SUMMARY
Catamenial epilepsy is a multifaceted neuroendocrine condition in which seizures are clustered around specific points in the menstrual cycle, most often around perimenstrual or periovulatory period. Generally, a two-fold or greater increase in seizure frequency during a particular phase of the menstrual cycle could be considered as catamenial epilepsy. Based on this criteria, recent clinical studies indicate that catamenial epilepsy affects 31 – 60% of the women with epilepsy. Three types of catamenial seizures (perimenstrual, periovulatory and inadequate luteal) have been identified. However, there is no specific drug available today for catamenial epilepsy, which has not been successfully treated with conventional antiepileptic drugs. Elucidation of the pathophysiology of catamenial epilepsy is a prerequisite to develop specific targeted approaches for treatment or prevention of the disorder. Cyclical changes in the circulating levels of estrogens and progesterone play a central role in the development of catamenial epilepsy. There is emerging evidence that endogenous neurosteroids with anticonvulsant or proconvulsant effects could play a critical role in catamenial epilepsy. It is thought that perimenstrual catamenial epilepsy is associated with the withdrawal of anticonvulsant neurosteroids. Progesterone and other hormonal agents have been shown in limited trials to be moderately effective in catamenial epilepsy, but may cause endocrine side effects. Synthetic neurosteroids, which enhance the tonic GABA-A receptor function, might provide an effective approach for the catamenial epilepsy therapy without producing hormonal side effects.
Keywords: Epilepsy, neurosteroid, allopregnanolone, THDOC, androstanediol, GABA-A receptor, progesterone withdrawal, menstrual cycle, ganaxolone, catamenial seizures, ovarian hormones
DEFINITION AND PREVALENCE OF CATAMENIAL EPILEPSY
Introduction
Epilepsy is one of the most common chronic neurological disorders characterized by the unpredictable occurrence of seizures. However, there is a form of epilepsy, called catamenial epilepsy, which does not adhere to this lack of pattern. Catamenial epilepsy, derived from the Greek word katomenios, meaning “monthly”, is characterized by seizures that cluster around specific points in the menstrual cycle (Fig. 1). Catamenial epilepsy affects from 10 – 70% of women with epilepsy (Dickerson, 1941; Rosciszewska, 1980; Tauboll et al., 1991; Duncan et al., 1993; Towanabut et al., 1998; Herzog et al., 2004; Gilad et al., 2008). The large variation in prevalence of catamenial epilepsy is partly because of methodological differences such as the criteria used for defining seizure exacerbation in relation to menstrual cycle, patients self-reporting, diaries, and other inaccurate records of seizures relating to menses (Duncan et al., 1993; Herzog et al., 2004; Bazan et al., 2005; El-Khayat et al., 2008). Despite such high incidence and increased awareness, there is no widely accepted definition of catamenial epilepsy.
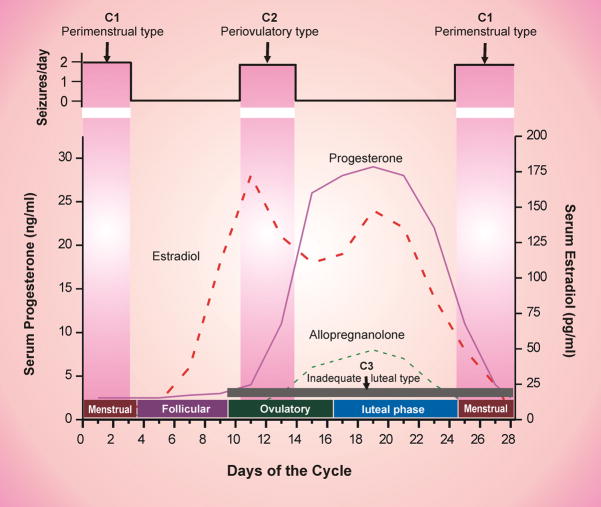
Fig. 1. Temporal relationship between ovarian hormones and occurrence of catamenial seizures during the menstrual cycle.
The upper panel illustrates the strong relationship between seizure frequency and estradiol/progesterone levels. The lower panel illustrates the three types of catamenial epilepsy. The vertical gray bars (left and right) represents the likely period for the perimenstrual (C1) type, while the vertical gray bar (middle) represent the likely period for the periovulatory (C2) type. The horizontal dark gray bar (bottom) represent the inadequate luteal (C3) type that likely occur starting early ovulatory to menstrual phases.
Definition of catamenial epilepsy
Catamenial epilepsy is commonly defined as the cyclical increase in seizures around the time of menses or at other phases of the menstrual cycle. According to Duncan et al., (1993), catamenial epilepsy is defined based upon the criteria of having at least 75% of the seizures during a 10-day period of the menstrual cycle beginning 4 days before menstruation. In the seminal study, Herzog et al. (1997) defined catamenial epilepsy as a greater than average seizure frequency during perimenstrual or periovulatory periods in normal ovulatory cycles and during the luteal phase in anovulatory cycles. Based on the review of a vast clinical experience, Newmark and Penry (1980) defined perimenstrual catamenial epilepsy as epileptic seizures occurring in women of fertile age exclusively or significantly more often during a 7-day period of the menstrual cycle, beginning 3 days before menstruation and ending 4 days after its onset. In recent study, Tuveri et al., (2008) utilized a fractional change method to calculate the catamenial change in seizure frequency. These are simple definitions for a rapid clinical assessment of subjects with catamenial epilepsy, but are arbitrary, quite variable, and there is little consensus in the clinical scientific literature for unified definition. Catamenial seizure exacerbations also can occur at other phases of the menstrual cycle but the wealth of information is limited. In general, a two-fold or greater increase in seizure frequency during a particular phase of the menstrual cycle could be considered as catamenial epilepsy (Reddy, 2004a; 2007). This simple definition can be used as standard criterion in study designs for the investigation of the pathophysiology and treatment of catamenial epilepsy.
Prevalence of catamenial epilepsy
Based on this criterion, recent studies confirmed that catamenial epilepsy affects 31–60% of women with epilepsy (Herzog et al., 2004; Bazan et al., 2005; El-Khayat et al., 2008). Thus, there is a need to reconcile these differences on the prevalence rate of catamenial epilepsy. In the latest study by Herzog et al., (2004), the frequency of catamenial epilepsy was assessed in 87 women who chartered seizures and menses during three cycles. They found that 39% of the women had catamenial epilepsy. In the more recent study by El-Khayat group (2008), a total of 31% of women with epilepsy showed catamenial seizures. The discrepancy may be due to the fact that the women included in these studies represent a select population and may not reflect the prevalence of catamenial seizures in the general epileptic population. Overall, these studies support the prevailing notion that at least 1 in every 3 women with epilepsy show catamenial seizure exacerbation.
Epilepsy affects an estimated 1.3 million women in the United States (Kaplan et al., 2007; Pennell, 2008). Based on the above prevalence rate, approximately 400,000 women with epilepsy in the United States are experiencing catamenial seizures in their active reproductive years. Catamenial seizures are often quite resistant to available drug treatments; some attacks induce impairment of consciousness, thereby limiting performance of many normal activities. This is primarily because there is no effective prevention or cure for catamenial epilepsy. There is a large gap in our understanding of what changes occur in the brain in relation to the hormonal fluctuations associated with catamenial epilepsy and how these changes alter sensitivity to anticonvulsant drugs. Thus, a detailed understanding of the patterns and pathophysiology is essential for the development of rational approaches for the prevention or treatment of catamenial epilepsy.
This article describes the role of endogenous neurosteroids in seizure susceptibility and the pathophysiology of catamenial epilepsy, with an emphasis on the therapeutic potential of neurosteroid-based strategies for treatment of catamenial seizures.
PATTERNS AND DIAGNOSIS OF CATAMENIAL EPILEPSY
Types of catamenial epilepsy
Studies on the frequency of catamenial epilepsy demonstrate the complexity of the condition and the difficulty in studying it. The need to develop better diagnostic tools of catamenial epilepsy and catamenial types has been widely recognized. Herzog and colleagues (1997) have described three distinct types of catamenial epilepsy: perimenstrual (C1), periovulatory (C2), and inadequate luteal-phase (C3) catamenial seizures (Table 1). However, these authors observed that the conventional perimenstrual form is the most common clinical type. Perimenstrual and periovulatory types are illustrated in Figure 1. The specific pattern of catamenial epilepsy can be identified simply by charting menses and seizures and obtaining a mid-luteal phase serum progesterone level to distinguish between normal and inadequate luteal phase cycles (Herzog et al., 2008). Several clinical markers can be used to check ovulation (see next paragraph). Overall, catamenial epilepsy designation can be made if a two-fold or greater increase in seizure frequency is observed during a particular phase of the menstrual cycle. In the primary clinical type, perimenstrual catamenial epilepsy, women with epilepsy experience an increase in seizure activity before, during or after the onset of menstruation (Newmark and Penry, 1980; Reddy, 2004a). Because of these types or the potential for multiple types, the precise diagnosis of menstrual cycle related seizures is complex and needs some background neuroendocrine information.
Table 1.
Three type of catamenial epilepsy proposed by Herzog (1997).
| Type | Characteristics |
|---|---|
| Perimenstrual (C1) | Characterized by a greater average daily seizure frequency during the menstrual phase (day −3 to +3) compared with the midfollicular (day 4 to 9) and midluteal (day −12 to 14) phases in normal ovulatory cycles. Incidence: 71%* |
| Periovulatory (C2) | Characterized by a greater average daily seizure frequency during the ovulatory phase (day 10 to −13) compared with the midfollicular and midluteal phases in normal ovulatory cycles. Incidence: 71%** |
| Inadequate luteal (C3) | Characterized by a greater seizure frequency during the ovulatory, luteal, and menstrual phases than during the midfollicular phase in women with inadequate luteal-phase cycles. This seizure exacerbation may extend from day 9 of one cycle to day 2 of the following cycle. Incidence: 78%** |
71% of women with normal ovulatory cycles had perimenstrual or periovulatory type.
78% of women with inadequate luteal-phase cycle showed the luteal type.
Ovulatory and anovulatory cycles
Catamenial epilepsy is observed in women with ovulatory or anovulatory cycles. Women with ovulatory cycles could experience either the perimenstrual or periovulatory catamenial types or even both within a single cycle (Bauer et al., 1998; Bauer, 2001). About 16.5% of cycles in study subjects are found to be anovulatory (Herzog et al., 2004), and these women showed a third type, referred to as inadequate luteal-phase or anovulatory luteal seizures. About 71% of women with normal ovulatory cycles had perimenstrual or periovulatory patterns, while 78% of women with anovulatory cycle showed the inadequate luteal type catamenial seizures (Table 1). In a separate study among patients showing catamenial epilepsy, 53% had the perimenstrual type, while the remaining showed inadequate luteal type (El-Khayat et al., 2008). Cortical excitability is found to be different in women during ovulatory and anovulatory cycles (Hattemer et al., 2007). There is new information on the distribution of catamenial seizure exacerbation patterns in relation to ovulation status (Herzog, 2008). As expected, the perimenstrual and periovulatory types occurs more frequently with ovulatory than anovulatory cycles, but inadequate luteal type can also occur in ovulatory cycles if they have high midluteal estradiol/progesterone ratios.
The diagnosis of ovulatory or anovulatory cycles is often made by estimating the midluteal phase progesterone levels. Progesterone levels lower than 5 ng/ml during days 20 through 22 of the cycle would certainly indicate inadequate luteal phase. Biopsy evidence of an underdeveloped secretory endometrium in 8 to 10 days postovulation is a much robust marker to assess inadequate luteal phase cycles. Subjects can be examined by pelvic-abdominal ultrasound to measure size of mature graffian follicle as a sign of ovulation. The simple, household approach to track ovulation is to record the lowest body temperature of the day usually taken each morning before getting out of bed. The lowest morning temperature of the month occurs just prior to ovulation.
Diagnosis of catamenial epilepsy
The diagnosis of catamenial epilepsy is mainly based on the assessment of menstruation and seizure records (Foldvary-Schaefer and Falcone, 2003; Herzog, 2006). The simple approach of evaluation of catamenial epilepsy, that is, whether the patient’s seizures tend to worsen at certain points of the menstrual cycle, is to record seizure diary in relation to menstrual cycle. Using the first day of menstrual bleeding as the first day of the cycle, the menstrual cycle is divided into four phases: (a) menstrual phase, days −3 to +3; (b) follicular phase, days +4 to +9; (c) ovulatory phase, days +10 to +16; and (d) luteal phase, days +17 to −4 (see Fig. 1). The number of seizures in each phase is counted for at least 2 cycles and a two-fold or greater increase in frequency during a particular phase of the menstrual cycle can be used as diagnostic criteria of catamenial epilepsy.
Record of detailed diary of seizures and the menstrual cycle would be important for accurate diagnosis of catamenial epilepsy. As shown in Table 1, the perimenstrual catamenial type is characterized by a greater average daily seizure frequency during the menstrual phase compared with the midfollicular and mid-luteal phases (Herzog et al., 1997). The periovulatory type is characterized by a greater average daily seizure frequency during the ovulatory phase, compared with the midfollicular and mid-luteal phases in normal ovulatory cycles. In women with anovulatory cycles, the inadequate luteal catamenial type is characterized by a greater seizure frequency during the ovulatory, luteal, and menstrual phases than during the midfollicular phase. Therefore, inadequate luteal type is more difficult to identify compared to the other two types that occur in normal menstrual cycles. Overall, the seizure frequency-menstrual days chart in women with catamenial epilepsy would show greater catamenial exacerbation than the point of inflection of the S-shaped distribution of the seizure exacerbation curve (Herzog et al., 2004).
PATHOPHYSIOLOGY OF CATAMENIAL EPILEPSY
Association of catamenial seizures and epileptic syndromes
Catamenial seizures are more common among women with focal epilepsy, especially temporal lobe epilepsy, compared with those who have generalized epilepsy but it is associated with every epilepsy syndrome (Marques-Assis, 1981; Morrel, 1999; Foldvary-Schaefer and Falcone, 2003). Catamenial seizures are seen in women with idiopathic, cryptogenic or symptomatic epilepsy and in subjects showing focal and generalized seizures (El-Khayat et al., 2008). Catamenial seizures are observed in women who are treated with first-generation and second-generation AEDs. There is no compelling evidence for specific association of catamenial seizures with any AED. However, as seen in the next section (see “AEDs”), estrogens and progesterone are both susceptible to drug interactions with some AEDs. Some AEDs are categorized as enzyme-inducing (Table 2), and thereby cause enhanced metabolism of steroid hormones that share common metabolic pathways.
Table 2.
List of AEDs that do and do not induce hepatic enzymes.
| Enzyme-inducing AEDs | Enzyme non-inducing AEDs |
|---|---|
| Carbamazepine (Tegretol) | Clonazepam (Rivotril) |
| Felbamate (Felbatol) | Ethosuximide (Zarontin) |
| Lamotrigine (Lamictal)* | Gabapentin (Neurontin) |
| Oxcarbazepin (Trileptal) | Levetiracetam (Keppra) |
| Phenobarbital (Luminal) | Pregabalin (Lyrica) |
| Phenytoin (Dilantin) | Tiagabine (Gabitril) |
| Topiramate (Topamax) | Valproate (Epilim) |
| Zonisamide (Zonegran) |
weak enzyme inducer
Pharmacoresistance and catamenial seizures
Catamenial epilepsy is form of intractable epilepsy. Many women with catamenial epilepsy show cyclical increase in seizure exacerbations despite treatment with AEDs, and therefore, catamenial epilepsy can be considered as a form of intractable or pharmacoresistant epilepsy. Despite with the best available drug treatment, many women do not respond or failed to exhibit resolution of catamenial seizures, while some women show substantial improvement in their seizure at certain times during the menstrual cycle. Therefore, women with catamenial epilepsy have intractable seizures and some may exhibit state-dependent pharmacoresistance (Reddy and Rogawski, 2009).
Reproductive status and catamenial seizures
Studies on the influence of gonadal hormones on seizure susceptibility shows a complex and predictable pattern. Changes in seizure activity in women can occur during changes in reproductive status (i.e. entering puberty, during pregnancy or after menopause). Although there is no overall consensus, puberty can affect the course of epilepsy. A significant increase in the incidence of generalized tonic-clonic seizures is observed in adolescents with epilepsy during puberty as compared with before puberty (Niijima and Wallace, 1989; Rosciszewka, 1987). Catamenial seizures can originate in women at puberty or in adolescent females with regular menstrual cycles. During puberty, the level of steroid hormones increases and menstrual periods begin. Because steroid hormones influence seizure susceptibility, seizure types may change as females with epilepsy go through puberty. Pregnancy affects seizure frequency and drug metabolism, raising special concerns in women with epilepsy (Pennell, 2008). However, such issues are beyond the scope of this article.
Currently there is little information on the relationship between epilepsy and menopause. Natural reductions in steroid hormones around perimenopause and menopause are associated with alterations in frequency or severity of seizures in women with epilepsy (Abbasi et al., 1999; Harden et al., 1999; 2006). There is emerging clinical evidence suggesting that menopause is associated with the increase in seizures in about 30% of women with epilepsy, but there is no consensus on these findings. Some women going through menopause have fewer seizures and many experience no change at all. Hormone replacement therapy is significantly associated with an increase in seizure frequency during menopause, and this is more likely in women with a history of catamenial epilepsy (Harden et al., 2006; Harden, 2008). It has been suggested that seizures may improve after menopause, especially in the women with catamenial epilepsy (Roste et al., 2008). One way to resolve this problem is to use animal models, in which the impact of reproductive senescence on ovarian hormones and frequency of seizures can be studied under more controlled conditions.
Potential causes of catamenial seizures
The exact cause of catamenial epilepsy remains unclear. Catamenial epilepsy is among the oldest neurological disorders known with early reports in 1881 (Gowers, 1881), yet the molecular mechanisms involved in the pathophysiology of catamenial epilepsy are not well understood. There is presently no specific treatment, and, often, conventional therapies have a disappointing lack of effect (Reddy, 2004a). Research in this field is focused on several basic questions such as: (i) What hormonal changes are responsible for catamenial seizure exacerbations? (ii) What changes occur in the brain in relation to the hormonal fluctuations associated with menstrual cycle? (iii) How these changes alter sensitivity to anticonvulsant drugs? (iv) Can an understanding of the pathophysiology of catamenial seizures be used to develop specific targeted approaches for prevention or treatment of the disorder? Investigations focused on the above issues would help elucidate the physiological basis of catamenial epilepsy and identify molecular targets essential for developing specific approaches for treatment of the disorder.
Catamenial epilepsy is a multifaceted condition attributed to numerous causes. Epilepsy typically develops due to certain genetic defect or often after a presumed initiating injury. Current hypothesis about the pathogenesis of epilepsy involves three stages: (i) the initial precipitating event; (ii) the latent period; and (iii) the chronic period with spontaneous seizures. Catamenial epilepsy, in many cases, is assumed to be an acquired disorder and currently there is no clear evidence of genetic components. A variety of mechanisms such as fluctuations in antiepileptic drug levels, changes in water and electrolyte balance, and physiological variation in ovarian hormone secretion have been proposed as causes for catamenial epilepsy (McQuarrie and Peeler, 1931; Ansell and Clarke, 1956; Shavit et al., 1984; Rosciszewska et al., 1986; Kumar et al., 1988; Narbone et al., 1990; Herzog, 1991; Herkes et al., 1993; Rodriguez-Macias, 1996; Harden et al., 1999; Tuveri et al., 2008). Overall, cyclical changes in the circulating levels of estrogens and progesterone are now widely accepted to play a central role in the development of this condition (Fig. 1). Generally, estrogens are found to be proconvulsant, while progesterone has powerful antiseizure effect and reduces seizures, and thus they play a central role in the pathophysiology of catamenial epilepsy. There is emerging evidence that stress-induced adrenal steroid hormones and androgens also influence seizure susceptibility (Joels, 1997; Reddy and Rogawski, 2002; Reddy, 2003a; 2004a; Rhodes et al., 2004) (see “Hormones and Neurosteroids”).
Menstrual cycle-related fluctuations in steroid hormones
The natural pattern of estrogen and progesterone production during the menstrual cycle is illustrated in Figure 1. In general, the female reproductive cycle is estimated to last 29 days. Day 1 is the onset of menstruation, and ovulation occurs 14 days before the onset of menstruation. The menstrual cycle is divided into four phases: (i) menstrual phase, days −3 to +3; (ii) follicular phase, days +4 to +9; (iii) ovulatory phase, days +10 to +16; and (iv) luteal phase, days +17 to −4. The early follicular phase is associated with low levels of estrogens and progesterone. The synthesis and secretion of estrogens and progesterone from the ovaries is controlled primarily by the hypothalamic GnRH and pituitary gonadotropins, FSH and LH. As ovulation approaches, the level of estrogen rises and triggers the release of a large surge of LH leading to ovulation. Following ovulation, the ruptured follicle luteinizes and forms a corpus luteum that secretes progesterone and estrogen. Estradiol begins to rise during the early follicular phase with the surge around midcycle that precedes ovulation. Then estradiol levels fall slightly at the beginning of luteal phase and increase to another peak at midluteal phase followed by a drop around perimenstrual phase. The progesterone levels starts to rise following ovulation, reaching sustained elevated levels throughout the luteal phase and decline before menstruation begins (Speroff et al., 1999). The neurosteroid allopregnanolone is increased in parallel to its precursor, progesterone (Reddy, 2004a; Tuveri et al., 2008) (see “Hormones and Neurosteroids”).
Reproductive abnormalities and AEDs
Reproductive disorders that affect the normal ovarian cycle function are implicated in catamenial epilepsy. In women with epilepsy, both seizures and antiepileptic drugs can disturb menstrual cycle (Morrell and Montouris, 2004). Seizures can profoundly affect steroid hormone secretion and regulation in women with epilepsy and are the leading cause of increased incidence of menstrual disturbances in epilepsy. For example, seizures can alter the release of hypothalamic and pituitary hormones such as LS/FSH secretion, while some antiepileptic drugs alter concentrations of sex steroid hormones. Reproductive dysfunction is common among patients with epilepsy and studies have found reduced fertility rates among women with epilepsy (Morrell and Montouris, 2004; Herzog, 2008). Women with epilepsy are at increased risk for polycystic ovary syndrome, which is characterized by enlargement of the ovaries with thickened stroma and numerous subcapsular follicular cysts, hirsutism, acne and obesity (Bilo and Meo, 2006). Epilepsy, AEDs and endocrine abnormalities have been casually linked to reproductive dysfunction in women. These are complex issues, seizure-induced disruptions or reproductive disorders that can affect the delicate normal mechanisms regulating ovarian cycle (e.g. ovulation, progesterone secretion) and thereby could adversely impact catamenial seizures.
Some AEDs are linked to the exacerbation of catamenial seizures. AED use is associated with changes in the serum levels of biologically active steroid hormones. AEDs can be divided into two groups, enzyme-inducing and non-enzyme-inducing AEDs (Table 2). AEDs such as phenytoin, carbamazepine and phenobarbital are potent inducers of liver cytochrome P450 isoforms (Rogawski and Loescher, 2004). The CYP3A4 and the other cytochrome P450 isoenzymes metabolize AEDs to a more water-soluble form, rendering them available for renal excretion. Because of common metabolic pathways, the AED-induced enzyme induction leads to enhanced metabolism of steroid hormones (Isojarvi et al, 2005) which may play a role in breakthrough seizures in women. Conversely, hepatic enzyme inhibitors like sodium valproate can increase the active steroid hormone levels. However, there is no direct clinical data available regarding the occurrence of catamenial seizures due to use of enzyme-inducing AEDs. In addition, the use of the enzyme-inducing AEDs phenobarbital, phenytoin and carbamazepine increases serum sex hormone-binding globulin concentrations in women with epilepsy, which may ultimately result in diminished concentrations of “free” or “biologically active” forms of steroid hormones. However, it remains to be determined to what extent such mechanisms contribute to catamenial epilepsy.
Contraceptive use and catamenial seizures
There is no evidence that the use of contraceptives increase the risk of seizures in women with epilepsy. Although some reports suggest that oral contraceptives (OCs) might exacerbate seizures, most studies show no effect of estrogen-based contraceptives on seizure frequency (Betts et al., 2003; Harden and Leppik, 2006; Velísková, 2007). In contrast, OC administration may alleviate the menstrually-related seizures. Provera (medroxyprogesterone acetate) was reported as a 3α-hydroxysteroidoxidoreductase inhibitor which implies that this agent might enhance synthesis of inhibitory neurosteroids in the brain (Belelli and Herd, 2003). However, there are many factors to consider in the choice of AED therapy and hormonal contraception since some AEDs can reduce the efficacy of OCs due to pharmacokinetic interactions (Crawford, 2005; Harden and Leppik, 2006). The enzyme-inducing AEDs cause enhanced metabolism of either or both the estrogenic or progestogenic component of OCs, thereby reducing their efficacy in preventing pregnancy. Moreover, there is evidence that OCs can also decrease the concentrations of AEDs such as lamotrigine and thereby increase the risk of seizures (Sabers et al., 2003; Sabers and Gram, 2006). Thus, these enzyme-inducing AEDS are likely to affect contraception and non-enzyme-inducing AEDs are unlikely to affect contraception.
ROLE OF HORMONES AND NEUROSTEROIDS IN CATAMENIAL EPILEPSY
Steroid hormones play a key role in the neuroendocrine control of neuronal excitability and brain function. As illustrated in Figure 1, cyclical changes of ovarian hormones estrogens and progesterone are now widely believed to be important in the pathogenesis of catamenial epilepsy. Generally, progesterone is anticonvulsant, while estrogen is proconvulsant. There is emerging evidence that changes in endogenous neurosteroids, including those derived from adrenal steroid hormones and circulating androgens, could substantially influence seizure susceptibility (Reddy, 2004a; 2006), and catamenial epilepsy (El-Khayat et al., 2008; Tuveri et al., 2008).
Estrogens
The role of estrogens in seizure susceptibility is highly complex. In general, estrogens have proconvulsant and epileptogenic properties in animals and humans. There are also studies that support protective effects of estrogens and it may also be anticonvulsant under some circumstances. The profile of estrogens in preclinical and clinical studies is described below.
Preclinical studies
There are three biologically active estrogens: estrone (E1), estradiol (E2), and estriol (E3). Estradiol is the major estrogen in premenopausal women, while estriol and estrone are less abundant than estradiol. In pregnancy, however, estriol is quantitatively the major estrogen. Postmenopausally, estrone is the principal estrogen and estradiol is made primarily via metabolism of estrone. There are two isomers of estradiol, 17β-estradiol and 17α-estradiol. 17β-Estradiol is the major isomer with bioactivity in menstruating women. Estradiol has been widely investigated in animal epilepsy models. However, the effect of estrogens on seizure susceptibility is highly variable and depends on factors such as treatment duration, dosage, hormonal status and the seizure model (Veliskova, 2007).
Early studies of estradiol administration to ovariectomized rats revealed proconvulsant effects (Logothetis and Harner, 1960; Wooley and Timiras, 1962a,b). Estrogens applied to cortex could increase cortical electrographic activity and/or elicit seizures (Marcus et al., 1966; McQueen and Woodbury, 1975; Julien et al., 1975). Studies using stimulus-evoked seizure threshold in rats showed that estrogen decreased seizure threshold or afterdischarge threshold (Woolley and Timiras, 1962a; Stitt and Kinnard, 1986). Subsequently, estradiol has been shown to facilitate kindling and audiogenic seizures in animals (Werboff and Havlena, 1963; Teresawa and Timiras, 1968; Hom and Buterbaugh, 1986; Buterbaugh, 1989; Buterbaugh and Hudson, 1991; Edwards et al., 1999). Estradiol also potentiates seizures induced by chemoconvulsants, pentylenetetrazol and kainic acid (Nicoletti et al., 1985; Woolley, 2000) (see review Woolley and Schwartzkroin, 1998). Acute administration of estradiol enhances the frequency and severity of PTZ-induced seizures (Reddy, 2004b), an effect consistent with its activity in several experimental models of partial and limbic seizures (Nicoletti et al., 1985; Hom and Buterbaugh, 1986). The proconvulsant-like activity of estradiol is most consistently demonstrated after chronic treatment in male rodents (Pericic et al., 1996; Saberi and Pourgholami, 2003). Further, studies in slices supported the idea that estrogen facilitates hippocampus excitability (Wong and Moss, 1994; Tauboll et al., 1991; Joels, 1997, see review Scharfman and MacLusky, 2006). In view of the above reports, there is a general consensus that estradiol is “proconvulsant” and facilitates seizure activity, although there are exceptions as described below. It is argued that focal seizures are more sensitive to estradiol than seizures induced by systemic chemoconvulsants.
The effect of estrogens on hippocampus excitability and seizure susceptibility is controversial. While estradiol has been shown to be proconvulsant in several studies, there is also evidence that support lack of effect or protective effect of estrogens. There are several studies using chronic estrogen administration in females that show either anticonvulsant or no effect of estrogen on seizures (Hoffman et al., 2003; Kalkbrenner and Standley, 2003; Reibel et al., 2000; Veliskova et al., 2000; Veliskova and Velisek, 2007). In low doses, estradiol can produce neuroprotective effects (see review, Velísek and Velísková, 2008). The estrogen-induced neuroprotection has been first demonstrated independently by Veliskova and colleagues (2000) and Reibel and colleagues (2000) in status epilepticus models. The neuroprotective activity of estrogens is then confirmed by several subsequent studies (Hoffman et al., 2003; Kalkbrenner and Standley, 2003; Veliskova and Velisek, 2007). Estradiol can protect neurons from seizure-induced damage. For example, estradiol regulation of the hippocampal expression of glutamic acid decarboxylase (GAD), the principal enzyme for the synthesis of inhibitory neurotransmitter GABA (Weiland, 1992; Joh et al., 2006), and estradiol modulation of neuropeptide Y (NPY) expression (Nakamura et al., 2004; Ledoux et al., 2009), especially estradiol increase in dentate gyrus inhibition via augmentation of hilar NPY (Veliskova and Velisek, 2007), could possibly be relevant to inhibition of seizures. Overall, there is new perception that both proconvulsant and neuroprotective features apply only under specific conditions and may be separated by therapy taking into account the dosage paradigm, timing, sex of the subjects and their gonadal hormone status. For example, the neuroprotective effect was observed following estradiol therapy in ovariectomized female rats (Veliskova, 2006) or aromatase inhibition in cultured hippocampal neurons (Zhou et al., 2007).
Clinical studies
Estradiol has been known to play a role in the exacerbation of seizures in women with epilepsy (Logothetis et al., 1959; Backstrom, 1976; Jacono and Robinson, 1987; Morrell, 1999). Plasma estradiol levels are found to increase during both the follicular and luteal phase of the normal menstrual cycle (Fig. 1). Backstrom (1976) was the first investigator to characterize the relationship between seizures and steroid hormones. In women with epilepsy, a positive correlation between seizure susceptibility and the estrogen-to-progesterone ratio was observed, peaking in the premenstrual and preovulatory periods and declining during the midluteal phase. Logothetis and colleagues (1959) have demonstrated that intravenous infusions of estrogen were associated with rapid interictal epileptiform activity in women with epilepsy and seizures were exacerbated when estrogen was given premenstrually. Therefore, it is hypothesized that estrogens may facilitate some forms of catamenial seizures observed during these phases. The periovulatory catamenial exacerbation has been attributed to the midcycle surge of estrogen that is relatively unopposed by progesterone until early luteal phase (Logothesis et al., 1959). An increase in the ratio of estrogen-to-progesterone levels during perimenstrual period (described below) might at least partly contribute to the development of perimenstrual seizure exacerbation (Bonuccelli et al., 1989; Herzog et al., 1997).
Interestingly, the serum concentration of estradiol in women with catamenial epilepsy is similar during the entire menstrual cycle to that of control subjects (Tuveri et al., 2008; El-Khayat et al., 2008). As expected, estradiol levels in women with catamenial epilepsy are lower in perimenstrual phase (~35 pg/ml) than the midluteal (~104 pg/ml) and follicular phases (~151 pg/ml). In the perimenstrual phase, progesterone levels are lower and the estrogen-to-progesterone ratio was higher in women with catamenial epilepsy (El-Khayat et al., 2008). In many patients with catamenial epilepsy, a marked increase in spike and wave discharges are observed during menstruation (Lin et al., 1952; El-Khayat et al., 2008). In addition, estradiol may play a prominent role in anovulatory cycles (Hattemer et al., 2007). However, the exact relationship between circulating estrogens and the perimenstrual or anovulatory catamenial seizures remains unclear.
Mechanisms of estrogens
The mechanisms involved in the excitatory effects of estrogens are highly complex. The biological effects of estrogens are mediated by two distinct estrogen receptors – ERα and ERβ (Lewandowski and Kaczmarek, 2002; Matthews and Gustafsson, 2004). Estrogen binding to the “classic” ERα or ERβ in the nucleus leads to cascade of events ultimately leading to modulation of target genes. ERα is widely distributed in the brain and reproductive organs of both females and males, whereas ERβ is more widely distributed in the female brain. Some cellular effects of estrogens are thought to be mediated by the ERX, a “membrane receptor” for estrogen that is not blocked by pharmacological antagonists of nuclear estrogen receptors (Ramirez and Zheng, 1996; Toran-Allerand et al., 2002). However, ERX is not completely characterized in the brain. Apart from classical estrogen receptor-mediated effects, estradiol affects neuronal excitability due to its organizational effects on synaptic structure and function (Pozzo-Miller et al., 1999; Maclusky et al., 2005). This mechanism may be apparent in estradiol’s ability to enhance glutamate receptor-mediated excitatory neurotransmission (Smith et al., 1988; Wong and Moss, 1994) and decrease GABAergic inhibition (Murphy et al., 1998). Estradiol acts on neurons within the limbic system, cerebral cortex and other regions important for seizure susceptibility. Both direct effects on glutamate receptor subtypes, and indirect effects through an increase in dendritic spine density of hippocampal N-methyl-D-aspartate (NMDA) receptor have been shown to be involved in estradiol modulation of the NMDA receptor function (Woolley and McEwen, 1994; Woolley et al., 1997; Rudick and Woolley, 2001). Chronic exposure of rats to estradiol increases the number and density of dendritic spines and excitatory synapses on hippocampal neurons that could increase the synchronization of synaptically driven neuronal firing in the hippocampus. This mechanism could be relevant to estradiol’s proconvulsant effects in animal models.
Recently, novel mechanisms underlying the estrogen regulation of hippocampal seizure activity have been proposed. There is emerging evidence of estrogen and neurotrophin interactions (Scharfman and MacLusky, 2006). Brain-derived neurotrophic factor (BDNF) is upregulated by estrogen in the mossy fiber pathway, because estrogen has a response element on the BDNF gene. BDNF, which is an agonist at trkB receptors, increases glutamatergic transmission in the hippocampus with resulting hyperexcitability and proconvulsant effects. Scharfman and colleagues (2003) have demonstrated that BDNF mediates estrogen actions on hippocampus excitability, thus explaining the proconvulsant actions of estrogen. Interestingly, trkB knockout mice are highly resistant to kindling epileptogenesis (He et al., 2004). Seizures induce dramatic increases in BDNF expression in both animals and patients with epilepsy (Murray et al., 2000). Taken together, BDNF and trkB receptors are important signaling systems in the estrogen-induced hippocampus excitability. The normal rise in estrogen during the periovulatory period can elevate BDNF, thereby playing a role in periovulatory catamenial epilepsy.
Progesterone
Progesterone plays a vital role in catamenial epilepsy. Unlike estrogen, the role of progesterone is clearer with consistent anticonvulsant and antiepileptic properties in animals and humans. Presently progesterone is undergoing a multicenter clinical trial in women with epilepsy (Herzog et al., 2008). The protective effects of progesterone and its molecular mechanism of action are described below.
Preclinical studies
Progesterone is secreted by the corpus luteum in the ovary. Hans Selye (1942) was the first to report the anticonvulsant properties of progesterone in the PTZ test. Consequently, progesterone has long been known to have antiseizure activity in a variety of animal models of epilepsy (Craig, 1966; Landgren et al., 1978). In recent years, numerous studies have confirmed the powerful anticonvulsant activity of progesterone in diverse animal seizure models (Landgren et al., 1978; Holmes and Weber, 1984; Mohammad et al., 1998; Kokate et al., 1999a; Frye and Scalise, 2000). Progesterone inhibits seizures in the PTZ, kindling and maximal electroshock tests (Kokate et al., 1999a; Lonsdale and Burnham, 2003; Reddy et al., 2004). Like neurosteroids, progesterone is inactive or requires high sedative doses to protect against seizures induced by glutamate receptor agonists (Kokate et al., 1996; Hoffman et al., 2003; Reddy et al., 2004). The seizure threshold elevation following progesterone administration is dose-dependent and lasts up to 2 hours post progesterone. Recent studies in our lab confirm the antiepileptogenic effects of progesterone in the kindling model of epileptogenesis (Briyal and Reddy, unpublished observations). Consequently, seizure susceptibility is very low during physiological conditions associated with high progesterone.
Clinical studies
In clinical studies progesterone has been found to reduce seizures (Backtrom et al., 1984; Herzog, 1995; 1999). Thus, natural cyclic variations in progesterone during the menstrual cycle could influence catamenial seizure exacerbation in women with epilepsy (Fig. 1). Seizures decrease in the mid-luteal phase when serum progesterone levels are high and increase premenstrually when progesterone levels fall and there is a decrease in the serum progesterone-to-estrogen ratio (Backstrom, 1976; Bonucelli et al., 1989; Herzog et al., 2001). Changes in progesterone levels have been directly correlated with catamenial seizures (Tuveri et al., 2008; El-Khayat et al., 2008). In patients with catamenial epilepsy, the midluteal phase serum progesterone levels are significantly lower (~9.4 ng/ml), compared to control subjects (~15 ng/ml), while estradiol levels are similar in both groups. Consequently, the estradiol-to-progesterone ratio increased significantly in the patients. In patients with perimenstrual catamenial seizures, progesterone levels are markedly lower (~0.75 ng/ml) compared to their levels in the control subjects (~1.6) (El-Khayat et al., 2008). Progesterone levels changed significantly throughout the menstrual cycle in both control and catamenial groups without significant difference between groups (Tuveri et al., 2008), which could be due to differences in study protocols. However, patients with inadequate luteal type seizures show significantly lower progesterone levels in the midluteal (~2.7 ng/ml) and menstrual phases (~0.6 ng/ml) compared to patients with noncatamenial seizures (~15.6 ng/ml) or to patients with the perimenstrual type (~6.6 ng/ml) (El-Khayat et al., 2008). Despite some methodological limitations, these findings provide evidence that disruption in normal ovarian cycle-related fluctuations in progesterone can be correlated to catamenial seizure exacerbation.
Although estrogens could potentially activate catamenial seizures, there is strong evidence implicating progesterone in the etiology of catamenial epilepsy. In 1956, Laidlaw first suggested that premenstrual seizure exacerbations could be explained on the basis of “rapid decline” or the “withdrawal” of the antiseizure effects of progesterone (Laidlaw, 1956), which was confirmed later in humans (Backstrom, 1976) and animal studies (Voiculescu et al., 1994; Smith et al., 1998a, b; Moran and Smith, 1998). These studies clearly indicate that catamenial seizures are associated with a rapid decline in progesterone at immediately before, during, and after menstruation. Consequently “synthetic” and “natural” progestin therapy has proved to be beneficial for catamenial epilepsy (Mattson et al., 1984; Herzog, 1995; 1999).
Mechanisms of progesterone
The potential mechanisms involved in the antiseizure effects of progesterone are illustrated in Figure 2. Physiological actions of progesterone are mediated by progesterone receptors (PR), a member of the nuclear receptor superfamily of transcription factors (Tsai and O’Malley, 1994). In progesterone-responsive target cells, progesterone binds to cytoplasmic PRs and the hormone-nuclear receptor complexes translocate to the cell nucleus where they activate or silence the transcription of downstream gene networks, thus affecting the physiological response of the target cell. There are two PR subtypes, PR-A and PR-B, which are transcribed from the same gene. PR-A and PR-B exhibit different physiological properties (Mulac-Jericevic et al., 2000). Several proteins referred as coactivators or corepressors enhance or inhibit PR-dependent target transcription. Estradiol induces PR expression, and therefore, progesterone sensitivity is dependent on prior estrogen exposure. Moreover, a ligand-independent pathway of PR activation via phosphorylation mechanisms (e.g. by dopamine activation of PR) has been characterized in the brain (Mani et al., 1996; 2001).
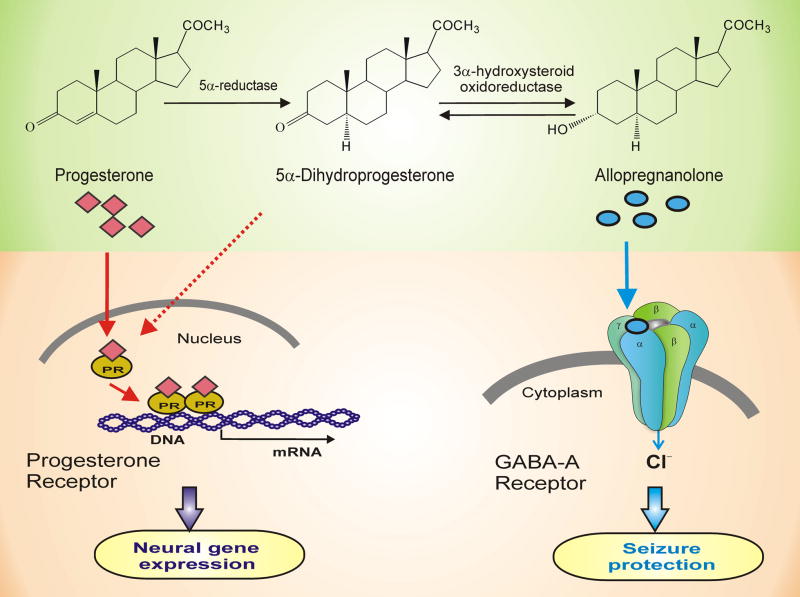
Fig. 2. Molecular mechanisms of progesterone and neurosteroids in the brain.
The two mechanisms by which progesterone affects seizure susceptibility are (i) binding to progesterone receptors (PRs) (left panel) and (ii) metabolism to GABAA receptor-modulating neurosteroids (right panel). Progesterone binding to PRs could lead to activation of neural gene expression in the brain. Neurosteroid allopregnanolone is synthesized from progesterone by two sequential A-ring reductions both in peripheral tissues and in the brain. Allopregnanolone binds and potentiate the GABAA receptor function leading protective effects against seizures. GABA-A receptors are believed to be pentameric with five protein subunits that form the chloride ion channel pore. The allopregnanolone binding site is thought to be at the “neurosteroid binding site”, which is distinct from sites for GABA, benzodiazepines and barbiturates. There are seven different classes of subunits with multiple variants; most GABAA receptors are believed to be composed of α, β and γ or δ subunits. Although allopregnanolone binds poorly to PRs, it could indirectly activate PRs by re-conversion to dihydroprogesterone, which is a moderately potent PR agonist. Moreover, progesterone and neurosteroids are shown to affect GABA-A receptor expression. Thus, there may be an interaction between genomic and non-genomic actions of progesterone in modulation of seizure activity.
The central nervous system is an important target for progesterone. PRs are widely distributed in the brain and involved in progesterone-mediated reproductive behavior (Parsons et al., 1982; Mani et al., 1996). However, there is strong evidence that the antiseizure effects of progesterone are not related to interactions with classical PR. First, the antiseizure effects of progesterone occur rapidly (within minutes), which is inconsistent with delayed genomic actions of the hormone. Second, the antiseizure activity of progesterone is not blocked by the PR antagonist RU486 (Mohammad et al., 1998). Finally, the antiseizure activity of progesterone was undiminished in PR knockout (PRKO) mice (Reddy et al., 2004), which are generated by a null mutation of the PR gene that abrogates both the PR-A and PR-B subtypes. Because 5α-reductase isoenzymes catalyze the rate-limiting step in the conversion of progesterone to allopregnanolone, it has been possible to use 5α-reductase inhibitors to examine whether the anticonvulsant activity of progesterone is dependent upon conversion to reduced metabolites (Frye et al., 1998; Kokate et al., 1999a). These studies, along with additional experiments using mice that lack the type I 5α-reductase isoenzyme (Frye et al., 2002), support the concept that 5α-reduced metabolites of progesterone, particularly allopregnanolone, are responsible for the seizure protection conferred by the parent hormone.
Molecular pathways involved in the actions of progesterone have been recently characterized utilizing the PRKO mouse model (Reddy et al., 2004; 2005; Reddy and Apanites, 2005; Reddy and Zeng, 2007a). Progesterone was tested in three distinct models of epilepsy: the PTZ test, amygdala kindling, and maximal electroshock tests. In all three models, the anticonvulsant potency of progesterone was undiminished in PRKO mice, compared with control wild-type mice (Reddy et al., 2004). Indeed, progesterone has enhanced anticonvulsant potency in PRKO mice in the PTZ and amygdala kindling model of epilepsy. The antiseizure activity of progesterone in PRKO mice was reversed by pretreatment with finasteride, a 5α-reductase inhibitor that blocks the metabolism of progesterone to allopregnanolone. It is, therefore, suggested that the PR is not required for the antiseizure effects of progesterone in these models. However, it cannot be concluded that PRs do not participate in the regulation of seizure susceptibility in the clinical setting. Though PRs do not contribute directly to the protective effects of progesterone, they could indirectly affect seizure susceptibility by a variety of signalling mechanisms (McEwen, 1994; Mani, 2003). Several rapid signaling mechanisms for PRs in the brain have been identified recently. They include non-genomic “membrane associated PRs” and short-latency effects of progesterone (Ramirez et al., 1996; Edwards et al., 2002; Li and O’Malley, 2003). However, the role of this membrane PR in seizure susceptibility has not been explored. To date, a rapid effect of progesterone has been reported in the hippocampus slice excitability that was blocked by the PR antagonist RU486 (Edwards et al., 2000).
Progesterone-derived neurosteroids
Neurosteroids are defined as steroids that are synthesized locally within the brain, and that rapidly modulate neural excitability mainly by targeting membrane receptors. Neurosteroids such as allopregnanolone are synthesized de novo in the brain from cholesterol (Kulkarni and Reddy, 1995). Circulating steroid hormones serve as precursors for the synthesis of neurosteroids in the brain (Schumacher et al., 2003). Several endogenous neurosteroids are identified with anticonvulsant or proconvulsant properties (Reddy, 2003a; 2006; 2008) (Table 3). Neurosteroids that are derived from ovarian, adrenal and gonadal sources have received increased attention because of their potential role in the pathophysiology of catamenial epilepsy.
Table 3.
Endogenous neurosteroids with anticonvulsant and proconvulsant properties.
| Neurosteroid | Potential mechanism(s) |
|---|---|
| Anticonvulsant neurosteroids: | |
| Progesterone | Precursor for neurosteroid allopregnanolone synthesis |
| Allopregnanolone | Potentiation of GABA-A receptor function |
| Pregnanolone | Potentiation of GABA-A receptor function |
| Dihydroprogesterone | Precursor for allopregnanolone synthesis |
| Androstanediol | Potentiation of GABA-A receptor function |
| Dihydrotestosterone | Precursor for THDOC synthesis |
| Deoxycorticosterone | Precursor for neurosteroid THDOC synthesis |
| Allotetrahydrodeoxycorticosterone (THDOC) | Potentiation of GABA-A receptor function |
| Proconvulsant neurosteroids: | |
| Estradiol | Hippocampal dendritic spine density Enhanced NMDA receptor function Induction of neurotrophin BDNF |
| Pregnenolone sulfate | Potentiation of NMDA receptor function Inhibition of GABA-A receptor function |
| DHEA sulfate | Potentiation of NMDA receptor function |
| Cortisol | Corticosteroid receptors and plasticity |
Preclinical studies
Progesterone is a prohormone for neurosteroid synthesis. Increasing evidence suggests that the antiseizure activity of progesterone is mediated by its metabolic conversion to allopregnanolone (5α-pregnan-3α-ol-20-one) (Fig. 2) (Belelli et al., 1989; Kokate et al., 1999a; Frye and Bayon, 1998; Reddy and Kulkarni, 2000; Mellon et al., 2001; Stoffel-Wagner, 2003). The conversion of progesterone into allopregnanolone occurs both in peripheral tissues and also locally within the brain (Corpechot et al., 1993). This conjecture was proved in animals using 5α-reductase enzyme inhibitors that block allopregnanolone synthesis (Kokate et al., 1999a; Frye and Bayon, 1998) and more recently confirmed in female mice with an induced null mutation in a 5α-reductase gene (Frye et al., 2002). The neurosteroid-mediated anticonvulsant activity of progesterone is also confirmed in PRKO mice (Reddy et al., 2004), providing strong evidence that allopregnanolone mediates anticonvulsant activity of progesterone.
The antiseizure properties of allopregnanolone have been most extensively studied in animal models of epilepsy. The antiseizure profile of allopregnanolone is summarized in Table 4. Allopregnanolone is a potent, broad-spectrum anticonvulsant agent. In animals, it protects against seizures induced by GABA-A receptor antagonists, pilocarpine, and kindling seizures (Belleli et al., 1989; Devaud et al., 1996; Kokate et al., 1994; 1996; Frye, 1995; Carter et al., 1997; Reddy et al., 2004; Lonsdale D, Burnham, 2007). At very high doses, allopregnanolone also protects mice against maximal electroshock-induced seizures. Allopregnanolone protects animals against partial seizures in the 6-Hz model (Kaminiski et al., 2004). However, it is inactive against seizures induced by glutamate receptor agonists at doses that protect against seizures induced by PTZ (Kokate et al., 1996). In rats undergoing neurosteroid withdrawal, allopregnanolone has enhanced antiseizure effects (Reddy et al., 2001). Allopregnanolone is effective in reducing spontaneous seizures in the rat model of temporal lobe epilepsy induced by pilocarpine or kainic acid (Reddy et al., 2007). It also protects against seizures in immature animals (Mares, 2005; Mares et al., 2006). Allopregnanolone elicits anxiolytic and behavioral effects that resemble the benzodiazepines and barbiturates (Reddy, 2003a). Pregnanolone, the 5β-stereoisomer of allopregnanolone, also has anticonvulsant activity but is less potent than allopregnanolone (Kokate et al., 1994).
Table 4.
Antiseizure profile of three major neurosteroids in animal models of epilepsy.
| Antiseizure potency (ED50)* |
|||
|---|---|---|---|
| Seizure Model | Allopregnanolone | THDOC | Androstanediol |
| GABA-A receptor antagonists: | |||
| Pentylenetetrazol | 12 (10–15) | 19 (77–122) | 40 (27–60) |
| Bicuculline | 12 (10–15) | 12 (10–15) | ND |
| Picrotoxin | 10 (5–19) | 10 (5–19) | 44 (24–81) |
| DMCM | ND | ND | 39 (21–74) |
| Glutamate receptor agonists: | |||
| Kainic acid | >40** | >40** | >200** |
| N-methyl-D-aspartate | >40** | >40** | >200** |
| Kindling models: | |||
| Amygdala kindling | 14 (8–23) | 15 (10–30) | ND |
| Electroshock models: | |||
| Maximal electroshock | 29 (19–44) | 48 (35–66) | 224 (182–274) |
| 6-Hz model | 14 (10–19) | ND | 29 (16–52) |
| Status epilepticus models: | |||
| Pilocarpine | 7 (4–13) | 7 (4–13) | 105 (48–232) |
| Temporal lobe epilepsy models: | |||
| Pilocarpine model | 5# | ND | ND |
| Kainic acid model | 5# | ND | ND |
ED50 is the dose in mg/kg producing seizure protection in 50% of animals. Values in parentheses are 95% confidence limits. ND, not determined.
Considered as inactive because of such highly sedative doses.
Significant decrease in spontaneous seizure frequency was observed at 5 mg/kg dose, given thrice daily.
Clinical studies
Although menstrual cycle-related fluctuations in allopregnanolone could play a critical role in the pathogenesis of catamenial seizures, there are few studies to date showing a direct correlation between allopregnanolone levels and seizure exacerbation. In an anecdotal case report, Herzog found the first clinical evidence that progesterone therapeutic activity in catamenial epilepsy requires conversion to 5α-reduced metabolites such as allopregnanolone (Herzog and Frye, 2003). In humans, progesterone is also converted to pregnanolone. Because progesterone’s actions on seizure susceptibility largely depend on its metabolic conversation to neurosteroids allopregnanolone and pregnanolone, natural fluctuations in allopregnanolone levels follow closely with that of progesterone (Wang et al., 1996) (Fig. 1). Recently, Tuveri and colleagues (2008) determined the serum concentrations of allopregnanolone in women with catamenial epilepsy and age-matched control subjects. The plasma levels of allopregnanolone during the follicular and luteal phases did not differ between the control subjects and women with catamenial epilepsy (Fig. 3) (Tuveri et al., 2008). Similar levels are noted in women with partial epilepsy and catamenial seizures (Murri and Galli, 1997). However, the levels of neurosteroids are not available on a continuous daily basis throughout the menstrual cycle, which is a major limitation in interpretation of this study. Highly sensitive assay is required for accurate analysis of endogenous allopregnanolone levels. Although neurosteroids are highly lipophilic and can readily cross the blood-brain barrier, the actual brain levels could be different due to local synthesis or accumulation within the brain (Stoffel-Wagner, 2003). Nevertheless, like abrupt decrease in progesterone levels around the perimenstrual phase, allopregnanolone levels would be expected to drop to baseline around menses. Thus, a relative deficiency or “withdrawal” from chronic allopregnanolone synthesis at the end of the luteal phase just before, or at, the onset of menses is a particularly vulnerable time for seizure exacerbation.
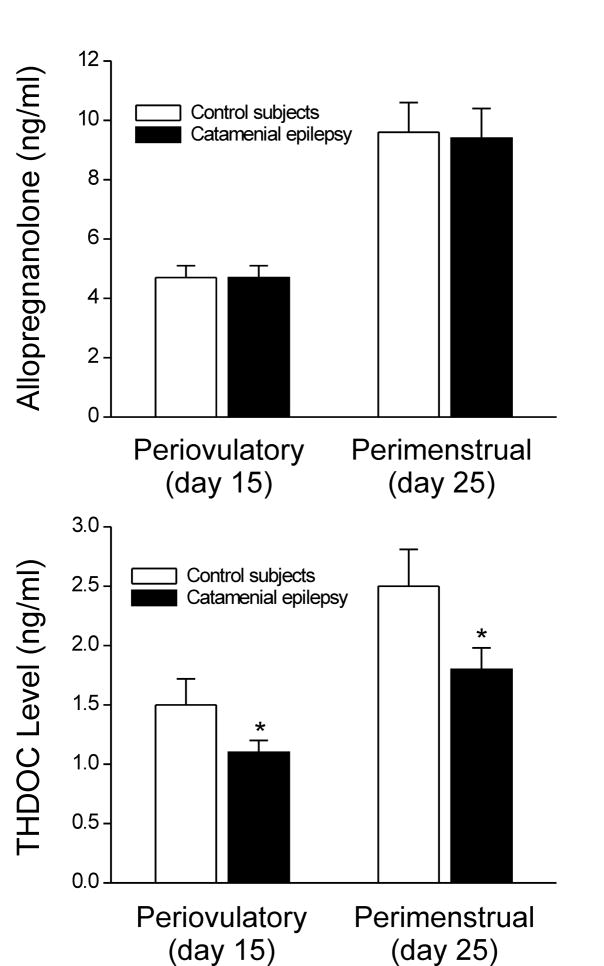
Fig. 3. Mean serum concentrations of two major GABA-A receptor-modulating neurosteroids, allopregnanolone and THDOC, during menstrual cycle in women with catamenial epilepsy and in control subjects.
Allopregnanolone levels are similar, but THDOC levels are significantly (p < 0.05) reduced in women with catamenial epilepsy during periovulatory, perimenstrual period and throughout the menstrual cycle (data from Tuveri et al., 2008).
Mechanisms of neurosteroids
As illustrated in Figure 2, allopregnanolone and related neurosteroids interact with postsynaptic GABA-A receptors. Allopregnanolone is a potent positive allosteric modulator of GABAA receptors (Harrison et al., 1987; Majewska et al., 1986; Lambert et al., 2003). GABA is the major inhibitory neurotransmitter in the brain. GABAA receptors mediate the bulk of synaptic inhibition in the brain and in the hippocampus play a critical role in the epileptogenesis. Allopregnanolone has specific binding sites on the GABAA receptor chloride ion channel that are distinct from the binding sites for GABA, benzodiazepines, and barbiturates (Gee et al., 1988; Turner et al., 1989; Lambert et al., 2003). Although the exact mode of how neurosteroids interact with the GABA-A receptor is unclear, two discrete binding sites have been identified in the receptor’s transmembrane domains that mediate the potentiating and direct activation effects of neurosteroids (Hosie et al., 2006). Thus, neurosteroids have two separate actions on GABA-A receptors: they potentiate the action of GABA and directly activate the receptor at two distinct sites that are different from the benzodiazepine or barbiturate sites (Hosie et al., 2006). Although neurosteroids modulate both synaptic and extrasynaptic GABAA receptors, their modulatory action is enhanced for extrasynaptic GABA-A receptor isoforms that contain a δ subunit (Herd et al., 2007). There is strong evidence that allopregnanolone at physiological concentrations (2–4 nM during luteal phase) activate GABAA receptors (Rapkin et al., 1997; Wang et al., 1996; Genazzani et al., 1998; Cooper et al., 1999; Belelli et al., 2002). The actual brain levels of allopregnanolone are slightly higher than in plasma because of local synthesis and accumulation (Bixo et al., 1997; Stoffel-Wagner, 2003). Therefore, endogenous allopregnanolone levels in brain are sufficiently high to have an ongoing modulatory influence on GABAA receptor-mediated synaptic inhibition and seizure susceptibility.
Allopregnanolone and related neurosteroids can interact with both classical genomic steroid receptors and membrane receptors. Generally, chronic effects of neurosteroids are due to both genomic (classical intracellular steroid receptors) and non-genomic rapid actions (ion channels and membrane receptors) in the brain. However, the genomic effects of neurosteroids are mainly due to their metabolic interconversion to steroids that binds to classical steroid receptors (Rupprecht et al., 1993; 1996). 5α-Reduced metabolites of allopregnanolone produced by intracellular oxidation of the 3α-hydroxyl group may nevertheless bind to progesterone receptors (Fig. 2). Further, there may be a cross-talk between genomic and non-genomic effects of neurosteroids in the brain.
Proconvulsant neurosteroids
The ovary secretes the sulfated neurosteroid pregnenolone sulfate (PS), which may also arise from local synthesis in the brain. Unlike allopregnanolone, PS is a proconvulsant steroid and can induce seizures and status epilepticus when administered systemically or directly into the brain (Reddy and Kulkarni, 1998; Kokate et al., 1999b; Williamson et al., 2004). PS inhibits the GABAA receptor function, and is also moderately potent allosteric agonist at NMDA receptors (Wu et al., 1991; Majewska, 1992). The proconvulsant or convulsant actions of PS are evident at high micromolar concentrations, which are 100 to 500-fold higher than its levels in the brain. Thus, it is highly unlikely that endogenous PS by itself can trigger seizures. However, PS can decrease GABAergic inhibitory transmission at physiological concentrations via a presynaptic action (Teschemacher et al., 1997; Mtchedlishvili and Kapur, 2003). Allopregnanolone blocks the seizure facilitating effects of PS, and consequently, PS could contribute to seizure susceptibility when allopregnanolone levels are low. Complete understanding of PS functions in catamenial seizure activity remains an important but formidable task.
Adrenal-derived neurosteroids
Stress can alter seizure susceptibility by releasing corticosteroids cortisol and deoxycorticosterone (DOC). Stress causes activation of hypothalamic-putuitary-adrenal axis that leads to release of corticosteroid hormones (cortisol in humans; corticosterone in rodents) (Joëls et al., 2007). Cortisol is an excitatory steroid with proconvulsant or seizure facilitating properties (Joels, 1997), while deoxycorticosterone has been shown to inhibit seizures (Reddy, 2003b). The adrenal cortex contains two anatomically and functionally distinct compartments: the outer zona glomerulosa, which secretes the mineralocorticoid aldosterone, and the inner zonae fasciculate/reticularis, which secrete the glucocorticoid cortisol as well as the adrenal androgens. DOC, a mineralocorticoids precursor with anesthetic and antiseizure properties, is also produced in the adrenal zona fasciculate. DOC has been shown to be a precursor for the synthesis of neurosteroids in the brain (Reddy, 2006).
Preclinical studies
DOC is an anticonvulsant steroid (Aird, 1944; Aird and Gordan, 1951). However, its mechanism of action in the brain was not clearly understood until recently. Recent studies suggest that DOC’s antiseizure effects are mediated by the neurosteroid allotetrahydrodeoxycorticosterone (THDOC, 5α-pregnane-3α,21-diol-20-one) (Reddy and Rogawski, 2002; Reddy, 2006). Unlike allopregnanolone, there is no evidence for the de novo synthesis of THDOC in the brain. Plasma THDOC concentration rises rapidly after systemic administration of DOC or following stress (Purdy et al., 1991; Reddy and Rogawski, 2002). Inhibition of 5α-reductase or 3α-HSOR completely prevents THDOC production, demonstrating that THDOC is generated through this biosynthetic pathway. Adrenalectomy has no effect on the conversion of DOC into THDOC, suggesting that the conversion mainly occurs at extraadrenal sites, principally in the liver and brain. In females, the pattern of THDOC secretion emulates that of allopregnanolone over the menstrual cycle, during pregnancy, and during conditions of stress (Purdy et al., 1991; Reddy, 2003a), suggesting that some amount of THDOC is also produced from ovarian sources.
In animals, THDOC elicits anxiolytic, antiseizure and behavioral effects that resemble the benzodiazepines and barbiturates (Crawley et al., 1986; Muller-Preuss et al., 2002; Reddy, 2003b). THDOC share common pharmacological effects with other neurosteroids. The antiseizure profile of THDOC is summarized in Table 4. THDOC is a broad-spectrum anticonvulsant. It protects against seizures induced by GABAA receptor antagonists, pilocarpine, and kindling (Kokate et al., 1996; Reddy and Rogawski, 2002). At very high doses, THDOC also protects mice against maximal electroshock-induced seizures. Unexpectedly, THDOC is highly effective in protecting seizures elicited due to the withdrawal of ethanol, cocaine, diazepam, and neurosteroids (Devaud et al., 1996; Tsuda et al., 1997; Reddy and Rogawski, 2001). Thus, it is expected to play an important role in catamenial seizures.
Clinical Studies
At present, there is very limited clinical information on the role of THDOC in catamenial epilepsy. The antiseizure properties of DOC in humans were first described in 1944 (Aird, 1944; Aird and Gordan, 1951). Tuveri and colleagues (2008) determined the serum concentrations of steroid hormones and neurosteroids in women with catamenial epilepsy (N=17) and age-matched control subjects (N=13). Serum levels of THDOC and other neurosteroids are measured during the follicular and luteal phases of the menstrual cycle. Patients showed a two-fold or greater increase in seizure frequency around the time of menstrual period, which is consistent with diagnosis of perimenstrual catamenial epilepsy. The serum levels of THDOC are lower by 30% in women with catamenial epilepsy than in the control subjects (Fig. 3), providing the first evidence that the adrenal neurosteroid THDOC could play a role in the pathophysiology of catamenial epilepsy. The reduced serum levels of THDOC are found in both the follicular and luteal phase indicating that these fluctuations are not related to a specific phase of the menstrual cycle. Although plasma levels might only partially reflect brain concentrations, THDOC deficiency could contribute to general decrease in seizure threshold and thereby enhance susceptibility to catamenial seizures in women at risk. Unlike allopregnanolone that has multiple sources including local synthesis within the brain, THDOC is derived almost exclusively from the adrenal gland (Reddy and Rogawski, 2002). Therefore, specific reduction in serum THDOC in women with catamenial epilepsy has important pathophysiological implications. It is likely that neurosteroid synthesizing enzymes may be altered in women with catamenial epilepsy, such as reported in patients with major depression and premenstrual syndrome (Strohle et al., 2000; Rasgon et al., 2001).
Mechanisms of THDOC
The post-synaptic GABAA receptor is a major target of neurosteroid THDOC. At the cellular level, corticosteroids act via two receptor types - mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) – that are highly expressed in limbic areas such as the hippocampus. However, these corticosteroid receptors are not involved in the anticonvulsant actions of THDOC because they occur rapidly (within minutes) and even in steroid receptor knockout mice (Reddy et al., 2004). Like other neurosteroids, THDOC is an extremely potent positive allosteric modulator of GABA-A receptor function (Reddy, 2003b). THDOC was first shown to be a potent barbiturate-like ligand of the GABA-A receptor (Majewska et al., 1986). THDOC has specific binding sites on the GABA-A receptor chloride channel, which are distinct from the sites for GABA, benzodiazepines, and barbiturates (Morrow et al., 1990; Gee et al., 1995). The THDOC enhancement of submaximal GABA-A receptor currents occurs through increases in both channel open frequency and open duration. THDOC therefore allows GABA-A receptors to remain open longer and permits more chloride ion flux. The sustained hyperpolarization resulting from increased chloride ion entry results in a net increase in membrane conductance of the neuron, thereby effectively shunting the influence of excitatory currents that would otherwise depolarize the neuron. Plasma levels of THDOC in rats normally fluctuate between 1 and 5 nM but increase to 15–30 nM following acute stress (Purdy et al., 1991; Reddy and Rogawski, 2002) and can reach 40–60 nM in pregnancy (Concas et al., 1998). Thus, physiological levels of THDOC can enhance the GABA-A receptor function.
THDOC and related neurosteroids modulates GABA-A receptor isoforms formed from diverse subunits (Lambert et al., 2003). Unlike benzodiazepines, neurosteroids do not show a stringent subunit-specificity for potentiation of GABA-A receptors because they can modulate most receptor isoforms formed from diverse subunits. Recent studies indicate that δ subunit-containing GABA-A receptors exhibit increased sensitivity to THDOC (Mihalek et al., 1999; Wohlfarth et al., 2002; Vicini et al., 2002). Attenuated behavioral sensitivity to neurosteroids has been reported for mice deficient in the GABA-A receptorδ subunit, suggesting for the first time a specific role of the δ subunit in neurosteroid modulation of GABA-A receptors. It is now recognized that there are two types of GABA-A receptor-mediated inhibition, namely “phasic” and “tonic” inhibition, which are mediated by different isoforms of the receptor (Richerson, 2004). Postsynaptic GABAA receptors are mainly responsible for transient phasic inhibition, while extrasynaptic GABAA receptors appear to contribute to a continuous tonic inhibition that controls levels of the excitability of neurons. Many of these extrasynaptic GABA- receptors contain the δ subunit. It is now clear that these δ subunit containing GABA- receptors are tonically activated by the low levels of GABA normally present in the extracellular space and play a physiological role in tonic inhibition in the hippocampal pyramidal neurons (Stell et al., 2003). Thus, these extrasynaptic δ subunit-rich GABA- receptors could be an important target for THDOC and related neurosteroids.
DHEAS and cortisol as proconvulsants
Dehydroepiandrosterone sulfate (DHEAS) is a sulfated neurosteroid from adrenal sources. Unlike THDOC, DHEAS is a proconvulsant steroid (Table 3) and can induce seizures and status epilepticus when administered systemically or directly into the brain (Reddy and Kulkarni, 1998; Kokate et al., 1999b; Williamson et al., 2004). DHEAS is moderately potent allosteric agonist at NMDA receptors (Wu et al., 1991; Majewska, 1992). Tuveri et al (2008) reported significant reduction of the DHEAS/cortisol ratio in women with perimenstrual catamenial epilepsy. Although there was no change in the levels of DHEAS, but DHEAS/cortisol ratio was lower during the follicular and luteal phases. Thus, this appears to be highly inconsistent with the possibility that it contributes to reduced seizure susceptibility around menstruation. Future studies using highly sensitive assay methods may clarify whether sulfated neurosteroids exacerbate catamenial seizures.
Cortisol is an excitatory corticosteroid and causes proconvulsant effects (Joëls, 1997). This could explain the seizure-precipitating effects of stress (Stein-Behrens et al., 1992; Frucht et al., 2000). Therefore, it is possible that stress could precipitate catamenial seizures in women with epilepsy. However, it is not clear how stress impacts the catamenial seizure exacerbation because of lack of controlled studies on the relationship between stress and catamenial epilepsy.
Androgenic neurosteroids
Testosterone is the primary circulating androgen and a prohormone for neurosteroid synthesis. Testosterone is abundant in the ovary and is essential precursor for estradiol synthesis. It is synthesized in the thecal interstitial cells of the ovary, and then metabolized to estradiol in the granulosa cells of the primordial follicle. Like estrogens, this raises the possibility that testosterone levels fluctuate with ovarian cycle in females (Rush and Blake, 1982). Testosterone is metabolized to neurosteroids via two distinct pathways: androgen pathway and estrogen pathway. In the androgen pathway, 3α-androstanediol is synthesized from testosterone by two sequential A-ring reductions (Martini, 1992; Martini et al., 1993). In the estrogen pathway, testosterone is converted into estradiol by the aromatase enzyme. The androstanediol and estradiol are synthesized in peripheral tissues and the brain (Martini, 1992; Jin and Penning, 2001).
Preclinical studies
There is emerging experimental evidence that testosterone-derived “androgenic neurosteroids”, androstanediol and estradiol, mediate the testosterone effects on neural excitability and seizure susceptibility (Edwards et al., 1999; Reddy, 2004b; 2008). Testosterone significantly lowers the seizure threshold, increases PTZ seizures, and enhances the development of amygdala-kindled seizures (Edwards et al., 1999; Reddy, 2004c). Thus, testosterone may exacerbate catamenial seizure activity because testosterone secretion increases about four-fold at around the time of ovulation (Rush and Blake, 1982). Androgens may affect spine synapse density in the hippocampus in females and contribute to plastic changes over the course of the menstrual cycle (Leranth et al., 2004). Since androgen and corticosteroid levels go up in the late follicular phase, they could contribute to some of the changes in excitability observed in the periovulatory phase. Therefore, androgenic neurosteroids could play a role in catamenial seizures.
The androstanediol is an emerging neurosteroid in the brain. Preclinical studies in animal models of epilepsy strongly support that androstanediol is a powerful antiseizure and neuroprotective agent (Reddy, 2008). Like allopregnanolone, androstanediol has powerful protective activity against seizures induced by several GABA-A receptor antagonists (Reddy, 2004b,c), pilocarpine and the maximal electroshock model (Kaminski et al., 2004, 2005). The anticonvulsant profile of androstanediol is shown Table 4. In intravenous PTZ test, it causes a dose-dependent elevation of seizure threshold (Reddy, 2004c), suggesting that it acts partly by elevating seizure threshold.
Clinical studies
Although androstanediol is present in abundant amount in the brain, there are no clinical studies to date investigating its role in catamenial epilepsy. The effect of androstanediol on specific regions involved in seizure control in the brain (Kaminiski et al., 2004) could be relevant for catamenial seizures. It is thought that testosterone modulation of seizure activity is dependent on its conversion to androstanediol (anticonvulsant) and estradiol (proconvulsant). Therefore, aromatase inhibitors, which block the synthesis of estradiol from testosterone, are proposed as adjunct treatment for epilepsy (Harden and MacLusky, 2005). Aromatase inhibition affects testosterone metabolism with a variable effect on estradiol and could elevate androstanediol levels. Nevertheless, it is conceivable that changes in androstanediol synthesis or activity could have an impact on the seizure sensitivity in women with catamenial epilepsy.
Mechanisms of androstanediol
Because androstanediol is structurally very similar to allopregnanolone, it is thought that its anticonvulsant actions are conferred by its selective interaction with GABA-A receptors (Frye and Reed, 1998). In electrophysiological studies, androstanediol has been shown to be a positive modulator of GABA-A receptors (Park-Chung et al., 1999). There is strong evidence that the effects of androstanediol are not related to interaction with classical androgen receptors (AR) because the anticonvulsant effects of androstanediol occur rapidly (within minutes) and the AR antagonists fail to prevent the antiseizure effects of androstanediol (Reddy, 2004c). Although androstanediol binds poorly to AR (Cunningham et al., 1979), it may indirectly affect AR by intracellular reduction to dihydrotestosterone, which is a potent AR agonist. Interestingly, dihydrotestosterone also protects against seizures (Reddy, 2004b).
ANIMAL MODELS OF CATAMENIAL EPILEPSY: IMPLICATIONS FOR UNDERSTANDING THE PATHOPHYSIOLOGY
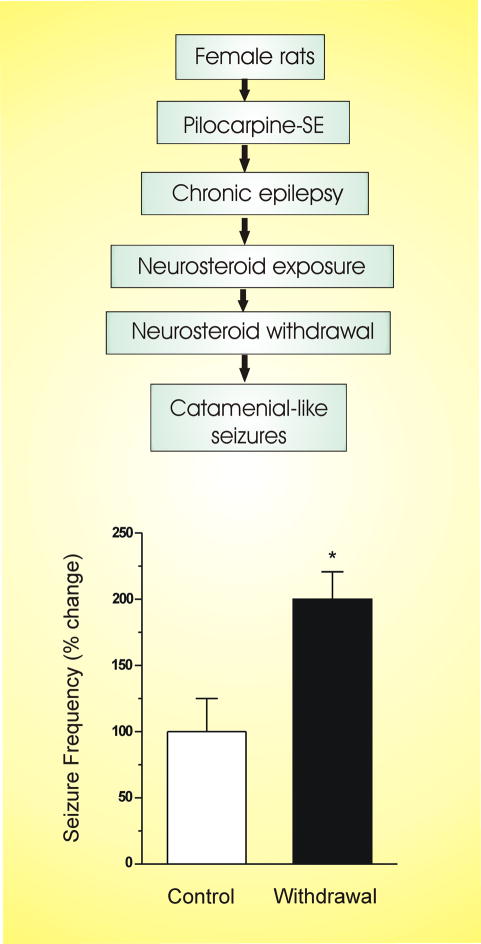
Animal models of epilepsy play a key role in the characterization of pathophysiology and discovery of AEDs. Conventional seizure models, which are largely based on the utilization of acutely induced seizures in naive animals, are not suitable because they do not allow testing of specific therapies that are targeted to catamenial epilepsy. These models are clearly different from such models as kindling, pilocarpine or chronic kainic acid that induce severe damage and remodeling response in the brain and thereby result in secondary seizures. Generally, animal models of catamenial epilepsy could be designed specifically to simulate the menstrual cycle and ovarian hormone-related changes in seizure susceptibility. During the luteal phase of the menstrual cycle, circulating levels of progesterone are increased for 10 to 12 days before declining (withdrawal) to low levels. Recently, three types of models have been described in animals that partially resemble catamenial seizure patterns. In the first category of models, attempts are made to mimic the luteal phase by inducing extended high levels of progesterone and estrogens followed by rapid decline to simulate the menstruation in normal rodents. These include pseudopregnancy, chronic progesterone, and progesterone (neurosteroid) withdrawal models (Smith et al., 1998a; Moran and Smith, 1998; Reddy et al, 2001). The second category of models are based on the naturally occurring estrous cycle or administration of exogenous hormones that simulate the specific stages of estrous cycle in ovariectomized rats (Frye et al., 1998; Frye and Bayons, 1998). These physiological models better mimic the normal ovarian cycle. In the third category of models, epilepsy animals are exposed to steroid hormones and neurosteroid withdrawal conditions, and the frequency and severity of spontaneous seizures are utilized as indices of catamenial-like seizure exacerbation (Reddy and Zeng, 2007b).
The pseudopregnancy model
Based on studies of progesterone and neurosteroids on seizure activity, Reddy et al. (2001) proposed the first model of perimenstrual catamenial epilepsy in pseudopregnant rats. It has been hypothesized that increased catamenial seizure susceptibility is caused by a sharp decline (“withdrawal”) in serum levels of progesterone and, consequently, of levels of allopregnanolone in the brain. In this model, after induction of persistently elevated progesterone in female rats, allopregnanolone was abruptly withdrawn by blocking its synthesis from progesterone using the 5α-reductase inhibitor finasteride. Acute withdrawal of allopregnanolone produced an increase in seizure susceptibility to PTZ, but long-term treatment with finasteride did not, mimicking the situation in catamenial epilepsy. In another model, withdrawal of progesterone is obtained by ovariectomy (Moran and Smith, 1998). This model is associated with decreased seizure threshold to picrotoxin and reduced sensitivity to benzodiazepines. The progesterone (neurosteroid) withdrawal-induced hyperexcitability fits well with clinical perimenstrual seizure patterns (Herzog et al., 1997), and also progesterone withdrawal-induced increase in seizure susceptibility in rats (Voiculescu et al., 1994; Frye and Bayons, 1998). The withdrawal model is consistent with a marked increase in seizure frequency that was observed when finasteride was concomitantly given with progesterone in women with epilepsy (Herzog and Frye, 2003).
The exogenous progesterone model
There are models in which progesterone was administered for extended periods using silastic implants or multiple daily injections (Costa et al., 1995; Smith et al., 1998a; Moran and Smith, 1998). These models mimic the high progesterone levels found during luteal phase of the menstrual cycle. Withdrawal of progesterone containing implants or cessation of chronic progesterone treatment induces an abrupt decline of progesterone (neurosteroids) levels that could simulate the hormonal milieu of menstruation. Akin to finasteride-induced neurosteroid withdrawal, withdrawal of progesterone has been associated with marked decrease in seizure threshold (Smith et al., 1998a; Moran and Smith, 1998). A similar predisposition to seizures is observed upon abrupt discontinuation of benzodiazepines (File, 1990) and ethanol (Kokka et al., 1993; Reilly et al., 2000), which also have the GABA-A receptor positive modulating properties.
The spontaneous model in female epilepsy rats
Previous models have utilized normal or healthy animals to study neurosteroid withdrawal. However, epilepsy animals are needed to study the influence of neurosteroid withdrawal on catamenial seizures. Currently available methods of epilepsy induction lead to severe reproductive dysfunction in female rats (Amado and Cavalheivo, 1998; Edwards et al., 1999). The pilocarpine model is widely used for inducing acute status epilepticus, followed by chronic spontaneous seizures after a latent period. The modified lithium-pilocarpine model of chronic epilepsy in rats may provide a stable epileptic condition and maintains reproductive function (Reddy et al., 2007). Reddy and Zeng (2007b) devised the neurosteroid-withdrawal model in rats with spontaneous seizures based on the hypothesis that prolonged exposure (like that of the luteal phase) followed by withdrawal (like that of menstruation) of progesterone, and therefore the “neurosteroid” allopregnanolone, mimics the hormonal state associated with heightened vulnerability to seizures. It is proposed that cyclic episodes of withdrawal would exacerbate seizure occurrence in epilepsy rats (Fig. 4). In this new model, chronic epilepsy with spontaneous recurrent seizures was induced in rats by treatment with pilocarpine. Rats with spontaneous seizure were then subjected to repeated pseudopregnancy-finasteride paradigm to model menstruation-like neurosteroid exposure and withdrawal. As shown in Fig. 4, neurosteroid withdrawal was associated with a significant ‘catamenial-like’ exacerbation of spontaneous seizures in epilepsy rats (Reddy and Zeng, 2007). The withdrawal period represents a crucial intervention phase for testing of potential treatments, and therefore, this chronic model may be useful for testing novel therapies for the perimenstrual catamenial epilepsy.
Fig. 4. Neurosteroid withdrawal-induced spontaneous seizures in female epilepsy rats.
The protocol for the catamenial model is illustrated in the top panel. Chronic epilepsy with spontaneous recurrent seizures was induced in rats by pilocarpine treatment. Pilocarpine-induced status epilepticus was terminated after 2 h with diazepam (5 mg/kg, ip) injections and animals with frequent spontaneous seizures (average 2 seizures daily) after 5 months post pilocarpine were utilized for neurosteroid withdrawal cycles. To simulate the hormonal state of the luteal-phase (high progesterone) and menstruation (withdrawal) in epilepsy rats, pseudopregnancy, which is associated with sustained secretion of progesterone and neurosteroids, was induced by gonadotropin regimen. Neurosteroid withdrawal was induced by treatment with finasteride, a 5α-reductase inhibitor that blocks the synthesis of allopregnanolone. Rats undergoing neurosteroid withdrawal exhibited significant (p<0.01) increase in daily seizure frequency as compared to control, non-withdrawing group (bottom panel), which is consistent with perimenstrual type catamenial epilepsy.
The molecular basis of withdrawal effects
Though the molecular basis of seizure susceptibility following neurosteroid withdrawal is not well understood, progesterone exposure and withdrawal has been shown to distinctly affect GABA-A receptor subunit plasticity. Chronic exposure of progesterone (or allopregnanolone) induces a down regulation of the γ2 subunit, and an up regulation of the α1 subunit, which consequently could result in alterations in the α1: α2 ratio of GABA-A receptor composition (Yu et al., 1996; Brussaard et al., 1997; Concas et al., 1998; Follesa et al., 1998; Foley et al., 2003). The most striking finding following progesterone withdrawal is marked up regulation of the α4 subunit and hyperexcitability of hippocampal neurons in progesterone withdrawal models (Smith et al., 1998a; 1998b; Guilinello et al., 2001 Guilinello et al., 2003). It has been suggested that the increased seizure susceptibility observed during the withdrawal period may be due to GABA-A receptor channel properties associated with the α4 subunit. Moreover, chronic exposure to the neurosteroid allopregnanolone also increases expression of the α4 subunit similar to progesterone withdrawal (Hsu and Smith, 2003; Smith et al., 2007). The α4 subunit has some unique properties: it renders GABAA receptors benzodiazepine insensitive; it commonly co-assembles with the γ2 or δ subunits; it accelerates decay of GABA-gated current; and it is expressed in the extrasynaptic sites in the hippocampus (Sur et al., 1999). In addition, progesterone withdrawal also increases expression of the δ subunit (Sundstrom-Poromaa et al., 2002), which is mainly expressed in extrasynaptic sites and confers benzodiazepine insensitivity when coassembled with the α4 subunit. Thus, increased expression of the α4 subunit might decrease the net GABA-A receptor-mediated inhibition and promote excitability leading to increased seizure exacerbation. However, there is no change in the expression of the α4 subunit in rat cerebral cortex and hippocampus during pregnancy or after delivery, which are associated with a massive fall in progesterone and allopregnanolone levels (Concas et al., 1998; Follesa et al., 1998). Moreover, the massive increase in progesterone-derived neurosteroids during pregnancy and their precipitous decline at parturition may have considerable effects on GABA-A receptors during pregnancy and postpartum. A marked downregulation of δ and γ2 subunits accompanied by significant decrease in tonic and phasic inhibitions are observed in pregnant mice, which rebounds immediately postpartum (Maguire and Mody, 2008).
The molecular basis of estrous cycle-related seizure susceptibility
The structure of the GABA-A receptor undergoes drastic alterations due to changing levels of progesterone during the ovarian cycle. Recently, Mody and colleagues demonstrated profound changes in the hippocampal GABA-A receptor subunit expression during different phases of the estrous cycle (Maguire et al., 2005). During the late diestrous phase (associated with high progesterone levels), expression of the δ subunit-containing GABA-A receptors was elevated, which was associated with an increase in tonic inhibition and diminished seizure susceptibility in mice. During the estrous phase (associated with low progesterone levels), tonic inhibition was reduced by 50% with corresponding increases in both seizure susceptibility and anxiety behavior in mice. These cyclic alterations in the γ subunit are also observed following exogenous progesterone treatment in ovariectomized female mice (Maguire and Mody, 2007). Unlike the phasic inhibition mediated by the δ subunit-containing GABA-A receptors, the δ subunit-containing GABA-A receptors are highly sensitive to neurosteroids (Mihalek et al., 1999; Stell et al., 2003). These findings are consistent with the possibility that deficiencies in regulatory mechanisms controlling normal cycling of the δ subunit-containing GABA-A receptors in the hippocampus could be a potential molecular mechanism for catamenial seizures. Thus, the δ subunit-containing GABA-A receptor is an important target for developing specific treatments for catamenial epilepsy.
The relevancy of animal models
The validation of animal models of catamenial epilepsy requires certain criteria to be met for them to be representative of the human condition. They should include close similarity in eliciting an epilepsy-like state, and a pathophysiology that mirrors the disease in human. The neurosteroid withdrawal model (Moran and Smith, 1998; Reddy et al., 2001; Reddy and Zeng, 2007b) partly meets these criteria and would certainly offer some advantages over the use of conventional seizure models. These models are based on similar physiological dynamics of ovarian progesterone secretion during the menstrual cycle, which cannot be simulated in the exogenous drug delivery models. In pseudopregnancy, secretion of progesterone by the luteinized ovaries occurs in a physiologically appropriate episodic fashion and leads to plasma progesterone levels that are within the physiologic range. Moreover, the 9-day elevation of progesterone and allopregnanolone levels in the pseudopregnancy model closely matches the 10-day increase in allopregnanolone levels during the luteal phase of the menstrual cycle (Wang et al., 1996; Rapkin et al., 1997). Although the exact etiology of catamenial epilepsy is not completely understood, the pseudopregnancy-neurosteroid withdrawal model better simulates changes in the allopregnanolone-to-estrogen ratio that is believed to be critical for perimenstrual catamenial epilepsy. Female rats with epilepsy showing spontaneous recurrent seizures would be more appropriate to model catamenial epilepsy. There are certain difficulties such as problems with consistent induction of epileptogenesis in female rats and potential abnormities in maintaining regular estrous cycles (Amado and Cavalheiro, 1998). However, the actual endocrine conditions that exist in the menstrual cycle are different from those observed in animal models of catamenial epilepsy. This is a major concern with most animal models developed in rodents. In rodents the estrous cycle duration is 4–7 days and the menstrual cycle in women lasts about 28 days.
Despite limitations from animal studies, it is suggested that the “withdrawal” of neurosteroids around the time of menstruation is likely to be most relevant to the enhanced excitability and greater seizure susceptibility in perimenstrual catamenial epilepsy. Therefore, the basis for enhanced seizure susceptibility in perimenstrual catamenial epilepsy is multifaceted and may include: (i) withdrawal of the anticonvulsant effects of neurosteroids, (ii) increased expression of α4 subunit following withdrawal resulting in reduced inhibition, (iii) overall reduction in GABA-A receptor-mediated inhibition, (iv) reduced expression of extrasynaptic (δ subunit) GABA-A receptors, and (v) other endocrine factors that occurs around the time of menstruation. All of these factors could be responsible for high incidence of catamenial seizure exacerbations.
THE HORMONAL AND NON-HORMONAL TREATMENT OF CATAMENIAL EPILEPSY
Presently there is no specific treatment for catamenial epilepsy. The conventional AEDs are the mainstay for the management of catamenial seizures in women. Approximately one third of women with epilepsy use more than one AED appropriate to their seizure type. This is partly because catamenial seizures are often refractory to conventional AEDs. Many of these drugs are prescribed for treatment of catamenial epilepsy without direct studies of effectiveness, with their use based primarily on empirical evidence (Guille et al., 2008). There are many factors to consider in the choice of hormonal or AED therapy. An ideal AED for catamenial epilepsy should not affect the dynamic changes in ovarian hormones but should be highly effective in controlling catamenial seizures without serious adverse effects.
Treatment of epilepsy in women must consider several important issues such as pharmacokinetics of AEDs, drug interactions, and contraceptive use (Crawford, 2005). Some AEDs can reduce the levels of ovarian hormones due to pharmacokinetic interactions. Estrogens and progestogens are metabolized by CYP34A. AEDs such as phenytoin, phenobarbital, carbamazepine, felbamate, topiramate, oxcarbazepine, and primidone induce CYP3A4 leading to enhanced metabolism of either or both the estrogen and progesterone, thereby influencing the dynamic equilibrium of ovarian hormones during the menstrual cycle (McAuley and Anderson, 2002). AEDs such as gabapentin, levetiracetam, tiagabine, zonisamide and pregabalin do not cause enzyme induction, and hence do not cause pharmacokinetic interactions with steroid hormones. Oral contraceptives also pose risk because they can decrease the concentrations of AEDs such as lamotrigine (Sabers et al., 2003) and thereby increase the risk of seizures.
Hormonal therapy
Endocrine treatment of seizures may rationally be aimed at those endocrinologic aspects of seizures that act either to exacerbate or to ameliorate them. Because progesterone has anticonvulsant effects and estrogen has proconvulsant effects, treatment with progesterone or estrogen antagonists may prove to be useful adjunctive treatments in appropriate patients. Table 5 lists an overview of various drugs investigated for the treatment of catamenial epilepsy. Many patients received these agents as supplements or adjunct drugs in a continuous or intermittent approach for inhibition of catamenial seizures (Zimmerman et al., 1973; Herzog, 1999). While these agents may be helpful for the treatment of catamenial seizures, each is based on small, unblinded studies or anecdotal reports.
TABLE 5.
List of hormonal and non-hormonal agents tested in catamenial epilepsy.
| Drug | Mechanism | Efficacy | Limitations |
|---|---|---|---|
| Hormonal Agents: | |||
| Medroxyprogesterone acetate | Progesterone analogue | Mixed | Reproductive dysfunction |
| Clomiphene | Estrogen receptor antagonist | Moderate | Reproductive dysfunction |
| Triptorelin | GnRH analogue | Moderate | Menopausal symptoms |
| Leuprolide | GnRH analogue | Moderate | Menopausal symptoms |
| Progesterone | Neurosteroid precursor | High | Sedation, depression, breast tenderness, vaginal bleeding, and weight gain |
| Non-hormonal agents: | |||
| Acetazolamide | Carbonic anhydrase inhibitor | Moderate | Tolerance |
| Clobazam | GABA-A receptor modulator | Moderate | Sedation/tolerance |
| Lamotrigine | Sodium channel blocker | Moderate | Dizziness, pilot study |
| Ganaxolone | GABA-A receptor modulator | High | Small pilot study |
Medroxyprogesterone acetate
Medroxyprogesterone acetate (MPA) is a widely investigated progestin-only contraceptive agent and is active by the parenteral and oral routes of administration. Zimmerman and colleagues (1973) used depot administration of MPA to treat a woman with catamenial seizures. Mattson et al (1984) found that MPA produces a 39% reduction in seizure frequency at a mean follow-up of 1 year. Suppression of seizures was evident when the patients were treated with parenteral MPA at dosages that were designed to eliminate menses. Therefore, it is conceivable that long-term MPA therapy is associated with undesirable reproductive disturbances. Although the mechanism of MPA action is not clearly understood, progesterone receptors appear to be important in its actions in catamenial epilepsy. Unlike progesterone, MPA (17-acetyloxy-6-methyl-pregn-4-ene-3,20-dione) is not extensively metabolized to GABA-A receptor-modulating neurosteroids. This could partly explain the moderate efficacy of MPA relative to progesterone, which is a prohormone for the synthesis of allopregnanolone in the brain.
Natural progesterone
Cyclic natural progesterone use has been demonstrated as an effective treatment for catamenial and non-catamenial seizures in women (Herzog, 1986; 1995; 1999). Cyclic natural progesterone supplement has been used in dosages that mimic physiological range levels. Progesterone is efficiently absorbed after oral administration as lozenges and rectal administration as suppositories. Progesterone was given at 100–200 mg, t.i.d. on days 15–28 of menstrual cycle. In a three-month investigation of cyclic natural progesterone therapy, 23 of 25 (92%) women with intractable seizures completed the trial. Average monthly seizure frequency was reportedly reduced by 54% to 68% during the 3-month treatment period (Herzog, 1995). A three-year follow-up report finds that 15 of the women continued on the same antiepileptic drug and progesterone protocol. These women continued to have a very substantial (62% to 74%) reduction in seizure frequency (Herzog, 1999). Two women who did not complete the three-month trial dropped out because of sedative (asthenia or depression) side effects all of which were resolved within a day of dose reduction. The safety profile of cyclic natural progesterone use has permitted it to be in a NIH clinical trial (Herzog et al., 2008).
High efficacy and ready availability make progesterone a valuable treatment for catamenial seizure exacerbation. It is yet to be recognized as an approved form of therapy for catamenial epilepsy. However, progesterone therapy in women may cause hormonal effects such as breakthrough vaginal bleeding and breast tenderness. At higher dosage, progesterone produces CNS side effects including sedation, emotional depression and asthenia (Herzog, 2008). Finasteride is contraindicated in women with epilepsy because finasteride prevents the antiseizure effectiveness of progesterone (Herzog and Frye, 2003). There are also concerns on the interpretation of progesterone efficacy in women with epilepsy because the studies are conducted by the open design and it is possible that the women who continued progesterone therapy possibly represent those who had the most favorable response. The outcome of ongoing NIH-sponsored multicenter trial (Herzog et al., 2008) examining the role of progesterone in women with epilepsy would confirm the efficacy and side effect profile of progesterone therapy.
Experimental evidence from studies in animal models (Kokate et al., 1999a; Frye et al., 2002; Reddy et al., 2004) and clinical data (Herzog, 1999; Herzog and Frye, 2003) is consistent with the possibility that the antiseizure effects of progesterone are due to its metabolic conversion to neurosteroids, principally allopregnanolone. The concurrent use of finasteride, an inhibitor of 5α-reductase required for the conversion of progesterone into allopregnanolone, with progesterone therapy has resulted in marked exacerbation of seizures in women with epilepsy (Herzog and Frye, 2003). However, not all effects of progesterone or synthetic progestins are due to neurosteroids. In fact, some authors have suggested that the effects of progesterone on seizure susceptibility involve interactions with PRs (Rupprecht et al., 1993; Edwards et al., 2000). However, northethisterone, a synthetic progestin that binds to PRs but does not metabolize to neurosteroids, has been found to be ineffective to improve seizure control in women with catamenial epilepsy (Dana-Haeri and Richens, 1983). Nevertheless, it is possible that the antiseizure effects of progesterone could involve both interaction with the PR and allopregnanolone formation.
GnRH and other hormonal agents
Hormonal preparations such as oral contraceptives, the synthetic gonadotropin-releasing hormone (GnRH) analogue triptorelin, and the estrogen receptor antagonist clomiphene have been found to be partly effective in a small number of women with catamenial epilepsy (Hall, 1977; Herzog, 1988; Dana-Haeri and Richens, 1983; Haider and Barnett, 1991; Baur et al., 1992). However, significant hormonal side effects as well as disturbances in reproductive health severely limit their utility as therapeutic agents for catamenial epilepsy. Addition of MPA and conjugated estrogens could limit some side effects of GnRH analogues such as goserolin (Reid and Gangar, 1992). In a study of nine women with catamenial epilepsy for four menstrual cycles, norethisterone (17α-ethinyl-19-nortestosterone) treatment was found to be ineffective in the control of perimenstrual catamenial seizures (Dana-Haeri and Richens, 1983). In patients with intractable catamenial epilepsy, ovariectomy may prevent or decrease catamenial seizures that occur due to cyclical changes in ovarian hormones or withdrawal from progesterone and neurosteroids.
Non-hormonal therapy
Acetazolamide
Acetazolamide is the prototype of a class of agents that are potent inhibitors of carbonic anhydrase, a key enzyme involved in NaHCO3 reabsorption and water balance in the kidney. Carbonic anhydrase is also present in the brain. Acetazolamide has been used empirically for years for the treatment of refractory (focal and generalized) and catamenial epilepsy (Ansell and Clark, 1956; Ross, 1958). However, there are few direct studies of its effectiveness in the management of catamenial seizures. Lim et al (2001) studied the efficacy of acetazolamide in 20 women with catamenial epilepsy. Almost 40% and 30% of subjects were confirmed with significant reduction in overall seizure frequency and severity, respectively. Loss of efficacy (tolerance) over 6–24 months was reported by 15% of women. Dose escalation or cyclical dosing may reduce the development of tolerance. The mechanism of acetazolamide’s antiseizure activity is obscure. The efficacy of acetazolamide in epilepsy has been attributed to production of metabolic acidosis; however, direct action of acetazolamide in the brain could contribute to its antiseizure action (Lombroso and Forxythe, 1960). Acetazolamide produces an accumulation of carbon dioxide in the brain that could be sufficient to prevent seizures in animals (Millichap et al., 1995). Like other sulfonamide derivatives, acetazolamide therapy may be associated with significant adverse effects such as drowsiness, paresthesias, sulfonamide-like renal lesions, and allergic hypersensitivity. However, development of antiseizure tolerance to acetazolamide makes it difficult to manage seizure control even with frequent increase in dosage.
Benzodiazepines
Benzodiazepines such as clonazepam and clobazam are potent, positive allosteric modulators of GABAA receptor and broad-spectrum antiseizure agents. Clonazepam is highly useful in the therapy of absence and myclonic seizures. However, development of tolerance to their antiseizure effects usually limits their clinical utility (Haigh and Feely, 1988). Clobazam has been found to be an effective agent for the treatment of catamenial epilepsy (Feely et al., 1982; Feely and Gibson, 1984). Clobazam (20 to 30 mg/day) was administered intermittently (from 2 to 4 days before menses) probably to avoid the tolerance usually associated with long-term continual therapy. Moreover, tolerance to its antiseizure effects was not evidenced after 6 to 13 months of clobazam therapy (Feely and Gibson, 1984). The most common adverse effects of clobazam are sedation and depression. However, cross-tolerance to benzodiazepines has been described in animal model due to chronic exposure to neuroactive steroids (Reddy and Rogawski, 2000a). This could further impact the clinical utility of benzodiazepines in catamenial epilepsy therapy.
Lamotrigine
Lamotrigine (Lamictal) is a second-generation AED that is useful for monotherapy and add-on therapy of partial and secondarily generalized seizures. Lamotrigine is reported anecdotally to have an effect on catamenial epilepsy (Ramaratham, 2003). Recently, Gilad et al. (2008) performed a prospective study for evaluation of the efficacy of lamotrigine in 18 women with catamenial epilepsy. Lamotrigine was given starting 25 mg daily, and the dosage gradually increased up to 100 mg, twice daily. Overall, lamotrigine had a beneficial effect of 66% (22% became seizure-free and 44% had a 50% reduction in number of seizures). Adverse effects observed include skin rash in one patient and dizziness and headaches in three other patients. Lamotrigine was ineffective in six patients (Gilad et al., 2008), indicating that lamotrigine may not be effective in all patients with catamenial epilepsy.
NOVEL TREATENT STRATEGIES FOR CATAMENIAL EPILEPSY
Neurosteroids
There is emerging evidence that neurosteroids represent a novel class of agents with potential utility in catamenial epilepsy therapy (Rogawski and Reddy, 2004). Neurosteroid allopregnanolone withdrawal-induced seizure susceptibility appears to simulate perimenstrual catamenial epilepsy (Reddy et al., 2001). During this seizure-prone state, the activity of conventional antiepileptic drugs, including diazepam and sodium valproate, is reduced in an animal model of catamenial epilepsy (Reddy and Rogawski, 2000a; 2001), possibly accounting for the clinical impression that catamenial seizures are unusually drug resistant. Unexpectedly, natural and synthetic neurosteroid analogs that positively modulate GABAA receptors actually have enhanced anticonvulsant potency in the catamenial model in rats. A similar increase in the anticonvulsant activity of neurosteroids is also observed during withdrawal from chronic ethanol (Devaud et al., 1996) as well as diazepam (Tsuda et al., 1997). While there is clearly increased sensitivity to the anticonvulsant effects of neurosteroids in the hyperexcitable states, the mechanisms underlying the enhanced sensitivity remain unclear. Since the δ subunit containing GABAA receptors confer increased sensitivity to neurosteroids (Mihalek et al., 1999; Wohlfarth et al., 2002), upregulation of the δ subunit expression may increase anticonvulsant sensitivity to neurosteroids during withdrawal states (Sundstrom-Poromaa et al., 2002). Hence, cyclical “neurosteroid replacement” during the perimenstrual period or continuous low dose administration could represent an effective and rational therapy for catamenial epilepsy (see review Reddy and Rogawski, 2009). However, natural neurosteroids are not suitable for therapeutic formulation for catamenial epilepsy because they are orally inactive, have a very short half-life (t1/2, 15–20 min), and could be converted to metabolites with undesired hormonal activity (Phillipps, 1975). For example, the 3α-hydroxyl group of allopregnanolone may undergo oxidation to a ketone (see Fig. 2), restoring activity at classical progesterone receptors (Rupprecht et al., 1993; 1996). Synthetic neurosteroid analogs may overcome these limitations.
Synthetic neuroactive steroids
The term “neuroactive steroid” has been widely used to represent both naturally occurring neurosteroids and their synthetic derivatives with rapid actions in the brain (Reddy, 2003b). The neurosteroid withdrawal hypothesis presents the possibility for novel, highly effective treatments. Using the catamenial epilepsy model, we evaluated the hypothesis that neurosteroid “replacement” is an effective and rational therapy for catamenial epilepsy (Reddy and Rogawski, 2000b; 2001). During this seizure-prone state, the activity of conventional antiepileptic drugs, including diazepam and sodium valproate, is reduced, possibly accounting for the clinical impression that catamenial seizures are unusually drug resistant. Unexpectedly, neurosteroids that positively modulate GABAA receptors actually have enhanced anticonvulsant potency in the model, providing support for a neurosteroid “replacement” approach to the treatment of catamenial epilepsy (Reddy and Rogawski, 2001). Overall, these observations suggest that neurosteroids represent a specific treatment approach for perimenstrual catamenial seizure exacerbations. It does suggest that cyclic replacement therapy would be highly effective. Unfortunately, natural neurosteroids are ineffective as treatments. They are orally inactive, have a very short (minutes) half-life and have the potential to metabolize to compounds that produce undesirable hormonal effects. The synthetic version of allopregnanolone called ganaxolone is available (Reddy and Woodward, 2004). Synthetic neuroactive steroids are designed to overcome the limitations of naturally occurring neurosteroids with significant improvements in pharmacokinetic and therapeutic properties.
Ganaxolone
Ganaxolone is a synthetic 3β-methyl analogue of allopregnanolone (Carter et al., 1997). The 3β-methyl substituent which minimizes metabolism at the 3α-hydroxyl group so ganaxolone is orally active, is not converted to the hormonally active 3-keto form, and hence lacks hormonal side effects. Ganaxolone was originally discovered at CoCensys in 1990s, and it was acquired recently by Marinus Pharmaceuticals for further development. Currently, ganaxolone is undergoing further evaluation in preclinical studies and clinical trials in patients with epilepsy (Nohria and Giller, 2007).
Ganaxolone is a potent positive allosteric modulator of GABA-A receptors and a broad-spectrum anticonvulsant agent (Carter et al., 1997; Monaghan et al., 1998). At relatively high concentrations (10 μM), ganaxolone, in the absence of GABA, directly activates the GABAA receptor chloride channel function. Animal studies indicate that ganaxolone is a potent broad-spectrum anticonvulsant agent (Reddy and Woodward, 2004). Ganaxolone protects against seizures induced by pentylenetrazol and other GABA-A receptor antagonists, kindling seizures, and flurothyl-induced seizures models (Carter et al., 1997; Gasior et al., 2000; Liptakova et al., 2000). Ganaxolone protected against partial seizures in the 6-Hz model (Kaminiski et al., 2004). At very high doses, it blocked tonic seizures induced by maximal electroshock. However, ganaxolone is inactive against tonic seizures induced by the glycine antagonist strychnine and limbic seizures induced by glutamate receptor agonist N-methyl-D-aspartate. As predicted due to its GABAergic actions, ganaxolone produces dose-dependent sedation in animals. Unlike diazepam, anticonvulsant tolerance does not develop to ganaxolone following chronic therapy (Reddy and Rogawski, 2000a), suggesting that ganaxolone is suitable for chronic treatment without losing its protective efficacy.
Ganaxolone has been evaluated in preclinical models of catamenial epilepsy. The anticonvulsant potency of ganaxolone is enhanced in the period following neurosteroid withdrawal in a rat model of catamenial epilepsy, while the potencies of two reference anticonvulsants, diazepam and valproate, are reduced (Reddy and Rogawski, 2000b). Consequently, ganaxolone appears to protect against catamenial seizures at plasma concentrations that are not anticonvulsant in control animals. Further studies suggest that anticonvulsant tolerance does not develop when ganaxolone was dosed repeatedly for up to one week (Reddy and Rogawski, 2000a), which is consistent with similar lack of tolerance to synthetic neuroactive steroids (Ramsey et al., 1974; Kokate et al., 1998) as well as progesterone therapy in women with epilepsy (Herzog, 1995; 1999). Neuroactive steroids may therefore avoid the problem of tolerance that severely limits the usefulness of benzodiazepines as anticonvulsants in long-term therapy. However, chronic ganaxolone treatment has been found to induce cross-tolerance to diazepam (Reddy and Rogawski, 2000b). This might impact the clinical utility of benzodiazepines in menstrual conditions (Sundstrom et al., 1997).
The efficacy of ganaxolone was further confirmed in a new, spontaneous model of perimenstrual catamenial epilepsy. In epileptic rats with neurosteroid withdrawal-induced catamenial-like seizures, ganaxolone treatment (7 mg/kg, sc) significantly (p<0.01) reduced the frequency of spontaneous seizures as compared to the vehicle control (Reddy and Rogawski, 2009, in press). These results raises the possibility that ganaxolone might provide a specific treatment for catamenial epilepsy.
To date, ganaxolone was exposed to a total of 961 human subjects (including 135 children) in safety and pharmacokinetic studies and various clinical trials (Nohria and Giller, 2007). Ganaxolone is well tolerated and has low drug-drug interactions (Monaghan et al., 1997). The main side effect is sedation, which is expected due to its GABAergic mechanism of action. Although there is no clear linear relationship between ganaxolone dose and plasma levels at these high doses, concentrations of 300 ng/ml of ganaxolone appears to be the threshold for a higher incidence of CNS side effects such as sedation and somnolence. Ganaxolone has significant efficacy in treating adult patients with refractory partial and generalized seizures (Laxer et al., 2000).
In a preliminary study, ganaxolone was evaluated in women with catamenial epilepsy (McAuley et al., 2001). Patients received oral ganaxolone (300 mg/day, bid) starting on day 21 of the menstrual cycle and continuing through the third full day following the beginning of menstruation. During the 4 months of this ganaxolone ‘pulse’ therapy, patients had a marked decrease in their catamenial seizures. Given these highly encouraging results, it is hoped that ganaxolone might provide a specific treatment option for catamenial epilepsy. Prospective clinical studies are clearly warranted to determine the efficacy of ganaxolone in women with catamenial epilepsy. Because of ganaxolone’s unique modulatory profile, ganaxolone could be an effective agent in cases where other GABA-A receptor modulators fail to offer seizure protection because ganaxolone modulates most GABA-A receptors with distinct subunits.
CONCLUSIONS AND FUTURE PERSPECTIVES
Catamenial epilepsy is a multifaceted neuroendocrine condition. Although ovarian hormones play a central role, the exact cause of catamenial epilepsy is unknown. Experimental studies to this point have indicated a clear role of estrogen, progesterone and endogenous neurosteroids in the pathophysiology of the three types of catamenial epilepsy (see summary, Table 6). Whether there are abnormalities in hormonal dynamics that predispose to catamenial epilepsy is not known. There is emerging evidence that neurosteroid withdrawal appears to be a critical factor for enhanced seizure susceptibility during perimenstrual periods. A variety of molecular mechanisms have been proposed for neurosteroid withdrawal-induced seizure exacerbation that include alterations in the subunit composition (α4 and δ subunits) and functional properties of GABA-A receptors. Recent clinical studies confirmed significant differences in the levels of neurosteroids in women with catamenial epilepsy. Further studies are clearly warranted to confirm these findings.
TABLE 6.
Overall summary of the potential changes in neurosteroid levels and seizure susceptibility in catamenial epilepsy.
| Type | Changes in neurosteroids | Neuronal excitability | Seizure susceptibility |
|---|---|---|---|
| C1 Perimenstrual* | Very low neurosteroids (withdrawal) Low estradiol |
Decreased GABAergic inhibition | Increased |
| C2 Periovulatory** | High estradiol Low neurosteroids |
Increased excitation Decreased GABAergic inhibition |
Increased |
| C3 Inadequate luteal*** | Very low neurosteroids Moderate estradiol |
Decrease in GABAergic inhibition Persistent excitation |
Increased |
Perimenstrual type occurs in women with normal menstrual cycle possibly due to a sharp decline (“withdrawal”) in the serum level of progesterone and, consequently, of the level of progesterone-derived anticonvulsant neurosteroids in the brain around perimenstrual period. The estradiol/neurosteroid ratio is highest during menstruation. Because neurosteroid potentiates GABA-A receptor-mediated inhibition, the rapid loss of neurosteroid-mediated inhibition, such as that occur before, during or after the onset of menses, could exacerbate seizures in many women with catamenial epilepsy.
Periovulatory type occurs in women with normal menstrual cycle possibly due to estradiol surge just before ovulation, and low neurosteroid levels do not offset the estradiol-induced excitation because the rise of anticonvulsant neurosteroid levels would not occur until after ovulation. Subsequently neurosteroid synthesis is strikingly high during the luteal phase and is long-lasting until they decline rapidly around perimenstrual period. The relatively low neurosteroid inhibition and marked estradiol excitation could lead to periovulatory seizures.
Inadequate luteal type occurs in women with anovulatory cycles possibly due to a loss of neurosteroid-mediated inhibition during luteal phase for a prolonged time. Progesterone secretion that occurs normally during the luteal phase is markedly decreased during anovulatory cycle resulting in abnormally low levels of neurosteroid in the brain. The midcycle surge in estradiol still occurs in anovulatory cycle at moderately reduced levels. Because neurosteroid potentiates GABA-A receptor-mediated inhibition, low levels or lack of sufficient neurosteroids may possibly result in minimal or no such potentiation which is accompanied by unopposed estradiol’s excitability. This imbalance in neurosteroid inhibition and estradiol excitation could ultimately trigger occurrence of C3 type seizures. In addition, the striking reductions in the levels of adrenally-derived neurosteroid THDOC during both follicular and luteal phases would most likely exacerbate type C1 and C2 seizures.
Despite the increased awareness, there is a large gap in our understanding of catamenial seizures and many questions remain unanswered: (i) What hormonal milieu is responsible for catamenial seizure exacerbations? (ii) How natural, cyclic variations in circulating estrogens and progesterone contribute to genesis of catamenial epilepsy? (iii) What changes occur in the brain in relation to the hormonal fluctuations associated with menstrual cycle? (iv) How these changes alter sensitivity to anticonvulsant drugs? (v) Do catamenial seizures actually represent an epileptogenic process or are merely an exacerbation of existing seizure disorder? (vi) Can an understanding of the pathophysiology of catamenial seizures be used to develop specific targeted approaches for prevention or treatment of the disorder? One of the main obstacles to answering these questions and developing therapeutic strategies for prevention or treatment of catamenial epilepsy has been the lack of animal models that reliably exhibit the clinical pathology of the condition. Allopregnanolone and related neurosteroids are commonly analyzed by radioimmunoassay and GC-MS, which are tedious and not readily available. Lack of simple and accessible technology for rapid analysis of neurosteroids is a major obstacle for the tardy progress in clinical investigations on neurosteroids.
Most patients with catamenial epilepsy are not successfully treated with conventional AEDs. The AEDs used to control epilepsy may also affect a woman’s hormones. Hormonal agents such as progesterone may provide effective therapy for catamenial epilepsy. However progesterone therapy is associated with undesirable hormonal side effects. The synthetic neuroactive steroids might provide an effective approach for catamenial epilepsy therapy without producing hormonal side effects. Ganaxolone has excellent efficacy in animal models of catamenial epilepsy and therefore, ganaxolone appears to be a specific drug for perimenstrual catamenial epilepsy. Although there are three types of catamenial epilepsy, it is not clear if different types respond differentially to treatment options. The outcome of NIH-sponsored multicentre trial examining the role of progesterone in women with epilepsy will further strengthen the case for neurosteroids. Future work should focus on specific neuroendocrine risk factor(s) and approaches for prevention of catamenial seizure exacerbation in women at risk. Application of advanced techniques, such as TMS, may accelerate efforts in further understanding of the neuroendocrine changes relevant to catamenial seizure exacerbation.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH/NINDS) grants NS051398 & NS052158 (to DSR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abassi F, Krumholz A, Kittner SJ, Langenberg P. Effects of menopause on seizures in women with epilepsy. Epilepsia. 1999;42:205–210. doi: 10.1111/j.1528-1157.1999.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Amado D, Cavalheiro EA. Hormonal and gestational parameters in female rats submitted to the pilocarpine model of epilepsy. Epilepsy Res. 1998;32:266–274. doi: 10.1016/s0920-1211(98)00057-6. [DOI] [PubMed] [Google Scholar]
- Ansell B, Clarke E. Acetazolamide in treatment of epilepsy. Brit Med J. 1956;1:650–661. doi: 10.1136/bmj.1.4968.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird RB. The effect of desoxycorticosterone in epilepsy. J Nerv Ment Dis. 1944;99:501–510. [Google Scholar]
- Aird RB, Gordan GS. Anticonvulsive properties of desoxycorticosterone. JAMA. 1951;145:715–719. doi: 10.1001/jama.1951.02920280027006. [DOI] [PubMed] [Google Scholar]
- Bäckström T, Zetterlund B, Blom S, Romano M. Effect of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurol Scand. 1984;69:240–248. doi: 10.1111/j.1600-0404.1984.tb07807.x. [DOI] [PubMed] [Google Scholar]
- Bäckström T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Bauer J. Interactions between hormones and epilepsy in female patients. Epilepsia. 2001;42 (Suppl 3):20–22. doi: 10.1046/j.1528-1157.2001.042suppl.3020.x. [DOI] [PubMed] [Google Scholar]
- Bauer J, Wildt L, Flugel D, Stefan H. The effect of a synthetic GnRH analogue on catamenial epilepsy: a study in ten patients. J Neurol. 1992;239:284–286. doi: 10.1007/BF00810354. [DOI] [PubMed] [Google Scholar]
- Bauer J, Burr W, Elger CE. Seizure occurrence during ovulatory and anovulatory cycles in patients with temporal lobe epilepsy: a prospective study. Eur J Neurol. 1998;5:83–88. doi: 10.1046/j.1468-1331.1998.510083.x. [DOI] [PubMed] [Google Scholar]
- Bazan AC, Montenegro MA, Cendes F, Min LL, Guerreiro CA. Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arg Neuropsiguiatr. 2005;63(3B):751–756. doi: 10.1590/s0004-282x2005000500006. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol. 1989;66:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABA-A receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts T, Yarrow H, Dutton N, Greenhill L, Rolfe T. A study of anticonvulsant medication on ovarian function in a group of women with epilepsy who have only ever taken one anticonvulsant compared with a group of women without epilepsy. Seizure. 2003;12:323–329. doi: 10.1016/s1059-1311(03)00065-7. [DOI] [PubMed] [Google Scholar]
- Bilo L, Meo R. Epilepsy and polycystic ovary syndrome: where is the link? Neurol Sci. 2006;27:221–30. doi: 10.1007/s10072-006-0675-y. [DOI] [PubMed] [Google Scholar]
- Bixo M, Andersson A, Winblad B, Purdy RH, Backstrom T. Progesterone, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Bonuccelli U, Melis GB, Paoletti AM, Fioretti P, Murri L, Muratorio A. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Res. 1989;3:100–106. doi: 10.1016/0920-1211(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Buterbaugh GG, Hudson GM. Estradiol replacement to female rats facilitates dorsal hippocampal but not ventral hippocampal kindled seizure acquisition. Exp Neurol. 1991;11:55–64. doi: 10.1016/0014-4886(91)90050-m. [DOI] [PubMed] [Google Scholar]
- Buterbaugh GG. Estradiol replacement facilitates the acquisition of seizures kindled from the anterior neocortex in female rats. Epilepsy Res. 1989;4:207–215. doi: 10.1016/0920-1211(89)90005-3. [DOI] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Folesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EJ, Johnston GA, Edwards FA. Effects of a naturally occurring neurosteroid on GABAA IPSCs during development in rat hippocampal or cerebellar slices. J Physiol. 1999;521:437–449. doi: 10.1111/j.1469-7793.1999.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, Mouren M, Prasad VV, Banner C, Sjovall J. Neurosteroids: 3α-hydroxy-5α-pregnan-20-one and its precursors in the brain, plasma and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Costa AM, Spence KT, Smith SS, ffrench-Mullen JM. Withdrawal from the endogenous steroid progesterone results in GABAA currents insensitive to benzodiazepine modulation in rat CA1 hippocampus. J Neurophysiol. 1995;74:464–469. doi: 10.1152/jn.1995.74.1.464. [DOI] [PubMed] [Google Scholar]
- Craig CR. Anticonvulsant activity of steroids: separability of anticonvulsant from hormonal effects. J Pharmacol Exp Ther. 1966;153:337–343. [Google Scholar]
- Crawford P. Best practice guidelines for the management of women with epilepsy. Epilepsia. 2005;46(Suppl 9):117–124. doi: 10.1111/j.1528-1167.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Glowa JR, Majewska MD, Paul SM. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 1986;398:382–5. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Cunningham GR, Tindall DJ, Means AR. Differences in steroid specificity for rat androgen binding protein and the cytoplasmic receptor. Steroids. 1979;33:261–276. doi: 10.1016/0039-128x(79)90003-5. [DOI] [PubMed] [Google Scholar]
- Dana-Haeri J, Richens A. Effect of norethisterone on seizures associated with menstruation. Epilepsia. 1983;24:377–381. doi: 10.1111/j.1528-1157.1983.tb04901.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Dickerson W. Effect of menstruation on seizures. J Nerv Ment Dis. 1941;94:160–169. [Google Scholar]
- Duncan S, Read CL, Brodie MJ. How common is catamenial epilepsy? Epilepsia. 1993;34:827–831. doi: 10.1111/j.1528-1157.1993.tb02097.x. [DOI] [PubMed] [Google Scholar]
- Edwards DP, Wardell SE, Boonyaratanakornkit V. Progesterone receptor interacting coregulatory proteins and cross talk with cell signaling pathways. J Steroid Biochem Mol Biol. 2002;83:173–186. doi: 10.1016/s0960-0760(02)00265-0. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Epps T, Carlen PL, MacLusky NJ. Progestin receptors mediate progesterone suppression of epileptiform activity in tetanized hippocampal slices in vitro. Neuroscience. 2000;101:895–906. doi: 10.1016/s0306-4522(00)00439-5. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Burnham WM, Mendonca A, Bowlby DA, MacLusky NJ. Steroid hormones affect limbic afterdischarge thresholds and kindling rates in adult female rats. Brain Res. 1999;838:136–150. doi: 10.1016/s0006-8993(99)01619-4. [DOI] [PubMed] [Google Scholar]
- El-Khayat HA, Soliman NA, Tomoum HY, Omran MA, El-Wakad AS, Shatla RH. Reproductive hormonal changes and catamenial pattern in adolescent females with epilepsy. Epilepsia. 2008;49:1619–1626. doi: 10.1111/j.1528-1167.2008.01622.x. [DOI] [PubMed] [Google Scholar]
- Feely M, Gibson J. Intermittent clobazam for catamenial epilepsy: tolerance avoided. J Neurol Neursurg Psychiatry. 1984;47:1279–1282. doi: 10.1136/jnnp.47.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy: a model for evaluating anticonvulsants. Lancet. 1982;2:71–73. doi: 10.1016/s0140-6736(82)91691-9. [DOI] [PubMed] [Google Scholar]
- File SE. The history of benzodiazepine dependence: a review of animal studies. Neurosci Biobehav Rev. 1990;14:135–146. doi: 10.1016/s0149-7634(05)80214-3. [DOI] [PubMed] [Google Scholar]
- Foldvary-Schaefer N, Falcone T. Catamenial epilepsy: pathophysiology, diagnosis, and management. Neurology. 2003;61 (Suppl 2):S2–15. doi: 10.1212/wnl.61.6_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- Foley CM, Stanton JJ, Price EM, Cunningham JT, Hasser EM, Heesch CM. GABAA α1 and α2 receptor subunit expression in rostral ventrolateral medulla in nonpregnant and pregnant rats. Brain Res. 2003;975:196–206. doi: 10.1016/s0006-8993(03)02635-0. [DOI] [PubMed] [Google Scholar]
- Follesa P, Floris S, Tuligi G, Mostallino MC, Concas A, Biggio G. Molecular and functional adaptation of the GABAA receptor complex during pregnancy and after delivery in the rat brain. Eur J Neurosci. 1998;10:2905–2912. doi: 10.1111/j.1460-9568.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- Frucht MM, Quigg M, Schwaner C, Fountain NB. Distribution of seizure precipitants among epilepsy syndromes. Epilepsia. 2000;41:1534–1539. doi: 10.1111/j.1499-1654.2000.001534.x. [DOI] [PubMed] [Google Scholar]
- Frye CA. The neurosteroid 3α,5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3α,5α-THP. Neuroendocrinology. 1998;68:272–280. doi: 10.1159/000054375. [DOI] [PubMed] [Google Scholar]
- Frye CA, Reed TA. Androgenic neuroactive steroids: anti-seizure effects in an animal model of epilepsy. Psychoneuroendocrinology. 1998;23:385–399. doi: 10.1016/s0306-4530(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Walf A, Harney J. Progesterone reduces pentylenetetrazol-induced ictal activity of wild-type mice but not those deficient in type I 5α-reductase. Epilepsia. 2002;43 (Suppl 5):14–17. doi: 10.1046/j.1528-1157.43.s.5.19.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Scalise TJ, Bayon LE. Finasteride blocks the reduction in ictal activity produced by exogenous estrous cyclicity. J Neuroendocrinol. 1998;10:291–296. doi: 10.1046/j.1365-2826.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Scalise TJ. Anti-seizure effects of progesterone and 3α,5α-THP in kainic acid and perforant pathway models of epilepsy. Psychoneuroendocrinology. 2000;25:407–420. doi: 10.1016/s0306-4530(99)00068-2. [DOI] [PubMed] [Google Scholar]
- Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM. Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology. 2000;39:1184–1196. doi: 10.1016/s0028-3908(99)00190-2. [DOI] [PubMed] [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- Gee KW, McCauley LD, Lan NC. A putative receptor for neurosteroids on the GABA-A receptor complex: the pharmacological properties and therapeutic potential of epalons. Crit Rev Neurobiol. 1995;9:207–227. [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A. Circulating levels of allopregnanolone in humans: gender, age and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Gilad R, Sadeh M, Rapoport A, Dabby R, Lampl Y. Lamotrigine and catamenial epilepsy. Seizure. 2008;17:531–534. doi: 10.1016/j.seizure.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Gowers WR. Their causes, symptoms, and treatment. London: J & A Churchill; 1881. Epilepsy and other chronic convulsive diseases; p. 197. [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guille C, Spencer S, Cavus I, Epperson CN. The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: implications for diagnosis and treatment. Epilepsy Behav. 2008;13:12–24. doi: 10.1016/j.yebeh.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the α4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. Eur J Neurosci. 2003;17:641–648. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider Y, Barnett DB. Catamenial epilepsy and goserelin. Lancet. 1991;338:1530–1530. doi: 10.1016/0140-6736(91)92354-5. [DOI] [PubMed] [Google Scholar]
- Haigh JRM, Feely M. Tolerance to the anticonvulsant effect of benzodiazepines. Trends Pharmacol Sci. 1988;9:361–366. doi: 10.1016/0165-6147(88)90255-6. [DOI] [PubMed] [Google Scholar]
- Hall SM. Treatment of menstrual epilepsy with a progesterone-only oral contraceptive. Epilepsia. 1977;18:235–236. doi: 10.1111/j.1528-1157.1977.tb04471.x. [DOI] [PubMed] [Google Scholar]
- Harden CL, Pulver MC, Ravdin L, Jacobs AR. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia. 1999;40:1402–1407. doi: 10.1111/j.1528-1157.1999.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Harden CL, MacLusky NJ. Aromatase inhibitors as add-on treatment for men with epilepsy. Expert Rev Neurother. 2005;5:123–127. doi: 10.1586/14737175.5.1.123. [DOI] [PubMed] [Google Scholar]
- Harden CL, Herzog AG, Nikolov BG, Koppel BS, Christos PJ, Fowler K, Labar DR, Hauser WA. Hormone replacement therapy in women with epilepsy: a randomized, double-blind placebo-controlled study. Epilepsia. 2006;47:1447–1451. doi: 10.1111/j.1528-1167.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- Harden CL, Leppik I. Optimizing therapy of seizures in women who use oral contraceptives. Neurology. 2006;67(Suppl 4):S56–S58. doi: 10.1212/wnl.67.12_suppl_4.s56. [DOI] [PubMed] [Google Scholar]
- Harden CL. Hormone replacement therapy: will it affect seizure control and AED levels? Seizure. 2008;17(2):176–80. doi: 10.1016/j.seizure.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interactions with the γ-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- Hattemer K, Knake S, Reis J, Rochon J, Oertel WH, Rosenow F, Hamer HM. Excitability of the motor cortex during ovulatory and anovulatory cycles: a transcranial magnetic stimulation study. Clin Endocrinol. 2007;66(3):387–93. doi: 10.1111/j.1365-2265.2007.02744.x. [DOI] [PubMed] [Google Scholar]
- He XP, Kotloski R, Nef S, Luikart B, Parada L, McNamara J. Conditional deletion of TrkB, but not BDNF, prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Herkes GK, Eadie MJ, Sharbrough F, Moyer T. Patterns of seizure occurrence in catamenial epilepsy. Epilepsy Res. 1993;15:47–52. doi: 10.1016/0920-1211(93)90008-u. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Intermittent progesterone therapy of partial complex seizures in women with menstrual disorders. Neurology. 1986;36:1607–1610. doi: 10.1212/wnl.36.12.1607. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Clomiphene therapy in epileptic women with menstrual disorders. Neurology. 1988;38:432–434. doi: 10.1212/wnl.38.3.432. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Reproductive endocrine considerations and hormonal therapy for women with epilepsy. Epilepsia. 1991;32(Suppl 6):S27–33. doi: 10.1111/j.1528-1157.1991.tb05889.x. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1600–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology. 1999;52:1917–1918. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Friedman MN, Freund S, Pascual-Leone A. Transcranial magnetic stimulation evidence of a potential role for progesterone in the modulation of premenstrual corticocortical inhibition in a woman with catamenial seizure exacerbation. Epilepsy Behav. 2001;2:367–369. doi: 10.1006/ebeh.2001.0232. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–391. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol. 2004;56:431–434. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Menstrual disorders in women with epilepsy. Neurology. 2006;66(6 Suppl 3):S23–28. doi: 10.1212/wnl.66.66_suppl_3.s23. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Disorders of reproduction in patients with epilepsy: primary neurological mechanisms. Seizure. 2008;17:101–110. doi: 10.1016/j.seizure.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM The NIH Progesterone Trial Study Group. Sensitivity and specificity of the association between catamenial seizure patterns and ovulation. Neurology. 2008;70:486–487. doi: 10.1212/01.wnl.0000278102.55701.d0. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Moore N, Fiskum G. Ovarian steroid modulation of seizure severity and hippocampal cell death after kainic acid treatment. Exp Neurol. 2003;183:124–34. doi: 10.1016/s0014-4886(03)00104-3. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Weber DA. The effect of progesterone on kindling: a developmental study. Brain Res. 1984;318:45–53. doi: 10.1016/0165-3806(84)90061-0. [DOI] [PubMed] [Google Scholar]
- Hom AC, Buterbaugh GG. Estrogen alters the acquisition of seizures kindled by repeated amygdala stimulation or pentyeletetrazol administration in ovariectomized female rats. Epilepsia. 1986;27:103–108. doi: 10.1111/j.1528-1157.1986.tb03510.x. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABA-A receptors through two discrete transmembrane sites. Nature. 2006;444(7118):486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Smith SS. Progesterone withdrawal reduces paired-pulse inhibition in rat hippocampus: dependence on GABAA receptor α4 subunit upregulation. J Neurophysiol. 2003;89:186–198. doi: 10.1152/jn.00195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isojarvi JI, Tauboll E, Herzog AG. Effect of antiepileptic drugs on reproductive endocrine function in individuals with epilepsy. CNS Drug. 2005;19:207–223. doi: 10.2165/00023210-200519030-00003. [DOI] [PubMed] [Google Scholar]
- Jacono JJ, Robinson J. The effects of estrogen, progesterone, and ionized calcium on seizures during the menstrual cycle in epileptic women. Epilepsia. 1987;28:571–577. doi: 10.1111/j.1528-1157.1987.tb03690.x. [DOI] [PubMed] [Google Scholar]
- Jin Y, Penning TM. Steroid 5α-reductases and 3α-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Baillieres Best Pract Res Clin Endocrinol Metab. 2001;15:79–94. doi: 10.1053/beem.2001.0120. [DOI] [PubMed] [Google Scholar]
- Joëls M. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- Joëls M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Joh HD, Searles RV, Selmanoff M, Alkayed NJ, Koehler RC, Hurn PD, Murphy SJ. Estradiol alters only GAD67 mRNA levels in ischemic rat brain with no consequent effects on GABA. J Cereb Blood Flow Metab. 2006;26:518–26. doi: 10.1038/sj.jcbfm.9600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien RM, Fowler GW, Danielson MG. The effects of antiepileptic drugs on estrogen-induced electrographic spike-wave discharge. J Pharmacol Exp Ther. 1975;193:647–656. [PubMed] [Google Scholar]
- Kalkbrenner KA, Standley CA. Estrogen modulation of NMDA-induced seizures in ovariectomized and non-ovariectomized rats. Brain Res. 2003;964:244–249. doi: 10.1016/s0006-8993(02)04065-9. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–867. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan PW, Norwitz ER, Ben Menachem E, Pennell PB, Druzin M, Robinson JN, Gordon JC. Obstetric risks for women with epilepsy during pregnancy. Epilepsy Behav. 2007;11:283–291. doi: 10.1016/j.yebeh.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1998;287:553–558. [PubMed] [Google Scholar]
- Kokate TG, Banks MK, Magee T, Yamaguchi S, Rogawski MA. Finasteride, a 5α-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J Pharmacol Exp Ther. 1999a;288:679–684. [PubMed] [Google Scholar]
- Kokate TG, Juhng KN, Kirkby RD, Llamas J, Yamaguchi S, Rogawski MA. Convulsant actions of the neurosteroid pregnenolone sulfate in mice. Brain Res. 1999b;831:119–124. doi: 10.1016/s0006-8993(99)01287-1. [DOI] [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcoholism. 1993;17:525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Reddy DS. Neurosteroids: a new class of neuromodulators. Drugs Today. 1995;31:433–455. [Google Scholar]
- Kumar N, Behari M, Ahuja GK, Jailkhani BL. Phenytoin levels in catamenial epilepsy. Epilepsia. 1988;29:155–158. doi: 10.1111/j.1528-1157.1988.tb04412.x. [DOI] [PubMed] [Google Scholar]
- Laidlaw J. Catamenial epilepsy. Lancet. 1956;271:1235–1237. doi: 10.1016/s0140-6736(56)90003-4. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Landgren S, Bäckström T, Kalistratov G. The effect of progesterone on the spontaneous interictal spike evoked by the application of penicillin to the cat’s cerebral cortex. J Neurol Sci. 1978;36:119–133. doi: 10.1016/0022-510x(78)90166-1. [DOI] [PubMed] [Google Scholar]
- Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL, Monaghan EP. Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Ganaxolone Presurgical Study Group. Epilepsia. 2000;41:187–1194. doi: 10.1111/j.1528-1157.2000.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide-Y to suppress hippocampus-dependent seizures. J Neurosci. 2009 doi: 10.1523/JNEUROSCI.4688-08.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NL. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski SKK, Kaczmarek L. Estrogen receptor. Potential functional significance of a variety of mRNA isoforms. FEBS Lett. 2002;524:1–5. doi: 10.1016/s0014-5793(02)03015-6. [DOI] [PubMed] [Google Scholar]
- Li X, O’Malley BW. Unfolding the action of progesterone receptors. J Biol Chem. 2003;278:39261–39264. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- Lin TY, Greenblatt M, Soloman HC. A polygraphic study of one case of peitit mal epilepsy: effects of medication and menstruation. Electroencephalogr Clin Neurophysiol. 1952;4:351–355. doi: 10.1016/0013-4694(52)90061-8. [DOI] [PubMed] [Google Scholar]
- Lim LL, Foldvary N, Mascha E, Lee J. Acetazolamide in women with catamenial epilepsy. Epilepsia. 2001;42:746–749. doi: 10.1046/j.1528-1157.2001.33600.x. [DOI] [PubMed] [Google Scholar]
- Liptakova S, Velisek L, Veliskova J, Moshe SL. Effect of ganaxolone on flurothyl seizures in developing rats. Epilepsia. 2000;41:788–793. doi: 10.1111/j.1528-1157.2000.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Logothetis J, Harner R, Morrel F. The role of estrogens in catamenial exacerbation of epilepsy. Neurology. 1959;9:352–360. doi: 10.1212/wnl.9.5.352. [DOI] [PubMed] [Google Scholar]
- Logothetis J, Harner R. Electrocortical activation by estrogens. Arch Neurol. 1960;3:290–297. doi: 10.1001/archneur.1960.00450030068007. [DOI] [PubMed] [Google Scholar]
- Lombroso CT, Forxythe I. A long-term follow-up of acetazolamide (Diamox) in the treatment of epilepsy. Epilepsia. 1960;1:493–500. doi: 10.1111/j.1528-1157.1959.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Lonsdale D, Burnham WM. The anticonvulsant effects of progesterone and 5α-dihydroprogesterone on amygdala-kindled seizures in rats. Epilepsia. 2003;44:1494–1499. doi: 10.1111/j.0013-9580.2003.59402.x. [DOI] [PubMed] [Google Scholar]
- Lonsdale D, Burnham WM. The anticonvulsant effects of allopregnanolone against amygdala-kindled seizures in female rats. Neurosci Lett. 2007;411:147–51. doi: 10.1016/j.neulet.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Maclusky NJ, Luine V, Hajszan T. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrnology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA-A receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA-A receptors: Relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA-A receptor plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–13. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD. Neuroactive steroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Progr Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]; Management Neurology. 61(6 Suppl 2):S2–15. doi: 10.1212/wnl.61.6_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- Mani SK. Signaling mechanisms in progesterone-neurotransmitter interactions. J Mol Endocrinol. 2003;30:127–137. doi: 10.1677/jme.0.0300127. [DOI] [PubMed] [Google Scholar]
- Mani SK, Allen JMC, Lydon JP, Mulac-Jericevic B, Blaustein JD, DeMayao FJ, Conneely O, O’Malley BW. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol. 1996;10:1728–1737. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- Mani SK, Mitchell A, O’Malley BW. Progesterone receptor and dopamine receptors are required in Delta 9-tetrahydrocannabinol modulation of sexual receptivity in female rats. Proc Natl Acad Sci USA. 2001;98:1249–1254. doi: 10.1073/pnas.031563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus EM, Watson CW, Goldman PL. Effects of steroids on cerebral electrical activity. Epileptogenic effects of conjugated estrogens and related compounds in the cat and rabbit. Arch Neurol. 1966;16:521–532. doi: 10.1001/archneur.1966.00470170075008. [DOI] [PubMed] [Google Scholar]
- Mares P. Anticonvulsant action of three neurosteroids against cortical epileptic afterdischarges in immature rats. Brain Res Bull. 2005;68:179–84. doi: 10.1016/j.brainresbull.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Mares P, Mikulecká A, Haugvicová R, Kasal A. Anticonvulsant action of allopregnanolone in immature rats. Epilepsy Res. 2006;70:110–117. doi: 10.1016/j.eplepsyres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Marques-Assis L. Influence of menstruation on epilepsy. Arq Neuropsiquiatr. 1981;39:390–395. doi: 10.1590/s0004-282x1981000400004. [DOI] [PubMed] [Google Scholar]
- Martini L. Androgen metabolism in the brain. Neuroendocrinol Lett. 1992;14:315–318. [Google Scholar]
- Martini L, Melcangi RC, Maggi R. Androgen and progesterone metabolism in the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1993;47:195–205. doi: 10.1016/0960-0760(93)90075-8. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ERα and ERβ. Mol Interv. 2004;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Mattson RH, Cramer JA, Caldwell BV, Siconolfi BC. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology. 1984;34:1255–1258. doi: 10.1212/wnl.34.9.1255. [DOI] [PubMed] [Google Scholar]
- McAuley JW, Moore JL, Reeves AL, Flyak J, Monaghan EP, Data J. A pilot study of the neurosteroid ganaxolone in catamenial epilepsy: clinical experience in two patients. Epilepsia. 2001;42:85–85. [Google Scholar]
- McAuley JW, Anderson GD. Treatment of epilepsy in women of reproductive age: pharmacokinetic considerations. Clin Pharmacokinet. 2002;41:559–79. doi: 10.2165/00003088-200241080-00002. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Steroid hormone actions in the brain: when is genome involved? Horm Behav. 1994;28:396–405. doi: 10.1006/hbeh.1994.1036. [DOI] [PubMed] [Google Scholar]
- McQuarrie I, Peeler DB. The effects of sustained pituitary antidiuresis and forced water drinking in epileptic children. A diagnostic and etiologic study. J Clin Invest. 1931;10:915–940. doi: 10.1172/JCI100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen JK, Woodbury DM. Attempts to produce spike-and-wave complexes in the electrocorticogram of the rat. Epilepsia. 1975;16:295–300. doi: 10.1111/j.1528-1157.1975.tb06060.x. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Rev. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–129010. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millichap JG, Woodbury DM, Goodman BS. Mechanism of the anticonvulsant action of acetazolmide, a carbonic anhydrase inhibitor. J Pharmacol Exp Ther. 1995;115:251–258. [PubMed] [Google Scholar]
- Mohammad S, Abolhassan A, Pourgholami MH. Evaluation of the anticonvulsant profile of progesterone in male amygdala-kindled rats. Epilepsy Res. 1998;30:195–202. doi: 10.1016/s0920-1211(98)00004-7. [DOI] [PubMed] [Google Scholar]
- Monaghan EP, McAculey JW, Data JL. Ganaxolone: a novel positive allosteric modulator of the GABAA receptor complex for the treatment of epilepsy. Exp Opin Invest Drugs. 1998;8:1663–1671. doi: 10.1517/13543784.8.10.1663. [DOI] [PubMed] [Google Scholar]
- Monaghan EP, Navalta LA, Shum L, Ashbrock DW, Lee DA. Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia. 1997;38:1026–1031. doi: 10.1111/j.1528-1157.1997.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Moran MH, Smith S. Progesterone withdrawal I: pro-convulsant effects. Brain Res. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- Morrell MJ. Epilepsy in women: the science of why it is special. Neurology. 1999;53 (Suppl 1):S42–S48. [PubMed] [Google Scholar]
- Morrell MJ, Montouris GD. Reproductive disturbances in patients with epilepsy. Cleve Clin J Med. 2004;71(Suppl 2):S19–24. doi: 10.3949/ccjm.71.suppl_2.s19. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Pace JR, Purdy RH, Paul SM. Characterization of steroid interactions with γ-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol Pharmacol. 1990;37:263–270. [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol Pharmacol. 2003;64:857–64. doi: 10.1124/mol.64.4.857. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Muller-Preuss P, Rupprecht R, Lancel M. The effects of the neuroactive steroid 3 alpha,5 alpha-THDOC on sleep in the rat. NeuroReport. 2002;13:487–490. doi: 10.1097/00001756-200203250-00026. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KD, Isackson PJ, Eskin TA, King MA, Montesinos SP, Abraham LA, Roper SN. Altered mRNA expression for brain-derived neurotrophic factor and type II calcium/calmodulin-dependent protein kinase in the hippocampus of patients with intractable temporal lobe epilepsy. J Comp Neurol. 2000;418:411–422. doi: 10.1002/(sici)1096-9861(20000320)418:4<411::aid-cne4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Murri L, Galli R. Catamenial epilepsy, progesterone and its metabolites. Cephalalgia. 1997;17(Suppl 20):46–47. doi: 10.1177/0333102497017S2014. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, Rosell DR, Akama KT, McEwen BS. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation-chloride cotransporters in the adult rat hippocampus. Neuroendocrinology. 2004;80:308–323. doi: 10.1159/000083657. [DOI] [PubMed] [Google Scholar]
- Narbone MC, Ruello C, Oliva A, Baviera G, D’Amico D, Bramanti P, Di Perri R. Hormonal disregulation and catamenial epilepsy. Funct Neurol. 1990;5:49–53. [PubMed] [Google Scholar]
- Newmark ME, Penry JK. Catamenial epilepsy: a review. Epilepsia. 1980;21:281–300. doi: 10.1111/j.1528-1157.1980.tb04074.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Speciale C, Sortino MA, Summa G, Caruso G, Patti F, Canonico PL. Comparative effects of estradiol benzoate, the antiestrogen clomiphene citrate and the progestin medroxyprogesterone acetate on kainic acid-induced seizures in male and female rats. Epilepsia. 1985;26:252–257. doi: 10.1111/j.1528-1157.1985.tb05414.x. [DOI] [PubMed] [Google Scholar]
- Niijima S, Wallace SJ. Effects of puberty on seizure frequency. Dev Med Child Neurol. 1989;31:174–180. doi: 10.1111/j.1469-8749.1989.tb03976.x. [DOI] [PubMed] [Google Scholar]
- Nohria V, Giller E. Ganaxolone. Neurotherapeutics. 2007;4:102–105. doi: 10.1016/j.nurt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennel PB. Antiepileptic drugs during pregnancy: What is known and which AEDs seem to be safest? Epilepsia. 2008;49(suppl 9):43–55. doi: 10.1111/j.1528-1167.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericic D, Manev H, Bujas M. Gonadal hormones and picrotoxininduced convulsions in male and female rats. Brain Res. 1996;736:174–179. doi: 10.1016/0006-8993(96)00677-4. [DOI] [PubMed] [Google Scholar]
- Phillipps GH. Structure-activity relationships in steroidal anaesthetics. J Steroid Biochem. 1975;6:607–613. doi: 10.1016/0022-4731(75)90041-2. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Inoue T, Murphy DD. Estradiol increases spine density and NMDA-dependent calcium transients in spines of CA1 pyramidal neurons from hippocampal slices. J Neurophysiol. 1999;81:1404–1411. doi: 10.1152/jn.1999.81.3.1404. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaratham S. Successful treatment of a patient with refractory catamenial epilepsy with lamotrigine. SYCP Epilepsy. 2003;10:18–20. [Google Scholar]
- Ramirez VD, Zheng J, Siddique KM. Membrane progesterone receptors for estrogen, progesterone and testosterone in the rat brain: fantasy or reality. Cell Mol Neurobiol. 1996;16:175–198. doi: 10.1007/BF02088175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez VD, Zheng J. Membrane sex-steroid receptors in the brain. Front Neuroendocrinol. 1996;17:402–439. doi: 10.1006/frne.1996.0011. [DOI] [PubMed] [Google Scholar]
- Ramsey MAE, Savege TM, Simpson BRJ, Goodwin R. Controlled sedation with alphaxolone-alphadalone. Br Med J. 1974;2:256–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;l90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Rasgon N, Serra M, Biggio G, Pisu MG, Fairbanks L, Tanavoli S, Rapkin A. Neuroactive steroid-serotonergic interaction: responses to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Eur J Endocrinol. 2001;145:25–33. doi: 10.1530/eje.0.1450025. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Proconvulsant effects of neurosteroids pregnenolone sulfate and dehydroepiandrosterone sulfate in mice. Eur J Pharmacol. 1998;345:55–59. doi: 10.1016/s0014-2999(98)00034-x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Development of neurosteroid-based novel psychotropic drugs. Progr Med Chem. 2000;37:135–175. doi: 10.1016/s0079-6468(08)70059-6. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000a;295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000b;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Kim YH, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:294–302. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:303–310. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulates GABAA receptor function and seizure susceptibility. J Neurosci. 2002;42:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol. 2003a;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol Sci. 2003b;24:103–6. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Role of neurosteroids in catamenial epilepsy. Epilepsy Res. 2004a;62:99–118. doi: 10.1016/j.eplepsyres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3α-androstanediol and 17-estradiol. Neuroscience. 2004b;128:000–000. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Anticonvulsant activity of the testosterone-derived neurosteroid 3α-androstanediol. NeuroReport. 2004c;15:515–518. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Woodward R. Ganaxolone: A prospective overview. Drugs Future. 2004;29:227–242. [Google Scholar]
- Reddy DS, Castenada DA, O’Malley BW, Rogawski MA. Antiseizure activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Apanites LA. Anesthetic effects of progesterone are undiminished in progesterone receptor knockout mice. Brain Res. 2005;1033:96–101. doi: 10.1016/j.brainres.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Reddy DS, O’Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Physiological role of adrenal deoxycorticosterone-derived neuroactive steroids in stress-sensitive conditions. Neuroscience. 2006;38:11–20. doi: 10.1016/j.neuroscience.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Zeng YC. Differential anesthetic activity of ketamine and the GABAergic neurosteroid allopregnanolone in mice lacking progesterone receptor A and B subtypes. Meth Find Exp Clin Pharmacol. 2007a;29:659–664. doi: 10.1358/mf.2007.29.10.1147766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Zeng YC. Effect of neurosteroid withdrawal on spontaneous recurrent seizures in a rat model of catamenial epilepsy. FASEB J. 2007b;21(6):A1179–1179. [Google Scholar]
- Reddy DS, Zeng YC, Dhanushkodi A, Hattiangady B, Shetty AK. Hippocampal interneuron damage, spontaneous recurrent seizures, mossy fiber sprouting, and neurosteroid efficacy in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia. 2007;48 (Suppl 6):291–292. [Google Scholar]
- Reddy DS. Perimenstrual catamenial epilepsy. Women’s Health. 2007;3:95–206. doi: 10.2217/17455057.3.2.195. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Mass spectrometric quantification and physiological-pharmacological activity of androgenic neurosteroids. Neurochem Internl. 2008;52:541–553. doi: 10.1016/j.neuint.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;5:00–000. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibel S, André V, Chassagnon S, André G, Marescaux C, Nehlig A, Depaulis A. Neuroprotective effects of chronic estradiol benzoate treatment on hippocampal cell loss induced by status epilepticus in the female rat. Neurosci Lett. 2000b;281:79–82. doi: 10.1016/s0304-3940(00)00784-9. [DOI] [PubMed] [Google Scholar]
- Reid B, Gangar KF. Catamenial epilepsy and goserelin. Lancet. 1992;339:253–253. doi: 10.1016/0140-6736(92)90066-c. [DOI] [PubMed] [Google Scholar]
- Reilly MT, Crabbe JC, Rustay NR, Finn DA. Acute neuroactive steroid withdrawal in withdrawal seizure-prone and withdrawal seizure-resistant mice. Pharmacol Biochem Behav. 2000;67:709–717. doi: 10.1016/s0091-3057(00)00416-0. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Harney JP, Frye CA. Gonadal, adrenal, and neuroactive steroids’s role in ictal activity. Brain Res. 2004;1000:8–18. doi: 10.1016/j.brainres.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Looking for GABA in all the wrong places: the relevance of extrasynaptic GABAA receptors to epilepsy. Epilepsy Curr. 2004;4:239–242. doi: 10.1111/j.1535-7597.2004.46008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Macias KA. Catamenial epilepsy: gynecological and hormonal implications. Five case reports. Gynecol Endocrinol. 1996;10:139–142. doi: 10.3109/09513599609097905. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Reddy DS. Neurosteroids: endogenous modulators of seizure susceptibility. In: Rho JM, Sankar R, Cavazos JE, editors. Epilepsy: Scientific Foundations of Clinical Practice. New York: Marcel Dekker; 2004. pp. 319–355. [Google Scholar]
- Rosciszewska D. Analysis of seizure dispersion during menstrual cycle in women with epilepsy. Monogr Neural Sci. 1980;5:280–284. [PubMed] [Google Scholar]
- Rosciszewska D. Epilepsy and menstruation. In: Hopkins A, editor. Epilepsy. London: Chapman & Hall; 1987. pp. 373–381. [Google Scholar]
- Rosciszewska D, Buntner B, Guz I, Zawisza L. Ovarian hormones, anticonvulsant drugs, and seizures during the menstrual cycle in women with epilepsy. J Neurol Neurosurg Psychiatry. 1986;49:47–51. doi: 10.1136/jnnp.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross IP. Acetazolamide therapy in epilepsy. Lancet. 1958;2:1308–1309. doi: 10.1016/s0140-6736(58)90578-6. [DOI] [PubMed] [Google Scholar]
- Roste LS, Tauboll E, Svalheim S, Gjerstad L. Does menopause affect the epilepsy? Seizure. 2008;17:172–175. doi: 10.1016/j.seizure.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Berning B, Hauser CAE, Holsboer F, Reul JMHM. Steroid receptor-mediated effects of neuroactive steroids: characterization of structure-activity relationship. Eur J Pharmacol. 1996;303:227–234. doi: 10.1016/0014-2999(96)00036-2. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglgansberger W, Holsboer F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–530. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- Rush ME, Blake CA. Serum testosterone concentrations during the 4-day estrous cycle in normal and adrenalectomized rats. Proc Soc Exp Biol Med. 1982;169:216–221. doi: 10.3181/00379727-169-41334. [DOI] [PubMed] [Google Scholar]
- Sabers A, Öhman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology. 2003;61:570–571. doi: 10.1212/01.wnl.0000076485.09353.7a. [DOI] [PubMed] [Google Scholar]
- Sabers A, Gram L. Lamotrigine pharmacokinetics during anticonception and pregnancy. Letters in Drug Design & Discovery. 2006;3:321–322. [Google Scholar]
- Saberi M, Pourgholami MH. Estradiol alters afterdischarge threshold and acquisition of amygdala kindled seizures in male rats. Neurosci Lett. 2003;340:41–44. doi: 10.1016/s0304-3940(03)00074-0. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71:3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Selye H. The antagonism between anesthetic steroid hormones and pentamethylenetetrazol (metrazol) J Lab Clin Med. 1942;27:1051–1053. [Google Scholar]
- Shavit G, Lerman P, Korczyn AD, Kivity S, Bechar M, Gitter S. Phenytoin pharmacokinetics in catamenial epilepsy. Neurology. 1984;34:959–961. doi: 10.1212/wnl.34.7.959. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABA-A receptor α4-subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA-A receptors: Focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Locally applied estrogens potentiate glutamate-evoked excitation of cerebellar Purkinje cells. Brain Res. 1988;475:272–282. doi: 10.1016/0006-8993(88)90615-4. [DOI] [PubMed] [Google Scholar]
- Speroff L, Glass RH, Kase NG. Clinical gynecologic endocrinology and infertility. 6. Lippincott Williams & Wilkins; Philadelphia: 1999. [Google Scholar]
- Stein-Behrens BA, Elliott EM, Miller CA, Schilling JW, Newcombe R, Sapolsky RM. Glucocorticoids exacerbate kainic acid-induced extracellular accumulation of excitatory amino acids in the rat hippocampus. J Neurochem. 1992;58:1730–1735. doi: 10.1111/j.1471-4159.1992.tb10047.x. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt SL, Kinnard WJ. The effects of certain progestins and estrogen on the threshold of electrically induced seizure patterns. Neurology. 1986;18:213–216. doi: 10.1212/wnl.18.3.213. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann NY Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- Strohle A, Pasini A, Romeo E, Hermann B, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Fluoxetine decreases concentrations of 3α,5α-tetrahydrodeoxycorticosterone (THDOC) in major depression. J Psychiatr Res. 2000;34:183–186. doi: 10.1016/s0022-3956(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Nyberg S, Bäckström T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology. 1997;17:370–381. doi: 10.1016/S0893-133X(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong Q, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the GABAA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Tauboll E, Lundervold A, Gjerstad L. Temporal distribution of seizures in epilepsy. Epilepsy Res. 1991;8:153–165. doi: 10.1016/0920-1211(91)90084-s. [DOI] [PubMed] [Google Scholar]
- Teresawa E, Timiras PS. Electrical activity during the estrous cycle of the rat: cyclic changes in limbic structures. Endocrinology. 1968;83:207–216. doi: 10.1210/endo-83-2-207. [DOI] [PubMed] [Google Scholar]
- Teschemacher A, Kasparov S, Kravitz EA, Rahamimoff R. Presynaptic action of the neurosteroid pregnenolone sulfate on inhibitory transmitter release in cultured hippocampal neurons. Brain Res. 1997;772:226–32. doi: 10.1016/s0006-8993(97)00872-x. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, Maclusky NJ. ER-X: a novel plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towanabut S, Chulavatnatol S, Suthisisang C, Wanakamanee U. The period prevalence of catamenial epilepsy at Prasat Neurological Institute, Bangkok. J Med Assoc Thai. 1998;81:970–977. [PubMed] [Google Scholar]
- Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Suzuki T, Misawa M. Modulation of the decrease in the seizure threshold of pentylenetetrazole in diazepam-withdrawn mice by the neurosteroid 5α-pregnan-3α,21-diol-20-one (alloTHDOC) Addiction Biol. 1997;2:455–460. doi: 10.1080/13556219772516. [DOI] [PubMed] [Google Scholar]
- Turner DM, Ransom RW, Yang JSJ, Olsen EW. Steroid anesthetics and naturally occurring analogs modulate the γ-aminobutyric acid receptor complex at a site distinct from barbiturates. J Pharmacol Exp Ther. 1989;248:960–966. [PubMed] [Google Scholar]
- Tuveri A, Paoletti AM, Orrù M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G, Concas A. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–1229. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Velisek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;41(Suppl 6):S30–35. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Veliskova J. The role of estrogens in seizures and epilepsy: the bad guys or the good guys? Neuroscience. 2006;138:837–844. doi: 10.1016/j.neuroscience.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Velísková J, Velísek L. Beta-estradiol increases dentate gyrus inhibition in female rats via augmentation of hilar neuropeptide-Y. J Neurosci. 2007;27:6054–6063. doi: 10.1523/JNEUROSCI.0366-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velíšková J. Estrogens and Epilepsy: Why are we so excited? Neuroscientist. 2007;13:77–88. doi: 10.1177/1073858406295827. [DOI] [PubMed] [Google Scholar]
- Velísek L, Velísková J. New avenue of research: antiepileptic drug and estradiol neuroprotection in epilepsy. Recent Patents CNS Drug Discov. 2008;3:128–37. doi: 10.2174/157488908784534577. [DOI] [PubMed] [Google Scholar]
- Vicini S, Losi G, Homanics GE. GABA-A receptor delta subunit deletion prevents neurosteroid modulation of inhibitory synaptic currents in cerebellar neurons. Neuropharmacology. 2002;43:646–650. doi: 10.1016/s0028-3908(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Voiculescu V, Hategan D, Manole E, Ulmeanu A. Increased susceptibility to audiogenic seizures following withdrawal of progesterone. Rom J Neurol Psychiatry. 1994;32:131–133. [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Bäckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- Weiland NG. Glutamic acid decarboxylase messenger ribonucleic acid is regulated by estradiol and progesterone in the hippocampus. Endocrinology. 1992;131:2697–2702. doi: 10.1210/endo.131.6.1446611. [DOI] [PubMed] [Google Scholar]
- Werboff LH, Havlena J. Audiogenic seizures in adult male rats treated with various hormones. Gen Comp Endocrinol. 1963;8:389–397. doi: 10.1016/0016-6480(63)90053-4. [DOI] [PubMed] [Google Scholar]
- Williamson J, Mtchedlishvili Z, Kapur J. Characterization of the convulsant action of pregnenolone sulfate. Neuropharmacology. 2004;46:56–864. doi: 10.1016/j.neuropharm.2003.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Moss RL. Patch-clamp analysis of direct steroidal modulation of glutamate receptor-channels. J Neuroendocrinol. 1994;6:347–355. doi: 10.1111/j.1365-2826.1994.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Schwartzkroin PA. Hormonal effects on the brain. Epilepsia. 1998;39 (Suppl 8):S2–S8. doi: 10.1111/j.1528-1157.1998.tb02601.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Estradiol facilitates kainic acid-induced, but not flurothyl-induced, behavioral seizure activity in adult female rats. Epilepsia. 2000;41:510–515. doi: 10.1111/j.1528-1157.2000.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DE, Timiras PS. The gonad-brain relationship: effects of female sex hormones on electroshock convulsions in the rat. Endocrinology. 1962a;70:196–209. doi: 10.1210/endo-70-2-196. [DOI] [PubMed] [Google Scholar]
- Woolley DE, Timiras PS. Estrous and circadian periodicity and electroshock convulsions in rats. Am J Physiol. 1962b;202:379–82. doi: 10.1152/ajplegacy.1962.202.2.379. [DOI] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- Yu R, Follesa P, Ticku MK. Downregulation of the GABA receptor subunits mRNA levels in the mammalian cultured cortical neurons following chronic neurosteroid treatment. Mol Brain Res. 1996;41:163–168. doi: 10.1016/0169-328x(96)00087-3. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW, Holder DT, Reiter EO, Dekaban AS. Medroxyprogesterone acetate in the treatment of seizures associated with menstruation. J Pediatr. 1973;83:959–963. doi: 10.1016/s0022-3476(73)80529-3. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lehan N, Wehrenberg U, Disteldorf E, von Lossow R, Mares U, Jarry H, Rune GM. Neuroprotection by estradiol: a role of aromatase against spine synapse loss after blockade of GABAA receptors. Exp Neurol. 2007;203:72–81. doi: 10.1016/j.expneurol.2006.07.020. [DOI] [PubMed] [Google Scholar]