Abstract
Hydropneumopericardium is a very rare complication of long-standing paraesophageal hernia, occurring as a result of rupture of the intrathoracic gastric volvulus into the pericardium. A chronic paraesophageal hernia that is complicated by gastric volvulus can develop into such surgical emergencies as acute gastric obstruction, strangulation, perforation, and rupture into adjacent structures. Subsequent hydropneumopericardium constitutes an acute emergency that requires immediate surgical treatment and pericardial drainage. Herein, we discuss what we believe to be the 1st reported case of hydropneumopericardium that presented as an acute coronary syndrome in a patient who had a chronic paraesophageal hernia (as a result of rupture of the gastric volvulus into the pericardium). The 80-year-old patient did not survive the condition.
Key words: Aged, 80 and over; diagnosis, differential; hernia, paraesophageal; pneumopericardium/complications/diagnosis/etiology/mortality/physiopathology/surgery/therapy; stomach volvulus/complications
Hydropneumopericardium is a very rare complication of long-standing paraesophageal hernia, occurring as a result of rupture of the intrathoracic gastric volvulus into the pericardium. A chronic paraesophageal hernia can be complicated by gastric volvulus and can present as various surgical emergencies, including acute gastric obstruction, strangulation, perforation, and rupture into adjacent structures.1,2 Subsequent hydropneumopericardium constitutes an acute emergency that requires immediate surgical treatment and pericardial drainage. The mortality rate of this condition has been reported to be as high as 58%.3 Here, we present the case of an 80-year-old woman whose hydropneumopericardium presented as an acute coronary syndrome (ACS).
Case Report
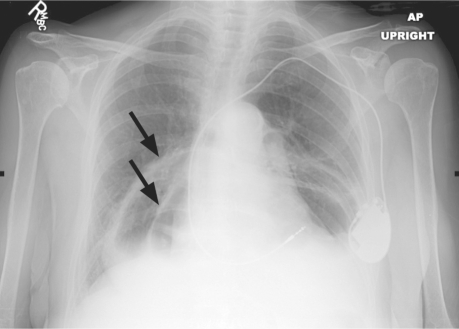
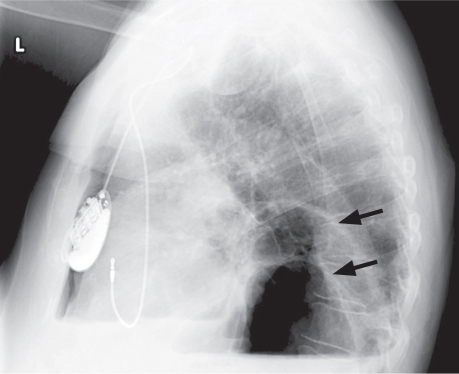
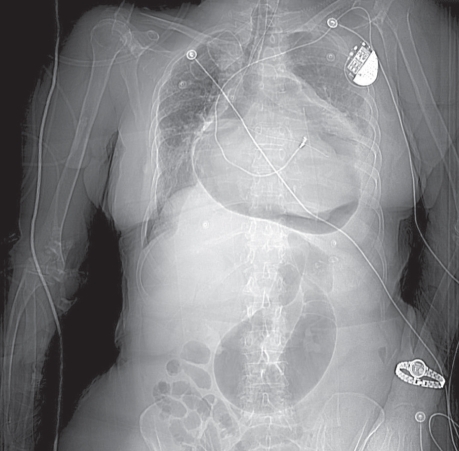
In April 2007, an 80-year-old woman presented at the emergency room with a 3-day duration of dyspnea and left-shoulder pain. She had a history of ischemic cardiomyopathy (left ventricular ejection fraction, 0.40), hypertension, chronic obstructive pulmonary disease, and atrial fibrillation treated by atrioventricular-node ablation and a permanent pacemaker. Physical examination revealed crackles in both lung bases and an oxygen saturation of 88% on room air. Chest radiographs taken upon her hospital admission showed 2 air–fluid levels within the mediastinum (Figs. 1 and 2). The more posterior air–fluid level was likely related to hiatal hernia; the cause of the anterior occurrence was not clear.
Fig. 1 Chest radiograph (anteroposterior view) shows a large paraesophageal hernia with 2 air-fluid levels (arrows).
Fig. 2 Chest radiograph (lateral view) shows 2 air-fluid levels and a large paraesophageal hernia (arrows).
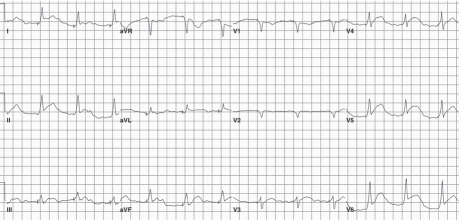
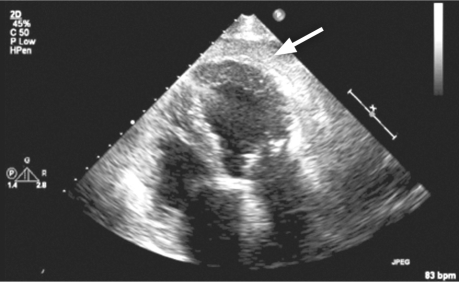
Fifteen hours after presentation, the patient experienced mild chest discomfort and suddenly became hypotensive (systolic blood pressure, 70 mmHg). Electrocardiography showed ST-segment elevation in the inferolateral leads (Fig. 3). Our cardiology team was urgently consulted. Bedside transthoracic echocardiography showed normal left ventricular systolic function and a hypokinetic inferior septum; right ventricular systolic function was normal. An echodense rim with a bright acoustic shadow, measuring 1.3 cm in diastole, surrounded the apical portion of the heart (Fig. 4). The patient's cardiac biomarkers peaked at a creatine kinase level of 104 IU/L and a troponin I level of 0.11 ng/mL. Emergency left-heart catheterization showed normal coronary arteries, and right-heart catheterization revealed low right-sided pressures (right atrium, 3/1 mmHg; right ventricle, 9/2 mmHg; pulmonary artery pressure, 11/6 mmHg; and mean pulmonary capillary wedge pressure, 4 mmHg). The possibility of extrinsic compression of the pericardium was considered, and a computed tomographic scan of the chest was ordered. The surgical team was urgently consulted, and contrast material was administered through the patient's nasogastric tube in order to rule out a fistulous connection. The contrast administration seemed to worsen the patient's breathing; her oxygen saturation dropped profoundly, and the nasogastric tube was connected to low wall suction.
Fig. 3 Electrocardiogram shows ST-segment elevation in the inferolateral leads.
Fig. 4 Transthoracic echocardiography (spontaneous contrast) shows an echodense rim with a bright acoustic shadow around the apical portion of the heart (arrow).
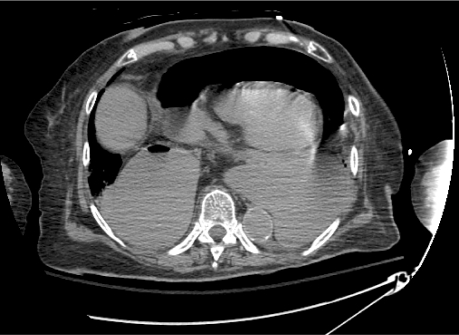
Computed tomography of the chest showed a large paraesophageal gastric hernia with volvulus and gastric infarction (Figs. 5 and 6). Intramural air was seen in the anterior aspect of the stomach. A large hydropneumopericardium was identified; it presumably originated from the dissection of the infarcted stomach into the pericardial sac. The tension of the cephalad herniation of the stomach caused traction on the duodenum, a short-loop small-bowel obstruction, and cephalization of the pancreas.
Fig. 5 Computed tomography of the chest shows a large paraesophageal gastric hernia with ruptured volvulus and gastric infarction, causing hydropneumopericardium and compression on the cardiac chambers.
Fig. 6 Computed tomography (coronal view) shows an air-filled pericardial cavity due to the rupture of an intrathoracic gastric volvulus into the pericardial cavity.
The patient experienced further oxygen desaturation and required intubation. The cardiothoracic team believed that the risk of operative death was high and that she had a poor prognosis in view of her multiple medical problems. The family chose palliative care, and the patient died 7 hours later.
Discussion
Paraesophageal hernia is a very common condition that is associated with an abnormal laxity of structures that normally prevent the displacement of the stomach. Because the stomach is fixed at the gastroesophageal junction, a herniated stomach tends to rotate around its longitudinal axis, resulting in an organoaxial volvulus. The course of paraesophageal hernia is progressive enlargement. Massive paraesophageal hernia with gastric volvulus1,2 is a serious condition that can result in strangulation and rupture into adjacent structures, leading to potentially fatal complications. Generally, this scenario is a late sequela of a long-standing paraesophageal hernia. We discovered that our patient had had this hernia (unreported upon this admission) for at least 10 years.
Bricketeau4 first described pneumopericardium in 1844. The causes of pneumopericardium can be divided into 5 broad categories: trauma, development secondary to procedures, fistulization from adjacent structures, barotraumas, and pericardial infections. The most common cause is trauma after penetrating or blunt chest injury.5 Pneumopericardium can be caused by invasive procedures, such as thoracotomy, tracheostomy, endotracheal intubation, pericardiectomy, esophagostomy, endoscopic perforation of the colon, coronary artery bypass grafting,6 pneumonectomy,7 and pneumoencephalography. Fistula into the pericardium from cancer of the esophagus, stomach, or bronchus, from an esophageal or gastric ulcer,8 from a lung abscess, and from pulmonary aspergillosis has been reported. Barotrauma is usually secondary to invasive and noninvasive positive-pressure ventilation and is most frequently seen in neonates. Pneumopericardium has also been reported in association with severe asthma, prolonged labor, cocaine inhalation,9 severe coughing, the Heimlich maneuver, and the Valsalva maneuver. It is postulated that a supra-atmospheric rise in airway pressure causes alveolar rupture, dissection leading to diversion of air to the pericardial reflection on the pulmonary vessels, and air entering the pericardium at this site. Rarely, pericardial infections causing purulent pericarditis, infections due to Histoplasma capsulatum, and gas-forming organisms have been reported to cause pneumopericardium.
The clinical manifestations, which are varied and which relate to the underlying causes, include chest pain, shoulder pain secondary to pericardial irritation (as in our patient), syncope, and breathlessness—and hemodynamic instability when cardiac tamponade occurs. Two distinctive clinical signs are associated with pneumopericardium. Bricketeau first described the splashing “mill-wheel” murmur or “bruit de moulin” in hydropneumopericardium. The 2nd sign is the presence of shifting tympany when the precordium of the patient is percussed in the recumbent and upright positions.10
Plain chest-radiographic findings of small pneumopericardium and pneumomediastinum can be similar; however, computed tomographic scanning helps to differentiate these conditions, because it shows pericardial air. In addition, such scanning may provide diagnostic clues regarding the ultimate origin of the pneumopericardium. A contrast swallow, although not without risk, has the potential to reveal an esophagopericardial fistula. In this context, the sensitivity of a barium swallow is reportedly 69% to 80%. Unfortunately, a negative result cannot completely exclude the diagnosis of fistula.
The reported mortality rate of pneumopericardium is high. A 57% all-cause mortality rate was noted in one of the largest collective reviews.3 A 72% mortality rate was noted in patients who experienced associated tamponade.3 In the absence of tamponade, treatment of the condition generally targets the specific cause. If signs of tamponade develop, urgent pericardiocentesis is required, and a pericardial catheter should be left in place in order to prevent the development of further tension. A cardiothoracic opinion should be sought in order to evaluate whether correction of the cause is indicated.
Typically, surgical treatment for paraesophageal hernia is reserved for patients who experience complications from associated gastroesophageal reflux disease, persistent symptoms despite medical treatment, or medication intolerance. Early operative repair of paraesophageal hernia should be considered in all eligible patients regardless of the presence of symptoms, because the operative mortality rate for emergent repairs approaches 50%, as opposed to less than 1% for elective repair.11
Although there have been several reports of gastric volvulus complicating paraesophageal hernia12 and also presenting as an ACS,13 we believe that ours is the 1st reported case of hydropneumopericardium that presented as an ACS in a patient who was known to have paraesophageal hernia (as a result of rupture of the gastric volvulus into the pericardium).
Conclusion
Gastric volvulus with hydropneumopericardium should be considered in patients with chronic paraesophageal hernia who present with characteristics that suggest ACS despite normal coronary arteries. Urgent imaging tests should be performed, because timely diagnosis and treatment of this life-threatening condition are important.
Footnotes
Address for reprints: Venkatesan Vidi, MD, Lahey Clinic Medical Center, 41 Mall Rd., Burlington, MA 01805. E-mail: Venki.Vidi@gmail.com
References
- 1.Beardsley JM, Thompson WR. Acutely obstructed hiatal hernia. Ann Surg 1964;159:49–62. [DOI] [PMC free article] [PubMed]
- 2.Brindley GV Jr. Complications of diaphragmatic hernia. AMA Arch Surg 1960;81(4):582–90.
- 3.Cummings RG, Wesly RL, Adams DH, Lowe JE. Pneumopericardium resulting in cardiac tamponade. Ann Thorac Surg 1984;37(6):511–8. [DOI] [PubMed]
- 4.Bricketeau M. Observation d'hydropneumopericarde accompagne d'un bruit de fluctuation perceptible a l'oreille [in French]. Arch Gen Med 1844;4:334–9.
- 5.Ladurner R, Qvick LM, Hohenbleicher F, Hallfeldt KK, Mutschler W, Mussack T. Pneumopericardium in blunt chest trauma after high-speed motor vehicle accidents. Am J Emerg Med 2005;23(1):83–6. [DOI] [PubMed]
- 6.Benedik J, Uchytil B, Cernosek J. Pneumopericardial tamponade after coronary artery bypass operation. Eur J Cardiothorac Surg 2002;21(3):585–6. [DOI] [PubMed]
- 7.Blum MG, Sundaresan RS. Giant hiatal hernia with gastric volvulus complicating pneumonectomy. Ann Thorac Surg 2006;81(4):1491–2. [DOI] [PubMed]
- 8.Gabor S, Woltsche M, Maier A, Smolle-Juttner FM. Pneumopericardium due to intrapericardial perforation of a gastric ulcer. Eur J Cardiothorac Surg 2003;23(1):131–3. [DOI] [PubMed]
- 9.Albrecht CA, Jafri A, Linville L, Anderson HV. Cocaine-induced pneumopericardium. Circulation 2000;102(22): 2792–4. [DOI] [PubMed]
- 10.James WB. Pneumopericardium. Am Med 1904;8:23–7.
- 11.Weiss CA 3rd, Stevens RM, Schwartz RW. Paraesophageal hernia: current diagnosis and treatment. Curr Surg 2002;59 (2):180–2. [DOI] [PubMed]
- 12.Sokol AB, Morgenstern L. Gastric volvulus complicating paraesophageal hiatal hernia. Calif Med 1972;117(1):66–9. [PMC free article] [PubMed]
- 13.Sivasankaran S, Kawamura A, Lombardi D, Nesto RW. Gastric volvulus presenting as an acute coronary syndrome. Tex Heart Inst J 2006;33(2):266–8. [PMC free article] [PubMed]