Summary
The formation of new blood vessels through the process of angiogenesis is critical in vascular development and homeostasis. Aberrant angiogenesis leads to a variety of diseases, such as ischemia and cancer. Recent studies have revealed important roles for miRNAs in regulating endothelial cell (EC) function, especially angiogenesis. Mice with EC-specific deletion of Dicer, a key enzyme for generating miRNAs, display defective postnatal angiogenesis. Specific miRNAs (angiomiRs) have recently been shown to regulate angiogenesis in vivo. miRNA-126, an EC-restricted miRNA, regulates vascular integrity and developmental angiogenesis. miR-378, miR-296, and the miR-17~92 cluster, contribute to tumor angiogenesis. Manipulating angiomiRs in the settings of pathological vascularization represents a new therapeutic approach.
Introduction
Endothelial cells (ECs) form the internal barrier of the vasculature, and play fundamental roles in vascular development and disease. During vasculogenesis, ECs differentiate from angioblast precursors, proliferate in situ, and coalesce to form a primitive vascular network. New vessels form from existing blood vessels by angiogenesis and subsequent remodeling. In response to angiogenic stimuli, ECs within blood vessels are activated to migrate and proliferate to form primary capillaries, which undergo remodeling through sprouting, branching and intussusception. In adult tissues, most blood vessels remain quiescent and function to conduct nutritive blood flow. However, postnatal angiogenesis occurs in response to physiological and pathological events, such as reproduction, inflammation, tissue regeneration and tumor growth. Aberrant angiogenesis leads to numerous disorders, such as cancer and ischemia. Angiogenic factors, such as VEGF and FGF, have been shown to be important for angiogenesis. Binding of these factors to their cell surface receptors activates mitogen-activated protein kinase (MAPK), phosphinositide 3-kinase (PI3K), and other pathways, which promote EC cell proliferation, migration and survival [1]. Molecules in VEGF signaling pathways have been advanced as targets for anti-angiogenic therapy.
MicroRNAs (miRNAs) represent a class of conserved non-coding small RNAs, which repress gene expression post-transcriptionally by targeting 3′-untranslated regions (3′UTRs) of mRNAs. Nearly 700 miRNAs have been identified in humans, and are predicted to regulate a third of protein coding genes [2]. The ability of one miRNA to target multiple mRNAs, especially those that function in the same intracellular pathway, as well as the possibility of targeting one mRNA by multiple miRNAs, adds a rich layer of regulation to gene expression. Mounting evidence indicates that miRNAs are important regulators of cardiovascular development and disease, as well as cancer (for review, [3–5]). Here we present a summary of recent advances in understanding the roles of miRNAs in angiogenesis and endothelial function.
Requirement miRNAs in angiogenesis as revealed by Dicer deletion
The importance of miRNAs in angiogenesis and endothelial function was revealed by disrupting the function of Dicer and Drosha—two key enzymes for miRNA biogenesis. Dicer hypomorphic mouse lines have defects in vascular remodeling during development or ovary angiogenesis [6,7]. Knockdown of Dicer or Drosha in vitro in human ECs results in a decrease in angiogenesis, assayed by endothelial tube formation in matrigel, although the effect for Drosha knockdown is less profound than for Dicer knockdown [8–10]. The distinct difference can be attributed to Drosha independent miRNA biogenesis [11,12], or functions of Dicer in addition to miRNA maturation, such as maintaining heterochromatin [13]. E-specific deletion of Dicer in mice provided direct in vivo evidence that endothelial miRNAs are required for postnatal angiogenesis in response to angiogenic stimuli [14]. In that study, Tie2-Cre or Tamoxifen-inducible VECad-Cre was used to achieve EC-specific inactivation of Dicer in mice. Both lines showed reduced postnatal angiogenic responses to a variety of stimuli, including exogenous VEGF, tumors, limb ischemia, and wound healing. Mechanistically, Dicer silencing leads to up-regulation of thrombospondin-1 (Tsp-1) [8,14], a potent inhibitor of angiogenesis, as well as altered expression of other key regulators of endothelial biology and angiogenesis, such as TEK/Tie2, KDR/VEGFR2, Tie-1, eNOS and IL-8 [10]. Tsp-1 is a predicted target of the Let-7 family and the miR-17~92 cluster. Inhibitors of Let-7f or the miR-17~92 cluster reduce EC sprouting and matrigel tube formation in vitro [8,14]. Conversely, transfection of ECs with components of the miR-17~92 cluster, especially miR-18a, rescued the induced expression of Tsp-1 and the defects in EC proliferation and morphogenesis caused by the loss of Dicer.
Expression and Regulation of miRNAs in ECs
miRNA profiling mainly in human umbilical vein endothelial cells (HUVECs) revealed that miR-221/222, miR-21, the let-7 family, the miR-17~92 cluster, the miRNA-23~24 cluster, and miR-126 are highly expressed in ECs [8,10,15–17]. miRNA-126 is the only miRNA known to be expressed specifically in the endothelial lineage and hematopoietic progenitor cells [18–23].
Hypoxia can stimulate angiogenesis, but also EC growth arrest and apoptosis. Hypoxia-regulated miRNAs (HRMs) have been identified in cancer cell lines or ECs by several groups [15,24,25]. Among the HRMs, miR-210 is induced by hypoxia in all cell types tested [15,24–28] and hypoxia-inducible factor-1α is necessary and sufficient for miR-210 activation. Sessa and colleagues found that VEGF induces time-dependent expression of miR-191, -155, -31, -17-5p, -18a, and miR-20a, with little change in miR-126 and miR-222 [14]. Interestingly, this set of VEGF-induced miRNAs is commonly overexpressed in human tumors, and has been implicated in the control of tumor growth, survival, and angiogenesis. Expression of miR-296 is elevated in primary tumor ECs isolated from human brain tumors compared to normal brain ECs [29]. VEGF, EGF, or conditioned medium from tumor cells was sufficient to upregulate miR-296 expression in ECs. Besides these, miR-130a can be strongly up-regulated by serum [30]; the miR-17-92 cluster is directly activated by c-Myc [31]; while miR-21 and miR-31 can be induced in ECs by the viral protein K15 [32]; miR-320 is upregulated in myocardial microvascular ECs from diabetic rats [33]. Interestingly, most of the signal-induced miRNAs are pro-angiogenic, likely contributing to the angiogenic action of different factors.
Another level of miRNA regulation in ECs involves the shuttling of miRNAs between cells through secretion in microvesicles [34]. Microvesicles have been shown to originate from tumor cells, platelets, monocytes, ECs or other cell types. EC-derived microvesicles were shown to be increased in hypertension patients [35]. How miRNA containing microvesicles regulate vascular disease is an interesting question for the future.
AngiomiRs: Functions and Targets
The functions of individual miRNAs in angiogenesis and tumor angiogenesis are just beginning to be revealed. Here we adopt a term “angiomiR” to name miRNAs that regulate angiogenesis either cell-autonomously (Fig. 1) or non-cell-autonomously (Fig. 2) [29]. Pro-angiomiRs promote angiogenesis by targeting negative regulators in angiogenic signaling pathways, while anti-angiomiRs inhibit angiogenesis by targeting positive regulators of angiogenesis. Recent progress toward understanding the functions of specific miRs in EC biology is summarized below (Table 1).
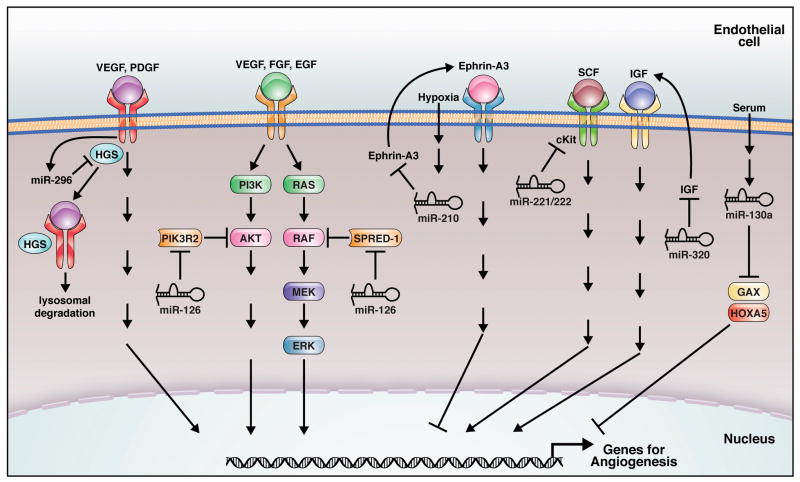
Fig. 1.
Cell-autonomous regulation of angiogenesis by miRNAs. Endothelial miRNAs regulate the angiogenic response to multiple growth factors by targeting angiogenic factors, receptors and signaling molecules. miR-126, miR-130a, miR-210, and miR-296 are considered as pro-angiomiRs; while miR-221/222, miR-320 are believed to be anti-angiomiRs. Regulation, targets and regulators for these miRNAs are indicated.
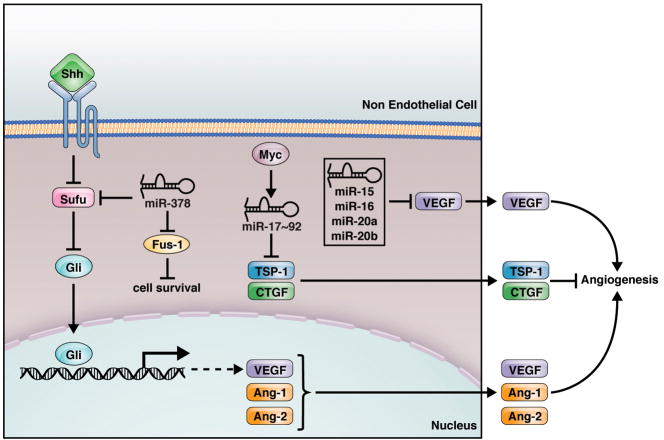
Fig. 2.
Non-cell-autonomous regulation of angiogenesis by miRNAs. Non EC cells, such as tumor cells, express miRNAs which can regulate the expression of angiogenic factors or inhibitors, thereby modulating angiogenesis in a non-cell-autonomous manner. The targets and regulators for these miRNAs are indicated.
Table 1.
microRNAs involved in angiogenesis
| miRs | Angiogenic Function | Relevant Targets | References |
|---|---|---|---|
| miR-17~92 | miR-17~92 overexpression in tumor cells promotes tumor angiogenesis | TSP-1 CTGF | [46] |
| miR-21, miR-31 | Required for viral protein induced endothelial cell migration | ? | [32] |
| miR-126 | Required for vascular integrity and angiogenesis in vivo | Spred-1, PIK3R2, | [18,23,36] |
| miR-130a | Antagonizes the anti-angiogenic activity of GAX and HOXA5 | GAX, HOXA5 | [30] |
| miR-210 | Enhances angiogenesis and survival response to Hypoxia in vitro | Ephrin-A3 | [15,28] |
| miR-296 | Required for tumor angiogenesis in vivo | HGS | [29] |
| miR-378 | Promotes tumor angiogenesis | Sufu, Fus-1 | [49] |
| miR-27b and let 7f | Required for angiogenesis in vitro | ? | [8] |
| miR-15b, miR-16, miR-20a, miR-20b | ? | VEGF | [24] |
| miR-221/222 | Impairs SCF induced angiogenesis | c-Kit | [17] |
| miR-320 | Inhibition of miR-320 improves angiogenesis in diabetic endothelial cells | IGF-1 | [33] |
A. AngiomiRs with in vivo evidences
miR-126
miR-126 is the only miRNA identified to date that shows EC-specific expression and the first vascular miRNA to be knocked out in mice. Loss-of-function studies in mice and zebrafish revealed an important function of miR-126 in governing vascular integrity and angiogenesis [18,23,36]. Targeted deletion of miR-126 in mice caused leaky vessels, hemorrhaging, and partial embryonic lethality, due to a loss of vascular integrity and defective angiogenesis [23,36]. miR-126−/− mice showed severely delayed vascularization during cranial vessel and retina development. Furthermore, miR-126−/− ECs are defective in angiogenesis in response to angiogenic factors, as shown by an aortic ring assay in vitro, and matrigel and corneal micropocket assays in vivo [23,36]. In zebrafish, knockdown of miR-126 resulted in hemorrhage, and collapse of the dorsal aorta and primary cardinal veins [18], indicating a conserved function of miR-126.
miR-126 is encoded by an intron of the Egfl7 gene, which encodes an EC-derived secreted peptide which acts as a chemoattractant and inhibitor of smooth muscle cell migration [37,38]. In vivo functional studies in mice and zebrafish indicate a role for Egfl7 in EC migration and vasculogenesis [39–41]. Intriguingly, miR-126−/− mice display similar vascular abnormalities to the previously reported Egfl7 knockout mice, such as edema, defective cranial vessel and retina vascularization [23,41]. This raised a controversy as to which molecule is responsible for the observed phenotype. In miR-126−/− mice, Egfl7 expression is not changed at either the mRNA or protein level [23]. However, miR-126 expression in Egfl7 knockout mice was not examined [41]. Very recently, floxed alleles of Egfl7 (Egfl7Δ) and miR-126 (miR-126Δ) were generated [36]. Egfl7Δ/Δ mice, in which miR-126 is not affected, are phenotypically normal; whereas miR-126Δ/Δ mice, in which Egfl7 is normally expressed, recapitulate numerous previously described embryonic and postnatal vascular phenotypes in Egfl7 knockout mice. These results clearly demonstrate that miR-126 is required for angiogenesis and maintenance of vascular integrity in mice. The in vivo functions of Egfl7 might be masked by its paralog Egfl8. This controversy highlights the importance of minimally disruptive gene targeting strategies because of the existence of intronic miRNAs in the genome.
Neoangiogenesis is essential for vascular regeneration in response to injury, such as myocardial infarction (MI). miR-126−/− mice also showed reduced survival and defective cardiac neovascularization following MI, suggesting a critical function of miR-126 in neoangiogenesis [23]. The proangiogenic action of miR-126 is mediated, at least in part, by promoting MAP kinase and PI3K signaling in response to VEGF and FGF, through targeting negative regulators of these signaling pathways, including the Sprouty-related EVH domain-containing protein Spred-1 and PI3K regulatory subunit 2 (PIK3R2/p85-β) (Fig. 1) [18,23,36]. Besides Spred-1 and PIK3R2, miR-126 also targets vascular cell adhesion protein 1 (VCAM-1), thereby regulating the adhesion of leukocytes to the endothelium [16], suggesting a role of miR-126 in vascular inflammation. miR-126 was also reported to inhibit tumorigenesis and to be downregulated in many cancer lines [42–44]. CT10 regulator of kinase (CRK) and PIK3R2 appear to be the relevant targets for miR-126 repression in cancer cells [42,43]. Why PIK3R2 represses PI3K-AKT signaling in ECs but enhances PI3K-AKT signaling in cancer cells is not known. However, these results indicate that miR-126 is a multi-functional miRNA with important roles in angiogenesis, tumor growth and invasion, and vascular inflammation.
The miR-17~92 cluster
The miR-17~92 cluster, also named OncomiR-1, was the first identified tumor promoting miRNA. This gene cluster encodes miR-17, miR-18, miR-19a, 20a, miR-19b-1 and miR-92-1. Two paralogs of miR-17~92, miR-106a~363 and miR-106b~25, also exist in mammals. miR-17~92 has been shown to cooperate with c-Myc to induce B cell lymphoma in mice [45]. Overexpression of miR-17~92 in Ras expressing cells promotes tumor angiogenesis in vivo in a non-cell autonomous manner [46]; while inhibition of miR-17~92 in vitro represses EC sprouting and tube formation in matrigel [14]. miR-17~92 promotes tumor angiogenesis by targeting anti-angiogenic proteins thrombospondin-1 (Tsp1) and connective tissue growth factor (CTGF) therefore regulating angiogenesis in a non- cell-autonomous manner. (Fig. 2) [14,46].
Despite the well-studied role of miR-17~92 in tumorigenesis and tumor angiogenesis, the physiological function of the miR-17~92 cluster during development was only recently described. Deletion of the miR-106a-363 and miR-106b-25 clusters, either alone or in combination, did not result in any obvious phenotype. In contrast, miR-17-92 knockout mice are smaller and die immediately after birth, likely due to severely hypoplastic lungs and ventricular septal defects [47]. The absence of miR-17~92 also inhibited B cell development at the pro-B to pre-B transition, indicating an essential role for miR-17~92 in B cell development. The precise role for miR-17~92 members in developmental angiogenesis remains unclear.
miR-378
miR-378 is enriched in CD34+ hematopoietic progenitor cells [48]. When overexpressed in cancer cell lines, miR-378 increased cell survival and reduced cell death [49]. When cells were transfected with a construct expressing an antisense sequence against miR-378, cell survival decreased significantly. Strikingly, nude mice injected with miR-378-transfected cancer cells form much larger tumors with larger blood vessels compared to GFP-transfected cells. This is consistent with a report that miR-378 promotes VEGF expression by competing with miR-125a for the same seed region in the VEGF 3′-UTR [24]. Suppressor of fused (Sufu) and Fus-1 are tumor suppressors which serve as targets for miR-378 repression (Fig. 2) [49]. Sufu functions as a negative regulator of Shh signaling. Shh induced vessels are characterized by distinct large-diameters. Shh promotes large-diameter vessel formation by inducing expression of angiogenic cytokines, including VEGF and angiopoietin-1 (Ang-1) and -2 (Ang-2) [50]. Therefore, miRNA-378 promotes cell survival by targeting Sufu and Fus-1, and regulates tumor angiogenesis by indirect upregulation of angiogenic factors.
miR-296
miR-296 was shown to be a pro-angiomiR by in vitro matrigel and scratch wound assays [29]. Intravenous injection of miR-296 antagomirs inhibited glioma angiogenesis in vivo. Hepatocyte growth factor-regulated tyrosine kinase substrate (HGS), is a miR-296 target that mediates its angiogenic function (Fig. 1). HGS is involved in the sorting of the VEGF and PDGF receptors for degradation. miR-296 is upregulated in tumor ECs from human gliomas, consistent with the lower HGS expression and upregulation of VEGFR2 and PDGFRβ in glioma blood vessels. These results support a role for miR-296 in promoting angiogenesis in tumors.
B. In vitro Evidence for AngiomiRs
miR-210, a hypoxia-induced miRNA, is a crucial regulator of angiogenesis and EC survival in response to hypoxia. miR-210 overexpression in normoxic ECs stimulated angiogenesis and VEGF-induced cell migration. Conversely, blockade of miR-210 via anti-miRNA transfection inhibited tube formation stimulated by hypoxia and cell migration in response to VEGF. miR-210 knockdown also inhibited cell growth and induced apoptosis in both normoxia and hypoxia. Ephrin-A3 is a relevant target of miR-210 in regulating the hypoxia response (Fig. 1) [15,28]. Overexpression of an Ephrin-A3 allele that is not targeted by miR-210 prevented miR-210-mediated stimulation of tube formation and EC migration.
miR-21 and miR-31 are potential pro-angiomiRs. miR-21 and miR-31 are up-regulated in various cancers, and miR-21 can stimulate invasion and metastasis in cancer [51]. Knockdown of miR-21 and miR-31 disrupted Virus protein K15M induced cell migration in ECs, suggesting that the upregulation of miR-21 and miR-31 contributes to the increased invasiveness of Kaposi’s sarcoma by K15M [32]. Let-7f and miR-27b are involved in angiogenesis based on the evidence that inhibitors against these two miRNAs reduce sprouting angiogenesis in vitro [8]. miR-130a, a miRNA induced by serum in ECs, is able to antagonize the antiangiogenic activity by its target gene GAX and HOXA5 (Fig. 1) [30].
Other miRNAs are believed to be anti-angiomiRs. Overexpression miR-221/222 in ECs impaired stem cell factor (SCF) induced angiogenesis and scratch wound healing [17]. C-Kit, the receptor for SCF, is a target for miR-221/222 repression, not only in ECs, but also in SMCs and HPCs (Fig. 1) [17,52,53]. miR-15b, miR-16, miR-20a and miR20b are potential anti-angiomiRs by targeting VEGF for repression (Fig. 2) [24]. Inhibition of miR-320 improved angiogenesis in diabetic ECs in vitro by targeting IGF-1 protein (Fig. 1) [33].
AngiomiR therapeutics
The identification of angiomiRs as key regulators of angiogenesis has opened a new avenue for therapeutics of vascular diseases. AngiomiR therapeutics provide a natural means of normalizing the expression of disease genes, potentially avoiding the toxicity or drug resistance caused by switching a single target on/off. Mimics of pro-angiomiRs, or antagomirs of anti-angiomiRs, can be used to elevate angiogenesis in the pathological setting of insufficient angiogenesis, such as MI and ischemia. Conversely, mimics of anti-angiomiRs, or antagomirs of pro-angiomiRs, may be efficacious in settings of pathological vascularization, such as tumor angiogenesis and retinopathy. Since miR-126 is necessary for neoangiogenesis after MI, miR-126 mimics might be utilized to enhance neoangiogenesis after MI or ischemia [23]. Potential challenges for angiomiR therapeutics include the efficiency of the delivery system, and the currently incomplete understanding of the biology of individual miRNAs.
Future directions
Future studies will be focused on elucidating the in vivo functions of the increasing angiomiR family members. Specific attention will be paid to miRNA target identification, cell-type specific functions of miRNAs, and combinatorial effects of related miRNAs. In the meantime, the miRNA signatures of different vascular disease models remain to be identified. These studies will contribute to emerging efforts for angiomiR therapeutics for numerous vascular disorders.
Acknowledgments
We thank Jose Cabrera for graphics. This work was supported by grants from the National Institutes of Health, the Donald W. Reynolds Clinical Cardiovascular Research Center, the Sandler Foundation for Asthma Research, and the Robert A. Welch Foundation to ENO. S.W. was supported by a fellowship grant from the American Heart Association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 2.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 3.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 4.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 5.van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circ Res. 2008;103:919–928. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, Han J. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118:1944–1954. doi: 10.1172/JCI33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 8.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 9.Shilo S, Roy S, Khanna S, Sen CK. Evidence for the involvement of miRNA in redox regulated angiogenic response of human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:471–477. doi: 10.1161/ATVBAHA.107.160655. [DOI] [PubMed] [Google Scholar]
- 10•.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. The authors showed that EC-specific deletion of Dicer in mice reduces postnatal angiogenesis in response to angiogenic stimuli. This is the first in vivo evidence that endothelial miRNAs are required for angiogenesis. [DOI] [PubMed] [Google Scholar]
- 11.Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol Cell. 2007;28:328–336. doi: 10.1016/j.molcel.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamura K, Hagen JW, Duan H, Tyler DM, Lai EC. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007;130:89–100. doi: 10.1016/j.cell.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suarez Y, Fernandez-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. This article showed that hypoxia induces miR-210 in ECs, and upregulation of miR-210 is a crucial element of endothelial cell response to hypoxia, affecting cell survival, migration, and differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 18••.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. The authors showed that knockdown of miR-126 in zebrafish results in loss of vascular integrity and hemorrhage during embryonic development. miR-126 regulates angiogenesis by targeting Spred-1 and PIK3R2, negative regulators of VEGF signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM. MicroRNA expression and function in cancer. Trends Mol Med. 2006;12:580–587. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS, et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2:219–229. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musiyenko A, Bitko V, Barik S. Ectopic expression of miR-126*, an intronic product of the vascular endothelial EGF-like 7 gene, regulates prostein translation and invasiveness of prostate cancer LNCaP cells. J Mol Med. 2008;86:313–322. doi: 10.1007/s00109-007-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. The authors demonstrated that knockout of an EC-specific microRNA miR-126 in mice caused edema, hemorrhaging, and partial embryonic lethality, due to loss of vascular integrity and defective angiogenesis. miR-126−/− mice showed reduced survival and defective neoangiogenesis following myocardial infarction. This is the first report of vascular miRNA to be knocked out in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–1348. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- 27.Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, Li C, O’Brien-Jenkins A, Katsaros D, Weber BL, et al. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7:255–264. doi: 10.4161/cbt.7.2.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulkkinen K, Malm T, Turunen M, Koistinaho J, Yla-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett. 2008;582:2397–2401. doi: 10.1016/j.febslet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 29••.Wurdinger T, Tannous BA, Saydam O, Skog J, Grau S, Soutschek J, Weissleder R, Breakefield XO, Krichevsky AM. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14:382–393. doi: 10.1016/j.ccr.2008.10.005. The authors demonstrated that miR-296 is required for tumor angiogenesis. Inhibition of miR-296 with antagomirs reduces angiogenesis in tumor xenografts in vivo. miR-296 contributes to angiogenesis by regulating VEGF and PDGF receptor turnover. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111:1217–1226. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 32.Tsai YH, Wu MF, Wu YH, Chang SJ, Lin SF, Sharp TV, Wang HW. The M type K15 protein of Kaposi’s sarcoma-associated herpesvirus regulates microRNA expression via its SH2-binding motif to induce cell migration and invasion. J Virol. 2009;83:622–632. doi: 10.1128/JVI.00869-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 expression in myocardial microvascular endothelial cell and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2008 doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 34•.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. The authors showed that miRNAs can be shuttled between cells through secretion in micrcovesicles. This provides a new mechanism for miRNA regulation and function. [DOI] [PubMed] [Google Scholar]
- 35.Bakouboula B, Morel O, Faure A, Zobairi F, Jesel L, Trinh A, Zupan M, Canuet M, Grunebaum L, Brunette A, et al. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:536–543. doi: 10.1164/rccm.200706-840OC. [DOI] [PubMed] [Google Scholar]
- 36•.Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, Kuo CJ. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008 doi: 10.1242/dev.029736. By knocking out of Egfl7 and miR-126 in mice seperately, the authors demonstrated that the vascular phenotypes of mouse Egfl7 locus attributes to the microRNA miR-126. This highlights the importance of minimally disruptive gene targeting strategies because of the existence of intronic miRNAs in the genome. [DOI] [PubMed] [Google Scholar]
- 37.Campagnolo L, Leahy A, Chitnis S, Koschnick S, Fitch MJ, Fallon JT, Loskutoff D, Taubman MB, Stuhlmann H. EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury. Am J Pathol. 2005;167:275–284. doi: 10.1016/S0002-9440(10)62972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soncin F, Mattot V, Lionneton F, Spruyt N, Lepretre F, Begue A, Stehelin D. VE-statin, an endothelial repressor of smooth muscle cell migration. EMBO J. 2003;22:5700–5711. doi: 10.1093/emboj/cdg549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Maziere A, Parker L, Van Dijk S, Ye W, Klumperman J. Egfl7 knockdown causes defects in the extension and junctional arrangements of endothelial cells during zebrafish vasculogenesis. Dev Dyn. 2008;237:580–591. doi: 10.1002/dvdy.21441. [DOI] [PubMed] [Google Scholar]
- 40.Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt M, Paes K, De Maziere A, Smyczek T, Yang S, Gray A, French D, Kasman I, Klumperman J, Rice DS, et al. EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development. 2007;134:2913–2923. doi: 10.1242/dev.002576. [DOI] [PubMed] [Google Scholar]
- 42.Crawford M, Brawner E, Batte K, Yu L, Hunter MG, Otterson GA, Nuovo G, Marsh CB, Nana-Sinkam SP. MicroRNA-126 inhibits invasion in non-small cell lung carcinoma cell lines. Biochem Biophys Res Commun. 2008;373:607–612. doi: 10.1016/j.bbrc.2008.06.090. [DOI] [PubMed] [Google Scholar]
- 43.Guo C, Sah JF, Beard L, Willson JK, Markowitz SD, Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. The authors demonstrated that miR-17-92–transduced RAS expressing cells formed larger, better-perfused tumors in vivo. miR-17~92 promotes tumor angiogenesis by targeting Tsp-1 and CTGF, therefore regulating angiogenesis in a non- cell-autonomous manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruchova H, Yoon D, Agarwal AM, Mendell J, Prchal JT. Regulated expression of microRNAs in normal and polycythemia vera erythropoiesis. Exp Hematol. 2007;35:1657–1667. doi: 10.1016/j.exphem.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. The authors showed that cancer cells transfected with miR-378 form significantly larger tumor in vivo. miR-378 promotes tumor growth and angiogenesis by targeting tumor suppressors SuFu and Fus-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 51.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 52.Davis BN, Hilyard AC, Nguyen PN, Lagna G, Hata A. Induction of microrna-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2008 doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Felicetti F, Errico MC, Bottero L, Segnalini P, Stoppacciaro A, Biffoni M, Felli N, Mattia G, Petrini M, Colombo MP, et al. The promyelocytic leukemia zinc finger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanisms. Cancer Res. 2008;68:2745–2754. doi: 10.1158/0008-5472.CAN-07-2538. [DOI] [PubMed] [Google Scholar]