Abstract
Cancer cells contain multiple signal transduction pathways whose activities are frequently elevated due to their transformation, and that are often activated following exposure to established cytotoxic therapies including ionizing radiation and chemical DNA damaging agents. Many pathways activated in response to transformation or toxic stresses promote cell growth and invasion and counteract the processes of cell death. As a result of these findings many drugs, predominantly protein and lipid kinase inhibitors, of varying specificities, have been developed to block signaling by cell survival pathways in the hope of killing tumor cells and sensitizing them to toxic therapies. Unfortunately, due to the plasticity of signaling processes within a tumor cell, inhibition of any one growth factor receptor or signaling pathway frequently has only modest long term effects on cancer cell viability, tumor growth, and patient survival. As a result of this realization, a greater emphasis has begun to be placed on rational combinations of drugs that simultaneously inhibit multiple inter-linked signal transduction/survival pathways. This, it is hoped, will limit the ability of tumor cells to adapt and survive because the activity within multiple parallel survival signaling pathways has been reduced. This review will discuss some of the approaches that have been taken to combine signal transduction modulatory agents to achieve enhanced tumor cell killing.
Keywords: kinase, phosphatase, receptor, inhibitor, survival, drug combination, sorafenib, Cdk inhibitor, Cdk9, Chk1 inhibitor
1. Introduction on growth factor receptors, signal transduction pathways and small molecule inhibitors
Cancer cells have elevated activities within multiple signal transduction pathways due to (reviewed in Valerie et al., 2007; Dent et al, 2003):
the increased expression of paracrine ligands acting on growth factor receptors;
receptor over-expression;
oncogenic activating mutations of receptors and downstream RAS GTP binding proteins;
inactivation of tumor suppressor lipid and protein phosphatases;
oncogenic activation of downstream protein and lipid kinases.
As a result of these modifications, in comparison to non-transformed cells, cancer cells divide more rapidly, are more migratory and invasive and have a greater capacity to survive exposure to a variety of toxic stresses, including those from anticancer therapeutics.
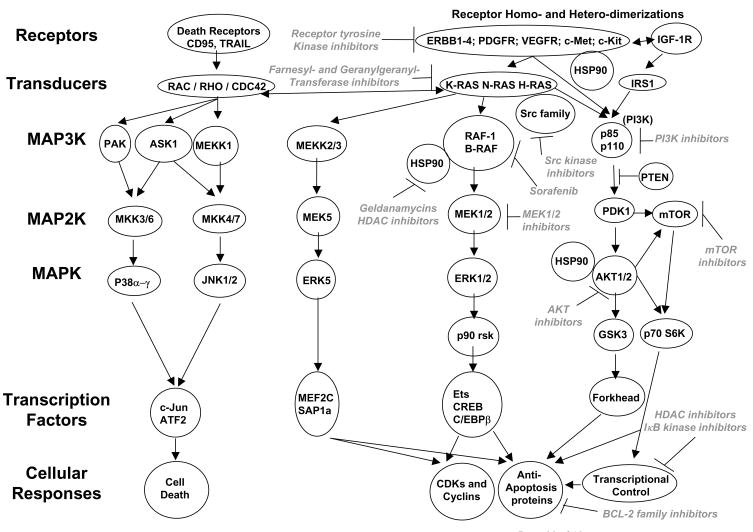
An overview of signal transduction pathway structure is provided to assist with understanding some of the concepts in this review, with emphasis on the original “mitogen activated protein (MAP) kinase” pathway. The p42 (ERK2) and p44 (ERK1) MAPKs are activated by dual tyrosine and threonine phosphorylation; they activate another protein kinase (p90rsk) by catalyzing its serine/threonine phosphorylation (Figure 1). When activated, ERK1/2 and p90rsk can migrate to the nucleus where they regulate transcription factors such as CREB, Elk-1 and Ets1 (Sturgill, 2008; Dent et al., 2003). ERK1/2 are phosphorylated and activated by MAPK kinases termed MEK1/2 (MAP2K). MEK1 and MEK2 are dual specificity tyrosine/threonine kinases and are activated by dual serine phosphorylation. The protein kinase responsible for catalyzing MEK1/2 activation was initially described as Raf-1 (C-Raf). Raf-1 is a member of a family of serine-threonine protein kinases termed Raf-1, B-Raf, and A-Raf; all “Raf” family members to varying extents phosphorylate and activate MEK1/2. Thus, the “Raf” protein kinases act at the level of a MAPK kinase kinase (MAP3K). The NH2 domain of Raf-1 reversibly interacts with RAS proteins in the plasma membrane, and the ability of Raf-1 to associate with RAS proteins is dependent on the RAS molecule being in the GTP-bound state (Ramos, 2008; McCubrey et al, 2008). Growth factor receptors, such as the epidermal growth factor receptor (EGFR) family (ERBB1–4), stimulate GTP for GDP exchange in RAS. Raf-1 activation is dependent on Raf-1 translocation to the plasma membrane followed by phosphorylation of Raf-1 S338 and Y341, and dephosphorylation of Raf-1 S259. Thus, a signaling pathway can be delineated from receptor tyrosine kinases through the activation of RAS proteins, translocation of Raf proteins to the plasma membrane, and the feeding of signals into MEK1/2-ERK1/2-p90rsk with resultant regulation of gene expression and modulation of apoptosis regulatory proteins. The activity of this pathway is elevated in many tumor cells, compared to their non-transformed counterparts, and as a result it was one of the first to be therapeutically targeted by small molecule kinase inhibitors at the level of the growth factor receptors (ERBB1, ERBB2), at the level of RAS proteins and at the level of MEK1/2 (Wong, 2009; Anjum and Blenis, 2008; Sebolt-Leopold and Herrera, 2008).
Figure 1. Signal transduction pathways in cancer cells and the enzymes which have been therapeutically targeted by drugs.
Tumor cells contain multiple signal transduction pathways, some of which mediate cell survival, such as the PI3K-AKT and RAF-MEK-ERK1/2 pathways. Of note, however, at every “level” of transduction, from the plasma membrane, to transcriptional and cell cycle control in the nucleus, specific enzymes have been targeted to block growth and facilitate tumor cell death. It is the combination of these drugs which will most likely yield the greatest therapeutic benefit.
Additional comparative cloning studies in mammalian and yeast cells showed that the original “MAPK” pathway was in fact one of many MAPK pathways. Thus, the ERK1/2-MAPK pathway has served as a template around which other MAPK family pathways have been described: the c-Jun NH2-terminal kinase (JNK) 1/2/3, the p38 MAPK, and the “big MAPK” MEK5-ERK5 pathways. In each pathway, a MAPK, a MAP2K, and a MAP3K have been shown to exist and behave phenomenologically in a similar manner to proteins described in the original ERK1/2 cascade (reviewed in Dent et al., 2003; Raman et al., 2007).
In a similar conceptual manner to MAP kinase pathways, the PI3K signaling pathways, PI3K-phosphoinositide-dependent kinase-1 (PDK1)-AKT-glycogen synthase kinase 3 and PI3K-phosphoinositide-dependent kinase-1-AKT-mammalian target of rapamycin (mTOR)-p70S6K, have also been delineated (Ihle and Powis, 2009; Memmott and Dennis, 2009). There are three AKT genes with AKT1 being most closely linked to proliferation and apoptosis resistance and AKT2 linked to insulin signaling, and AKT3 has been shown to act as a transforming kinase in melanoma (Steelman et al, 2008; Di Cosimo and Baselga, 2008; Stahl et al., 2004). The ability of PI3K to signal downstream to PDK1 is negatively regulated by the proto-oncogene phosphatase and tensin homologue on chromosome ten (PTEN); PTEN is frequently mutated, deleted or epigenetically silenced in prostate cancer, melanoma and glioblastoma (e.g. Carnero et al., 2008). PI3K p110 catalytic subunits are also noted in some tumors to be mutated to a partially active conformation (Gonzalez and De Groot, 2008; Knobbe et al., 2008). The activities of multiple MAPK pathways and of the PI3K pathway are variably elevated and/or suppressed in a variety of transformed cells compared to non-transformed cells, and studies have linked enhanced basal levels of ERK1/2, PI3K-AKT-mTOR, and ERK5 pathway activity in tumor cells to increased rates of proliferation and protection of cells from toxic stresses. Conceptually this heightened signaling activity could provide a therapeutic window for kinase inhibitors to selectively impact on tumor cell, but not non-transformed cell, growth and viability. Similarly, reduced basal activity levels or the abilities of stresses to activate the JNK1/2 and p38 MAPK pathways correlate with enhanced tumor cell viability.
Thus, based on data from several decades of research, a wide variety of signaling proteins and signaling pathways have been causatively linked in many tumor types to cell growth, invasion and survival that has in turn prompted multiple drug companies and synthetic chemists to generate therapeutically active inhibitors, predominantly of kinases, and in particular agents that bind within the ATP binding site(s) within the kinase domain.
Important examples are (see Table 1): small molecule inhibitors of growth factor receptors such as ERBB1–4 (erlotinib, canertinib, gefitinib, lapatinib) as well as inhibitory antibodies; PDGFR (nolitinib, sorafenib; ABT869), c-Met (PHA665752), and the IGF1R (BMS536924; and inhibitory antibodies); FLT3 (ABT869) (Chu and Small, 2009); Abl and c-Kit tyrosine kinases (imatinib, nolitinib); Src family tyrosine kinases (dasatinib, AZD0530); Checkpoint/Chk1 regulatory kinase (UCN-01, AZD7762); vascular endothelial growth factor receptors (sorafenib, sunitinib; AG13726 and inhibitory antibodies) (Broxterman and Georgopapadakou, 2005); Heat shock protein 90 (17AAG, 17DMAG); Aurora kinases (VE465, MK0457) (see Figure 2); cyclin dependent kinases (flavopiridol, R-roscovitine (CYC202)); phosphatidyl inositol 3 kinase (PX866, BEZ235, BGT226, XL147); mTOR (Rapamycin (sirolimus), RAD001 (everolimus) AP23573 (deforlimus), CI779 (temsirolimus), BEZ235, PI103) (Jiang and Liu, 2008); IκB kinase (IKI-1, AS602868); AKT (perifosine, GSK690693); MEK1/2 (AZD6244, PD184352, PD0325901); Raf kinases (sorafenib, PLX4032), Ras farnesylation/geranylgeranylation (lonafarnib, tipifarnib).
Table 1. Clinically relevant therapeutic drugs and their targets.
Below is a short list of some of the most widely used and novel cancer therapeutic drugs. Many of these drugs are now being combined in a rational manner in vitro and in pre-clinical animal models to achieve a synergy of drug-induced tumor cell death and growth arrest.
| Growth factor Receptor Tyrosine Kinases |
| ERBB1–4 family: erlotinib, canertinib, gefitinib, lapatinib; and inhibitory antibodies. |
| PDGFR: nolitinib, sorafenib, ABT869. |
| c-Met: PHA665752. |
| IGF1R: BMS536924. |
| c-Kit: imatinib, nolitinib. |
| Vascular Endothelial Growth Factor Receptor family: sorafenib, sunitinib, AG13726, ABT869; and inhibitory antibodies. |
| Non-receptor Tyrosine Kinases |
| Abl: imatinib, nolitinib. |
| Src family tyrosine kinases: dasatinib, AZD0530. |
| Small GTPase Inhibitors |
| Ras family farnesylation/geranylgeranylation inhibitors: lonafarnib, tipifarnib. |
| Intracellular signal transduction intermediates |
| Phosphatidyl inositol 3 kinase: PX866, BEZ235, BGT226, XL147. |
| AKT: perifosine, GSK690693. |
| mTOR: Rapamycin (sirolimus), RAD001 (everolimus), AP23573 (deforlimus), CI779 (temsirolimus), BEZ235, PI103. |
| Raf family kinases: Sorafenib, PLX4032. |
| MEK1/2: AZD6244, PD184352, PD0325901. |
| Heat shock protein 90: 17AAG, 17DMAG. |
| Regulation of Transcription |
| IκB kinase: IKI-1, AS602868. |
| Histone deacetylase inhibitors: vorinostat, LBH589, MS275, sodium valproate. |
| Inhibition of proteasome degradative activity: bortezomib, carfilzomib. |
| Regulation of cell cycle progression and genomic stability |
| Aurora kinases: VE465, MK0457. |
| Cyclin dependent kinases: flavopiridol, R-roscovitine (CYC202). |
| Checkpoint/Chk1 regulatory kinase: UCN-01, AZD7762. |
| ATM/ATR: KU55933 |
| PARP1: PJ34, AZD2281 (KU59436) |
| Regulation of mitochondrial function |
| Bcl-2 family protein inhibitors: ABT-737, GX15-070, Gossypol |
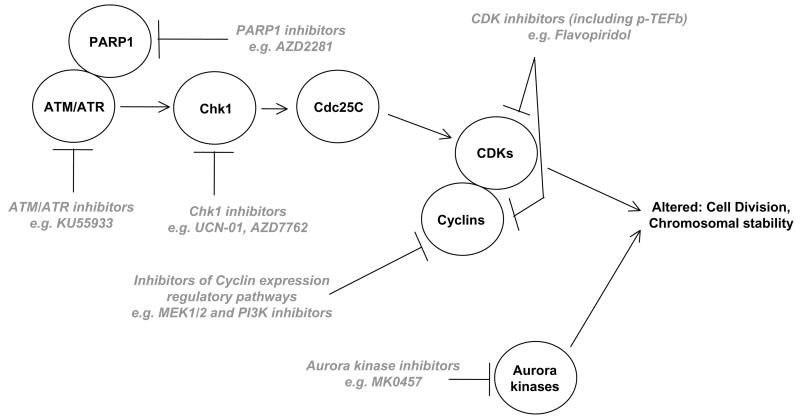
Figure 2. Therapeutic drugs that target the cell cycle.
Kinase inhibitors that modulate the cell cycle have frequently been combined with toxic drugs and/or radiation which cause DNA damage. This will lead to inappropriate cell cycle progression with damage DNA and result in tumor cell death. Inhibition of PARP1 ADP ribosylation activity suppresses the ability of DNA damage to activate ATM/ATR as well as increase DNA repair processes.
In addition to agents that target kinase activities, other therapeutic drugs that have been developed recently to modify the biology and/or kill tumor cells include those that: modify protein acetylation (histone deacetylase inhibitors, HDACIs: vorinostat, LBH589, MS275, sodium valproate); modify the activity of protective Bcl-2 family proteins in the mitochondrion (ABT-737, GX15-070, gossypol) and agents that inhibit proteasome degradative activity (bortezomib, carfilzomib). (Grant 2008a; Tímár and Döme, 2008; Ihle and Powis, 2009; Memmott and Dennis, 2009; Sebolt-Leopold and Herrera, 2008; McCubrey et al., 2008; Steelman et al., 2008; Grant, 2008b; Grant and Dent, 2007; McConkey and Zhu, 2008). Some of the above agents have already been combined in vitro and in animal models to achieve a synergistic increase in tumor cell killing e.g. (Table 2).
Table 2. Clinically relevant combinations of therapeutic drugs: pre-clinical and clinical testing.
Below is a short list of some of the published/tested combinations of novel cancer therapeutic drugs.
| Growth factor Receptor Tyrosine Kinases |
| ERBB1–4 family inhibitors + mTOR inhibitors |
| ERBB1–4 family inhibitors + c-Met inhibitors |
| ERBB1–4 family inhibitors + IGF1R inhibitors |
| ERBB1–4 family inhibitors + Vascular Endothelial Growth Factor Receptor family inhibitors |
| PDGFR/VEGFR/FLT3 inhibitors + mTOR inhibitors |
| Non-receptor Tyrosine Kinases |
| Abl kinase inhibitors + Src family tyrosine kinase inhibitors |
| Tyrosine kinase inhibition(s) + Inhibition of proteasome degradative activity |
| Small GTPase Inhibitors |
| Ras family farnesylation/geranylgeranylation inhibitors + Checkpoint/Chk1 regulatory kinase inhibitors |
| Intracellular signal transduction intermediates |
| Phosphatidyl inositol 3 kinase inhibitors + MEK1/2 inhibitors |
| Phosphatidyl inositol 3 kinase inhibitors + mTOR inhibitors |
| AKT inhibitors + mTOR inhibitors |
| Inhibitors of heat shock protein 90 + MEK1/2 inhibitors |
| Regulation of Transcription |
| Histone deacetylase inhibitors + Sorafenib |
| Inhibition of proteasome degradative activity + Histone deacetylase inhibitors |
| IKK inhibitors + Histone deacetylase inhibitors |
| Inhibition of proteasome degradative activity + Cyclin dependent kinase inhibitors |
| Regulation of cell cycle progression and genomic stability |
| Cyclin dependent kinase inhibitors + Histone deacetylase inhibitors |
| Checkpoint/Chk1 regulatory kinase inhibitors + MEK1/2 inhibitors |
| Inhibitors of heat shock protein 90 + Cyclin dependent kinase inhibitors |
| Cyclin dependent kinase inhibitors + Phosphatidyl inositol 3 kinase inhibitors |
| Regulation of mitochondrial function |
| Bcl-2 family protein inhibitors + Inhibition of proteasome degradative activity |
2. Oncogene addiction
In vitro, numerous tumor cell types have been shown to exhibit growth reduction following inhibition of growth factor receptors, e.g. ERBB1 or inhibition of (MEK1/2) signaling pathways. However, in many such studies the primary effect of a single kinase inhibitory agent at low “target specific” doses on tumor cells was cyto-static, rather than cyto-toxic (e.g. Carter et al., 1998; Benavente et al., 2009; Tsai et al., 2008; Smalley et al., 2008; Yacoub et al., 2006; Yacoub et al., 2006a; Martin et al., 2008). In contrast to the relatively encouraging findings from preclinical in vitro work, clinical studies using many of the above mentioned inhibitors as single agents frequently did not demonstrate any form of tumor growth control (e.g. Hida et al., 2009). As a result of the patient findings with kinase inhibitors as single agents, a large body of literature has developed demonstrating in preclinical models that inhibition of growth factor receptors and/or downstream signaling molecules can promote cell death induced by a wide variety of established cytotoxic therapies including ionizing radiation, microtubule targeted agents, and topoisomerase inhibitors and other DNA damaging agents (e.g. Harari et al., 2007; Yacoub et al., 2006a; Takigawa et al., 2007). Thus when combined with established cytotoxic therapies, some of the kinase inhibitors can enhance their toxicity and have shown tumor control in patients, with subsequent FDA approval for their use, for example with ionizing radiation and cisplatin, and with capecitabine (Ryan et al., 2008; Loeffler-Ragg et al., 2008).
Where single receptor-targeted agent-induced anticancer responses were particularly pronounced in patients, such as for imatinib in the treatment of Bcr-Abl+ CML, it was hypothesized and proven that the tumor control effect was due to CML cells being exquisitely “addicted” to the kinase activity of the Bcr-Abl fusion protein for growth and survival (Druker, 2008). Similar findings were made for imatinib in gastro-intestinal tumors that express a mutated active form of c-Kit (Antonescu, 2008). On the contrary, in non-small cell lung cancer (NSCLC), despite the tumors of ~70% of patients are overexpressing ERBB1, only a small subpopulation of patients (~10%) responded to ERBB1 inhibitors and these individuals statistically tended to be non-smokers and with an Asian/female genetic background (Ladanyi and Pao, 2008). Subsequently it was shown in responsive NSCLC patients, in a conceptually parallel manner to data from Bcr-Abl+ cells, that ERBB1 was mutated to become a constitutively active kinase, with such NSCLC cells being addicted to the survival signals emanating from the mutated receptor (Johnson and Janne, 2006; Le Tourneau et al., 2008).
Thus only a minority of tumor cell types appear to present with a relatively simple single oncogene activating mutation/survival signaling addiction that would predict for effectiveness of a single kinase inhibitory drug. These findings make clear that the rational development of approaches which simultaneously target multiple signal transduction pathways to kill tumor cells will more likely have broad therapeutic usefulness. Despite this growing body of knowledge, the testing of combinations of multiple kinase inhibitory agents has only within the last 5 years truly begun to be explored. In part, such approaches have often been hampered by the lack of (commercial) availability of clinically relevant drugs for testing in academic institutions and by intellectual property issues that preclude the combination of proprietary agents from different pharmaceutical companies.
3. Redundant survival signaling pathways and the combinations of multiple kinase inhibitors
3.1. Receptors
Exposure of tumor cells expressing a mutated active form of ERBB1, but generally not an over-expressed wild type ERBB1, to kinase domain inhibitors results in growth arrest and tumor cell death (Pao et al., 2004; Sordella et al., 2004). Over the course of many months’ kinase inhibitor exposure, secondary mutations in the receptor kinase domain develop which render the receptor resistant to the kinase inhibitor. A more rapid mechanism of resistance to ERBB receptor inhibitors as single agents, prior to the development of secondary mutations, is the compensatory activation of growth factor receptors such as c-MET (+c-Src), and the IGF1R which can act in parallel to provide survival signaling (Figure 1) (Jin and Esteva, 2008; Mueller et al., 2008; Bean et al., 2007). These receptors can provide a survival signal in their own right as receptor tyrosine kinases as well as causing trans-phosphorylation of inhibited ERBB receptors, thereby permitting the ERBB receptors to act as docking sites for e.g. RAS GTP exchange factors. Combinations of ERBB receptor inhibitors with inhibitors of c-Met or of the IGF1R have proven efficacious in promoting cell death and reverting significantly the ERBB inhibitor resistant phenotype (Huang et al, 2009; Arteaga, 2007). Others have noted lower levels of the pro-apoptotic protein Bim in ERBB1 inhibitor resistant cells (Deng et al., 2008). In cells that are oncogene addicted to mutated active forms of ERBB1, the use of Bcl-2 family inhibitors such as ABT-737 can promote drug toxicity demonstrating that these receptors, in part, regulate cell survival through maintaining mitochondrial stability (Cragg et al., 2007). We have noted that resistance to the ERBB1/ERBB2 inhibitor lapatinib can be mediated by increased expression of protective Mcl-1 and Bcl-XL with reduced expression of pro-apoptotic Bax (Martin et al., 2008). The Bcl-2 family antagonist gossypol can also partially circumvent this resistance mechanism (unpublished findings).
One profound problem in many cancer cell types, for the use of single agent or combinations of multiple receptor tyrosine kinase inhibitors is that oncogenic mutations downstream of growth factor receptors have the potential to abrogate any long-term anti-proliferative effect of receptor inhibition, e.g. mutations of RAS proteins, B-Raf, p110 PI3K or of PTEN. Thus tumor cell types which have a high penetrance of such downstream oncogenic mutations e.g. K-Ras in pancreatic cancers, PTEN in glioblastoma, PI3K p110 mutation in breast and colorectal cancers, will be a priori predicted to be relatively refractory to toxic and/or cyto-static effects caused by inhibition of one or multiple growth factor receptor function(s) such as ERBB1 (Koliopanos et al., 2008; Gonzalez and De Groot, 2008; Zhao and Vogt, 2008; Sartore-Bianchi et al, 2009). Hence, inhibitors of multiple signaling pathways downstream of the growth factor receptors, and in all likelihood at or below the level of RAS and PTEN, will need to be rationally combined.
3.2. RAS proteins
RAS proteins were initially envisaged as a prime target for therapeutic intervention and drugs that blocked farnesylation of RAS proteins were developed. However, possibly due to a redundancy of lipid modification where RAS proteins can be geranylgeranylated and still maintain plasma membrane localization and activity, the direct targeting of RAS by farnesyltransferase inhibitors has not significantly progressed as a single agent therapeutic in the clinic. In the laboratory, inhibitors of RAS farnesylation have been shown to interact with cytotoxic drugs such as cisplatin, ionizing radiation and with anti-estrogens to kill breast cancer cells (Rengan et al., 2008; Sizemore et al., 1999; Martin et al., 2007). We have noted in both carcinomas and hematological malignancies that inhibitors of RAS farnesylation synergistically interact with Chk1 inhibitors in vitro and in vivo to cause cell killing by blocking Chk1 inhibitor –induced activation of the ERK1/2 pathway (see below) (Dai et al., 2008; Hamed et al., 2008).
3.3. MEK1/2 and PI3K-AKTmTOR signaling pathways
Based on knowledge that both the PI3K-AKT/PI3K-mTOR and MEK1/2-ERK1/2 pathways can provide survival signals downstream of RAS proteins and PTEN, recent studies and elegantly presented data in a genetically defined mouse model of lung cancer utilizing induction of a mutated K-RAS protein that inhibition of both PI3K and MEK1/2 can have a profound anti-tumor effect in vitro and in vivo (Engelman et al., 2008). Nonetheless, not all tumor cells exhibit an exquisite sensitivity to combined inhibition of both PI3K and MEK1/2, at least at concentrations where the actions of the inhibitory drugs can be considered specific (e.g. Yacoub et al., 2003). This could be due to additional survival pathways that can compensate for loss of PI3K and MEK1/2 signaling e.g. via NFκB. Inhibitors of the PI3K-related kinase mTOR (e.g. rapamycin; PI-103 CI779) have been examined as single agents in patient studies with sometimes excellent results e.g. in renal carcinoma, sometimes modest and sometimes confounding results showing increased tumor growth, such as in breast cancer (Kroog and Motzer, 2008; Carracedo et al, 2008). This latter undesirable effect may be due to feedback activation of AKT via TORC2 or due to activation of ERK1/2 caused by the mTOR inhibitor providing a survival signal through PI3K (Grant, 2008; O’Reilly et al, 2006; Carracedo et al, 2008). Inhibition of the compensatory activation of ERK1/2 following treatment with an mTOR inhibitor results in a synergistic induction of tumor cell killing combining mTOR and MEK1/2 inhibitors (Kinkade et al., 2008; Frost et al., 2009; Lasithiotakis et al., 2008). In NSCLC cells that express a mutant active RAS, and that are resistant to ERBB1 inhibitors, it has been shown that inhibition of PI3K or AKT results in tumor cell killing in vitro and in vivo (Janmaat et al., 2006; Ihle et al., 2005; Festuccia et al., 2008). However, in colon cancer cells, mutational activation of RAS predicts for resistance to PI3K inhibitors as single agents (Ihle et al., 2009a). Thus, at a “simplistic” level, inhibition of only two survival signaling pathways probably also will not always result in a profound enhancement of tumor cell killing.
Data from melanoma cells can also be particularly enlightening to address the concept of dual or multi-pathway inhibition in how we could develop approaches to enhance tumor cell killing by kinase inhibitors. Malignant melanoma cells frequently have oncogenic activating mutations in RAS proteins or in B-Raf (V600E), or inactivation of PTEN function. In melanoma cells expressing a mutant active B-Raf, inhibition of B-Raf or MEK1/2 generates only a cyto-static response (Haass et al., 2008). Similarly, in melanoma cells with RAS mutation or loss of PTEN, inhibition of PI3K or mTOR suppresses growth without causing profound cytotoxicity (Stahl et al., 2004; Howes et al., 2007). The combination of Raf/MEK inhibition with inhibition of PI3K and mTOR, however, causes both a growth arrest and a cyto-toxic response (Bedogni et al., 2006; Smalley et al., 2006). In this respect, several of the recently developed mTOR inhibitors also strongly inhibit PI3K p110 enzymes, thus the combination of a MEK1/2 inhibitor with, e.g. the dual specificity mTOR/PI3K inhibitors BEZ235 or PI-103, represents in fact a multi-pathway inhibitory approach that will circumvent possible re-activation of AKT due to mTOR/TORC2 signaling (Engelman et al., 2008; Fan et al., 2007). As inhibition of mTOR also results in a rapid reduction in the expression of short half-lived anti-apoptotic proteins such as Mcl-1 and c-FLIP-s, this drug combination may mediate cell killing through a varied and complex range of mechanisms (Adams and Cooper, 2007).
In vitro, inhibitors of mTOR such as rapamycin, PI-103 and CCI779 have been shown using a wide variety of tumor cell types to synergize with ERBB receptor inhibitors to promote tumor cell death (McCubrey et al., 2008; Azarriti et al., 2008; Wang et al., 2008; Wang et al., 2008a; Fan et al., 2007). This is likely due to the fact that inhibition of mTOR reduces protein synthesis resulting in a rapid reduction in the expression of short half-lived protective proteins as well as potentially reducing receptor/cognate ligand expression, which will result in a lower apoptotic threshold. The combination of mTOR inhibitors with growth factor receptor inhibitors other than those which inhibit ERBB1, e.g. the PDGFR/VEGFR/FLT3 inhibitor ABT869, has also been shown to synergize to kill hepatocellular carcinoma cells in vitro and in vivo (Jasinghe et al., 2008). Several of the published studies combining ERBB1 inhibitors with mTOR inhibitors have also demonstrated excellent tumor cell responses in mouse models, and based on these findings, phase I trials are presently underway combining ERBB1 inhibitors with rapamycin in glioblastoma and NSCLC (e.g. Jimeno et al., 2007; LoPiccolo et al., 2008). Thus resistance to ERBB receptor inhibitors as well as those of other receptors can be overcome by the addition of agents that block signaling through multiple downstream pathways.
The use of agents which simultaneously target multiple additional pathways/regulatory protein molecules together with those of greater specificity has also been shown to have value, for example combining HSP90 inhibitors (17AAG/17DMAG) which suppress the expression/activity of multiple oncogenic signaling HSP90 client proteins with inhibitors of MEK1/2. This combination blocks signaling by the PI3K-AKT and MEK1/2-ERK1/2 pathways, as well as other survival pathways e.g. ERBB-STAT and NFκB pathways, generates reactive oxygen species, and causes cell death in hepatoma, pancreatic cancer and leukemia cells (Park et al., 2008; Nguyen et al., 2006). Similar data in myeloma cells using the AKT inhibitor perifosine when combined with 17DMAG have also been published (Huston et al., 2008). Collectively, these data on mTOR inhibitors or 17AAG demonstrates that three-pathway or a multi-pathway inhibitory approach of kinase inhibitors is required to kill a broad spectrum of tumor cell types.
3.3.1. The case of sorafenib
As noted above, many kinase inhibitors are not highly specific for one particular protein kinase, which is most likely due to their targeting the kinase ATP binding site. An outstanding example is sorafenib (Nexavar®), which was developed as an inhibitor of Raf-1 and B-Raf. Indeed, initial in vitro studies suggested that the agent would have activity against tumors that are oncogene addicted to mutant activated B-Raf, such as in some melanoma and colon cancer cells (Lee and McCubrey, 2003). Of note are some studies suggesting that low doses of sorafenib can actually activate ERK1/2 suggesting that the biological actions of this drug are much more complicated (Tochizawa et al., 2008). In agreement with this proposition, the clinical activity of sorafenib appeared to be only partially dependent on modulation of the Raf-MEK-ERK pathway, with the majority of anti-tumor effects now being thought to be mediated by inhibition of class III receptor tyrosine kinases that regulate tumor angiogenesis (Takimoto and Awada, 2008). Inhibition of these growth factor receptors would also be predicted to reduce activity within the ERK1/2, PI3K-AKT-mTOR and NFκB pathways in both endothelial and tumor cells.
More recently, sorafenib has been shown to cause an endoplasmic reticulum stress response in tumor cells that in part can account for its toxicity due to PKR-like endoplasmic reticulum kinase (PERK) – eIF2α mediated suppression of protein translation (Zhang et al., 2008; Park et al., 2008a; Rahmani et al., 2005; Rahmani et al., 2007). Thus the expression of short-lived anti-apoptosis proteins, such as Mcl-1, XIAP and c-FLIP-s, could be lowered after sorafenib exposure through both losses of ERK1/2, PI3K-AKT-mTOR and NFκB signaling by reduced transcription as well as by ER stress signaling through eIF2α through reduced translation. Sorafenib has been shown to synergize with the mTOR inhibitor rapamycin to reduce angiogenesis in vitro and in vivo in both hepatoma and melanoma models (Wang et al., 2008; Lasithiotakis et al., 2008). As discussed previously with regard to 17DMAG and mTOR inhibitors, the interactions of sorafenib with other kinase inhibitors to synergistically kill tumor cells may represent a highly complex multi-factorial induction of many pro-apoptotic signals due to reduced expression of survival proteins and survival signaling.
Data from our laboratories and from others has shown that sorafenib synergizes with histone deacetylase inhibitors to cause cell death in hepatoma, cholangiocarcinoma and leukemia cells (Park et al., 2008a; Zhang et al., 2008; Baradari et al., 2007; Dasmahapatra et al., 2007). Histone deacetylase inhibitors have a multi-factorial mode of action, including disruption of co-repressor complexes regulating transcription, for example with increased expression of death receptors and their ligands; the induction of reactive oxygen species; the generation of toxic lipids such as ceramide; inhibition of HSP90 chaperone function; and activation of NFκB (Emanuele et al., 2008). Our data argued that sorafenib and histone deacetylase inhibitors interacted to cause death through ceramide-dependent activation of the death receptor CD95 in parallel to an ER stress response that inhibited expression of multiple protective BCL-2 family proteins as well as c-FLIP-s. Clearly, the interaction of sorafenib with histone deacetylase inhibitors again represents the simultaneous inhibition and activation of multiple signal transduction pathways which ultimately facilitates increased levels of tumor cell killing.
3.4. Cdk inhibitors
The use of cyclin-dependent kinase inhibitors as anticancer therapeutics was originally founded on the belief that blocking Cdk function would block cell cycle progression, thereby slowing tumor cell growth and perhaps leading to differentiation and/or cell death (Senderowicz, 1999) (see Figure 2). A prototype Cdk inhibitor is flavopiridol, a drug that at clinically relevant concentrations with a modified infusion schedule has been shown to generate objective responses in CLL patients (Phelps et al., 2009). It has been appreciated that flavopiridol and other clinically relevant Cdk inhibitors such as R-roscovitine (CYC202) are excellent modulators of the apoptotic threshold in tumor cells, resulting in greater cell killing when combined with a variety of other agents. Flavopiridol has shown activity in refractory solid tumors when combined with established chemotherapeutic drugs such as taxanes and gemcitabine (Fornier et al., 2007; Shapiro, 2004). In part, the actions of Cdk inhibitors may be related to inhibition of Cdk7 (CAK1) and Cdk9/Cyclin T (p-TEFb) (Canduri et al., 2008). Inhibition of Cdk7 blocks regulatory phosphorylation of multiple Cdk proteins at T161. Inhibition of Cdk9 (p-TEFb) suppresses protein levels due to loss of RNA polymerase II transcription. Thus the expression of short-lived anti-apoptosis proteins, such as Mcl-1, XIAP and c-FLIP-s, Cyclin proteins, and growth factor receptors such as c-Met, can be rapidly reduced by flavopiridol (Moghul et al., 1994). Furthermore, both flavopiridol and CYC202 have been shown to inhibit IKK enzymes, thereby suppressing NFκB function (Takada and Agarwal, 2004; Gao et al., 2004). Thus the clinically relevant Cdk inhibitors have a large number of putative cellular targets by which they can modulate the apoptotic threshold and the proliferative index of a tumor cell. In this respect it is interesting to note that histone deacetylase inhibitors, which promote NFκB activation via acetylation and IκB degradation synergize with flavopiridol or roscovitine to kill breast cancer and leukemic cells (Gao et al., 2004; Mitchell et al., 2007). Similar data using CYC202 and the histone deacetylase inhibitor MS275 were also recently noted in hepatoma cells (Gahr et al., 2008). Inhibition of Cdk9 has also been shown to promote the toxicity of PI3K/AKT inhibitors that could potentially occur indirectly through inhibition of NFκB (Mohapatra et al., 2009). Prior work from our laboratories has suggested a direct connection between Cdk inhibitor toxicity in leukemic cells and modulation of PI3K pathway activity (Yu et al., 2003). Thus the inhibition of the p-TEFb pathway which has pleiotropic downstream targets and together with agents that target protein acetylation or signaling pathway activities represents a highly tractable approach to treating a wide variety of malignancies.
Cdk inhibitors have also been shown to interact with inhibitors of growth factor receptors. CYC202 enhances the toxicity of inhibitors of ERBB1 or ERBB2 in a synergistic fashion in some tumor cell types, though in tumor cells expressing mutant active RAS proteins or with altered PTEN function, the toxic combination effect of both drugs was additive or less than additive (Fleming et al., 2008). We have similar synergistic drug interaction data combining the ERBB1/ERBB2 inhibitor lapatinib with flavopiridol in a variety of breast cancer cell lines (unpublished findings). Farnesyltransferase inhibitors have been shown to enhance the toxicity of roscovitine (Wesierska-Gadek et al., 2007). The less than additive effect of the CYC202/ERBB inhibitor drug combination in killing tumor cell types expressing onco-proteins downstream of the growth factor receptors to some extent provides further confirmation of the hypothesis that mutation of oncogenes downstream of growth factor receptors suppresses the ability of growth factor receptor inhibitors to have therapeutic efficacy, even in combination with other signal transduction –inhibitory agents.
4. Rapid compensatory activation of inter-linked survival signaling
In several cell systems we have noted that inhibition of one intracellular protective signaling protein/pathway rapidly results in the rapid compensatory activation of another protective signaling protein/pathway. The best example we have found to date being inhibition of the cell cycle regulatory checkpoint kinase, Chk1 (Figure 2). Deletion of Chk1 is embryonically lethal and Chk1 activation downstream of ATR/ATM plays a key role in regulating Cdc25C and Cdc2 activity, and thus cell cycle progression, after DNA damage. We discovered that in addition to its established cell cycle regulatory targets; inhibition of Chk1 rapidly lead to activation of ERK1/2 in multiple solid and blood tumor cell types, but not in their non-transformed cell counterparts (Dai et al., 2002; McKinstry et al., 2002). Activation of ERK1/2 was in part dependent on Chk1 inhibitor –induced DNA damage, as inhibition of PARP1 blocked γH2AX and ERK1/2 phosphorylation. Inhibition of MEK1/2 blocks Chk1 inhibitor –stimulated ERK1/2 activation and causes a profound increase in tumor cell killing both in vitro and in vivo. Similar data were obtained using a PARP1 inhibitor (Dent, unpublished observations). This synergistic killing effect has been noted with combinations of multiple Chk1 and MEK1/2 inhibitors (Dai et al., 2008; Hamed et al., 2008). Cell killing is mediated through mitochondrial dysfunction and the use of agents which inhibit Bcl-2/Bcl-XL, e.g. HA14-1, profoundly enhance MEK/Chk –inhibitor toxicity and circumvent the protective effect of Bcl-XL protein over-expression (Hamed et al., 2008). In myeloma cells, this drug combination appears to mediate tumor cell death through a Bim-dependent mechanism (Pei et al., 2007). Thus, this finding suggests that, unlike for earlier discussed combinations, for these two closely interrelated survival signaling pathways, a combination of only two specific kinase inhibitors can have a profound anti-tumor effect. The combination of MEK1/2 and Chk1 inhibitors has yet to be evaluated in the clinic.
5. Conclusions and perspective
In comparison to non-transformed cells, tumor cells over-express growth factor receptors and express mutated proteins that facilitate the oncogenic phenotype, resulting in the activation of multiple signal transduction pathways. There are profound levels of survival signaling redundancy and of signaling plasticity which permit tumor cells to adapt and overcome multiple environmental and therapeutic stresses. Collectively this implies that the use of highly specific targeted therapies, often using single inhibitory agents as was initially employed with ERBB1 inhibitors, was almost guaranteed to fail. In a few specific tumor cell types, the single agent cytotoxicity of a kinase inhibitor is evident due to very specific oncogene addiction of those cells. However, many of the most promising signal transduction modulatory drug combinations involve agents that both act to suppress survival signaling within multiple pathways and act to suppress expression of multiple apoptosis inhibitory proteins. Thus agents that inhibit mTOR and suppress expression of many proteins; those that destabilize multiple activated signaling proteins through HSP90 inhibition; those that block Cdk9 (p-TEFb) function and suppress expression of multiple proteins and those that inhibit multiple receptor tyrosine kinases appear to represent the best avenues for therapeutic efficacy in multiple malignancies.
It is also becoming apparent that simply combining more (and more) inhibitors of kinase proteins together can result in less tumor control in patients due to antagonistic interactions between the agents e.g. (Tol et al., 2009). In the Tol et al. study, the addition of the inhibitory ERBB1 antibody cetuximab to bevacizumab + capecitabine + oxaliplatin therapy led to shorter survival of colorectal cancer patients. We have also noted that inhibition of ERBB1 suppresses the toxicity of single agent Chk1 inhibitor treatment (McKinstry et al., 2002). One reason for these findings could be the possibility that ERBB1 signaling not only generates cyto-protective signals but has also been linked to the tyrosine phosphorylation and activation of the CD95 death receptor (Reinehr et al., 2003). Over-stimulated ERBB1 signaling can also enhance radiosensitivity (Yacoub et al., 2006). These findings emphasize the need for careful preclinical evaluations of drug combination mechanism(s) of action to provide the foundation for any subsequent clinical translation. Whether it will be possible to bring all of the most promising combinations of such kinase inhibitory agents from different pharmaceutical companies through to the clinic in a timely fashion is at present an open question (Table 2).
Acknowledgments
Support for the present study was provided; to P.D. from PHS grants (P01-CA104177, R01-CA108325, R01-DK52825), The Jim Valvano “V” foundation, and The Goodwin Foundation; to S.G. from PHS grants (R01-CA63753; R01-CA77141) and a Leukemia Society of America grant 6405-97; to PBF from PHS grants (P01-CA104177, R01-CA097318; R01-CA098172; P01-NS031492), the Samuel Waxman Cancer Research Foundation and the National Foundation for Cancer Research. P.D. is The Universal Inc. Professor in Signal Transduction Research and P.B.F. is a SWCRF Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams KW, Cooper GM. Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. J Biol Chem. 2007;282:6192–6200. doi: 10.1074/jbc.M610643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- Antonescu CR. Targeted therapies in gastrointestinal stromal tumors. Semin Diagn Pathol. 2008;25:295–303. doi: 10.1053/j.semdp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Arteaga CL. HER3 and mutant EGFR meet MET. Nat Med. 2007;13:675–677. doi: 10.1038/nm0607-675. [DOI] [PubMed] [Google Scholar]
- Azzariti A, Porcelli L, Gatti G, Nicolin A, Paradiso A. Synergic antiproliferative and antiangiogenic effects of EGFR and mTor inhibitors on pancreatic cancer cells. Biochem Pharmacol. 2008;75:1035–1044. doi: 10.1016/j.bcp.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Baradari V, Höpfner M, Huether A, Schuppan D, Scherübl H. Histone deacetylase inhibitor MS-275 alone or combined with bortezomib or sorafenib exhibits strong antiproliferative action in human cholangiocarcinoma cells. World J Gastroenterol. 2007;13:4458–4466. doi: 10.3748/wjg.v13.i33.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci (USA) 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni B, Welford SM, Kwan AC, Ranger-Moore J, Saboda K, Powell MB. Inhibition of phosphatidylinositol-3-kinase and mitogen-activated protein kinase kinase 1/2 prevents melanoma development and promotes melanoma regression in the transgenic TPRas mouse model. Mol Cancer Ther. 2006;5:3071–3077. doi: 10.1158/1535-7163.MCT-06-0269. [DOI] [PubMed] [Google Scholar]
- Benavente S, Huang S, Armstrong EA, Chi A, Hsu KT, Wheeler DL, Harari PM. Establishment and characterization of a model of acquired resistance to epidermal growth factor receptor targeting agents in human cancer cells. Clin Cancer Res. 2009;15:1585–1592. doi: 10.1158/1078-0432.CCR-08-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman HJ, Georgopapadakou NH. Anticancer therapeutics: “Addictive” targets, multi-targeted drugs, new drug combinations. Drug Resist Updates. 2005;8:183–197. doi: 10.1016/j.drup.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Canduri F, Perez PC, Caceres RA, de Azevedo WF., Jr CDK9 a potential target for drug development. Med Chem. 2008;4:210–218. doi: 10.2174/157340608784325205. [DOI] [PubMed] [Google Scholar]
- Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S, Auer KL, Reardon DB, Birrer M, Fisher PB, Valerie K, Schmidt-Ullrich R, Mikkelsen R, Dent P. Inhibition of the mitogen activated protein (MAP) kinase cascade potentiates cell killing by low dose ionizing radiation in A431 human squamous carcinoma cells. Oncogene. 1998;16:2787–2796. doi: 10.1038/sj.onc.1201802. [DOI] [PubMed] [Google Scholar]
- Chu SH, Small D. Mechnanisms of resistance to FLT3 inhibitors. Drug Resist Updat. 2009;12 doi: 10.1016/j.drup.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681–1689. doi: 10.1371/journal.pmed.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Chen S, Pei XY, Almenara JA, Kramer LB, Venditti CA, Dent P, Grant S. Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood. 2008;112:2439–2449. doi: 10.1182/blood-2008-05-159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Landowski TH, Rosen ST, Dent P, Grant S. Combined treatment with the checkpoint abrogator UCN-01 and MEK1/2 inhibitors potently induces apoptosis in drug-sensitive and -resistant myeloma cells through an IL-6-independent mechanism. Blood. 2002;100:3333–3343. doi: 10.1182/blood-2002-03-0940. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra G, Yerram N, Dai Y, Dent P, Grant S. Synergistic interactions between vorinostat and sorafenib in chronic myelogenous leukemia cells involve Mcl-1 and p21CIP1 down-regulation. Clin Cancer Res 2007. 2007;13:4280–4290. doi: 10.1158/1078-0432.CCR-07-0835. [DOI] [PubMed] [Google Scholar]
- Deng J, Shimamura T, Perera S, Carlson NE, Cai D, Shapiro GI, Wong KK, Letai A. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007;67:11867–11875. doi: 10.1158/0008-5472.CAN-07-1961. [DOI] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- Di Cosimo S, Baselga J. Targeted therapies in breast cancer: where are we now? Eur J Cancer. 2008;44:2781–2790. doi: 10.1016/j.ejca.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808–4817. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele S, Lauricella M, Tesoriere G. Histone deacetylase inhibitors: apoptotic effects and clinical implications. Int J Oncol. 2008;33:637–646. [PubMed] [Google Scholar]
- Fan QW, Cheng CK, Nicolaides TP, Hackett CS, Knight ZA, Shokat KM, Weiss WA. A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007;67:7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming IN, Hogben M, Frame S, McClue SJ, Green SR. Synergistic inhibition of ErbB signaling by combined treatment with seliciclib and ErbB-targeting agents. Clin Cancer Res. 2008;14:4326–4335. doi: 10.1158/1078-0432.CCR-07-4633. [DOI] [PubMed] [Google Scholar]
- Fornier MN, Rathkopf D, Shah M, Patil S, O’Reilly E, Tse AN, Hudis C, Lefkowitz R, Kelsen DP, Schwartz GK. Phase I dose-finding study of weekly docetaxel followed by flavopiridol for patients with advanced solid tumors. Clin Cancer Res. 2007;13:5841–5846. doi: 10.1158/1078-0432.CCR-07-1218. [DOI] [PubMed] [Google Scholar]
- Frost P, Shi Y, Hoang B, Gera J, Lichtenstein A. Regulation of D-cyclin translation inhibition in myeloma cells treated with mammalian target of rapamycin inhibitors: rationale for combined treatment with extracellular signal-regulated kinase inhibitors and rapamycin. Mol Cancer Ther. 2009;8:83–93. doi: 10.1158/1535-7163.MCT-08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festuccia C, Gravina GL, Muzi P, Millimaggi D, Dolo V, Vicentini C, Bologna M. Akt down-modulation induces apoptosis of human prostate cancer cells and synergizes with EGFR tyrosine kinase inhibitors. Prostate. 2008;68:965–974. doi: 10.1002/pros.20757. [DOI] [PubMed] [Google Scholar]
- Gahr S, Peter G, Wissniowski TT, Hahn EG, Herold C, Ocker M. The histone-deacetylase inhibitor MS-275 and the CDK-inhibitor CYC-202 promote anti-tumor effects in hepatoma cell lines. Oncol Rep. 2008;20:1249–1256. [PubMed] [Google Scholar]
- Gao N, Dai Y, Rahmani M, Dent P, Grant S. Contribution of disruption of the nuclear factor-kappaB pathway to induction of apoptosis in human leukemia cells by histone deacetylase inhibitors and flavopiridol. Mol Pharmacol. 2004;66:956–963. doi: 10.1124/mol.104.002014. [DOI] [PubMed] [Google Scholar]
- Gonzalez J, de Groot J. Combination therapy for malignant glioma based on PTEN status. Expert Rev Anticancer Ther. 2008;8:1767–1779. doi: 10.1586/14737140.8.11.1767. [DOI] [PubMed] [Google Scholar]
- Grant S, Dent P. Simultaneous interruption of signal transduction and cell cycle regulatory pathways: implications for new approaches to the treatment of childhood leukemias. Curr Drug Targets. 2007;8:751–759. doi: 10.2174/138945007780830764. [DOI] [PubMed] [Google Scholar]
- Grant S. Cotargeting survival signaling pathways in cancer. J Clin Invest. 2008a;118:3003–3006. doi: 10.1172/JCI36898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S. Is the focus moving toward a combination of targeted drugs? Best Pract Res Clin Haematol. 2008b;21:629–637. doi: 10.1016/j.beha.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass NK, Sproesser K, Nguyen TK, Contractor R, Medina CA, Nathanson KL, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res. 2008;14:230–239. doi: 10.1158/1078-0432.CCR-07-1440. [DOI] [PubMed] [Google Scholar]
- Hamed H, Hawkins W, Mitchell C, Gilfor D, Zhang G, Pei XY, Dai Y, Hagan MP, Roberts JD, Yacoub A, Grant S, Dent P. Transient exposure of carcinoma cells to RAS/MEK inhibitors and UCN-01 causes cell death in vitro and in vivo. Mol Cancer Ther. 2008;7:616–629. doi: 10.1158/1535-7163.MCT-07-2376. [DOI] [PubMed] [Google Scholar]
- Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25:4057–4065. doi: 10.1200/JCO.2007.11.8984. [DOI] [PubMed] [Google Scholar]
- Hida T, Ogawa S, Park JC, Park JY, Shimizu J, Horio Y, Yoshida K. Gefitinib for the treatment of non-small-cell lung cancer. Expert Rev Anticancer Ther. 2009;9:17–35. doi: 10.1586/14737140.9.1.17. [DOI] [PubMed] [Google Scholar]
- Howes AL, Chiang GG, Lang ES, Ho CB, Powis G, Vuori K, et al. The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Mol Cancer Ther. 2007;6:2505–2514. doi: 10.1158/1535-7163.MCT-06-0698. [DOI] [PubMed] [Google Scholar]
- Huang F, Greer A, Hurlburt W, Han X, Hafezi R, Wittenberg GM, et al. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69:161–170. doi: 10.1158/0008-5472.CAN-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston A, Leleu X, Jia X, Moreau AS, Ngo HT, Runnels J, Anderson J, et al. Targeting Akt and heat shock protein 90 produces synergistic multiple myeloma cell cytotoxicity in the bone marrow microenvironment. Clin Cancer Res. 2008;14:865–874. doi: 10.1158/1078-0432.CCR-07-1299. [DOI] [PubMed] [Google Scholar]
- Ihle NT, Powis G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol Cancer Ther. 2009;8:1–9. doi: 10.1158/1535-7163.MCT-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle NT, Lemos R, Jr, Wipf P, Yacoub A, Mitchell C, Siwak D, Mills GB, Dent P, Kirkpatrick DL, Powis G. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res. 2009a;69:143–150. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle NT, Paine-Murrieta G, Berggren MI, Baker A, Tate WR, Wipf P, Abraham RT, et al. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol Cancer Ther. 2005;4:1349–1357. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmaat ML, Rodriguez JA, Gallegos-Ruiz M, Kruyt FA, Giaccone G. Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. Int J Cancer. 2006;118:209–214. doi: 10.1002/ijc.21290. [DOI] [PubMed] [Google Scholar]
- Jasinghe VJ, Xie Z, Zhou J, Khng J, Poon LF, Senthilnathan P, Glaser KB, Albert DH, Davidsen SK, Chen CS. ABT-869, a multi-targeted tyrosine kinase inhibitor, in combination with rapamycin is effective for subcutaneous hepatocellular carcinoma xenograft. J Hepatol. 2008;49:985–997. doi: 10.1016/j.jhep.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updates. 2008;11:63–76. doi: 10.1016/j.drup.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, Kulesza P, Wheelhouse J, Chan A, Zhang X, Kincaid E, et al. Dual EGFR and mTOR targeting in squamous cell carcinoma models, and development of early markers of efficacy. Br J Cancer. 2007;96:952–959. doi: 10.1038/sj.bjc.6603656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Esteva FJ. Cross-talk between the ErbB/HER family and the type I insulin-like growth factor receptor signaling pathway in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:485–498. doi: 10.1007/s10911-008-9107-3. [DOI] [PubMed] [Google Scholar]
- Johnson BE, Jänne PA. Epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Cancer Res. 2005;65:7525–7529. doi: 10.1158/0008-5472.CAN-05-1257. [DOI] [PubMed] [Google Scholar]
- Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobbe CB, Lapin V, Suzuki A, Mak TW. The roles of PTEN in development, physiology and tumorigenesis in mouse models: a tissue-by-tissue survey. Oncogene. 2008;27:5398–5415. doi: 10.1038/onc.2008.238. [DOI] [PubMed] [Google Scholar]
- Koliopanos A, Avgerinos C, Paraskeva C, Touloumis Z, Kelgiorgi D, Dervenis C. Molecular aspects of carcinogenesis in pancreatic cancer. Hepatobil Pancreat Dis Int. 2008;7:345–356. [PubMed] [Google Scholar]
- Kroog GS, Motzer RJ. Systemic therapy for metastatic renal cell carcinoma. Urol Clin North Am. 2008;35:687–701. doi: 10.1016/j.ucl.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Mod Pathol. 2008;21 (S2):S16–22. doi: 10.1038/modpathol.3801018. [DOI] [PubMed] [Google Scholar]
- Lasithiotakis KG, Sinnberg TW, Schittek B, Flaherty KT, Kulms D, Maczey E, Garbe C, Meier FE. Combined inhibition of MAPK and mTOR signaling inhibits growth, induces cell death, and abrogates invasive growth of melanoma cells. J Invest Dermatol. 2008;128:2013–2023. doi: 10.1038/jid.2008.44. [DOI] [PubMed] [Google Scholar]
- Lee JT, McCubrey JA. BAY-43-9006 Bayer/Onyx. Curr Opin Investig Drugs. 2003;4:757–756. [PubMed] [Google Scholar]
- Le Tourneau CL, Vidal L, Siu LL. Progress and challenges in the identification of biomarkers for EGFR and VEGFR targeting anticancer agents. Drug Resist Updates. 2008;11:99–109. doi: 10.1016/j.drup.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Loeffler-Ragg J, Schwentner I, Sprinzl GM, Zwierzina H. EGFR inhibition as a therapy for head and neck squamous cell carcinoma. Expert Opin Investig Drugs. 2008;17:1517–1531. doi: 10.1517/13543784.17.10.1517. [DOI] [PubMed] [Google Scholar]
- LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updates. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AP, Miller A, Emad L, Rahmani M, Walker T, Mitchell C, et al. Lapatinib resistance in HCT116 cells is mediated by elevated MCL-1 expression and decreased BAK activation and not by ERBB receptor kinase mutation. Mol Pharmacol. 2008;74:807–822. doi: 10.1124/mol.108.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LA, Head JE, Pancholi S, Salter J, Quinn E, Detre S, et al. The farnesyltransferase inhibitor R115777 (tipifarnib) in combination with tamoxifen acts synergistically to inhibit MCF-7 breast cancer cell proliferation and cell cycle progression in vitro and in vivo. Mol Cancer Ther. 2007;6:2458–2467. doi: 10.1158/1535-7163.MCT-06-0452. [DOI] [PubMed] [Google Scholar]
- McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–179. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Milella M, Tafuri A, Martelli AM, Lunghi P, et al. Targeting the Raf/MEK/ERK pathway with small-molecule inhibitors. Curr Opin Investig Drugs. 2008;9:614–630. [PubMed] [Google Scholar]
- McKinstry R, Qiao L, Yacoub A, Dai Y, Decker R, Holt S, Hagan MP, Grant S, Dent P. Inhibitors of MEK1/2 interact with UCN-01 to induce apoptosis and reduce colony formation in mammary and prostate carcinoma cells. Cancer Biol Ther. 2002;1:243–253. doi: 10.4161/cbt.75. [DOI] [PubMed] [Google Scholar]
- Memmott RM, Dennis PA. Akt-dependent and independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21:178–190. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Park MA, Zhang G, Yacoub A, Curiel DT, Fisher PB, Roberts JD, Grant S, Dent P. Extrinsic pathway- and cathepsin-dependent induction of mitochondrial dysfunction are essential for synergistic flavopiridol and vorinostat lethality in breast cancer cells. Mol Cancer Ther. 2007;6:3101–3112. doi: 10.1158/1535-7163.MCT-07-0561. [DOI] [PubMed] [Google Scholar]
- Moghul A, Lin L, Beedle A, Kanbour-Shakir A, DeFrances MC, Liu Y, Zarnegar R. Modulation of c-MET proto-oncogene (HGF receptor) mRNA abundance by cytokines and hormones: evidence for rapid decay of the 8 kb c-MET transcript. Oncogene. 1994;9:2045–2052. [PubMed] [Google Scholar]
- Mohapatra S, Chu B, Zhao X, Djeu J, Cheng JQ, Pledger WJ. Apoptosis of metastatic prostate cancer cells by a combination of cyclin-dependent kinase and AKT inhibitors. Int J Biochem Cell Biol. 2009;41:595–602. doi: 10.1016/j.biocel.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Hunter LA, Ethier SP, Boerner JL. Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells. Cancer Res. 2008;68:3314–3322. doi: 10.1158/0008-5472.CAN-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TK, Rahmani M, Gao N, Kramer L, Corbin AS, Druker BJ, Dent P, Grant S. Synergistic interactions between DMAG and mitogen-activated protein kinase kinase 1/2 inhibitors in Bcr/abl+ leukemia cells sensitive and resistant to imatinib mesylate. Clin Cancer Res. 2006;12:2239–2247. doi: 10.1158/1078-0432.CCR-05-2282. [DOI] [PubMed] [Google Scholar]
- O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci (USA) 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Zhang G, Mitchell C, Rahmani M, Hamed H, Hagan MP, et al. Mitogen-activated protein kinase kinase 1/2 inhibitors and 17-allylamino-17-demethoxy geldanamycin synergize to kill human gastrointestinal tumor cells in vitro via suppression of c-FLIP-s levels and activation of CD95. Mol Cancer Ther. 2008;7:2633–2648. doi: 10.1158/1535-7163.MCT-08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther. 2008a;7:1648–1662. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei XY, Dai Y, Tenorio S, Lu J, Harada H, Dent P, Grant S. MEK1/2 inhibitors potentiate UCN-01 lethality in human multiple myeloma cells through a Bim-dependent mechanism. Blood. 2007;110:2092–2101. doi: 10.1182/blood-2007-04-083204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps MA, Lin TS, Johnson AJ, Hurh E, Rozewski DM, Farley KL, et al. Clinical response and pharmacokinetics from a phase I study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009 doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43–9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217–35227. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Davis EM, Crabtree TR, Habibi JR, Nguyen TK, Dent P, Grant S. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol Cell Biol. 2007;27:5499–5513. doi: 10.1128/MCB.01080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Graf D, Häussinger D. Bile salt-induced hepatocyte apoptosis involves epidermal growth factor receptor-dependent CD95 tyrosine phosphorylation. Gastroenterology. 2003;125:839–853. doi: 10.1016/s0016-5085(03)01055-2. [DOI] [PubMed] [Google Scholar]
- Rengan R, Cengel KA, Hahn SM. Clinical target promiscuity: lessons from ras molecular trials. Cancer Metastasis Rev. 2008;27:403–414. doi: 10.1007/s10555-008-9133-z. [DOI] [PubMed] [Google Scholar]
- Ryan Q, Ibrahim A, Cohen MH, Johnson J, Ko CW, Sridhara R, Justice R, Pazdur R. FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist. 2008;13:1114–1119. doi: 10.1634/theoncologist.2008-0816. [DOI] [PubMed] [Google Scholar]
- Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–1857. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- Senderowicz AM. Flavopiridol: the first cyclin-dependent kinase inhibitor in human clinical trials. Invest New Drugs. 1999;17:313–320. doi: 10.1023/a:1006353008903. [DOI] [PubMed] [Google Scholar]
- Shapiro GI. Preclinical and clinical development of the cyclin-dependent kinase inhibitor flavopiridol. Clin Cancer Res. 2004;10:4270s–4275s. doi: 10.1158/1078-0432.CCR-040020. [DOI] [PubMed] [Google Scholar]
- Sizemore N, Cox AD, Barnard JA, Oldham SM, Reynolds ER, Der CJ, Coffey RJ. Pharmacological inhibition of Ras-transformed epithelial cell growth is linked to down-regulation of epidermal growth factor-related peptides. Gastroenterology. 1999;117:567–576. doi: 10.1016/s0016-5085(99)70449-x. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Lioni M, Palma MD, Xiao M, Desai B, Egyhazi S, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008;7:2876–2883. doi: 10.1158/1535-7163.MCT-08-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- Steelman LS, Stadelman KM, Chappell WH, Horn S, Bäsecke J, Cervello M, et al. Akt as a therapeutic target in cancer. Expert Opin Ther Targets. 2008;12:1139–1165. doi: 10.1517/14728222.12.9.1139. [DOI] [PubMed] [Google Scholar]
- Sturgill TW. MAP kinase: it’s been longer than fifteen minutes. Biochem Biophys Res Commun. 2008;371:1–4. doi: 10.1016/j.bbrc.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Takada Y, Aggarwal BB. Flavopiridol inhibits NF-κB activation induced by various carcinogens and inflammatory agents through inhibition of IκBα kinase and p65 phosphorylation: abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J Biol Chem. 2004;279:4750–4759. doi: 10.1074/jbc.M304546200. [DOI] [PubMed] [Google Scholar]
- Takigawa N, Takeyama M, Kozuki T, Shibayama T, Hisamoto A, Kiura K, et al. Combination of SN-38 with gefitinib or imatinib overcomes SN-38-resistant small-cell lung cancer cells. Oncol Rep. 2007;17:983–987. [PubMed] [Google Scholar]
- Takimoto CH, Awada A. Safety and anti-tumor activity of sorafenib (Nexavar) in combination with other anti-cancer agents: a review of clinical trials. Cancer Chemother Pharmacol. 2008;61:535–548. doi: 10.1007/s00280-007-0639-9. [DOI] [PubMed] [Google Scholar]
- Tímár J, Döme B. Antiangiogenic drugs and tyrosine kinases. Anticancer Agents Med Chem. 2008;8:462–469. doi: 10.2174/187152008784533035. [DOI] [PubMed] [Google Scholar]
- Tochizawa S, Masumori N, Yanai Y, Ohmoto Y, Yabuuchi Y, Tsukamoto T. Antitumor effects of a combination of interferon-alpha and sorafenib on human renal carcinoma cell lines. Biomed Res. 2008;29:271–278. doi: 10.2220/biomedres.29.271. [DOI] [PubMed] [Google Scholar]
- Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. New Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci (USA) 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- Wang X, Hawk N, Yue P, Kauh J, Ramalingam SS, Fu H, Khuri FR, Sun SY. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors’ anticancer efficacy. Cancer Biol Ther. 2008;7:1952–1958. doi: 10.4161/cbt.7.12.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhou J, Fan J, Qiu SJ, Yu Y, Huang XW, Tang ZY. Effect of rapamycin alone and in combination with sorafenib in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res. 2008a;14:5124–5130. doi: 10.1158/1078-0432.CCR-07-4774. [DOI] [PubMed] [Google Scholar]
- Wesierska-Gadek J, Maurer M, Schmid G. Inhibition of farnesyl protein transferase sensitizes human MCF-7 breast cancer cells to roscovitine-mediated cell cycle arrest. J Cell Biochem. 2007;102:736–747. doi: 10.1002/jcb.21325. [DOI] [PubMed] [Google Scholar]
- Wong KK. Recent developments in anti-cancer agents targeting the Ras/Raf/MEK/ERK pathway. Recent Pat Anticancer Drug Discov. 2009;4:28–35. doi: 10.2174/157489209787002461. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Miller A, Caron RW, Qiao L, Curiel DA, Fisher PB, Hagan MP, Grant S, Dent P. Radiotherapy-induced signal transduction. Endocr Relat Cancer. 2006;13 (S1):S99–114. doi: 10.1677/erc.1.01271. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Gilfor D, Hawkins W, Park MA, Hanna D, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. MEK1/2 inhibition promotes taxotere lethality in mammary tumors in vivo. Cancer Biol Ther. 2006a;5:1332–9. doi: 10.4161/cbt.5.10.3215. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Mitchell C, Lister A, et al. Melanoma differentiation-associated 7 (interleukin 24) inhibits growth and enhances radiosensitivity of glioma cells in vitro and in vivo. Clin Cancer Res. 2003;9:3272–3281. [PubMed] [Google Scholar]
- Yu C, Rahmani M, Dai Y, Conrad D, Krystal G, Dent P, Grant S. The lethal effects of pharmacological cyclin-dependent kinase inhibitors in human leukemia cells proceed through a phosphatidylinositol 3-kinase/Akt-dependent process. Cancer Res. 2003;63:1822–1833. [PubMed] [Google Scholar]
- Zhang G, Park MA, Mitchell C, Hamed H, Rahmani M, Martin AP, et al. Vorinostat and sorafenib synergistically kill tumor cells via FLIP suppression and CD95 activation. Clin Cancer Res. 2008;14:5385–5399. doi: 10.1158/1078-0432.CCR-08-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene 2008. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]