Abstract
Nitric oxide (NO•) competitively inhibits oxygen consumption by mitochondria at cytochrome c oxidase and S-nitrosates thiol proteins. We developed mitochondria-targeted S-nitrosothiols (MitoSNOs) that selectively modulate and protect mitochondrial function. The exemplar MitoSNO1, produced by covalently linking an S-nitrosothiol to the lipophilic triphenylphosphonium cation, was rapidly and extensively accumulated within mitochondria, driven by the membrane potential, where it generated NO• and S-nitrosated thiol proteins. MitoSNO1-induced NO• production reversibly inhibited respiration at cytochrome c oxidase and increased extracellular oxygen concentration under hypoxic conditions. MitoSNO1 also caused vasorelaxation due to its NO• generation. Infusion of MitoSNO1 during reperfusion was protective against heart ischemia-reperfusion injury, consistent with a functional modification of mitochondrial proteins, such as complex I, following S-nitrosation. These results support the idea that selectively targeting NO• donors to mitochondria is an effective strategy to reversibly modulate respiration and to protect mitochondria against ischemia-reperfusion injury.
Keywords: nitric oxide, S-nitrosation
There is considerable interest in the interaction of nitric oxide (NO•) with mitochondria as it can regulate, protect, or damage mitochondria, depending on the context (1). Physiological levels of NO• competitively inhibit mitochondrial oxygen (O2) consumption by cytochrome c oxidase (complex IV), thereby slowing respiration at low O2 concentrations (2). While the physiological significance of this is unclear, it may occur in vivo during hypoxia, enabling NO• to prevent anoxia and the activation of O2-sensitive transcription factors (3). The metabolism of NO• can also lead to the S-nitrosation of thiol proteins, thereby reversibly regulating their activity (1, 4). Although the mechanism of S-nitrosation in vivo is obscure (5), there is evidence for the S-nitrosation of mitochondrial proteins (1, 6–10). In particular, complex I, a major entry point for electrons into the respiratory chain, can be S-nitrosated (6–10), which may alter its activity and thereby protect against damage during ischemia-reperfusion injury (9, 10).
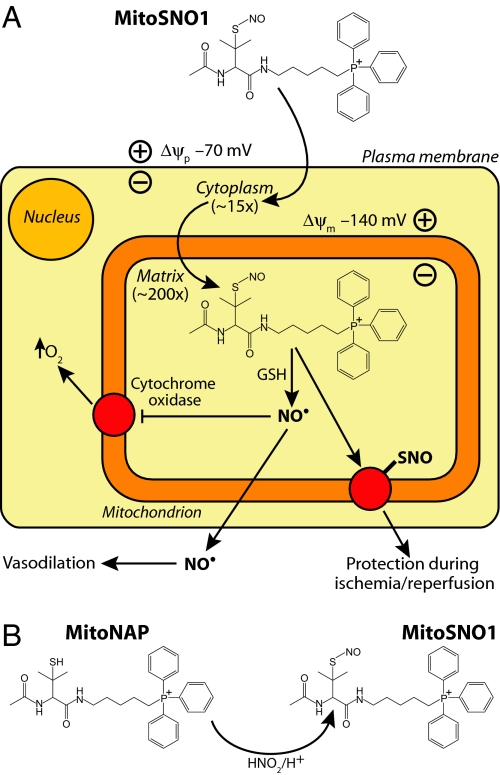
Here we report on a small molecule approach to selectively generate NO• and S-nitrosothiols within mitochondria using mitochondria-targeted S-nitrosothiols (MitoSNOs). S-Nitrosothiols (SNOs) release NO• slowly under the oxidizing conditions that prevail in the extracellular environment, but should release NO• rapidly within mitochondria, probably following transnitrosation of glutathione (GSH), although the detailed mechanism of NO• release is uncertain (5). In addition, SNOs readily transfer NO+ to transnitrosates protein thiols (7). To target SNOs to mitochondria, we attached a lipophilic triphenylphosphonium (TPP) cationic moiety, which has been used to target molecules to mitochondria both in vitro and in vivo (11). The delocalized positive charge and lipophilic surface enables TPP cations to cross biological membranes rapidly without a requirement for a carrier protein, and to accumulate several hundred-fold in energized mitochondria in vivo driven by the membrane potential (Δψ) (12). The anticipated possible modes of action of MitoSNOs are shown in Fig. 1A.
Fig. 1.
Rationale for developing mitochondria-targeted S-nitrosothiols (MitoSNOs). (A) The Nernst equation dictates that lipophilic TPP cations should be accumulated approximately 10-fold for every 60 mV of membrane potential (Δψ), with values of the plasma Δψ (≈70 mV) and mitochondrial Δψ (≈140 mV) for the heart leading to approximately 3,000-fold concentration of MitoSNO1 within mitochondria. In the oxidizing extracellular environment, MitoSNO1 should be relatively stable. In contrast, glutathione (GSH) within mitochondria should release NO• from MitoSNO1, mainly by by transnitrosation to GSNO followed by reduction to release NO• by mechanisms that are currently uncertain. The high concentration of NO• generated will compete with O2 for the active site of cytochrome c oxidase, particularly when the O2 concentration is low, and slow respiration while elevating the cytosolic concentration of O2. MitoSNO1 will also S-nitrosate mitochondrial thiol proteins by transferring a nitrosonium group (NO+) to protein thiolates, modifying their function and may lead to protection during ischemia-reperfusion injury. (B) The structure and synthesis of MitoSNO1 by the S-nitrosation of the free thiol precursor MitoNAP.
Results and Discussion
Synthesis and Stability of MitoSNO1.
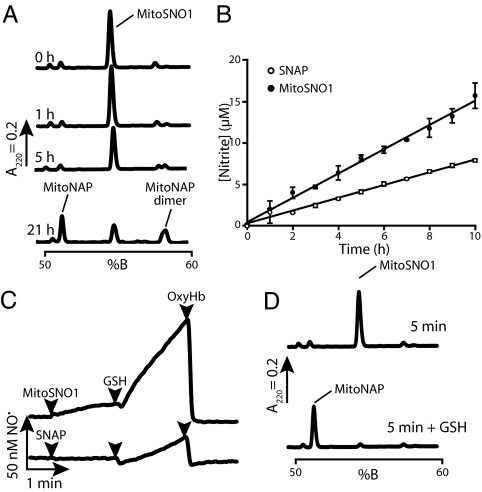
A mitochondria-targeted SNO (MitoSNO1) based on the stable S-nitroso-N-acetylpenicillamine (SNAP) was prepared by the S-nitrosation of the thiol precursor mito-N-acetylpenicillamine (MitoNAP) (Fig. 1B). As a mitochondria-specific NO• donor, MitoSNO1 should be relatively stable in aqueous solution but react rapidly with intramitochondrial thiols such as GSH to release NO• (Fig. 1A). Reverse-phase liquid chromatography (RP-HPLC) showed that there was negligible decay of MitoSNO1 after 1 h (Fig. 2A). After 21 h, MitoSNO1 had decayed by approximately 50 ± 11% (mean ± SEM, n = 4), giving 2 new peaks by RP-HPLC (Fig. 2A). Electrospray mass spectrometry (ESMS) showed that 1 product was a symmetric disulfide dimer of MitoNAP (Fig. S1), an expected product of SNO decomposition (5), and the other MitoNAP. The accumulation of nitrite from the breakdown of MitoSNO1 showed that MitoSNO1 decays about 2-fold faster than SNAP with half-lives of 23 ± 3 h and 44 ± 3 h (n = 2, mean ± range), respectively (Fig. 2B). Examination of solutions of MitoSNO1 or SNAP with an NO• electrode showed low levels of NO• (Fig. 2C), consistent with the stability evident in Fig. 2 A and B. Addition of GSH dramatically increased NO• formation from MitoSNO1 (Fig. 2C), probably by transnitrosation of GSH which then decays to release NO• (5). Confirmation that the electrode was detecting NO• was obtained by adding oxyhemoglobin (OxyHb) to scavenge NO• (Fig. 2C). RP-HPLC analysis showed that GSH rapidly converted MitoSNO1 to MitoNAP (Fig. 2D), presumably by transnitrosation of GSH and reduction of any MitoNAP dimer. These results demonstrate that MitoSNO1 is relatively stable in aqueous buffer, slowly decaying to a disulfide and NO•, but that it should rapidly react with GSH inside mitochondria, probably by transnitrosation, leading to NO• production, as outlined in Fig. 1A.
Fig. 2.
Stability of MitoSNO1 and its decomposition to produce NO•. (A) Stability of MitoSNO1 (5 μM) in KCl buffer measured by RP-HPLC. The identities of the RP-HPLC peaks for MitoNAP and the disulfide dimer of MitoNAP obtained at 21 h were confirmed by electrospray mass spectrometry (ESMS). (B) Stability of MitoSNO1 (50 μM) and SNAP (50 μM) in KCl buffer measured by nitrite accumulation. Data are means of triplicate determinations. (C) Release of NO• from MitoSNO1 (5 μM) or SNAP (5 μM) on reaction with GSH measured using an NO• electrode. Where indicated GSH (200 μM) or oxyhemoglobin (OxyHb, 5 μM) was added. (D) Reduction of MitoSNO1 (5 μM) to MitoNAP by GSH (500 μM) analyzed by RP-HPLC.
Membrane Potential-Dependent Mitochondrial Uptake of MitoSNO1 and NO• Release.
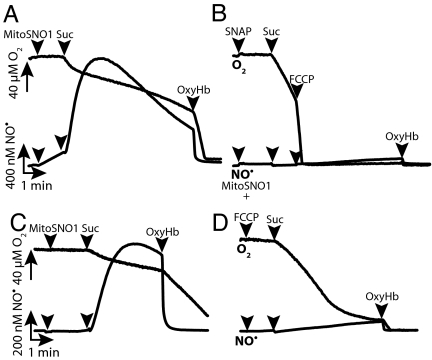
A significant Δψ-dependent uptake of MitoSNO1 into isolated mitochondria was observed (Fig. S2). Within mitochondria, MitoSNO1 was anticipated to interact with thiols such as GSH to release NO• that should then affect respiration by competitively inhibiting O2 consumption at cytochrome c oxidase. This was demonstrated by using NO• and O2 electrodes to measure respiration rate and NO• concentration simultaneously (Fig. 3). Mitochondria were incubated with the complex I inhibitor rotenone to prevent Δψ generation (Fig. 3A). Addition of the respiratory substrate succinate initiated O2 consumption and generated a Δψ, leading to a rapid increase in NO• production due to the accumulation of MitoSNO1 within mitochondria and its reaction with GSH (Fig. 3A). The respiration rate slowed as NO• accumulated, and this inhibition was reversed by degrading NO• with OxyHb, indicating that the respiratory inhibition was due to the reversible competition between NO• and O2 at cytochrome c oxidase (Fig. 3A). MitoNAP had no effect on respiration or the NO• electrode response. The amount of NO• generated by SNAP in the presence of energized mitochondria was negligible (Fig. 3B), presumably because it was not selectively taken up by mitochondria. To explore further the Δψ-dependence of NO• production by MitoSNO1, studies with decreased concentrations and fewer mitochondria were undertaken (Fig. 3 C and D). The initial production of NO• from MitoSNO1 was negligible, but subsequent mitochondrial energization with succinate led to a dramatic increase in NO• production (Fig. 3C). The build-up of NO• decreased respiration, and this was reversed by OxyHb (Fig. 3C). Inclusion of the uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) resulted in decreased NO• production as the Δψ was largely abolished, preventing the uptake of MitoSNO1 into mitochondria (Fig. 3D). The decreased accumulation of NO• in the presence of FCCP still inhibited respiration, but inhibition occurred at a lower O2 concentration (Fig. 3D). The metabolism of MitoSNO1 by mitochondria was investigated by analyzing the amounts of MitoSNO1 and MitoNAP in the supernatant by RP-HPLC and showed that energized mitochondria rapidly converted MitoSNO1 to MitoNAP within 2–3 min. Therefore MitoSNO1 is a Δψ-dependent source of intramitochondrial NO• that can reversibly modulate mitochondrial O2 consumption, particularly at low O2 concentrations.
Fig. 3.
Membrane potential-dependent MitoSNO1 mitochondrial uptake and NO• generation. (A–D) Mitochondria were incubated with rotenone and electrodes were used to measure the concentrations of O2 and NO• simultaneously. Where indicated succinate (10 mM), OxyHb (5 μM), or FCCP (500 nM) were added. (A and B) Two milligrams mitochondrial protein/mL and 20 μM MitoSNO1 (A) or SNAP (B) were used. (C and D) One-half milligram mitochondrial protein/mL and 5 μM MitoSNO1 were used.
Uptake of MitoSNO1 by Cells Leads to NO• Generation and Respiratory Inhibition.
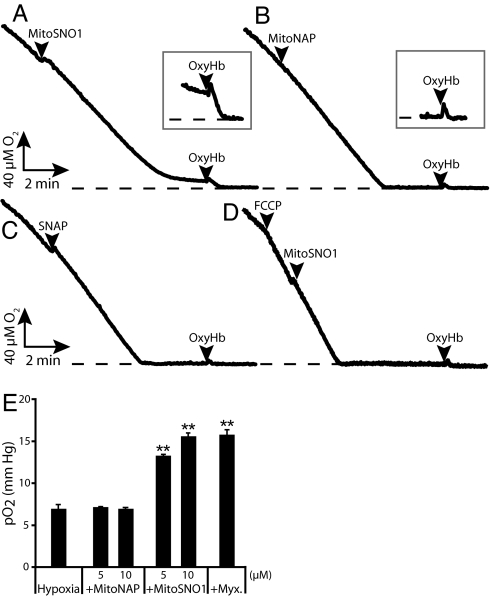
The effect of MitoSNO1 on respiration by Jurkat cells was measured using an O2 electrode (Fig. 4). There was no initial effect on respiration rate after addition of MitoSNO1, but as the cells consumed more O2, the respiration rate decreased and approached zero even though there was still O2 remaining (Fig. 4A). This inhibition was reversible and was due to NO•, as degradation with OxyHb restored the maximal respiration rate and allowed the O2 concentration to reduce to zero (Fig. 4A). At low O2 concentrations, the NO• generated by MitoSNO1 prevented mitochondrial oxygen consumption almost entirely and maintained a low O2 concentration. This is illustrated in the expanded O2 electrode trace in Fig. 4A, which shows that addition of OxyHb restores respiration by degrading NO•. In contrast, neither MitoNAP (Fig. 4B) nor SNAP (Fig. 4C) affected respiration. Addition of the uncoupler FCCP before MitoSNO1 prevented the inhibition of respiration (Fig. 4D). The effective Km of cytochrome c oxidase for O2 is very low (< 1 μM) (13), illustrated by the sharp transition from maximal O2 consumption to zero respiration in Fig. 4B. This contrasts with the situation in the presence of MitoSNO1 (Fig. 4A) where there is a gradual decline in O2 consumption rate. These findings are consistent with the Δψ-dependent uptake of MitoSNO1 into mitochondria within cells facilitating NO• release, which slows respiration through the competitive inhibition between NO• and O2 at cytochrome c oxidase and becomes more effective as the O2 concentration decreases. We next determined whether the reversible inhibition of cytochrome c oxidase by NO• from MitoSNO1 could increase O2 bioavailability during hypoxic conditions. HeLa cells were maintained at 1% O2 for 60 min and then the effects of a further 30-min incubation with MitoSNO1, MitoNAP, or the mitochondrial inhibitor myxathiazol on extracellular pO2 was assessed by fluorescence quenching oximetry (Fig. 4E). MitoSNO1 and myxathiazol both increased extracellular O2 during hypoxia compared with controls, while MitoNAP had no effect (Fig. 4E). This demonstrates that NO• production by MitoSNO1 within the mitochondria of hypoxic cells can slow respiration sufficiently to modulate cytosolic O2 concentration.
Fig. 4.
Effect of MitoSNO1 on respiration in cells. (A–D) Jurkat cells were incubated with 5 μM MitoSNO1 (A), MitoNAP (B), SNAP (C), or MitoSNO1 and FCCP (500 nM) (D). Where indicated 5 μM OxyHb was added. Sections of the O2 electrode trace where OxyHb was added have been expanded 4-fold in the vertical dimension. The dashed line indicates anaerobiosis. (E) Effect of MitoSNO1 on O2 bioavailability in hypoxia. HeLa cells were incubated at 1% O2 concentration with 5% CO2 and the balance N2 for 60 min before the indicated treatments. Cellular pO2 values, measured by fluorescence quenching oximetry and expressed as mean ± SD, are shown. (**, P < 0.001 compared to hypoxia alone and to MitoNAP treatments by ANOVA).
S-Nitrosation of Mitochondrial Protein Thiols by MitoSNO1.
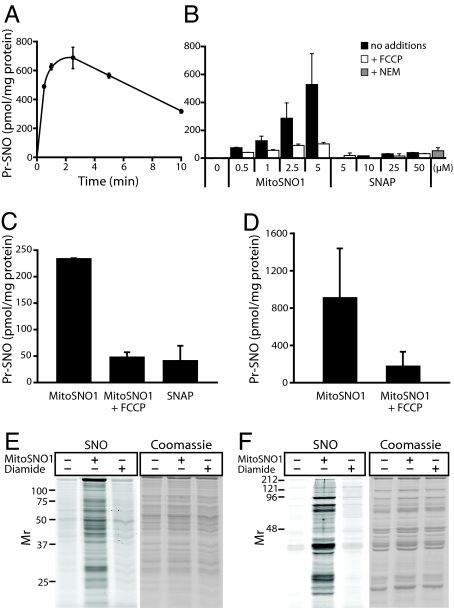
To evaluate the S-nitrosation of mitochondrial thiol proteins by MitoSNO1 (Fig. 1A), MitoSNO1 was incubated with isolated mitochondria, and a sensitive chemiluminescence assay was used to measure SNOs (14). S-nitrosation of mitochondrial proteins by MitoSNO1 was found to peak over 2–3 min and then gradually decline (Fig. 5A), consistent with the metabolism of MitoSNO1 seen in Fig. 3. Incubation of mitochondria with MitoSNO1 for 2.5 min showed extensive, concentration-dependent S-nitrosation of proteins that was largely blocked by abolishing the Δψ with the uncoupler FCCP (Fig. 5B). In contrast, SNAP, even at 50 μM, led to negligible S-nitrosation (Fig. 5B). Blocking protein thiols with the alkylating agent N-ethyl maleimide (NEM) prevented S-nitrosation by MitoSNO1 (Fig. 5B). Incubations with MitoSNO1 in the absence of free NO•, achieved by excess OxyHb, still led to extensive mitochondrial S-nitrosation (Fig. S3). Incubation of mitochondria with 500 μM of 3,3-bis(aminoethyl)-1-hydroxy-2-oxo-1-triazene (Deta-NONOate), which generates a 5- to 10-fold greater NO• concentration than 5 μM MitoSNO1 (Fig. S4A), but does not transnitrosate thiols (7), gave approximately 20 pmol SNO/mg protein, around 30-fold less than that caused by 5 μM MitoSNO1 (Fig. S4B). Thus the contribution of free NO• to S-nitrosation by MitoSNO1 is minor. Therefore MitoSNO1 leads to the extensive S-nitrosation of mitochondrial thiol proteins following its Δψ-dependent uptake into the matrix, and this S-nitrosation is predominantly due to the direct transfer of NO+ from MitoSNO1 to thiolates within the matrix.
Fig. 5.
S-nitrosation of mitochondrial thiols by MitoSNO1. (A) Time course of S-nitrosation of mitochondrial proteins by MitoSNO1. Mitochondria were incubated with rotenone and succinate in the presence of 5 μM MitoSNO1. Data are means ± range of duplicate samples. (B) S-nitrosation of mitochondrial proteins by different concentrations of MitoSNO1 and SNAP. Mitochondria were incubated withMitoSNO1 or SNAP for 2.5 min ± 500 nM FCCP. For the +NEM sample, 10 mM NEM was added followed 1 min later by 5 μM MitoSNO1 and then incubated for a further 2.5 min. Data are means ± range of duplicate samples. (C) S-nitrosation of cellular protein thiols by MitoSNO1. C2C12 cells were preincubated for 5 min with either 20 μM FCCP or DMSO carrier, then 5 μM MitoSNO1 or SNAP was added and incubated for 5 min. Data are means ± range of duplicate determinations. (D) S-nitrosation of a mitochondria-enriched cell subfraction. C2C12 cells were incubated as in (C), and the cells were then processed to isolate a mitochondria-enriched fraction. Data are mean ± SEM of triplicate determinations, or mean ± range of duplicate determinations (+FCCP). (E and F) Visualisation of S-nitrosated mitochondrial proteins. Liver (E) or heart (F) mitochondria were incubated with rotenone and succinate in the presence of no additions, 10 μM MitoSNO1 or 500 μM diamide for 5 min. S-nitrosated thiol proteins were then selectively tagged with Cy3 maleimide and separated by SDS PAGE gel. The Cy3 fluorescence (SNO) and coomassie staining are shown.
Incubation of C2C12 cells with MitoSNO1 led to protein S-nitrosation that peaked after 5–10 min (Fig. S5). The extent of S-nitrosation by MitoSNO1 after 5 min was considerably greater than that by SNAP and was decreased by the uncoupler FCCP (Fig. 5C). When a mitochondria-enriched fraction was isolated from cells that had been incubated with MitoSNO1 the mitochondrial proteins were extensively S-nitrosated and this was decreased by abolishing the Δψ with FCCP (Fig. 5D). These data demonstrate the accumulation of MitoSNO1 within mitochondria inside cells where it selectively S-nitrosates thiol proteins.
The interaction of MitoSNO1 with mitochondrial thiol proteins may lead to other modifications, such as disulfide formation, glutathionylation, sulfenylation, or sulfenylamide formation, in addition to persistent S-nitrosation (7). To assess these, the content of reactive, solvent-exposed thiols on native mitochondrial proteins was measured. Under control conditions, there were 43.2 ± 3.8 nmol thiols/mg protein, and after incubation with 5 μM MitoSNO1 for 5 min, this did not change significantly (46.7 ± 9.6; means ± SEM, n = 3). This is consistent with the finding that the extent of mitochondrial protein S-nitrosation by 5 μM MitoSNO1 (≈500 pmol SNO/mg protein; Fig. 5) was only approximately 1% of the total protein thiols available. Incubation of mitochondria with 10 μM MitoSNO1 for 10 min showed no loss of GSH or accumulation of GSSG. While there was a small amount of MitoSNO1 binding to thiol proteins through disulfide bonds (Fig. S6), protein-glutathione mixed disulfides in mitochondria incubated with MitoSNO1 were not detectable (Fig. S7). To visualize S-nitrosated mitochondrial proteins, free thiols are first alkylated, then S-nitrosothiols are selectively degraded with ascorbate and copper to generate an exposed thiol that is labeled with a fluorescently-tagged Cy3 maleimide (15). This technique showed that exposure of liver (Fig. 5E) or heart (Fig. 5F) mitochondria to MitoSNO1 led to the S-nitrosation of a large number of different proteins. The thiol oxidant diamide did not label proteins, indicating that this technique is selective for SNOs over other thiol modifications. To summarize, MitoSNO1 does not lead to bulk changes in free protein thiol content or the GSH pool, but instead causes the S-nitrosation of a small proportion of mitochondrial protein thiols that persists in the presence of a reduced mitochondrial glutathione pool, indicating that the local environments of the particular cysteine residues affected may help stabilize these S-nitrosothiols (4).
Effect of MitoSNO1 on Complex I Activity and S-nitrosation.
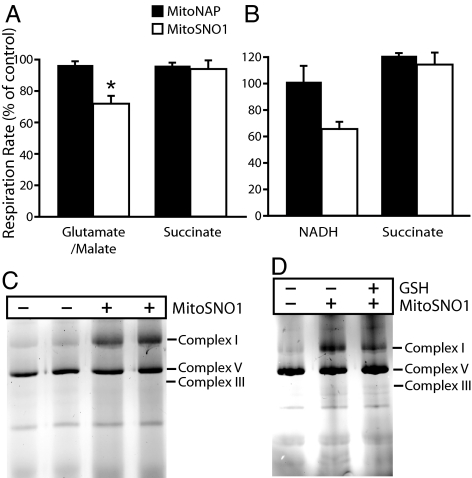
Complex I, the major entry point for electrons into the respiratory chain, can be S-nitrosated, and this correlates with potentially important alterations to its activity (6–10). MitoSNO1 inhibited respiration by about 30% on the complex I-linked substrates glutamate/malate, but had little effect on respiration with the complex II substrate succinate (Fig. 6A). As this inhibition occurred in the presence of high O2 concentrations, did not vary with the O2 concentration, and did not affect respiration on succinate, it was not due to NO• inhibition at cytochrome c oxidase (Fig. 3). MitoSNO1 is either inhibiting NADH oxidation by complex I or affecting NADH supply. To distinguish between these possibilities, we investigated respiration by mitochondrial membranes that directly oxidize both succinate and NADH (Fig. 6B). Results showed that MitoSNO1 inhibited NADH respiration by about 35% while succinate was unaffected (Fig. 6B), strongly pointing to a specific effect of MitoSNO1 on complex I.
Fig. 6.
Inhibition and S-nitrosation of complex I by MitoSNO1. (A) Effect of MitoSNO1 on mitochondrial respiration. Heart mitochondria were incubated in an oxygen electrode with 5 mM glutamate and 5 mM malate, or succinate, and incubated with 10 μM MitoSNO1, 10 μM MitoNAP or carrier for 2–3 min, then 250 μM adenosine diphosphate (ADP) was added and the rate of respiration measured. Respiration +MitoSNO1 is expressed as a percentage of that with MitoNAP, and are means ± SEM from 3 mitochondrial preparations; *P < 0.05 by Student's paired t test. (B) Effect of MitoSNO1 on respiration by mitochondrial membranes. Bovine heart mitochondrial membranes were incubated in an O2 electrode with 75 μM MitoSNO1 or MitoNAP, or ethanol carrier for 5 min ± rotenone, then 1 mM NADH or 10 mM succinate was added and respiration measured. Data are expressed as respiration as a percentage of the appropriate controls and are means ± range of 2 separate experiments. (C) S-nitrosation of complex I by MitoSNO1. Bovine heart mitochondrial membranes were incubated with 75 μM MitoSNO1 or carrier for 5 min. Then protein SNOs were labeled by maleimide-Cy3 and respiratory complexes were separated by BN-PAGE and scanned for Cy3 fluorescence. The locations of respiratory complexes I, III, and V were determined by immunoblotting. (D) Effect of GSH on S-nitrosation of complex I by MitoSNO1. Bovine heart mitochondrial membranes were incubated and processed as in (C) except that after incubation ± MitoSNO1 the membranes were incubated ± 1 mM GSH for 15 min.
As the inhibition of complex I by MitoSNO1 in mitochondria occurred in the presence of a reduced GSH pool, we assessed the effect of GSH on complex I inhibition and S-nitrosation by MitoSNO1. Incubating MitoSNO1-treated membranes with 1 mM GSH for 10–15 min did not reverse the inhibition of NADH respiration. Incubation of membranes with MitoSNO1 under the conditions described in Fig. 6B led to the formation of 4.8 ± 0.9 nmol SNOs/mg protein (mean ± SD, n = 4), and GSH treatment only decreased the SNO content by approximately 50%. To confirm that complex I was S-nitrosated, we incubated membranes with MitoSNO1 for 5 min and then selectively tagged S-nitrosothiols with Cy3-maleimide (15). Complex I was then isolated by blue native polyacrylamide gel electrophoresis (BN-PAGE) and showed that complex I was S-nitrosated (Fig. 6C). Incubation of membranes with 1 mM GSH for 15 min did not reverse this complex I S-nitrosation (Fig. 6D). Thus, the inhibition of complex I activity on MitoSNO1 treatment may result from S-nitrosation that is relatively resistant to reversal by GSH. Therefore, MitoSNO1 persistently S-nitrosates complex I, and the continued inhibition of complex I by MitoSNO1, even in the presence of GSH, correlates with its S-nitrosation.
Vasorelaxation of Blood Vessels by MitoSNO1.
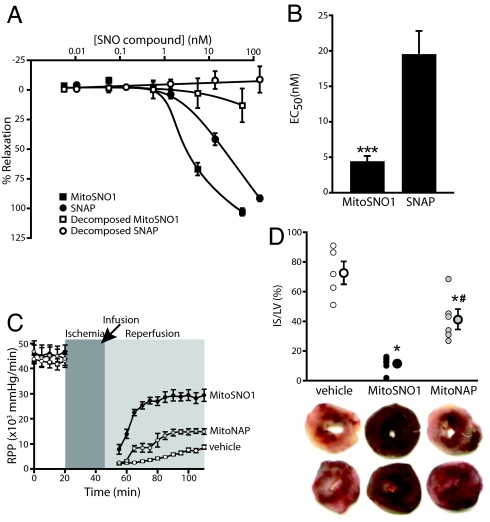
The uptake of MitoSNO1 into mitochondria within vascular tissue and the subsequent NO• release may make MitoSNO1 a potent vasodilator (Fig. 1A). This was evaluated by determining whether MitoSNO1 could relax the smooth muscle in blood vessel walls. Sections of rat thoracic aorta in a myograph were precontracted, and then cumulative amounts of MitoSNO1 were added (Fig. 7A). This showed that MitoSNO1 was a vasodilator, and that pretreating MitoSNO1 to degrade its SNO moiety rendered it ineffective (Fig. 7A). In this assay, MitoSNO1 was a more potent vasodilator than SNAP (Fig. 7B). MitoSNO1 was also an effective vasodilator in endothelium-denuded rat resistance mesenteric arteries (Fig. S8). These data demonstrate MitoSNO1 is an effective endothelium-independent vasodilator acting via NO• release within tissues. As TPP compounds are rapidly taken up from the circulation by the heart following i.v. injection, this may indicate more rapid ways of targeting pathologies such as angina, while also avoiding the problem of nitrate tolerance that impedes the use of nitroglycerin.
Fig. 7.
Vasodilatory and tissue protective effects of MitoSNO1. (A) Dose-response curves for the effect of MitoSNO1 and related compounds on blood vessel relaxation. Data are means ± SEM, n = 8 (MitoSNO1) or n = 4 (decomposed MitoSNO1 and SNAP). (B) EC50 for the effect of MitoSNO1 and SNAP on blood vessel relaxation from (A) above. ***, P < 0.001 by Student's unpaired t test. (C and D) MitoSNO1 protects against I/R injury in Langendorff-perfused mouse hearts. Hearts were subjected to 25 min of ischemia followed by 1 h of reperfusion. MitoSNO1 or MitoNAP (100 nM final), or vehicle carrier, was added during reperfusion. Hearts were then stained with 2,3,5-triphenyltetrazolium chloride (TTC) to visualize infarct. (C) Protection of heart function by MitoSNO1 during I/R injury. Data show rate pressure product (RRP). The arrow indicates where infusion with MitoSNO1 or MitoNAP was initiated. Data are means ± SEM, n = 6–7. (D) Decreased cardiac infarct size following infusion with MitoSNO1. Upper panel shows typical heart staining. Pale white staining is necrotic infarct, while live tissue stains deep red. Lower panel shows quantitation of infarct size versus area at risk. Data are means ± SEM, n = 6–7. *, P < 0.05 versus vehicle control group; #, P < 0.05 versus vehicle control group (ANOVA).
MitoSNO1 Protects Against Cardiac Ischemia-reperfusion Injury.
There is considerable evidence pointing to mitochondrial damage during the reperfusion phase of cardiac ischemia-reperfusion (I/R) injury (6, 9, 10). This damage can be decreased by ischemic preconditioning (IPC), whereby previous exposure to short periods of I/R protects against subsequent I/R injury (6, 9, 10). While the nature of the protection afforded by IPC remains obscure, mitochondrial NO• and NO2− metabolism may play a role, perhaps through the S-nitrosation of mitochondrial proteins, particularly complex I, protecting mitochondrial function on reperfusion (9, 10, 16). As MitoSNO1 is rapidly taken up by mitochondria within cells where it S-nitrosates proteins, including complex I (Figs. 5 and 6), it may also protect heart mitochondria from I/R damage. When mouse hearts were Langendorff perfused and subjected to 25 min of global normothermic ischemia followed by 1 h of normoxic reperfusion, there was extensive damage, as indicated by the decrease in function (rate pressure product, RPP) (Fig. 7C) and by the large infarcted area (Fig. 7D). When MitoSNO1 was perfused into the heart during reperfusion, there was significantly less damage, as indicated by the better recovery of function (Fig. 7C) and the decrease in infarct size (Fig. 7D). Infusion of MitoNAP during the reperfusion phase was far less protective than MitoSNO1 (Fig. 7 C and D). A small degree of protection was also observed when MitoSNO1 was delivered before the I/R injury, similar to that observed with the parent compound MitoNAP (Fig. S9). The rapid metabolism of MitoSNO1 in cells may be such that pretreatment does not induce S-nitrosation at the critical time point of reperfusion, and the mild protection seen during pretreatment is therefore likely to be due to residual antioxidant activity of the N-acetylpenicillamine function on MitoNAP. These findings support the S-nitrosation of mitochondrial proteins as a therapeutic strategy in minimizing I/R injury and is also consistent with the S-nitrosation of mitochondrial proteins such as complex I contributing to both IPC and to the protection of mitochondrial function by NO• and NO2− during I/R injury.
Conclusion
Here we made a mitochondria-targeted NO• donor and S-nitrosating agent by conjugating an SNO to a lipophilic TPP cation. This compound, MitoSNO1, is rapidly accumulated within energized mitochondria in cells and tissues driven by the Δψ. One MitoSNO has been explored in detail and future work will be aimed at modifying the structure around the SN rationally to change the rate of NO• release and to alter the relative extent of NO• formation and S-nitrosation within mitochondria. The NO• released from MitoSNO1 reversibly inhibits respiration at cytochrome c oxidase at low O2 concentrations and should prove useful when the intention is to manipulate the local O2 concentration, the redox state of the mitochondrial respiratory chain or the Δψ. MitoSNO1 was shown to rapidly S-nitrosate mitochondrial proteins, and future work will relate to the proteins and the cysteine residues affected, helping to unravel the physiological significance of mitochondrial S-nitrosation. A particularly important finding was that MitoSNO1 protected against heart I/R injury. This protection is seen as likely to be a consequence of the persistent S-nitrosation of complex I and other mitochondrial proteins, a modification that is also seen during IPC and during cardioprotection by NO2− (9, 10, 16). The mechanism by which mitochondrial S-nitrosation is protective during I/R is not known, but MitoSNO1 will be a useful tool in elucidating this point. The fact that MitoSNO1 was most protective when administered during reperfusion is particularly significant, as most cardioprotective agents have to be administered before I/R injury. This finding raises the possibility of mitochondria-targeted NO• donors being used as therapies for myocardial infarction, where these compounds would have to be administered iv after the ischemic event had occurred. The feasibility of this is supported by the fact that TPP compounds are very rapidly (< 5 min) taken up by the heart and other organs following i.v. administration.
In conclusion, we have shown that it is possible to target an SNO to mitochondria in cells and in tissues where it releases NO• and S-nitrosates protein thiols. This approach can be used to selectively modulate mitochondrial O2 consumption and to protect mitochondrial function in ways that are useful therapeutically. The development of mitochondria-targeted NO• donors reported here adds to the growing pool of mitochondria-targeted molecules that can be used to manipulate mitochondrial function and suggests that there is scope to develop many other mitochondria-targeted bioactives and pharmacophores.
Materials and Methods
Chemical Syntheses.
MitoSNO1 was synthesized from the thiol precursor mito-N-acetyl penicillamine (MitoNAP) by S-nitrosation with acidic nitrite (Fig. 1B). Mito-N-acetyl penicillamine (MitoNAP), was synthesized by the addition of 5-aminopentyltriphenylphosphonium salt to the thietane derived from penicillamine. Details of the synthetic procedures are available in SI Methods.
Mitochondrial Preparations and Incubations.
Rat liver mitochondria were prepared in 250 mM sucrose, 5 mM Tris-HCl, and 1 mM EGTA, pH 7.4, and heart mitochondria in the same buffer supplemented with 0.1% BSA (17). Mitochondrial membranes were prepared from bovine heart mitochondria. Protein concentration was determined by the biuret assay using BSA as a standard or by the bicinchoninic acid assay. Mitochondrial incubations were usually in KCl buffer [120 mM KCl, 10 mM Hepes, 1 mM EGTA, 100 μM N,N-bis(2-bis[carboxymethyl]aminoethyl) glycine (DTPA), and 10 μM neocuproine, pH 7.2] with 10 mM succinate and 4 μg/mL rotenone in the dark at 37 °C, unless stated otherwise.
Heart Ischemia-reperfusion Injury.
Male C57BL6 mice (30–35 g) were obtained from Harlan and were maintained with food and water available ad libitum. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication #85–23, 1996). Isolated mouse hearts were retrograde reperfused in Langendorff mode under constant flow (4 mL/min), essentially as described for rat hearts (18).
Additional methods are available in SI Methods.
Supplementary Material
Acknowledgments.
We thank Dr Helena M. Cochemé for assistance with the figures and comments on the manuscript and Dr Gunter Kuhnle for assistance with the NO• analyser. This work was supported by the Medical Research Council (U.K.) and the Research Committee of the University of Otago. Work by P.S.B. is supported by a grant from the US National Institutes of Health (#HL-071158). Work by C.T.T. and J.F.G. is supported by Science Foundation Ireland and the Health Research Board of Ireland.
Footnotes
Conflict of interest statement: M.P.M. and R.A.J.S. have submitted a patent on the technology described in this manuscript.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903250106/DCSupplemental.
References
- 1.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nature Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 2.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Letts. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 3.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 4.Janssen-Heininger YM, et al. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogg N. The biochemistry and physiology of S-nitrosothiols. Annu Rev Pharmacol Toxicol. 2002;42:585–600. doi: 10.1146/annurev.pharmtox.42.092501.104328. [DOI] [PubMed] [Google Scholar]
- 6.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahm CC, Moore K, Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: Implications for the interaction of nitric oxide with mitochondria. J Biol Chem. 2006;281:10056–10065. doi: 10.1074/jbc.M512203200. [DOI] [PubMed] [Google Scholar]
- 8.Galkin A, Moncada S. S-nitrosation of mitochondrial complex I depends on its structural conformation. J Biol Chem. 2007;282:37448–37453. doi: 10.1074/jbc.M707543200. [DOI] [PubMed] [Google Scholar]
- 9.Shiva S, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: Effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 2007;42:812–825. doi: 10.1016/j.yjmcc.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 12.Smith RAJ, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci USA. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wikstrom M, Krab K, Saraste M. Cytochrome oxidase - a synthesis. London: Academic press; 1981. [Google Scholar]
- 14.Feelisch M, et al. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: Implications for the fate of NO in vivo. FASEB J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Kettenhofen NJ, Shiva S, Hogg N, Gladwin MT. Copper dependence of the biotin switch assay: Modified assay for measuring cellular and blood nitrosated proteins. Free Radic Biol Med. 2008;44:1362–1372. doi: 10.1016/j.freeradbiomed.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 17.Chappell JB, Hansford RG. Preparation of mitochondria from animal tissues and yeasts. In: Birnie GD, editor. Subcellular components: preparation and fractionation. London: Butterworths; 1972. pp. 77–91. [Google Scholar]
- 18.Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: Implications for pathology and cardioprotection. Biochem J. 2006;395:611–618. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.