Abstract
We sought to compare outcomes in patients ≥60 years of age with those of their younger counterparts who underwent ventricular assist device implantation intended as a bridge to cardiac transplantation and also to identify retrospectively additional pre- and postoperative factors that might portend adverse outcomes.
The medical records of 88 patients who were treated with bridge-to-transplantation ventricular assist devices from 1996 through 2007 were reviewed. Laboratory values, hemodynamic parameters, and the need for hemodynamic support were evaluated. Postoperative complications and bridge-to-transplantation success rates versus death rates were evaluated. Seventeen patients were ≥60 years old and 71 patients were <60 years old. In the older group, 59% of patients underwent successful bridging to transplantation, compared with 69% of the younger patients (P = 0.41). Multivariate analysis distinguished age ≥60, female sex, earlier time period of operation, higher mean pulmonary arterial and central venous pressures, need for preoperative intra-aortic balloon pumps, and postoperative respiratory failure as independent risk factors for death. After orthotopic heart transplantation, survival to hospital discharge was 100% in the older group and 93.9% in the younger patients. Median lengths of stay were similar in both age categories.
Multivariate analysis identified age as 1 of 6 independent risk factors for death in this study. Patients who successfully underwent cardiac transplantation, however, had similar survival statistics regardless of age category. Case-by-case evaluation is warranted when analyzing risk–benefit ratios of bridge-to-transplantation ventricular assist device therapy in the older patient population.
Key words: Aged; heart failure, congestive/surgery; heart-assist devices/statistics & numerical data; heart transplantation; multivariate analysis; retrospective studies; risk assessment; risk factors; treatment outcome
Congestive heart failure afflicts more than 5 million Americans and was responsible for more than 280,000 deaths in 2004.1 Orthotopic heart transplantation remains an effective method of treatment for a distinct subset of these patients, despite the oft-cited limitation of the finite donor pool. In accordance with current demographic trends, patients 60 years of age or older are one of the fastest growing segments of the heart-transplantation population in the United States, accounting for 213 cases in 1988 and for more than 1,000 in 2006.2
Ventricular assist devices (VADs) have been FDA-approved since 1994 as a bridge to transplantation (BTT) in certain critically ill patients who otherwise would not survive until an organ becomes available. While effective, the BTT VAD is a costly and labor-intensive therapy for both patient and physician and has significant associated morbidities. Because more centers are now considering older patients to be suitable candidates for heart transplantation, it is important to evaluate outcomes of BTT VADs in this patient population.
The purpose of this study was to assess the success of BTT VADs in both older and younger cohorts at our center, as well as to identify additional pre- and postoperative factors that might portend adverse outcomes.
Patients and Methods
We retrospectively reviewed the medical records, from 1996 through 2007, of 88 patients whose end-stage cardiomyopathy was treated at UCLA Medical Center with VADs that were intended as bridges to transplantation. We excluded patients who were bridged to recovery, who were undergoing VAD implantation as destination therapy, or who were being treated for postcardiotomy shock. Our indication for the placement of an assist device was the need for life-saving therapy among heart-transplantation candidates who were in imminent danger of worsening multiorgan failure, despite optimal medical management.
From 1996 through 2000, our center implanted exclusively the HeartMate VE® or IP® (Thermo Cardiosystems, now part of Thoratec Corporation; Woburn, Mass). Starting in 2000, our center implanted the Thoratec Paracorporeal VAD™ (Thoratec Corporation; Pleasanton, Calif); and from 2004 through 2007, we implanted either the Thoratec Paracorporeal VAD™ or the HeartMate® XVE (Thoratec), depending upon their availability.
Preoperative patient evaluation included the examination of blood urea nitrogen, creatinine, lactate, bicarbonate, total bilirubin, and international normalized ratio (INR) levels. The hemodynamic variables examined were left ventricular ejection fraction (LVEF) and cardiac index, in addition to right atrial, pulmonary arterial, and systemic pressures. In addition, patients were categorized with respect to the presence of 3 or more vasopressors, the use of an intra-aortic balloon pump (IABP), and the need for dialysis. Bridge-to-transplantation rates were also examined with respect to early or late time period: specifically, the years 1996 through 2001 were compared with the years 2002 through 2007.
The BTT rate was defined as the percentage of patients who underwent successful cardiac transplantation while on VAD support. Postoperative outcomes were defined as successful BTT or death: no VAD-support patients in this current study are still on the donor waiting list.
In addition, complications were defined as bleeding that required operative re-exploration; device thrombus that led to flow limitations or thromboembolism; culture-proven infection; stroke; and respiratory failure that required mechanical ventilation for longer than 72 hours, reintubation, or tracheostomy.
Statistical Analysis. Categorical data were analyzed using 2 × 2 contingency tables, with P values determined by the Fisher exact test. Continuous variables were compared using Student's t test. Mortality curves were estimated using Kaplan-Meier (actuarial) curves, with P values generated by the log-rank test (JMP, SAS Institute; Cary, NC). Multivariate analyses were performed with use of a proportional hazards regression model for competing risks (R, The R Foundation for Statistical Computing; Vienna, Austria). Two-tailed P values <0.05 were considered statistically significant.
Results
Group 1 comprised 17 patients 60 years of age or older, and group 2 comprised 71 patients 59 years of age or younger. The average age for each group was 64.1 ± 3.6 and 41.4 ± 15.5 years, respectively (P <0.0001). Men comprised 88.2% of the older group and 78.9% of the younger group (P = 0.51). Ischemic heart disease was the cause of heart failure in 64.7% of the older group and in 18.3% of the younger group (P = 0.0003). A specific breakdown of the causes of heart failure is shown in Figure 1.
Fig. 1 Cause of heart failure by age group, expressed as percentages.
CM = cardiomyopathy; CTx = chemotherapy; DCM = dilated cardiomyopathy; ICM = ischemic cardiomyopathy
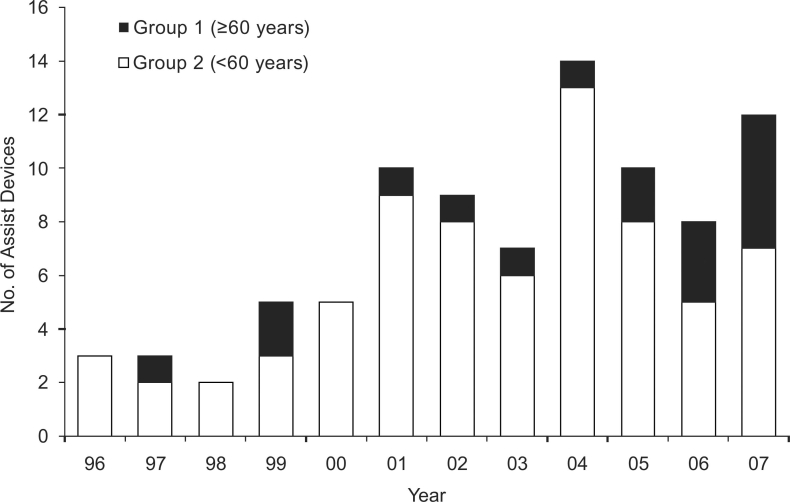
Biventricular assist devices (BiVADs) were placed in 57 patients (64.8%), whereas left ventricular assist devices (LVADs) were placed in 31 patients (35.2%). Most of the LVADs (20 of 31) were placed in the early time period (from 1996 through 2001). Annual VAD placement ranged from 2 to 14 in number; most older patients received VADs during the second 5-year period (Fig. 2).
Fig. 2 Number of ventricular assist devices placed per annum by age group. These figures include both biventricular assist devices and left ventricular assist devices.
The preoperative condition of the patients did not differ significantly between groups 1 (older) and 2 (younger). The respective mean laboratory values were: creatinine, 1.8 and 1.9 mg/dL; urea nitrogen, 42.2 and 46.6 mg/dL; and total bilirubin, 1.6 and 2.4 mg/dL. In the older and younger groups, respectively, the mean LVEFs were 0.21 and 0.19, the mean central venous pressures (CVPs) were 13.4 mmHg and 12.6 mmHg, the mean pulmonary arterial pressures were 34.6 mmHg and 34.4 mmHg, and the mean cardiac indices were 1.8 L/(min·m2) and 2.0 L/(min·m2). Forty-seven percent of the older patients required 3 or more vasoactive agents, compared with 28.6% of the younger patients. In addition, 29.4% of the older patients required temporary hemodialysis, compared with 28.6% of the younger patients. Intra-aortic balloon pump usage was also roughly equivalent, at 58.8% and 63.4% among the older and younger groups, respectively. None of these differences reached statistical significance. Differences in postoperative complication rates between groups 1 and 2 were also not statistically significant (Fig. 3).
Fig. 3 Percentage of postoperative complications by age group and overall.
NS = not significant
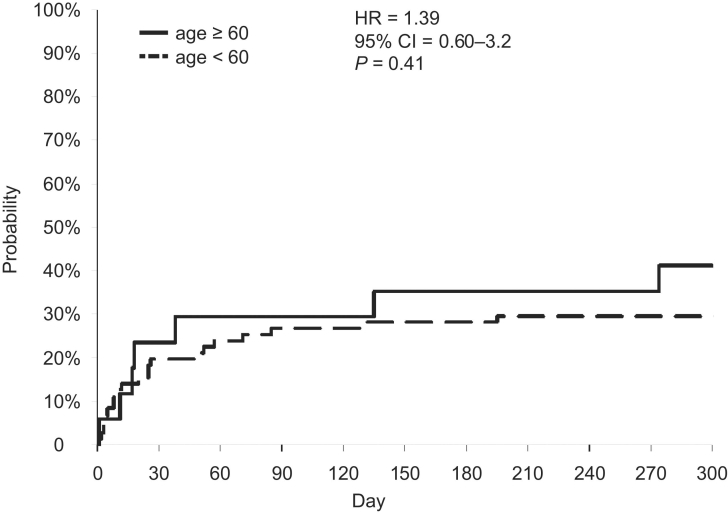
Fifty-nine percent (10 of 17) of patients in group 1 were successfully bridged to transplantation, compared with 69% (49 of 71) of the younger patients. Thus, the death rates for the older and younger groups were 41% and 31%, respectively. The hazard ratio was 1.39 (95% confidence interval = 0.60–3.2, P = 0.41) (Fig. 4). The most common cause of death in aggregate was multiple-system organ failure (65.5%), followed by stroke (24.1%). Other causes of death (10.4%) included ischemic bowel, pneumonia, and preoperative pulmonary embolism.
Fig. 4 Probability of death by age group (Kaplan-Meier mortality curve).
CI = confidence interval; HR = hazard ratio
The median duration of support for the older group was 50 days (range, 1–366 d), versus 53 days (range, 1–275 d) for the younger group. In aggregate, the median duration of support before BTT or death was 52 days. Controlling for the risk of death, we found that the median time to transplantation was 104 days (range, 13–236 d) for the older group and 98 days (range, 5– 355 d) for the younger group.
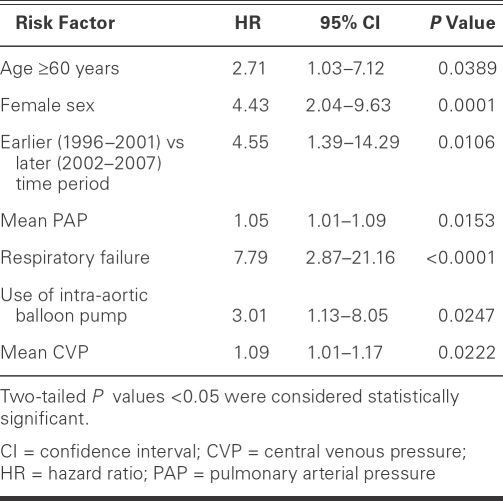
Table I shows the results of multivariate analysis of risk factors for death in patients who underwent VAD placement as BTT. Independent risk factors for death were age 60 years or older, female sex, earlier time period of operation, higher mean pulmonary arterial pressures and CVPs, need for preoperative IABP support, and postoperative respiratory failure.
TABLE I. Multivariate Analysis of Risk Factors for Death
Among patients who successfully underwent heart transplantation, survival rates to hospital discharge were 100% for the older group versus 93.9% for the younger group (P = 1.0). Overall survival was 94.9%. Median lengths of stay were similar: 80 days (23–188 d) for the older group and 91 days (24–265 d) for the younger group (P = 0.54). Median lengths of stay after orthotopic heart transplantation were also similar, at 14.5 days (9–63 d) for the older group versus 16 days (5–74 d) for the younger group (P = 0.97).
Discussion
Ventricular assist devices have become a common component of the armamentarium used to treat advanced congestive heart failure. As the U.S. population ages, a greater number of older patients will become candidates for this risky but life-saving treatment.
There has been a trend at our institution towards earlier intervention during the course of heart failure, before significant secondary organ dysfunction has occurred. However, elective or planned placement, as documented by others,3,4 is not yet in use at our center. This earlier intervention—along with improved patient selection, which eliminates from treatment consideration those patients with irrecoverable multi-organ failure—has led to improvements in BTT rates, as can be seen by comparing results from our earlier (1996–2001) and later (2002–2007) time periods.
In this study, multivariate analysis revealed 6 variables, in addition to age, that are independent risk factors for death—and therefore for unsuccessful BTT. These are female sex, surgery in the earlier time period, higher mean pulmonary arterial pressure, higher mean CVP, need for preoperative IABP, and postoperative respiratory failure. Many of these risk factors have been cited in previous studies.
Infection rates in the 2 groups were similar. Sepsis was the leading cause of infection (at 37% of all cases), followed by pneumonia and device-related wound infections (at 28% and 23%, respectively). Urinary tract infections and colitis each comprised 5% of the total infection rate. In accordance with the findings of other studies,5,6 the presence of infection did not appear to lower successful BTT rates. Although the development of LVAD endocarditis has been associated with increased mortality rates, there were no incidences of this catastrophic complication in our series. Current policy at our institution includes daily rounds by an infectious disease team dedicated to the management of heart failure patients. In general, broad-spectrum perioperative antibiotics—including vancomycin (or daptomycin if there is a documented history of vancomycin-resistant enterococcus) in addition to a 3rd-generation cephalosporin (or piperacillin/tazobactam, for previously hospitalized patients)—is continued throughout the patient's course in the intensive care unit. Subsequent positive cultures or signs of infection dictate additional antibiotic therapies.
Thrombus, as defined above (device thrombus that led to flow limitations or thromboembolism), occurred in only 4 of our 88 patients. Three of the 4 patients sustained neuroembolic strokes and the other patient experienced multiorgan failure, presumably secondary to systemic emboli. All 4 patients died. One of these patients had flow-limiting thrombus that required changeout of the pump unit. Mere fibrin strands, on the other hand, were ubiquitous findings, present by some estimates in up to 80% of our patients whose VAD support lasted over 1 month. These were clinically insignificant and were treated with intermittent, low systemic doses (2 mg) of tissue plasminogen activator, together with therapeutic INR maintenance via warfarin. Our current policy mandates the initiation of intravenous heparin infusion at 24 postoperative hours, if not contraindicated by perioperative hemorrhage. Conversion to oral warfarin (targeted INR, 2.5–3.5) is started when clinically feasible.
Two patients had heparin-induced thrombocytopenia (HIT), documented by positive antiplatelet-antibody and aggregation studies. This accounts for only 2.3% of our 2 study groups, which likely indicates an underdiagnosis of this clinical entity. Other studies have documented rates from 8.4% to 20% and have associated HIT, inexorably, with poorer outcomes.7 Additional studies that might clarify the true incidence of HIT in our patient population would be of interest. In addition, the avoidance of heparin use in this very susceptible patient population, by substituting such alternatives as argatroban or bivalrudin, may be warranted.8
Major stroke occurred in 11 of the 88 patients. All but 1 of these died, accounting for 11.4% of the overall deaths in this study. This compares with the 9.7% contribution of stroke to death in the REMATCH trial, which (similarly) used the HeartMate XVE.9 Although the 91% mortality rate seen in patients suffering a major stroke in our study seems at 1st glance to be an overwhelming risk factor for death, we chose, rather, to consider major stroke as a proxy for death, given that unrecoverable neurologic deterioration led to a withdrawal of care by patients' families in each of these cases.
We, as others,3,10 have found that women have significantly lower rates of successful BTT. It has been suggested that lower body mass index (BMI) may be a causal factor. One study11 found significantly higher mortality rates after VAD placement in patients who presented with lower BMI. The comparative BMIs for men and women in our study, however, were quite similar at 24.6 and 25.8, respectively (P = 0.40). The mean age of the women in our series, at 43.0 years, was also similar to that of the men, at 46.5 years (P = 0.44). Another study10 found that female patients presented with a higher VAD risk-factor score, which included such variables as ventilatory status, CVP >16 mmHg, and prolonged prothrombin time. This score became a significant predictor of success or failure in BTT. In our study, however, there was no laboratory or hemodynamic evidence to suggest that female patients were sicker at initial presentation than their male counterparts. Many studies have looked for causal associations between female sex and poorer outcomes in cardiac surgery in general. Increased comorbidities, such as diabetes mellitus, hypertension, peripheral vascular disease, and the more advanced stages of heart disease have been noted in female patients, and these may account for survival differences.12 In future studies, we will explore these factors in our female cohort.
In this study, patients who had BiVAD implantations experienced higher rates of BTT success than did patients on left ventricular support only. This counters the results of those who contend that patients who required BiVAD support had poorer outcomes because they were sicker.13 One reason for these variant results may be that most of the LVAD interventions (64.5%) in our experience occurred in the earlier years, before the current, more stringent exclusion criteria were put in place. The other, perhaps confounding, factor is our bias—shared by others14,15—that many patients in profound cardiogenic shock benefit inherently from biventricular support. Our group has enacted a rather proactive policy with regard to implanting BiVADs. Although low pulmonary artery pressures and high CVPs constituted definite evidence of the need for right ventricular support, the absence of such numbers did not summarily disqualify patients for biventricular support at our institution.
The link between respiratory failure and adverse outcomes in cardiac surgery has been well documented in the medical literature.16,17 In 1 study,18 the overall incidence of respiratory failure after cardiac surgery was 9.1%. However, there was substantial variance between procedures—for example, coronary artery bypass grafting (5.8%), multi-valve surgery (17%), and aortic arch surgery (22.4%).18 These rates are still significantly lower than the 47.7% respiratory-failure rate seen in our series. Filsoufi and colleagues18 identified several risk factors for respiratory failure, including congestive heart failure, LVEF <0.30, renal failure, hemodynamic instability, and IABP insertion, that apply well to our patient population and could explain our exceedingly high rates of respiratory failure. The link between postoperative respiratory failure and death has been attributed to associated prolonged intensive care unit stays, multi-organ failure, and sepsis.17 Because of respiratory failure's strong association with adverse outcomes among cardiac surgery patients, and among VAD recipients in particular, aggressive extubation protocols and other preemptive attempts to stave off the development of this complication should be pursued.
Our review corroborates the findings of previous studies that advanced age is an independent risk factor for death in patients undergoing VAD therapy as a BTT. Age of 60 years or greater conferred a risk of death that was 2.71 times the rate for patients under age 60, when simultaneous factors were controlled. To determine whether advanced age alone justifies withholding VAD therapy would require a nonexistent control group consisting of patients 60 years of age or older who were bridged to transplantation with medical therapy alone.
Although the REMATCH trial studied destination VADs in patients who were deemed non-transplantation candidates, many of those patients were excluded from orthotopic heart transplantation on the basis of age greater than 65 years. A subset of these patients might have been listed for transplantation at our center and at many other institutions that are now performing cardiac transplantation in older candidates. Therefore, REMATCH data9 showing that mechanically assisted patients enjoy increased quality of life in the 1st year, together with a 48% risk reduction for death, may justifiably be extrapolated to apply to many of our BTT patients. Despite the decreased survival rate of older patients in our series when compared with their younger peers, our current BTT success rates may be reasonable given the much bleaker prognosis for comparable patients who are medically treated. In addition, the older patient group in our series, having undergone transplantation, exhibited resilience similar to that of their younger counterparts with respect both to survival to discharge and to median length of stay.
Conclusion
The use of VADs requires a large investment of healthcare resources. As a consequence, patients' risk factors such as advanced age often face considerable scrutiny. Indeed, advanced age in this study was an independent risk factor for death, along with female sex, earlier time period of operation, pulmonary hypertension, higher central venous pressure, need for IABPs, and postoperative respiratory failure. Nevertheless, data from studies such as the REMATCH trial show enhanced survival with VADs in the older population, when compared with medical management alone. This is particularly true for the 1st year after implantation, when most BTT occurs. In addition, patients who underwent transplantation successfully in this study had similar survival statistics and lengths of hospital stays, regardless of age category. Careful evaluations should therefore be performed on a case-by-case basis, taking into account the unique risk factors inherent to each patient when making decisions in regard to the risk–benefit ratio of BTT VAD therapy in the older patient population.
Footnotes
Address for reprints: Murray H. Kwon, MD, 10833 Le Conte Ave., 62-182 CHS, Los Angeles, CA 90095. E-mail: mkwon@mednet.ucla.edu
This work was supported by funds from a Maurice Marciano Family Foundation Grant.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117(4):e25–146. [DOI] [PubMed]
- 2.Weiss ES, Nwakanma LU, Patel ND, Yuh DD. Outcomes in patients older than 60 years of age undergoing orthotopic heart transplantation: an analysis of the UNOS database. J Heart Lung Transplant 2008;27(2):184–91. [DOI] [PubMed]
- 3.Dang NC, Topkara VK, Kim BT, Mercando ML, Kay J, Naka Y. Clinical outcomes in patients with chronic congestive heart failure who undergo left ventricular assist device implantation. J Thorac Cardiovasc Surg 2005;130(5):1302–9. [DOI] [PubMed]
- 4.Deng MC, Weyand M, Hammel D, Schmid C, Kerber S, Schmidt C, et al. Selection and management of ventricular assist device patients: the Muenster experience. J Heart Lung Transplant 2000;19(8 Suppl):S77–82. [DOI] [PubMed]
- 5.Sinha P, Chen JM, Flannery M, Scully BE, Oz MC, Edwards NM. Infections during left ventricular assist device support do not affect posttransplant outcomes. Circulation 2000;102(19 Suppl 3):III194–9. [DOI] [PubMed]
- 6.Simon D, Fischer S, Grossman A, Downer C, Hota B, Heroux A, Trenholme G. Left ventricular assist device-related infection: treatment and outcome. Clin Infect Dis 2005;40(8): 1108–15. [DOI] [PubMed]
- 7.Koster A, Huebler S, Potapov E, Meyer O, Jurmann M, Weng Y, et al. Impact of heparin-induced thrombocytopenia on outcome in patients with ventricular assist device support: single-institution experience in 358 consecutive patients. Ann Thorac Surg 2007;83(1):72–6. [DOI] [PubMed]
- 8.Barzaghi N, Locatelli A, Ranucci M. Ventricular assist device implantation and the risk for heparin-induced thrombocytopenia. Ann Thorac Surg 2008;85(1):360–1. [DOI] [PubMed]
- 9.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med 2001; 345(20):1435–43. [DOI] [PubMed]
- 10.Morgan JA, Weinberg AD, Hollingsworth KW, Flannery MR, Oz MC, Naka Y. Effect of gender on bridging to transplantation and posttransplantation survival in patients with left ventricular assist devices. J Thorac Cardiovasc Surg 2004; 127(4):1193–5. [DOI] [PubMed]
- 11.Butler J, Howser R, Portner PM, Pierson RN 3rd. Body mass index and outcomes after left ventricular assist device placement. Ann Thorac Surg 2005;79(1):66–73. [DOI] [PubMed]
- 12.Fox AA, Nussmeier NA. Does gender influence the likelihood or types of complications following cardiac surgery? Semin Cardiothorac Vasc Anesth 2004;8(4):283–95. [DOI] [PubMed]
- 13.El-Banayosy A, Arusoglu L, Kizner L, Tenderich G, Boethig D, Minami K, Korfer R. Predictors of survival in patients bridged to transplantation with the Thoratec VAD device: a single-center retrospective study on more than 100 patients. J Heart Lung Transplant 2000;19(10):964–8. [DOI] [PubMed]
- 14.Magliato KE, Kleisli T, Soukiasian HJ, Tabrizi R, Coleman B, Hickey A, et al. Biventricular support in patients with profound cardiogenic shock: a single center experience. ASAIO J 2003;49(4):475–9. [PubMed]
- 15.Tsukui H, Teuteberg JJ, Murali S, McNamara DM, Buchanan JR, Winowich S, et al. Biventricular assist device utilization for patients with morbid congestive heart failure: a justifiable strategy. Circulation 2005;112(9 Suppl):I65–72. [DOI] [PubMed]
- 16.Cohen AJ, Katz MG, Frenkel G, Medalion B, Geva D, Schachner A. Morbid results of prolonged intubation after coronary artery bypass surgery. Chest 2000;118(6):1724–31. [DOI] [PubMed]
- 17.Kollef MH, Wragge T, Pasque C. Determinants of mortality and multiorgan dysfunction in cardiac surgery patients requiring prolonged mechanical ventilation. Chest 1995;107 (5):1395–401. [DOI] [PubMed]
- 18.Filsoufi F, Rahmanian PB, Castillo JG, Chikwe J, Adams DH. Predictors and early and late outcomes of respiratory failure in contemporary cardiac surgery. Chest 2008;133(3):713–21. [DOI] [PubMed]