Abstract
While many studies have focused on modulating the immune response and enhancing axonal regeneration after spinal cord injury (SCI), there is limited work being performed on evaluating the role of glial scar in SCI. We sought to evaluate the effects of glial scar resection in contusion models and dorsal hemisection models of SCI. At one week postinjury, 2mm of glial scar was excised from specimens in one of the two groups from each injury model. Functional outcome was measured weekly using the Basso, Beattie, Bresnahan (BBB) Locomotor Rating Scale along with histologic evaluation of spinal cord tracts to determine axonal regeneration. Within the dorsal hemisection model, there was no significant difference in recovery for animals that underwent glial scar excision versus animals that did not have scar excision (p=0.61). Animals subjected to the contusion model, however, demonstrated lower BBB scores in the glial resection group during the earlier postoperative periods (<4 weeks; p<0.05). Histological analysis revealed no axons within the glial resection contusion model, and moderate axonal growth within the nonresection contusion group and both hemisection groups (p>0.05 for differences among the three groups). While glial scar may serve to stabilize the preserved axonal tracts and thereby permit modest recovery in a contusion model of SCI, it may be of less importance with a dorsal hemisection model. These experiments highlight that basic biologic processes following SCI may vary tremendously based on the injury mechanism and that the role of glial scar in spinal cord regeneration must be elucidated.
Keywords: axon regeneration, astrocyte, glial scar, spinal cord injury
INTRODUCTION
Trauma to the adult spinal cord is particularly devastating because of the inability of the central nervous system (CNS) to regenerate after injury. Unlike in the peripheral nervous system, axonal recovery in the spinal cord is thwarted by two fundamental obstacles: the inherently weak regenerative ability of CNS axons and a powerfully inhibitive post-injury milieu of physical and chemical factors.1 The most potent of these factors is the glial scar that develops after any CNS injury.2–4 The glial scar is a collection of reactive cells (astrocytes, microglia, oligodendrocyte precursors, meningeal fibroblasts) and their expressed cell-surface and matrix molecules, which surround the area of injury and ultimately stymie the advancement of all regenerating axons.
The scar features a core zone of meningeal cells and oligodendrocyte precursors, and a lesion-surround zone of astrocytes, oligodendrocyte precursors, and microglia.5 The core zone is separated from the surround zone by a basement membrane composed mostly of type IV collagen.6 While some axons may regenerate through the surround zone, no axon can penetrate the core zone without some form of experimental manipulation. 7,8
The inhibitory effects of the scar are conferred by three classes of molecules, all of which are expressed by one or more of the reactive cells in the glial scar. These include the chondroitin sulphate proteoglycans (CSPGs) (NG2, brevican, phosphacan, neurocan, versican), semaphorin 3 proteins, and eph/ephrin tyrosine kinases. Although the precise mechanisms of their actions are unclear, the molecules exert their inhibitory effects either by directly or indirectly binding to the axon cell surface or by binding and deactivating trophic factors, cell adhesion molecules, and extracellular matrix molecules that are requisite for axonal growth and regeneration.9 The ultimate effect of the gliotic response to injury is the inhibition of axonal regeneration and remyelination by both physical and chemical means.10
There is tremendous therapeutic potential in the ability to modulate the gliotic scarring response to CNS injury.1 In-vitro and in-vivo studies to date, though relatively limited, have demonstrated enhancement of axonal regeneration and functional recovery after inhibition of specific glial scar constituents. Enzymatic digestion of the glycosoaminoglycan chains of CSPGs, for instance, stimulates axonal regeneration through the site of injury.10–12 Function-blocking antibodies to semaphorin receptors have allowed sensory axons to regenerate into the formidable core zone of the scar.13 Chelating agents that prevent collagen IV synthesis around the core zone have allowed successful axonal regeneration in animal models.14 Animals with clonal deletions of a certain eph molecule have almost no astroglial scar response and demonstrate unimpeded regeneration of motor axons through the zone of injury.15
Though these biochemical alterations of the gliotic response show promise, no study has rigorously investigated the effect of surgical manipulation of the glial scar on axonal regeneration after spinal cord injury. Here we evaluated the effect of surgical glial scar resection on recovery in the two most common models of spinal cord injury: cord contusion and dorsal cord hemisection. The objectives were to determine 1) whether glial scar resection at the site of cord injury results in enhanced axonal regeneration and functional recovery, and 2) whether the possible effects of glial scar resection are influenced by the particular mechanism of injury.
METHODS
Animals
Adult female Sprague-Dawley rats (160–180 gm; Charles River Laboratories) were used for all surgical procedures. Animals were housed according to the National Institutes of Health guidelines. The Institutional Review Board of the University of California at Irvine approved all animal procedures. Before surgery, animals were anaesthetized with a mixture of 50 mg/kg ketamine (Phoenix Pharmaceuticals Inc., St. Joseph, MO), and 2.6 mg/kg xylazine (Phoenix Pharmaceuticals Inc.) Corneal reflex and withdrawal to painful stimuli of the hindlimbs were used to establish proper anesthesia. The appropriate surgical site was shaved and aseptically prepared with betadine prior to incision. Body temperature was maintained at 37 ± 0.5°C throughout the operative and perioperative period by use of heating pads. Upon completion of the particular surgical procedure, animals were returned to their warmed cages with ad libitum access to food and water. Postoperative antibiosis with Enrofloxacin (2.5mg/kg s.c. q.d., Bayer Corp., Shawnee Mission, KS) and fluid resuscitation with lactated ringers (5ml/100g s.c.) were administered as necessary. Postoperative analgesia was achieved with buprenorphine (0.01 mg/kg s.c. q.d., Abbott Laboratories, Abbott Park, Illinois) as necessary.
Surgical Procedures
A moderate spinal cord contusion injury was created in 24 adult female Sprague-Dawley rats, and a dorsal hemisection injury was created in an additional 24 rats according to the procedures previously described.7,8 At one week post-injury creation, the spinal cords were re-exposed for all animals.16 The members of each spinal cord injury group (contusion versus hemisection) were randomly divided into two subgroups (n=12 for each group). In the first subgroup of each injury group, iridectomy scissors were used under 10× magnification to resect the glial scar overlying the area of injury. The glial scar was grossly distinct from the underlying cord in both color and friability. The length of scar excision was 2.0 mm in each specimen as measured by a ruler, with the midpoint overlying the geometric epicenter of injury as defined above. The resected specimen was then observed under 100× magnification to confirm that only glial scar tissue had been removed. The thickness of the resected specimen was also measured and found to be between 0.5mm and 0.6mm for each specimen. The underlying injured spinal cord was left intact. Animals in the second subgroup underwent a sham procedure in which no resection of gliotic scar was performed. All wounds were then closed following the procedures as mentioned previously. Identifying ear and tail markings were then made. Four groups were thus created: contusion injury with glial scar resection, contusion injury without glial scar resection, hemisection injury with glial scar resection, hemisection injury without glial scar exicision. The particular treatment condition for each animal was recorded by a member of the investigative team who was not involved in any further behavoral or histologic analysis, and who had exclusive knowledge of treatment for each animal until the conclusion of the study. Routine postoperative care including bladder expressions were continued as appropriate.
Outcome Assessment
Functional outcome was assessed using the Basso, Beattie, Bresnahan (BBB) Locomotor Rating Scale for rat hindlimb motor function.17 The BBB recordings were performed by two trained observers who were blinded to the experimental groupings and who scored the animals independently in a noise-free environment. Animals were assessed during the course of a 4-minute exposure to an open field arena consisting of a metal circular enclosure and a non-slip floor (1000 cm diameter, 18 cm wall height). BBB scores were recorded once preoperatively to establish a baseline control, again on the second postoperative day, and weekly thereafter to assess functional recovery. Animals were monitored for a total of seven postoperative weeks.
At the end of the sixth week of behavioral data collection, all the animals were subjected to either ascending or descending tract tracing using the surgical protocol discussed previously.7,8 One-half of the animals from each group underwent descending (corticospinal) tract tracing by stereotactic biotinylated dextran amine (BDA) injection in the sensorimotor cortex. To trace ascending sensory neurons, the remaining half of the animals in each group received BDA tracer injection in the fourth lumbar (L4) dorsal root ganglion. Following biotinylated dextran amine tracer injections, the animals were allowed to survive for two additional weeks, after which they were euthanized under pentobarbitone anesthesia and perfused intracardially using 4% paraformaldehyde in pH 7.4 0.1 M phosphate buffer. Intact spinal cords were harvested and post-fixed in 4% paraformaldehyde at 4°C for 4 hours, rinsed in 0.1M Na2HPO4 for one hour, and equilibrated overnight in 30% sucrose buffer at 4°C. These spinal cords were cut to produce three segments, one block extending 7 mm rostral and 7 mm cadual to the center of injury, and the others containing the sites rostral and caudal to the lesion. Each of these segments was submerged in TissueTek® (VWR International, West Chester, PA) and frozen using liquid nitrogen. The frozen tissue was sectioned using a cryostat to produce 25μm longitudinal sections of the lesion segment, and cross-sections of the rostral and caudal segment. Sections were thaw-mounted onto poly-lysine treated slides (2 mg/ml; Sigma-Aldrich, St. Louis, MO) and stored at −80°C. In preparation for staining, the slides were thawed and dried at room temperature for 3 hours to promote section adherence to slides. The specimens were then rinsed for 5 min in 1× phosphate buffered saline (PBS) and 30 min in PBS with 0.3% H2O2, and incubated overnight at 4°C with avidin and biotinylated horseradish peroxidase in 0.1% Triton in PBS. The following day, the tissue was rinsed for 1 hour in 1× PBS, 5 min in 0.1 M acetate buffer, and then reacted for 30 min in a 70 mL solution of 0.1 M acetate buffer containing 1.875 g nickel ammonium sulfate, 40 mg diaminobenzidine, 150 mg glucose, 30 mg ammonium chloride, and 0.6 mg glucose oxidase (all reagents from Sigma-Aldrich). Slides were then rinsed 2 times in 0.1 M acetate buffer, dehydrated, and coverslipped.
Sections were analyzed using light microscopy, with darkfield used for confirmation of axons. Axon quantification for labeled specimens was accomplished using a 100X objective by verifying the number of BDA+ axons intersecting a dorsoventral line along five sites of the lesion segment on the longitudinal sections.18 The sites assessed were the rostral and the caudal ends (7 mm away from the contusion epicenter in either direction), the lesion area, and the areas 3.5 mm rostral and caudal to the lesion area, allowing for data from the entire length of the section. These axon numbers were averaged for each treatment group and normalized to the average number of descending or ascending axons present at the rostral and caudal ends, respectively, to create an axonal index as a measure of axonal regeneration.19
Statistical Analysis
BBB Scores
Statistical significance of variations in scores between treatment groups and the effect of any interobserver variability within a particular treatment group were determined using one-way ANOVA, with BBB scores from each observer for each animal from a particular treatment group at each time point. Fisher’s F-distribution was then used to determine statistical significance for α=0.05.
Axonal Index
Axonal indices for each specimen were calculated as described above and then averaged for each treatment group. Variance between index means was analyzed using the paired Student’s T-test. Significance was determined for α=0.05.
RESULTS
Functional Recovery
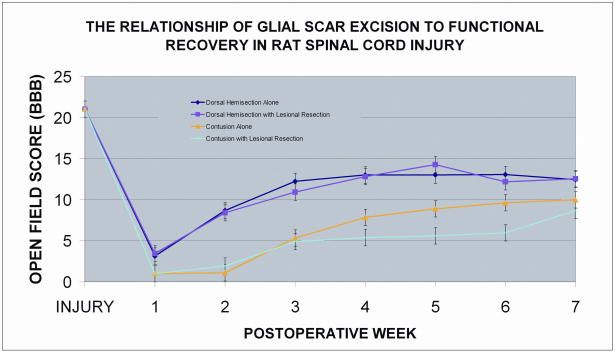
Functional recovery was quantified using the Basso, Beattie, Bresnahan (BBB) scale which is based on the rather predictable sequence of hindlimb locomotor recovery in rats after spinal cord contusion. The scale provides enough validity to distinguish the behavioral outcomes of different treatment groups and has been shown to predict the anatomical changes at the lesion site.17 Animals are rated on a scale of 0 (no observable hindlimb movement) to 21 (consistent coordinated gait, toe clearance, plantar stepping, and trunk stability). Within the dorsal hemisection group, there was no significant difference in BBB scores between specimens in which the glial scar was resected and nonresection controls (p = 0.61) (Figure 1). In contrast to the hemisection group, specimens in the contusion group that underwent scar resection demonstrated significantly worse functional scores relative to nonresection controls throughout most of the recovery period (p < 0.05). Interestingly, both hemisection groups (resected and nonresected) demonstrated significantly higher recovery scores relative to either of the contusion groups (p < 0.005 for all comparisons between hemisection and contusion groups) beyond the first postoperative week.
Figure 1.
BBB locomotor rating analysis of the four spinal cord injury groups: 1) contusion injury without glial scar resection, 2) contusion injury with glial scar resection, 3) dorsal hemisection injury without glial scar resection, 4) dorsal hemisection injury with glial scar resection. Within the dorsal hemisection group, there is no significant difference in BBB scores between specimens in which the glial scar was resected and nonresection controls (p = 0.61). Specimens in the contusion group that underwent scar resection demonstrated significantly worse functional scores relative to nonresection controls throughout most of the recovery period (p < 0.05). Both hemisection groups (resected and nonresected) demonstrated significantly higher recovery scores relative to either of the contusion groups (p < 0.005 for all comparisons between hemisection and contusion groups). Error bars represent standard error.
Axonal Regeneration
BDA+ axons were present in transverse spinal cord sections rostral and caudal to the area of contusion in all specimens, indicating successful labeling of descending and ascending tracts (Figure 2). Significant edema was not observed in any of the sections. Numerous intraneural injections into the L4 dorsal root ganglia failed to reveal any tracing of BDA+ axons within the ascending sensory tracts less than 7.0 mm caudal to the epicenter of cord contusion in any of the treatment groups (p < 0.05). This observation suggests that with these animals there was essentially no ascending axonal regeneration in the treatment groups. Tracing of the descending corticospinal tracts revealed the complete absence of BDA+ axons less than 7.0 mm proximal (rostral) to or at any point distal (caudal) to the center of injury in the contusion group in which resection of gliotic scar was performed (Figure 3). In both hemisection groups and in the nonresection contusion group, there was a minimal presence of BDA+ axons less than 7.0 mm rostral to the injury center, with statistically insignificant differences between the groups (p > 0.05 for all comparisons) (Figure 4). None of the groups demonstrated BDA+ axons at or caudal to the lesion epicenter.
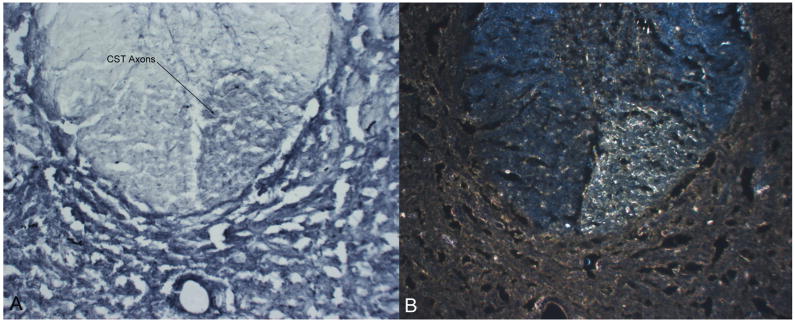
Figure 2.
Unilateral BDA-labeling of the corticospinal tract rostral to the site of lesion verifying darkly-stained axons under light microscopy (left) and confirmed using darkfield (right). cst: corticospinal tract
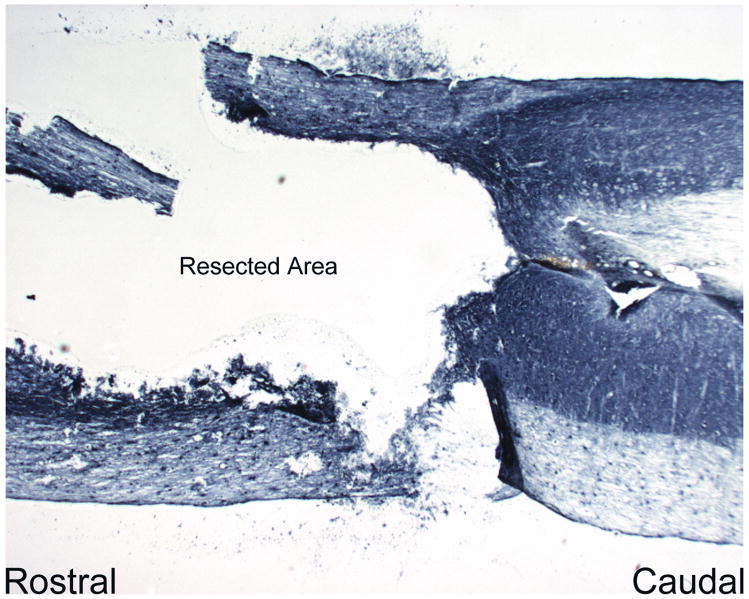
Figure 3.
Longitudinal image of the glial resection in the contusion injury model with complete absence of BDA+ axon labeling near the lesion site. (20× magnification)
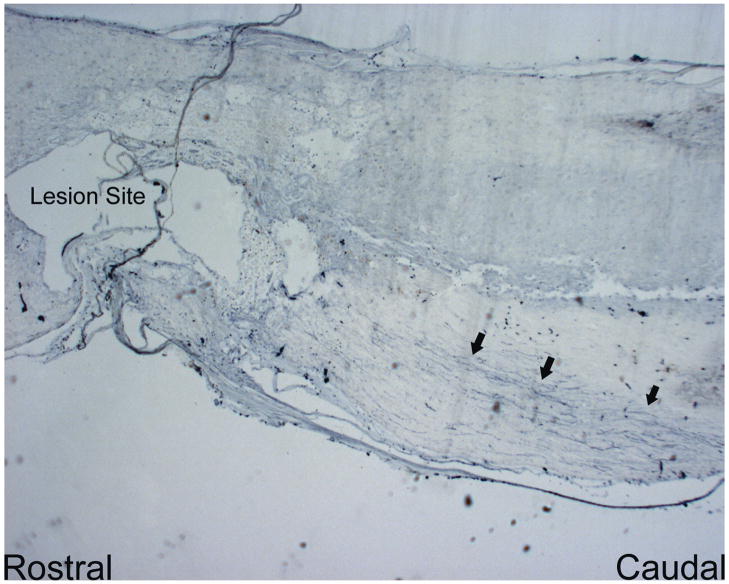
Figure 4.
Longitudinal image of the non-resected contusion injury lesion site showing modest BDA+ axons the dorsal portion of the spinal cord. (20× magnification)
DISCUSSION
Any successful treatment of spinal cord injury must address both the limited intrinsic regenerative capacity of CNS axons and the complex extrinsic mechanisms that impede their regeneration. Experimental approaches that enhance the axonal regenerative capability by introduction of so-called permissive elements to the injury site have had remarkable success. These pro-regenerative strategies include peripheral nerve and Schwann cell transplantation, olfactory-ensheathing cell transplantation, stem cell transplantation, direct gene therapy, and nerve trophic factor supplementation.8,20–24 Though necessary for axonal regeneration, application of these permissive elements alone is insufficient as a therapeutic modality.25 The intervention must also directly address the non-permissive, highly inhibitory substrata and milieu of the injured cord.26 As the majority of the inhibitory factors are contained by the glial scar, modulation of the gliotic response to cord injury is an inevitable objective of treatment.10
In this study, we investigated the effect of glial scar resection on spinal cord axonal regeneration and functional recovery. We found that although functional recovery was not affected by glial resection after bilateral dorsal hemisection injury in the adult rat, it was significantly worsened when the same procedure was performed after a moderate contusion injury. The difference in outcome relative to mechanism of injury suggests that the glial scar may serve variable purposes depending on the nature and extent of cord trauma.
Despite its multifaceted inhibitory influence, the glial scar must offer some protective benefit to the injured spinal cord.27 The role of the gliotic response in mitigating the extension of cord injury beyond the initial site of trauma, for instance, is well known.28 In a study of transgenic mice, selective ablation of reactive astrocytes in the glial scar after both contusion and penetrating spinal cord injury led to markedly increased tissue disruption, cellular degeneration, cystic changes, and profound and persistent motor deficits relative to nonablated controls.29 It is likely that both the cellular and extracellular matrix elements of the gliotic scar play a critical role in biochemical protection and structural stabilization of cord integrity, and thus function, after spinal cord injury. While this putative stabilization role of the scar may allow for modest axonal recovery in a contusion model of cord injury, its function is apparently less important in the particular hemisection model used in this study.
There are several possible explanations for this difference in glial scar function based on injury mechanism. The underlying severity of cell death and damage, and the ensuing requirement for fibrotic stabilization, may be vastly different between the two models of injury. Although the severity of the contusion injury can be precisely controlled, the extent of cord trauma compared to that in a hemisection injury at the same level is not well characterized. The field of injury and cystic degeneration after a moderate contusion injury are greater than after a hemisection injury that is limited mostly to the dorsomedial corticospinal tracts.27 The benefit of a stabilizing scar would then be proportionately different.
The potential for plasticity may be another source of the observed difference between the two groups. The hemisectioned cords may have a greater concentration of spared axons that could subsequently undergo compensatory sprouting and synapse formation with target motor units. Enhanced functional recovery would then be a function of this collateral sprouting rather than of regenerating axons.30 Greater plasticity due to collateral sprouting in the two hemisectioned groups may explain their superior functional recovery compared to the nonresection contusion group despite similar numbers of BDA+ axons at the lesion site among the three groups. Tract tracing schemes that better distinguish collateral fibers of spared axons from regenerating axons are currently under development.31 If the hemisectioned groups benefited from compensatory sprouting and the function-enhancing plasticity, this could potentially have offset any deleterious effects of glial scar resection.
The limitations of this investigation should be the focus of further studies. First, scar resection was performed at 1 week postinjury in all specimens. The glial scar is a dynamic entity with an evolving composition relative to the time of injury, it would be interesting to determine the effect, if any, of variable time points between injury and resection.10 Secondly, it is possible that the resection procedure itself may have caused further injury to the cord. If this were the case, one would have expected the scar-resected hemisection specimens to also have performed worse than, not identical to, the nonresection controls.
This study underscores the basic biological differences between the two types of spinal cord injury, which merit additional investigation. More importantly, it confirms the duality and profound complexity of the gliotic response to spinal cord injury. Contrary to prevailing dogma, the glial scar is not a uniformly deleterious structure: despite some of its nonpermissive features, it also plays an important and essential protective role after spinal cord injury. As the cellular and molecular components of the glial scar continue to be characterized, it will be possible to specifically neutralize the inhibitory constituents while preserving its pro-regenerative protective elements. The ability to modulate the behavior of the glial scar can enhance almost every existing modality of spinal cord injury treatment, from transplantation to gene therapy. Its conflicting role in recovery mandates further systematic studies.
Acknowledgments
Funding from NIH/NINDS NSO221 & NS049203 and the Roman Reed Foundation
References
- 1.Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–383. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- 2.Bahr M, Przyrembel C, Bastmeyer M. Astrocytes from adult rat optic nerves are nonpermissive for regenerating retinal ganglion cell axons. Exp Neurol. 1995;131:211–220. doi: 10.1016/0014-4886(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 3.Davies SJ, Goucher DR, Doller C, et al. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermanns S, Klapka N, Muller HW. The collagenous lesion scar--an obstacle for axonal regeneration in brain and spinal cord injury. Restor Neurol Neurosci. 2001;19:139–148. [PubMed] [Google Scholar]
- 5.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 6.Stichel CC, Muller HW. The cns lesion scar: New vistas on an old regeneration barrier. Cell Tissue Res. 1998;294:1–9. doi: 10.1007/s004410051151. [DOI] [PubMed] [Google Scholar]
- 7.Dinh P, Bhatia N, Rasouli A, et al. Transplantation of preconditioned schwann cells following hemisection spinal cord injury. Spine. 2007;32:943–949. doi: 10.1097/01.brs.0000261408.61303.77. [DOI] [PubMed] [Google Scholar]
- 8.Rasouli A, Bhatia N, Suryadevara S, et al. Transplantation of preconditioned schwann cells in peripheral nerve grafts after contusion in the adult spinal cord. Improvement of recovery in a rat model. J Bone Joint Surg Am. 2006;88:2400–2410. doi: 10.2106/JBJS.E.01424. [DOI] [PubMed] [Google Scholar]
- 9.Levi AD, Guenard V, Aebischer P, et al. The functional characteristics of schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J Neurosci. 1994;14:1309–1319. doi: 10.1523/JNEUROSCI.14-03-01309.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 11.McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 12.Smith-Thomas LC, Stevens J, Fok-Seang J, et al. Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J Cell Sci. 1995;108 ( Pt 3):1307–1315. doi: 10.1242/jcs.108.3.1307. [DOI] [PubMed] [Google Scholar]
- 13.Shearer MC, Niclou SP, Brown D, et al. The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signalling pathways. Mol Cell Neurosci. 2003;24:913–925. doi: 10.1016/j.mcn.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Klapka N, Hermanns S, Straten G, et al. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur J Neurosci. 2005;22:3047–3058. doi: 10.1111/j.1460-9568.2005.04495.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldshmit Y, Galea MP, Bartlett PF, et al. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24(45):10064–73. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bregman BS, Coumans JV, Dai HN, et al. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog Brain Res. 2002;137:257–273. doi: 10.1016/s0079-6123(02)37020-1. [DOI] [PubMed] [Google Scholar]
- 17.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 18.Keirstead HS, Hughes HC, Blakemore WF. A quantifiable model of axonal regeneration in the demyelinated adult rat spinal cord. Exp Neurol. 1998;151:303–313. doi: 10.1006/exnr.1998.6806. [DOI] [PubMed] [Google Scholar]
- 19.Zheng B, Ho C, Li S, et al. Lack of enhanced spinal regeneration in nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 20.Barakat DJ, Gaglani SM, Neravetla SR, et al. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 2005;14:225–240. doi: 10.3727/000000005783983106. [DOI] [PubMed] [Google Scholar]
- 21.Cao Q, Xu XM, Devries WH, et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill CE, Moon LD, Wood PM, et al. Labeled schwann cell transplantation: Cell loss, host schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338–343. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- 23.Pearse DD, Pereira FC, Marcillo AE, et al. Camp and schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 24.Plant GW, Christensen CL, Oudega M, et al. Delayed transplantation of olfactory ensheathing glia promotes sparing/regeneration of supraspinal axons in the contused adult rat spinal cord. J Neurotrauma. 2003;20:1–16. doi: 10.1089/08977150360517146. [DOI] [PubMed] [Google Scholar]
- 25.Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 26.Rabchevsky AG, Streit WJ. Grafting of cultured microglial cells into the lesioned spinal cord of adult rats enhances neurite outgrowth. J Neurosci Res. 1997;47:34–48. [PubMed] [Google Scholar]
- 27.Siegenthaler MM, Tu MK, Keirstead HS. The Extent of Myelin Pathology Differs following Contusion and Transection Spinal Cord Injury. J Neurotrauma. 2007;24:1631–1646. doi: 10.1089/neu.2007.0302. [DOI] [PubMed] [Google Scholar]
- 28.Yiu G, He Z. Glial inhibition of cns axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faulkner JR, Herrmann JE, Woo MJ, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagg T. Collateral sprouting as a target for improved function after spinal cord injury. J Neurotrauma. 2006;23:281–294. doi: 10.1089/neu.2006.23.281. [DOI] [PubMed] [Google Scholar]
- 31.Steward O, Zheng B, Tessier-Lavigne M. False resurrections: Distinguishing regenerated from spared axons in the injured central nervous system. J Comp Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]