G, Lakkis N, Hirch-Ginsburg C, et al. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer 2007;109(3):621–7. [DOI] [PubMed]
- 20.Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 2004; 109(25):3122–31. [DOI] [PubMed]
- 21.Romero L, de Virgilio C. Preoperative cardiac risk assessment: an updated approach. Arch Surg 2001;136(12):1370–6. [DOI] [PubMed]
- 22.Ho KT, Miller TD, Hodge DO, Bailey KR, Gibbons RJ. Use of a simple clinical score to predict prognosis of patients with normal or mildly abnormal resting electrocardiographic findings undergoing evaluation for coronary artery disease. Mayo Clin Proc 2002;77(6):515–21. [DOI] [PubMed]
- 23.Torres MR, Short L, Baglin T, Case C, Gibbs H, Marwick TH. Usefulness of clinical risk markers and ischemic threshold to stratify risk in patients undergoing major noncardiac surgery. Am J Cardiol 2002;90(3):238–42. [DOI] [PubMed]
- 24.Alkeylani A, Miller DD, Shaw LJ, Travin MI, Stratmann HG, Jenkins R, Heller GV. Influence of race on the prediction of cardiac events with stress technetium-99m sestamibi tomographic imaging in patients with stable angina pectoris. Am J Cardiol 1998;81(3):293–7. [DOI] [PubMed]