Abstract
Toxic alcohol effects on pancreatic acinar cells, causing the often fatal human disease acute pancreatitis, are principally mediated by fatty acid ethyl esters (non-oxidative products of alcohol and fatty acids), emptying internal stores of Ca2+. This excessive Ca2+ liberation induces Ca2+-dependent necrosis due to intracellular trypsin activation. Our aim was to identify the specific source of the Ca2+ release linked to the fatal intracellular protease activation. In 2-photon permeabilized mouse pancreatic acinar cells, we monitored changes in the Ca2+ concentration in the thapsigargin-sensitive endoplasmic reticulum (ER) as well as in a bafilomycin-sensitive acid compartment, localized exclusively in the apical granular pole. We also assessed trypsin activity in the apical granular region. Palmitoleic acid ethyl ester (POAEE) elicited Ca2+ release from both the ER as well as the acid pool, but trypsin activation depended predominantly on Ca2+ release from the acid pool, that was mainly mediated by functional inositol 1,4,5- trisphosphate receptors (IP3Rs) of types 2 and 3. POAEE evoked very little Ca2+ release and trypsin activation when IP3Rs of both types 2 and 3 were knocked out. Antibodies against IP3Rs of types 2 and 3, but not type 1, markedly inhibited POAEE-elicited Ca2+ release and trypsin activation. We conclude that Ca2+ release through IP3Rs of types 2 and 3 in the acid granular Ca2+ store induces intracellular protease activation, and propose that this is a critical process in the initiation of alcohol-related acute pancreatitis.
Keywords: calcium, inositol trisphopshate receptors, pancreatitis
The pancreatic acinar cell is potentially dangerous because it produces a range of precursor digestive enzymes (zymogens) that, if inappropriately activated inside the cells, cause autodigestion resulting in the often fatal human disease acute pancreatitis (1, 2).
Exocytotic secretion of zymogens is controlled by local cytosolic Ca2+ spikes in the apical granular region, generated by small quantities of Ca2+ released from internal stores (3, 4). In contrast, prolonged global cytosolic [Ca2+] elevations—associated with emptying the Ca2+ stores—cause intracellular trypsin activation and transform the normally electron dense zymogen granules (ZGs) into empty looking vacuoles (2, 5–7). The vacuoles are post-exocytotic, endocytic structures, and it is in these vacuoles that trypsin activation occurs (8).
The association between alcohol abuse and acute pancreatitis is well known (1, 2, 9), but the exact mechanism by which alcohol initiates the disease is unclear. Hypertriglyceridemia is also a recognized cause of pancreatitis (10) and the presence of high concentrations of fatty acid ethyl esters [FAEEs, non-oxidative products of alcohol and fatty acids (FAs)], particularly in the pancreas, was reported in a postmortem study of subjects intoxicated by alcohol at the time of death (11). FAEEs induce trypsin activation and vacuole formation (12) and also elicit global sustained [Ca2+]i elevations, due to emptying of intracellular Ca2+ stores, causing Ca2+-dependent necrosis (9, 13, 14). Although FAs alone can also—but rather slowly—release intracellular Ca2+ and elicit necrosis (13), they do not, unlike FAEEs, primarily liberate Ca2+ from internal stores, but act by inhibiting mitochondrial ATP synthesis. The reduced intracellular ATP level prevents Ca2+ pump function in both intracellular stores and the plasma membrane (9, 14).
Although the endoplasmic reticulum (ER) is the principal source of Ca2+ released from internal stores in response to neurotransmitter or hormonal stimulation (3, 15, 16), Ca2+ can also be liberated from acid stores (3, 15, 17–22). Several Ca2+-liberating agents release Ca2+ from both thapsigargin (TG)-sensitive and bafilomycin (Baf)-sensitive acidic stores (21, 22).
The purpose of this study was to test the hypothesis that the toxic FAEE action is due to trypsin activation linked to Ca2+ release from the acid store. Using 2-photon permeabilized acinar cells (21, 22), we show that palmitoleic acid ethyl ester (POAEE) releases Ca2+ from both the TG-sensitive ER and the Baf-sensitive acid store in the granular apical region. POAEE activates trypsin in pathophysiologically relevant concentrations and this activation depends on Ca2+ release from the apical acid store, mainly through functional IP3 receptors (IP3Rs) of types 2 and 3. Inhibition of IP3Rs of types 2 and 3, but not type 1, with specific antibodies reduces markedly POAEE-elicited Ca2+ release as well as trypsin activation, and there are only very small Ca2+ release and trypsin activation responses to POAEE when IP3Rs of type 2 and 3 are knocked out.
Results
Mechanism by which POAEE Induces Ca2+ Release.
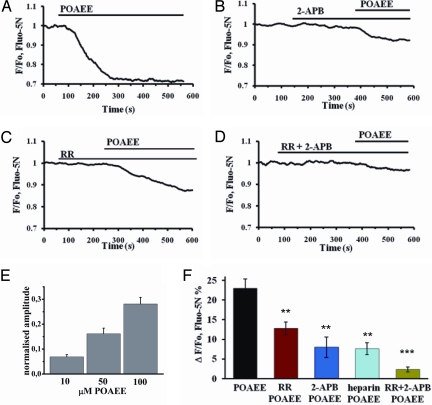
POAEE released Ca2+ from intracellular stores (Fig. 1). Fig. 1A shows the action of 100 μM POAEE, which elicits a sustained cytosolic [Ca2+] elevation in intact acinar cells (13, 14). POAEE (100 μM) elicited a marked reduction in [Ca2+]store [ΔF/F0 = 23.0 ± 2.3% (SEM), n = 9] (Fig. 1 A and F). The POAEE-elicited reduction in [Ca2+]store was concentration-dependent in the range of 10–100 μM (Fig. 1E), which is relevant pathophysiologically (11–13).
Fig. 1.
IP3Rs and RyRs play major roles in POAEE- induced Ca2+ release from intracellular stores. (A) POAEE (100 μM) evoked a marked reduction in [Ca2+]store in a Fluo-5N AM loaded permeabilized cell. (B) Inhibition of IP3Rs with 100 μM 2-APB reduced amplitude of POAEE-elicited reduction in [Ca2+]store. (C) The RyR antagonist ruthenium red (RR) (10 μM) also reduced the POAEE effect. (D) Combination of 2-APB (100 μM) and RR (10 μM) very markedly diminished the amplitude of the POAEE-elicited reduction in [Ca2+]store. (E and F) Comparisons of means of averaged amplitudes of POAEE-elicited reductions in [Ca2+]store measured 200 s after POAEE (100 μM in F) application. Error bars show S.E.M; P values (relating to results summarized in F) were calculated with 1-way ANOVA test in comparison to POAEE control (first black column in F), **, P < 0.01; ***, P < 0.001.
The principal Ca2+ release channels are IP3Rs and ryanodine receptors (RyRs) (3, 15, 16), and we investigated whether inhibitors of these channels influence the POAEE-elicited Ca2+ liberation (Fig. 1). The IP3R inhibitor 2-aminoethyldiphenyl borate (2-APB) (23) diminished markedly the POAEE-induced Ca2+ release (average response: 8.0 ± 2.6%, n = 5; Fig. 1 B and F). 2-APB also blocks store-operated Ca2+ channels in the plasma membrane (24). Although this is not a major concern in studies on permeabilized cells, we tested another IP3R inhibitor, heparin, which also reduced markedly the POAEE-induced Ca2+ release (7.7 ± 1.5%, n = 6; Fig. 1F). We blocked RyRs by preincubation with ruthenium red (RR) and this also reduced the POAEE-induced Ca2+ liberation (12.8 ± 1.7%, n = 5; Fig. 1 C and F), indicating a role for RyRs. Combined application of 2-APB and RR almost abolished the POAEE responses (2.4 ± 0.7%, n = 8) (Fig. 1 D and F).
Localizations and Characteristics of 2 Different Ca2+ Stores.
Our previous studies of 2-photon permeabilized acinar cells (21, 22) showed the existence of 2 separate Ca2+ stores, namely the ER and an acidic store. The ER maintains a high Ca2+ concentration by action of a Ca2+ pump, which can be specifically inhibited by TG (25) thereby depleting the ER of Ca2+ due to leaks in the ER membrane (3, 26). The acidic store does not possess this pump, but depends on a vacuolar-type H+ pump, which can be inhibited specifically by Baf (27). This store is exclusively present in the apical granular pole of the acinar cells (21, 22).
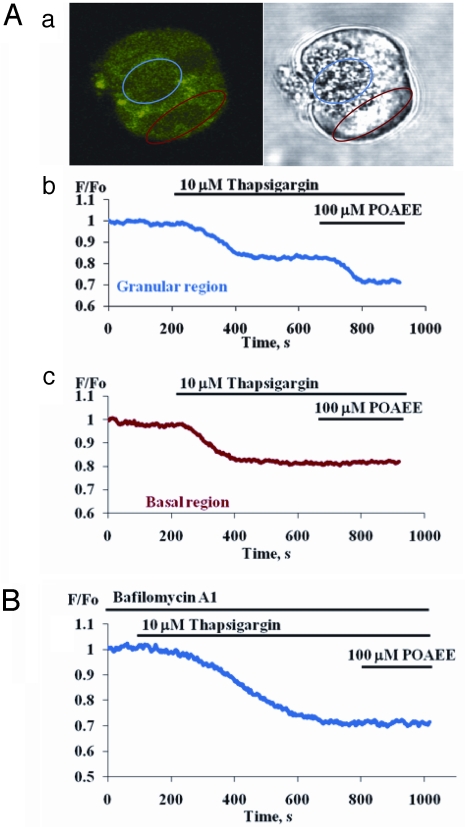
Fig. 2 illustrates the protocol used to investigate the POAEE action on the acid store. The ER was first emptied of Ca2+ by TG. Thereafter POAEE (100 μM) evoked a further reduction in [Ca2+]store in the granular, but not the basal, region (Fig. 2A; n = 5). Because TG reduced [Ca2+]store in both basal and apical areas, whereas after TG treatment POAEE only reduced [Ca2+]store in the apical granular pole, the functional ER seems to be present throughout the cell (28) whereas the acid TG-insensitive store seems to be confined to the apical part (21). Accumulation of Ca2+ into the acid store may depend on a Ca2+-H+ exchanger (3), because Baf reduces slowly [Ca2+]store in the apical granular pole (21). We re-investigated the action of Baf (100 nM) and found slowly developing reductions in [Ca2+]store restricted to the granular pole (n = 8). To speed up the responses, we used a combination of Baf (100 nM) and the Na+/H+ antiporter monensin (5 μM). This caused a more acute reduction in [Ca2+]store (14.5% ± 0.9; n = 6), which occurred exclusively in the granular region (Fig. S1). The TG-induced reduction in [Ca2+]store in the granular area was 25.4% ± 3.2 (n = 10). We tested whether the POAEE-elicited Ca2+ release in the granular pole, after emptying the ER elements of Ca2+ with TG, was from a Baf-sensitive store. When POAEE was added after TG treatment following a 30-min preincubation with Baf (100 nM), it failed to reduce [Ca2+]store in the granular region (Fig. 2B; n = 6). POAEE also released Ca2+ from ER elements in the apical pole. After preincubation with Baf, but without TG treatment, POAEE elicited a clear (but significantly smaller than without Baf, P < 0.02) reduction in [Ca2+]apical store (9.5 ± 0.7%, n = 5). We conclude that POAEE is capable of releasing Ca2+ from both the ER and an acid store in the apical pole.
Fig. 2.
POAEE releases Ca2+ from TG-insensitive, acidic store. (A) Panel (a) Fluorescent and transmitted light images of a permeabilized cell loaded with Fluo-5N AM. Two regions of interest from which measurements shown in b and c were obtained are outlined in blue (granular area) and red (baso-lateral area). Panel (b) The blue trace shows first that TG reduced [Ca2+]store to a lower stable level. Thereafter POAEE (100 μM) evoked a further reduction in [Ca2+]store in the granular area. Panel (c) Red trace shows that POAEE was unable to evoke further Ca2+ release in basal area after TG had reduced [Ca2+]store. (B) After preincubation with Baf (100 nM, 30 min), TG could still reduce [Ca2+]store, but thereafter POAEE failed to induce any further Ca2+ release in the granular area.
POAEE-elicited Ca2+ Release from the Acid Store Depends on Functional IP3Rs and RyRs.
In these experiments (Fig. S2), we investigated specifically the actions of Ca2+ release channel blockers on the effect of POAEE on the acid (non-TG-sensitive) Ca2+ stores. In control experiments, after TG treatment, POAEE evoked a marked reduction in [Ca2+]acid store (12 ± 0.7%, n = 5; Fig. S2D). After application of TG + the IP3R inhibitor 2-APB, POAEE only evoked a very minor reduction in [Ca2+]acid store (2.3 ± 0.2%, n = 5; Fig. S2 A and D) and a very similar result was obtained with another IP3R inhibitor, heparin (2.3 ± 3%, n = 6; Fig. S2D). RR also inhibited Ca2+ release markedly (4.0 ± 0.6%, n = 4; Fig. S2B and D). When TG was combined with both 2-APB and RR, subsequent POAEE stimulation failed to elicit any Ca2+ release (0.1 ± 0.05%, n = 6; Fig. S2 C and D). This was not due to the acid Ca2+ stores being empty, because subsequent addition of a Ca2+ ionophore and a protonophore elicited substantial further Ca2+ release (Fig. S2C) (n = 5). The complete inhibition of Ca2+ liberation by blockade of Ca2+ release channels indicates that POAEE does not release Ca2+ from the acid granular store by causing unspecific membrane permeabilization.
POAEE-elicited Ca2+ Release from Acidic Store Does Not Require Phospholipase C Activation.
Because the POAEE-elicited Ca2+ release from the acidic store is particularly dependent on functional IP3Rs, we tested whether POAEE acts by stimulating IP3 production. The most widely used inhibitor of phospholipase C (PLC) is the aminosteroid compound U73122 (29). U73122 is a powerful PLC inhibitor, but other effects—including release of Ca2+ from IP3-sensitive stores—have been noted (29, 30). We used U73122 at a concentration (10 μM) that abolishes cytosolic Ca2+ signal generation evoked by muscarinic receptor activation in many systems (29), including pancreatic acinar cells (31). The PLC inhibitor itself caused a reduction in [Ca2+]acid store (4.2 ± 0.3%, n = 4) and the subsequent POAEE-elicited reduction in [Ca2+]acid store was significantly diminished (6.6 ± 0.9%; n = 9) compared with normal control POAEE responses (12.0 ± 0.7%; n = 8) (P < 0.0003) (Fig. S3 A and B). Importantly, U73122 did not abolish the POAEE-evoked Ca2+ release in any of the 8 experiments carried out. The reduced POAEE response in the presence of U73122 is most likely explained by the fact that U73122 had already itself reduced [Ca2+] in the store.
POAEE induces Trypsin Activation.
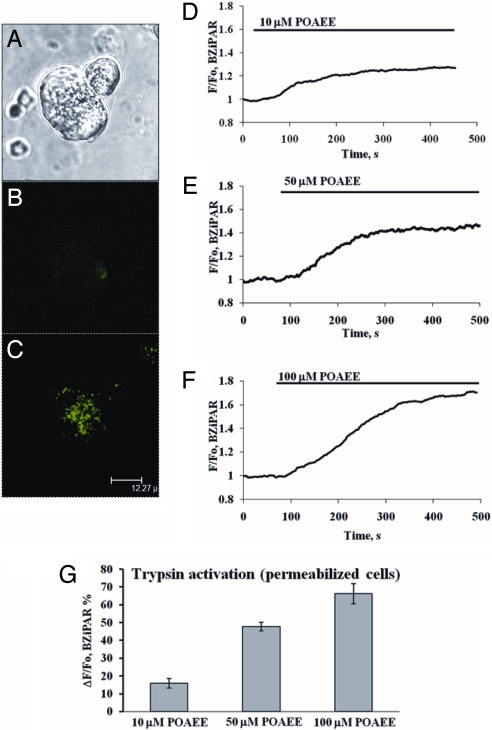
Intra-acinar activation of zymogens is a key event in the initiation of acute pancreatitis (1, 2) and we therefore tested whether POAEE could elicit trypsin activation. We monitored, in real time, trypsin activation using a probe (BZiPAR) that becomes fluorescent when trypsin cleaves the 2 oligopeptide side chains (32). POAEE activated trypsin in the apical granular pole (Fig. 3 A–G). Before POAEE application, there was virtually no fluorescence, whereas after POAEE, the substrate had been cleaved preferentially in the granular area (Fig. 3 B and C). POAEE caused trypsin activation in a concentration-dependent manner, within a range (10–100 μM) (Fig. 3 D–F) that is pathophysiologically relevant (11–13). These data are summarized in Fig. 3G.
Fig. 3.
POAEE activates trypsin in granular area. (A) Transmitted light image of 2 cells. Left cell is permeabilized and granular area is clearly visible in the right part [for scale bar (12.27 μm) see (C)]. (B) Virtual absence of BZiPAR fluorescence in permeabilized, but unstimulated, cell shown in A. (C) POAEE (100 μM) has evoked trypsin activation in granular region as seen by BZiPAR fluorescence (green). (D–F) Fluorescence traces showing time courses of trypsin activation evoked by POAEE (10, 50, and 100 μM). (G) Summary of the results of POAEE-induced trypsin activation, at 3 different concentrations (mean ± SEM, n = 5–8 for each column).
Trypsin activation may, at least in part, be mediated by the lysosomal cysteine protease cathepsin B (8, 9, 33). We therefore tested the effect of the cathepsin B inhibitor CA74Me (8, 34) which, at a concentration of 50 μM, markedly reduced POAEE (100 μM)-elicited trypsin activation from the control level of ≈70% (Fig. 3G) to 14.8 ± 2.1% (n = 6).
POAEE-induced Trypsin Activation Depends on Ca2+ Release from the Acid Store via IP3Rs.
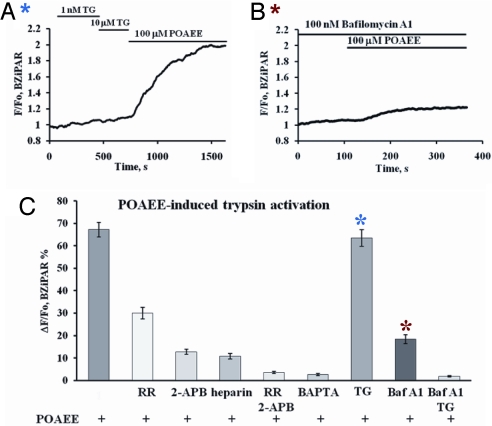
We tested the effects of inhibiting the POAEE-elicited Ca2+ release on the ability of POAEE to activate trypsin (Fig. 4 A–C). Inhibition of RyRs with 10 μM RR reduced POAEE-elicited trypsin activation (Fig. 4C), but stronger inhibitions were observed with the IP3R inhibitors 2-APB or heparin (Fig. 4C). When inhibitors of both IP3Rs and RyRs were combined, POAEE failed to evoke trypsin activation (Fig. 4C). These data indicate that POAEE cannot activate trypsin without releasing Ca2+ from internal stores and points to IP3Rs as the most important elements, although RyRs also play a role.
Fig. 4.
POAEE-induced trypsin activation is inhibited by agents interfering with Ca2+ transport functions. (A) Emptying ER Ca2+ store by TG did not inhibit POAEE-induced trypsin activation. (B) Preincubation with 100 nM Baf reduced very markedly POAEE-induced trypsin activation. (C) Summary of results concerning the effects of RR, 2-APB, heparin, BAPTA, TG, and Baf on POAEE-induced trypsin activation (mean ± SEM; n = 5–8 in each case).
Depletion of intracellular stores may be important, but a local rise in the cytosolic [Ca2+] could also be significant. To test this, we clamped the cytosolic (bath) [Ca2+] at the physiological resting level by incubating the permeabilized cells in a solution containing a high concentration of a Ca2+/BAPTA (Ca2+ chelator) mixture. Under this condition, POAEE failed to induce trypsin activation (Fig. 4C).
Finally, we tested whether any particular Ca2+ store was of special importance for trypsin activation, using TG and Baf as tools to discriminate between the ER and the acid stores (Fig. 4 A–C). Emptying the ER store using TG—using a protocol designed to minimize the risk of Ca2+-induced Ca2+ release (Fig. 4A) —had virtually no effect on the POAEE-induced trypsin activation, which remained very similar to what was obtained under control conditions (Fig. 4 A and C). On the other hand, preincubation with Baf, which empties slowly the acid store of Ca2+ (21), reduced markedly the subsequent POAEE-induced trypsin activation (Fig. 4 B and C). Combining TG and Baf abolished the POAEE response (Fig. 4C).
Procedures other than POAEE stimulation, which releases Ca2+ from the granular store, might also be expected to evoke trypsin activation. As already mentioned, Baf does reduce [Ca2+]acid store, but only slowly. We therefore tested the effect of Baf (100 nM) and found that trypsin activation did occur (13.7 ± 1.1%, n = 6).
POAEE-induced Ca2+ Release Depends Mainly on IP3Rs of types 2 and 3: Studies with IP3R Antibodies.
The physiologically most important IP3Rs in the pancreas are types 2 and 3 (35). To test which types are involved in the POAEE-elicited Ca2+ release from the critical acid stores, we used antibodies against different IP3R types (Fig. S4 A and B). First, we tested whether IP3-elicited Ca2+ release in our preparation was blocked by antibodies against type 2 and 3 IP3Rs. When both antibodies were combined, IP3 did not evoke any Ca2+ release (Fig. S4B), whereas in the presence of an antibody against type 3 alone, there was a markedly diminished IP3-elicited reduction of [Ca2+] in the acid store (Fig. S4B).
Antibodies against type 2 and 3 IP3Rs markedly reduced the Ca2+ release evoked by POAEE (Fig. S4A). Further addition of antibodies against type 1 IP3Rs did not produce any stronger inhibition [Fig. S4B, no significant difference (n.s.) between the degree of inhibition produced by antibodies against types 2 and 3 and the extent of inhibition caused by antibodies to all 3 subtypes], suggesting that types 2 and 3 are the main IP3Rs involved in the POAEE-induced Ca2+ release from the acidic stores. Antibodies against type 3 IP3Rs reduced the Ca2+ release responses more than antibodies against type 2 receptors (P < 0.02), but the difference was minor (Fig. S4B). Antibodies against type 1 IP3Rs did not inhibit the POAEE-induced Ca2+ release; the response in the presence of the type 1 antibody was not significantly different from control (Fig. S4B). Fig. S4B summarizes all of the data using single antibodies, combinations of antibodies as well as controls.
POAEE-induced Trypsin Activation Depends on IP3Rs of types 2 and 3: Studies with IP3R Antibodies.
To investigate which types of IP3Rs are involved in the POAEE-elicited trypsin activation, we used antibodies against different types of IP3Rs. Antibodies against type 1 IP3Rs did not reduce the extent of trypsin activation evoked by POAEE (Fig. S5 A and E). Antibodies against type 2 IP3Rs partially inhibited trypsin activation by POAEE (Fig. S5E), but antibodies against type 3 IP3Rs inhibited trypsin activation more markedly (Fig. S5 B and E). The inhibition by type 3 antibodies was significantly stronger (P < 0.02) than that exerted by the type 2 antibodies.
When antibodies against IP3Rs of both types 2 and 3 were used together, the POAEE-elicited trypsin activation was reduced to a low level (Fig. S5 C and E), but no further inhibition was observed by a combination of antibodies against all IP3R types (1, 2, and 3) (Fig. S5 D and E). The absence of any significant difference between these 2 data sets suggests that it is mainly IP3Rs of types 2 and 3 that are involved in the POAEE-induced trypsin activation. Control antibodies did not change significantly trypsin activation induced by POAEE (Fig. S5E).
POAEE-elicited Ca2+ Release from the Acid Store and Trypsin Activation Depend on Functional IP3Rs of Types 2 and 3: Knock Out of Type 2 and 3 IP3Rs.
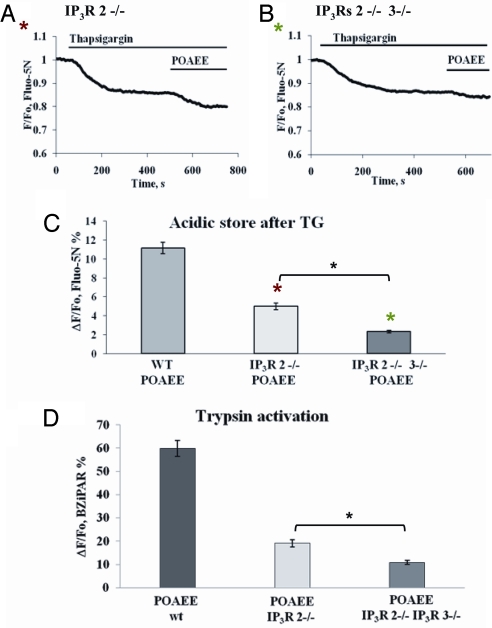
The most direct approach to determining which types of IP3Rs are involved in POAEE-elicited Ca2+ release and trypsin activation is to compare the results from mice in which specific types of IP3Rs have been knocked out with those from the appropriate wild-type controls (35). As seen in Fig. 5 A–C, POAEE evoked a much reduced Ca2+ release from the acidic store in acinar cells from IP3R2−/− mice (5.0 ± 0.4%; n = 7) compared with wild-type controls (11.1 ± 0.6%; n = 6). A stronger reduction in the POAEE-elicited Ca2+ release from the TG-insensitive store was observed in acinar cells isolated from mice in which both the type 2 and the type 3 IP3Rs had been knocked out (2.3 ± 0.1%; n = 8) (Fig. 5 B and C). The Ca2+ release response from the double KO (IP3R2−/−IP3R3−/−) mice was significantly smaller (P < 0.0001) than from the single KO (IP3R2−/−) mice.
Fig. 5.
Inhibition of POAEE-elicited Ca2+ release and trypsin activation in pancreatic acinar cells in which the type 2 IP3R has been knocked out (IP3R2−/−) and in which both types 2 and 3 IP3Rs have been knocked out (IP3R2−/−,IP3R3−/−). (A) Diminished POAEE-elicited reduction in [Ca2+]acid store in permeabilized cells from IP3R2−/− mice. (B) POAEE only elicited a tiny reduction in [Ca2+]acid store from IP3R2−/−-3−/− double knockout mice. (C) Summary of results concerning effects of IP3R subtypes 2 and 3 knockouts (in comparison with experiments on cells isolated from wild-type mice) on POAEE-elicited reductions in [Ca2+]acid store (mean ± SEM, n = 6–8 in each case). (D) Summary of data concerning effects of knocking out IP3R subtypes 2 and 3 on POAEE-elicited trypsin activation as compared to control data from wild-type mice (mean ± SEM, n = 4 in each case).
In a separate series of experiments, the POAEE-elicited trypsin activation was tested in permeabilized pancreatic acinar cells from wild type, IP3R2−/− and IP3R2−/−,IP3R3−/− mice (Fig. 5D). The POAEE-elicited trypsin activation was markedly reduced in the experiments on acinar cells from IP3R2−/− mice (19.1 ± 0.6%; n = 4) as compared to controls (59.9 ± 3.5%; n = 4) and even more reduced in the experiments on cells from the double KO (IP3R2−/−,IP3R3−/−) mice (10.9 ± 0.9%, n = 4). POAEE-elicited trypsin activation was significantly lower in the double KO experiments compared with the single KOs (P < 0.004) (Fig. 5D).
Discussion
Our results show that the fatal intracellular trypsin activation, which initiates acute pancreatitis (1, 2), depends mainly on intracellular Ca2+ release through IP3Rs of types 2 and 3 from an acid granular store.
ZGs constitute a major Ca2+ store in the apical granular pole with a high [Ca2+] (17, 28). IP3 releases Ca2+ from isolated ZGs, as well as ZGs in intact cells, whereas specific ER Ca2+ pump inhibition with TG cannot liberate Ca2+ from these organelles (17, 36).
ZGs are not the only acid Ca2+ stores. Important stores, from which Ca2+ can be mobilized, have also been demonstrated in lysosomes and endosomes (17–21, 36–39). Trypsin activation takes place in acid Baf-sensitive postexocytotic endocytic structures, which are, at least partially, co-localized with lysosomes (8). This may be an important part of the acid Ca2+ store involved in the Ca2+ release and trypsin activation responses to POAEE characterized in this study. Our result showing that inhibition of the lysosomal enzyme cathepsin B markedly reduces POAEE-elicited trypsin activation is in agreement with this hypothesis.
How could Ca2+ release from intracellular stores promote trypsin activation? Our data indicate that POAEE-elicited Ca2+ release from the acid granular pool is more important for zymogen activation than Ca2+ liberation from the ER. However, our results also show that clamping the cytosolic [Ca2+] at the normal resting level prevents zymogen activation. Most likely, zymogen activation depends both on a reduction in [Ca2+]acid store and an increase in [Ca2+] in the apical cytosolic environment. This would agree with the ion exchange concept of Verdugo (19, 39) in which replacement of Ca2+ in the matrix of secretory granules by K+ causes matrix disaggregation. In the case of ZGs or post-exocytotic vacuoles, this would favor toxic enzyme activation. Ca2+-activated opening of K+ channels in the ZG could play an important role in this process (2, 19, 39).
How does POAEE, and presumably other FAEEs, activate Ca2+ release? POAEE stimulation could activate PLC and thereby generate IP3, but our results with the PLC inhibitor U73122 do not provide evidence for this and furthermore indicate that even if this process did occur it may not be essential for POAEE-evoked Ca2+ release. POAEE clearly does not act specifically to open type 2 and 3 IP3Rs in the acid pool, because Ca2+ release can also be activated from the ER and via both IP3Rs and RyRs. Most likely, the functionally dominant Ca2+ release channels in the membranes of the acid stores are mainly IP3Rs of types 2 and 3, and these molecules would therefore be the principal mediators of the quantitatively important Ca2+ release that appears to be chiefly responsible for trypsin activation.
Coffee drinking (caffeine) has some protective effect against alcohol-related pancreatitis (40), and we have previously shown that caffeine reduces POAEE-induced Ca2+ signal generation (14). Our finding, that specific inhibition or knock out of type 2 and 3 IP3Rs very markedly reduces POAEE-elicited trypsin activation, provides fresh evidence indicating that such inhibition could be of potential benefit. Therefore, the inhibitory effect of caffeine on IP3Rs (41, 42) could be a useful starting point for therapeutic considerations. Unfortunately, other caffeine effects, for example activation of RyRs mediating Ca2+ release from the sarcoplasmic reticulum in the heart, potentially causing serious cardiac arrhythmias (43), limit the usefulness of caffeine itself as a drug for pancreatitis treatment.
Our results provide direct evidence at the molecular level demonstrating that reduced IP3R operation can protect against alcohol-related, and probably also hypertriglyceridemic, pancreatitis. This should encourage development of membrane-permeable agents, possibly related to caffeine, with specific inhibitory actions on IP3Rs of types 2 and 3.
Materials and Methods
Isolation of Pancreatic Acinar Cells.
Single pancreatic acinar cells and clusters of 2 or 3 acinar cells were isolated from the pancreas of adult mice by collagenase digestion and mechanical disruption as described previously (21, 22). We mostly used CD 1 male mice, but in the IP3R knock-out experiments, control or mutant male or female mice with C57BL/6JJmsSIc origin were used. All experiments were carried out with freshly isolated cells, attached to the coverslip of the perfusion chamber at room temperature (23 °C).
[Ca2+]store and Trypsin Measurements in Permeabilized Cells.
Cells to be permeabilized were loaded with 5–7.5 μM Fluo-5N AM, for 45 min at 36.5 °C, and then transferred to polyL-lysine coated coverslips in a flow chamber. Cells were first washed with an intracellular solution based on K-Hepes, containing (mM): KCl, 127; NaCl, 20; Hepes KOH, 10; ATP, 2; MgCl2, 1; EGTA, 0.1; CaCl2 0.05; pH 7.2; 291 mosmol/L. Thereafter, cells were permeabilized using a 2-photon microscope, as previously described (21). We used the intracellular K-Hepes-based solution already described, except in the [Ca2+] clamp experiments when 10 mM BAPTA and 2 mM CaCl2 were included. Cells were observed using a Leica SP2 MP dual 2-photon microscope. Fluo-5N AM was excited at 476 nm, and emission at 500–600 nm wavelengths was collected. For trypsin measurements, the trypsin substrate BZiPAR [rhodamine 110, bis (CBZ-L-isoleucyl-L-prolyl-L-arginine amide)] (10 μM) was added to the experimental chamber after permeabilization for the duration of the experiment. Antibodies to IP3Rs were applied after permeabilization (dilution 1:100) and incubated for 30 min before measurements.
Reagents.
Chemicals, unless otherwise indicated, were obtained from Sigma, Calbiochem, Merck. Thapsigargin, ryanodine, and ruthenium red were purchased from Tocris Biosciences. Palmitoleic acid ethyl ester (POAEE) was from MP Biomedicals. All fluorescent dyes including BZiPAR were purchased from Molecular Probes and Invitrogen, and FFP-18 (K+) salt from TEF Labs. Antibodies against IP3Rs and control antibodies were from Chemicon International (AB3000, AB9076, and CBL600B) and Insight Biotechnology (sc-28614).
Transgenic Mice.
IP3R2 knockout mice, IP3R2/IP3R3 double knockout mice and wild-type control mice were generated in the Laboratory of Developmental Neurobiology (Brain Science Institute, RIKEN, Japan) (35).
Supplementary Material
Acknowledgments.
This work was supported by a Medical Research Council (MRC) Program Grant and by the Japanese Science and Technology Agency Calcium Oscillation Project. OHP is an MRC Professor. G.L. is a Wellcome Trust Prize PhD student. We thank RIKEN BSI-Olympus Collaboration Center for the technical assistance with confocal image acquisition.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904818106/DCSupplemental.
References
- 1.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: Bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: Effects of alcohol, bile and coffee. Trends Pharmacol Sci. 2006;27:113–120. doi: 10.1016/j.tips.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 4.Murphy JA, et al. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology. 2008;135:632–641. doi: 10.1053/j.gastro.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Parekh AB. Calcium signaling and acute pancreatitis: Specific response to a promiscuous messenger. Proc Natl Acad Sci USA. 2000;97:12933–12934. doi: 10.1073/pnas.97.24.12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raraty M, et al. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA. 2000;97:13126–13131. doi: 10.1073/pnas.97.24.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruger B, Albrecht E, Lerch MM. The role of intracellular calcium signalling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157:43–50. doi: 10.1016/S0002-9440(10)64515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherwood MW, et al. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA. 2007;104:5674–5679. doi: 10.1073/pnas.0700951104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen OH, et al. Fatty acids, alcohol and fatty acid ethyl esters: Toxic Ca2+ signal generation and pancreatitis. Cell Calcium. 2009 doi: 10.1016/j.ceca.2009.02.005. in press (doi: 10.1016/j.ceca.2009.02.005) [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, et al. Enhanced susceptibility to pancreatitis in severe hypertriglyceridemic lipoprotein lipase deficient mice and agonist-like function of pancreatic lipase in pancreatic cells. Gut. 2009;58:422–430. doi: 10.1136/gut.2007.146258. [DOI] [PubMed] [Google Scholar]
- 11.Laposata EA, Lange LG. Presence of nonoxidative ethanol-metabolism in human organs commonly damaged by ethanol abuse. Science. 1986;231:497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- 12.Werner J, et al. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology. 1997;113:286–294. doi: 10.1016/s0016-5085(97)70106-9. [DOI] [PubMed] [Google Scholar]
- 13.Criddle DN, et al. Ethanol toxicity in pancreatic acinar cells: Mediation by nonoxidative fatty acid metabolites. Proc Natl Acad Sci USA. 2004;101:10738–10743. doi: 10.1073/pnas.0403431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Criddle DN, et al. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology. 2006;130:781–793. doi: 10.1053/j.gastro.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 16.Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signalling concepts. J Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- 17.Gerasimenko OV, Gerasimenko JV, Belan PV, Petersen OH. Inositol trisphosphate and cyclic ADP-ribose-mediated release of Ca2+ from single isolated pancreatic zymogen granules. Cell. 1996;84:473–480. doi: 10.1016/s0092-8674(00)81292-1. [DOI] [PubMed] [Google Scholar]
- 18.Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol. 1998;8:1335–1338. doi: 10.1016/s0960-9822(07)00565-9. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen T, Chin WC, Verdugo P. Role of Ca2+/K+ ion exchange in intracellular storage and release of Ca2+ Nature. 1998;395:908–912. doi: 10.1038/27686. [DOI] [PubMed] [Google Scholar]
- 20.Churchill GC, et al. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 21.Gerasimenko JV, Sherwood M, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP, cADPR and IP3 all release Ca2+ from the endoplasmic reticulum and an acidic store in the secretory granule area. J Cell Sci. 2006;119:226–238. doi: 10.1242/jcs.02721. [DOI] [PubMed] [Google Scholar]
- 22.Gerasimenko JV, et al. Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors. J Biol Chem. 2006;281:40154–40163. doi: 10.1074/jbc.M606402200. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-permeable modulator of Ins(1,4,5)P-3 induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 24.Bakowski D, Glitsch MD, Parekh AB. An examination of the secretion-like coupling model for the activation of the Ca2+ release-activated Ca2+ current I-CRAC in RBL-1 cells. J Physiol. 2001;532:55–71. doi: 10.1111/j.1469-7793.2001.0055g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerasimenko JV, et al. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol. 2003;163:271–282. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nature Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 28.Gerasimenko OV, et al. The distribution of the endoplasmic reticulum in living pancreatic acinar cells. Cell Calcium. 2002;32:261–268. doi: 10.1016/s0143416002001938. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz LF, Hirdes W, Suh B-C, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: Activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mogami H, Mills CL, Gallacher DV. Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5)P3-mediated Ca2+ release and directly activates ion channels in mouse pancreatic acinar cells. Biochem J. 1997;324:645–651. doi: 10.1042/bj3240645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yule DI, Williams JA. U73122 inhibits Ca2+ oscillations in response to cholecystokinin and carbachol, but not to JMV-180 in rat pancreatic acinar cells. J Biol Chem. 1992;267:13830–13835. [PubMed] [Google Scholar]
- 32.Kruger B, Lerch MM, Tessenow W. Direct detection of premature protease activation in living pancreatic acinar cells. Laboratory Investigation. 1998;78:763–764. [PubMed] [Google Scholar]
- 33.Halangk W, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumgartner HK, et al. Caspase-8-mediated apoptosis induced by oxidative stress is independent of the intrinsic pathway and dependent on cathepsins. Am J Physiol Gastrointest Liver Physiol. 2007;293:G296–307. doi: 10.1152/ajpgi.00103.2007. [DOI] [PubMed] [Google Scholar]
- 35.Futatsugi A, et al. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki S, Nakagaki I, Kondo H, Hori S. Changes in element concentrations induced by agonist in pig pancreatic acinar cells. Pflügers Arch. 1996;432:538–545. doi: 10.1007/s004240050167. [DOI] [PubMed] [Google Scholar]
- 37.Yamasaki M, et al. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J Biol Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 38.Menteyne A, Burdakov A, Charpentier G, Petersen OH, Cancela JM. Generation of specific Ca2+ signals from Ca2+ stores and endocytosis by differential coupling to messengers. Curr Biol. 2006;16:1931–1937. doi: 10.1016/j.cub.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 39.Quesada I, Chin WC, Verdugo P. ATP-independent luminal oscillations and release of Ca2+ and H+ from mast cell secretory granules: implications for signal transduction. Biophys J. 2003;85:963–970. doi: 10.1016/S0006-3495(03)74535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morton C, Klatsky AL, Udaltsova N. Smoking, coffee, and pancreatitis. Am J Gastroenterol. 2004;99:731–738. doi: 10.1111/j.1572-0241.2004.04143.x. [DOI] [PubMed] [Google Scholar]
- 41.Wakui M, Osipchuk YV, Petersen OH. Receptor-activated cytosolic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2+-induced Ca2+ release. Cell. 1990;63:1025–1032. doi: 10.1016/0092-8674(90)90505-9. [DOI] [PubMed] [Google Scholar]
- 42.Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+ release channels. Trends Pharmacol Sci. 1994;15:145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 43.Balasubramaniam R, Chawla S, Grace AA, Huang CL. Caffeine-induced arrhythmias in murine hearts parallel changes in cellular Ca2+ homeostasis. Am J Physiol Heart Circ Physiol. 2005;289:H1584–H1593. doi: 10.1152/ajpheart.01250.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.