Abstract
Although the biological function of sleep remains uncertain, the consequences of sleep deprivation are well-described and are reported to be detrimental to cognitive function and affective well-being. Sleep deprivation also is strongly associated with elevated risk factors for cardiovascular disease. We used a mouse model of cardiac arrest/cardiopulmonary resuscitation to test the hypothesis that acute sleep deprivation would exacerbate neuroinflammation and neurodegeneration after global ischemia. The resulting data led to a rejection of our hypothesis that sleep deprivation is necessarily detrimental. Indeed, acute sleep deprivation (ASD) was associated with a reduction in ischemia-induced interleukin 1β (IL-1β) gene expression and attenuation of neuronal damage in the hippocampus. Further, sleep deprivation increased gene expression of two anti-inflammatory cytokines, IL-6 and IL-10 that are associated with improved ischemic outcome. To determine whether the anti-inflammatory properties of ASD were specific to ischemia, mice were treated systemically with lipopolysaccharide (LPS), a potent inflammogen. Acute sleep deprivation attenuated the central and peripheral increase in tumor necrosis factor-α (TNFα) and increased IL-10 expression. Together, the ischemia and LPS data suggest that, ASD produces an anti-inflammatory bias that could be exploited to improve medical procedures that are compromised by inflammation.
Keywords: Global ischemia, sleep deprivation, glucocorticoids, inflammation, cytokines, mifepristone, lipopolysaccharide, biological rhythms
Introduction
Difficulty with sleep is reported by a substantial minority of Americans (Ohayon, 2002). Decreased quantity and quality of sleep, as well as disruption in circadian rhythmicity, has been attributed to changes in lifestyle, the proliferation of shift work, international travel, and also to exposure to light at night (Navara and Nelson, 2007, Rajaratnam and Arendt, 2001). Importantly, sleep disturbances occur with even greater frequency among individuals at elevated risk for cardiovascular disease including the aged, obese, hospital in-patients, and individuals with a history of cardiovascular disease (Bliwise, 1993, Katz and McHorney, 1998, Leung and Douglas Bradley, 2001, Vgontzas, et al., 1994, Wolk, et al., 2005). The link between disordered sleep and cardiovascular disease is especially important in light of mounting evidence that sleep problems may act as a risk factor for the development of cardiovascular disease.
The epidemiological link between cardiovascular disease and disordered sleep is not entirely surprising given the bidirectional interactions between the two systems, particularly via immunological and neuroendocrine processes. Sleep deprivation can produce a proinflammatory and prooxidative environment while also activating classical stress responses which are all associated with poor ischemic outcomes (DeVries, et al., 2007, Lorton, et al., 2006, Silva, et al., 2004, Toth, 1995). Thus, we predicted that sleep deprivation would establish a physiological milieu in which relatively minor ischemic injuries could produce significant neurologic damage. The data obtained lead to the rejection of our hypothesis and countered the dogma that sleep deprivation necessarily impairs health.
The overall goals of this experiment were: (1) to assess the modulatory action of acute sleep deprivation on experimental cardiac arrest outcome; specifically, we investigated the consequences of prior sleep deprivation for behavioral, inflammatory, and histological outcomes following global cerebral ischemia. (2) To determine whether elevated glucocorticoids during the sleep deprivation period were necessary for sleep deprivation-induced changes in ischemic outcomes. (3) To determine whether sleep deprivation-induced alterations in inflammatory responses were specific to ischemic injuries in the CNS by measuring inflammatory responses to the bacterial endotoxin lipopolysaccharide (LPS) in the periphery. (4) Finally, we sought to determine whether the elevated glucocorticoids during the sleep deprivation period were necessary for the immunomodulatory effects of acute sleep deprivation.
Methods
An overview of the experimental design is provided in Figure 1, while specific details of experimental design are provided below
Figure 1. Timeline of experimental procedures.
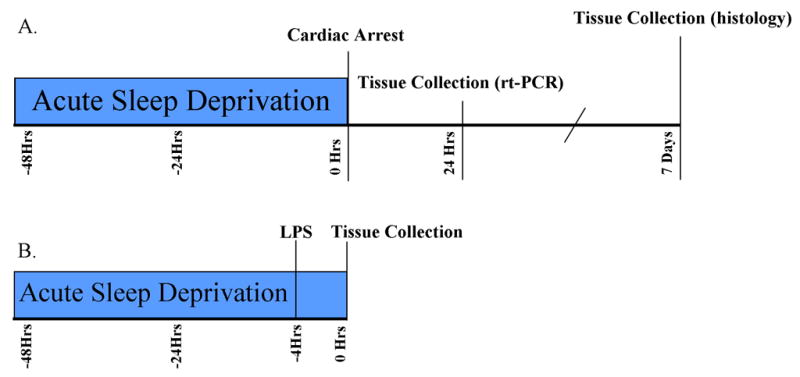
Timeline of experimental events in the cardiac arrest (A) and LPS (B) studies.
Animals
Adult male C3H/e mice used in these experiments were purchased from Harlan (Indianapolis, IN). Mice were individually-housed upon arrival in our laboratory and were allowed to acclimate for at least a week prior to the onset of any experimental procedures. All mice had ad libitum access to food (Harlan Teklad #8640) and filtered tap water. Animals were housed in polypropylene cages, in colony rooms with constant temperature (21 ± 4 °C) and humidity (50 ± 10%). The experimental conditions were approved by the Ohio State University Institutional Lab Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines.
Sleep deprivation
Mice were sleep deprived with a slightly modified version of the multiple platform procedure originally developed for use with rats (Nunes and Tufik, 1994). Briefly, standard polycarbonate micro-isolator cages were filled with water up to 1.5 cm below the top of five plastic platforms (3 cm in diameter) that were affixed to the bottom of the cages. In this manner, mice were able to move freely between platforms, but the platforms were not sufficiently close to allow the animals to sleep across them. On the morning of surgery mice were briefly anesthetized with isoflurane vapors and a blood sample collected from the retro-orbital sinus. All mice were placed in a standard cage for approximately 20 min before surgery to allow them to recover from anesthesia and to normalize body temperatures, and no steps were taken to prevent sleep during this period. Standard housing conditions were similar except the water and platforms were replaced with standard corncob bedding. All animals were housed in standard conditions after surgery.
An additional group of mice were either sleep-deprived or maintained in standard conditions and then treated twice daily with mifepristone (RU486, Sigma Aldrich; 50mg/kg in sesame oil i.p.) coincident with the transfer to sleep deprivation chambers and concluding 12 h before treatments. Mice were then injected with LPS or saline vehicle and tissue collected as below.
Cardiac Arrest/CPR Procedure
Mice were anesthetized with 3% halothane in air, intubated, and maintained on 1.5% halothane. A temperature probe was placed in the temporalis muscle on the left side of the head. Temporalis muscle temperature was used as an index of brain temperature. We have previously demonstrated in mice that brain temperature and temporalis temperature are highly correlated (r2 = 0.94) over the range of temperatures experienced during our cardiac arrest/CPR procedure (24 to 39.5 °C) (Neigh, et al., 2004). Head temperature was manipulated independently of body temperature through the use of a double lumen coil that was placed around the head and filled with circulating water to achieve a brain temperature of 37° C (normothermic), or 27° C (hypothermic); the hypothermic head temperature is completely protective against ischemia-induced neuronal damage, thereby serving as a control (Neigh, et al., 2004). A second temperature probe was placed to monitor rectal temperature. A PE10 catheter was inserted into the right jugular vein for potassium chloride (KCl) and epinephrine (EPI) administration. A cannula (Fine Science, Foster City, CA) was inserted into the right femoral artery, and connected to a blood pressure transducer (Columbus Instruments, Columbus, OH) to allow continuous monitoring of arterial blood pressure. The intubation tube was connected to a ventilator (Columbus Instruments, Columbus, OH) and mice were ventilated with a tidal volume of 150 μl and a respiratory rate of 160 breaths per minute. Mice were stabilized for 10 min during which time blood pressure and temperatures were recorded at one min intervals. At the end of the acclimation period, body temperature was decreased to 27° C by circulating cold water through a coil system beneath the animal and placement of an alcohol patch on the ventrum. To induce cardiac arrest, KCl (50 μl, 0.5 M, 4°C) was injected via the jugular catheter. The mice were detached from the ventilator. Slow rewarming via heating lamp and thermal blanket begin when body temperature reaches 27° C after ~4 min of arrest. At 7 min 45 sec into the arrest period, the mouse was reattached to the ventilator and ventilated with 100% oxygen with a tidal volume of 150 μl and a respiratory rate of 160 breaths/min. Eight min after injection of KCl, CPR was initiated via injection of epinephrine (16 μg EPI in 0.6 cc saline, 37° C) into the jugular vein catheter and chest compressions (300/min). Additional EPI was administered in increments of 0.5 μg in conjunction with continued chest compressions until the mouse is resuscitated (maximal dose of EPI: 32 μg). Mice were maintained on 100% oxygen for 25 min following resuscitation of spontaneous circulation. Catheters were then removed and wounds sutured.
Tissue Collection, Processing, and Analysis
Seven days post-reperfusion, a blood sample was collected from the retro-orbital sinus, and then the mice were euthanized with sodium pentobarbital. Mice were then perfused transcardially with ice-cold 0.1 M PBS and 4% paraformaldehyde. Brains were removed, postfixed overnight, cryoprotected and then frozen on dry ice. Brains were cut at 14 μm on a cryostat and thaw-mounted onto Super Frost Plus slides (Fisher, Hampton, NH). Sections were stored at −20° C until further processing.
Fluoro Jade C histochemistry
Fluoro-Jade C (FJ) is a fluorescein derivative that labels degenerating neurons. Mounted 14-μm sections were stained according to established protocols. Briefly, slides were dried at room temperature, immersed in a basic ethanol solution (80% containing 1% sodium hydroxide) and then rinsed in 70% ethanol and distilled water (dH20). Slides were then treated with potassium permanganate (0.06% in (dH20) for 10 min, rinsed with water, and then incubated in Fluoro Jade C (0.0001% in a 1% acetic acid solution); sections were rinsed in dH20, and thoroughly dried on a slide-warmer, cleared for 1 min in xylene, and coverslipped with DPX (Sigma, St. Louis, MO).
Fluoro Jade positive cells were counted in multiple hippocampal regions (approximately 2 mm caudal to bregma; CA1, CA2, CA3, dentate gyrus, dentate hilus, and subiculum), by an experimenter unaware of the experimental conditions associated with each sample. Black and white images of fluorescently-stained sections were captured with a digital camera (Axiocam, Zeiss, Thornwood, NY) connected to a fluorescent microscope using Axiovision software (Zeiss).
Real Time PCR
Twenty-four hours after CA/CPR surgery or 4 h after LPS, fresh tissue was collected from additional animals/time point/head temperature. At that point, mice were euthanized, a trunk blood sample was collected, and then brains and spleen (LPS study only) were removed using aseptic techniques, and stored in RNAlater RNA stabilization solution (Ambion, Austin, TX) overnight at 4° C. Tissue samples for PCR analysis were dissected out and total RNA was extracted from >30 mg of individual hippocampi using a homogenizer (Ultra-Turrax T8, IKA Works, Wilmington, NC) and an RNeasy Mini Kit according to manufacturer’s protocol (Qiagen, Valencia, CA). Extracted RNA was suspended in 30 μl RNase-free water and RNA concentration was determined by spectrophotometer (Nanodrop-1000, Nanodrop Technologies, Wilmington, DE). All RNA samples were stored at −70° C until further analysis. cDNA was created via reverse transcription of 2 μg of RNA from each sample with MMLV Reverse Transcriptase enzyme (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol.
IL-1β and TNFα primers and probes (Overbergh, et al., 1999) were synthesized as follows, with probes labeled with 6-FAM (fluorescent dye) and MGB (non-fluorescent quencher) at the 5′ and 3′ ends, respectively: IL-1β forward 5′-CAACCAACAAGTGATATTCTCCATG-3′, IL-1β reverse 5′-AGATCCACACTCTCAGCTGCA-3′, IL-1β probe 5′-CTGTGTAATGAAAGACGGCACACCCACC -3′; TNFα forward 5′-CATCTTCTCAAAATTCGAGTGACAA-3′, TNFα reverse 5′-GGGAGTAGACAAGGTACAACCC-3′, TNFα probe 5′-CACGTCGTAGCAAACCACCAAGTGA -3′. A TaqMan 18S Ribosomal RNA primer and probe set (labeled with VIC fluorescent dye; Applied Biosystems, Foster City, CA) were used as the control gene for relative quantification of IL-6 and IL-10. Amplification was performed on an ABI 7000 Sequence Detection System by using Taqman® Universal PCR Master Mix. The universal two-step RT-PCR cycling conditions used were: 50° C for 2 min, 95° C for 10 min, followed by 40 cycles of 95° C for 15 sec and 60° C for 1 min. Relative gene expression of individual samples run in duplicate was calculated by comparison to a relative standard curve and standardized by comparison to 18s rRNA signal. The other half of the spleen from the LPS study was homogenized in RIPA buffer containing protease inhibitors (Pierce, Rockford, IL). Splenic tissue lysates and serum samples were assayed using a sandwich ELISA kit (BD Biosciences, San Jose, CA) for TNFα according to the manufacturer’s protocol.
Radioimmunoassay Procedures
Blood samples were collected into heparinized microcapillary tubes and then kept on ice until they were spun for 30 min at 3500rpm. The resultant supernatant was removed and stored at −80°C. All blood samples were assayed for total circulating corticosterone, in a single assay, using a double antibody 125I radioimmunoassay (MP Biomedicals Irvine, CA). The assay was conducted following the guidelines set by the manufacturer. These kits are highly specific and crossreactivity with other steroids is less than 1%. Intra-assay variance was less than 10% and the minimum detection threshold was 5 ng/ml.
Statistical analyses
In the cerebral ischemia study, cell death and cytokine expression data were analyzed with two-factor ANOVAs with head temperature and sleep conditions as the between subjects variable. Cell death data violated the equal variance assumption of parametric statistics and as such were log-transformed. Surgical parameters (e.g. head temperature) were analyzed with repeated measures ANOVA with group as the between subjects variable. In the LPS study, cytokine expression was analyzed with two-factor ANOVAs with sleep status and treatment (LPS vs. saline) as between subject variables. After significant F scores, multiple comparisons were conducted with Tukey’s honestly significant differences test. All mean differences were considered statistically significant if p<0.05.
Results
To determine the effects of acute sleep deprivation on neuroinflammation and neuronal damage, male mice were housed in a modified version of the multiple platform apparatus (Nunes and Tufik, 1994) or a standard cage for 48 h prior to induction of 8 min of cardiac arrest. Figure 1A illustrates the experimental timeline for the cardiac arrest experiment. The mice were resuscitated via epinephrine injection and chest compressions (CA/CPR) (Neigh, et al., 2004). The ischemic control group consisted of mice that underwent the same cardiac arrest procedure, but ischemic influences on the brain were prevented through the use of hypothermia (Neigh, et al., 2004). Sleep deprivation did not alter the surgical parameters or overall survival following CA/CPR (Supplemental Figure 1), but it did decrease the resulting cytokine gene expression and neuronal damage in the hippocampus.
Normothermic cardiac arrest significantly reduced the percentage of mice surviving seven days post reperfusion as compared to mice that underwent cardiac arrest with hypothermic heads (Χ2=8.20, p<0.05; Fig 2). However, sleep deprivation did not alter the survival proportions at seven days post-cardiac arrest.
Figure 2. Sleep deprivation does not alter cardiac arrest-induced mortality.
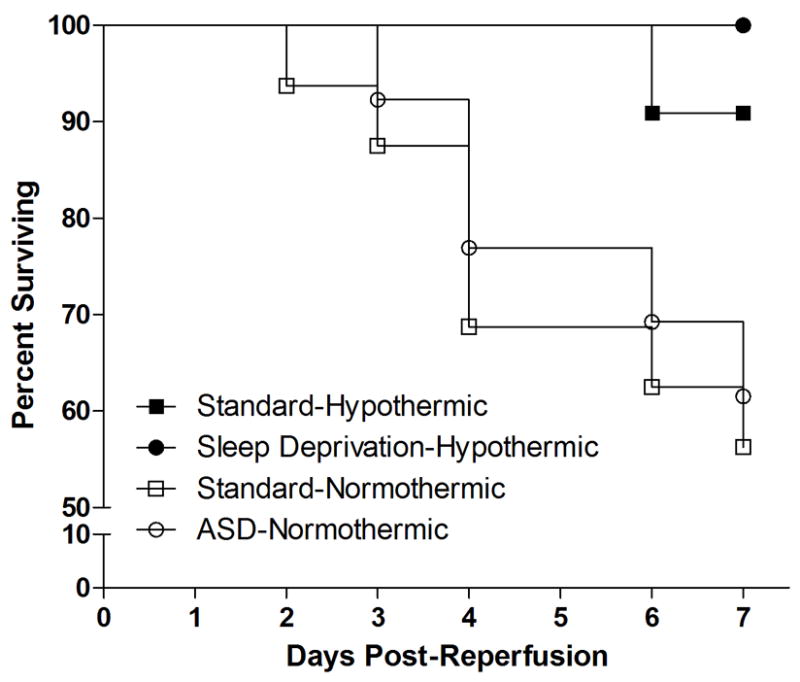
Normothermic cardiac arrest induced significant mortality but this effect was not altered by prior sleep deprivation.
As expected, histological analysis on post-operative day 7 revealed that normothermic cardiac arrest induced neuronal degeneration in the hippocampal formation, an effect that was attenuated by prior sleep deprivation. Hypothermia during cardiac arrest completely blocked the induction of neuronal damage, as indicated by a relative absence of Fluoro-Jade positive cells, in the hippocampus and surrounding regions (see Fig 3a–d). Mice that underwent normothermic cardiac arrest had significantly more Fluoro-Jade positive neurons than hypothermic control animals in the CA1 (F1,29=48.95, p<0.000001; see Fig 3e) and across the entire hippocampal formation (F1,29=33.01, p<0.00001; see Fig 3f). ASD reduced the number of Fluoro-Jade positive cells in all mice in the CA1 (F1,29=4.92, p<0.05), and the whole hippocampus (F1,29=2.07, p<0.05). There was also a significant interaction between sleep status and head temperature in the CA1 (F1,29=7.73, p<0.01) and entire hippocampus (F1,29=9.89, p<0.01) that was mediated in part by a slight increase in Fluoro-Jade positive cells in the sleep deprived-hypothermic group.
Figure 3. Cell death and inflammatory responses are inhibited by prior sleep deprivation.
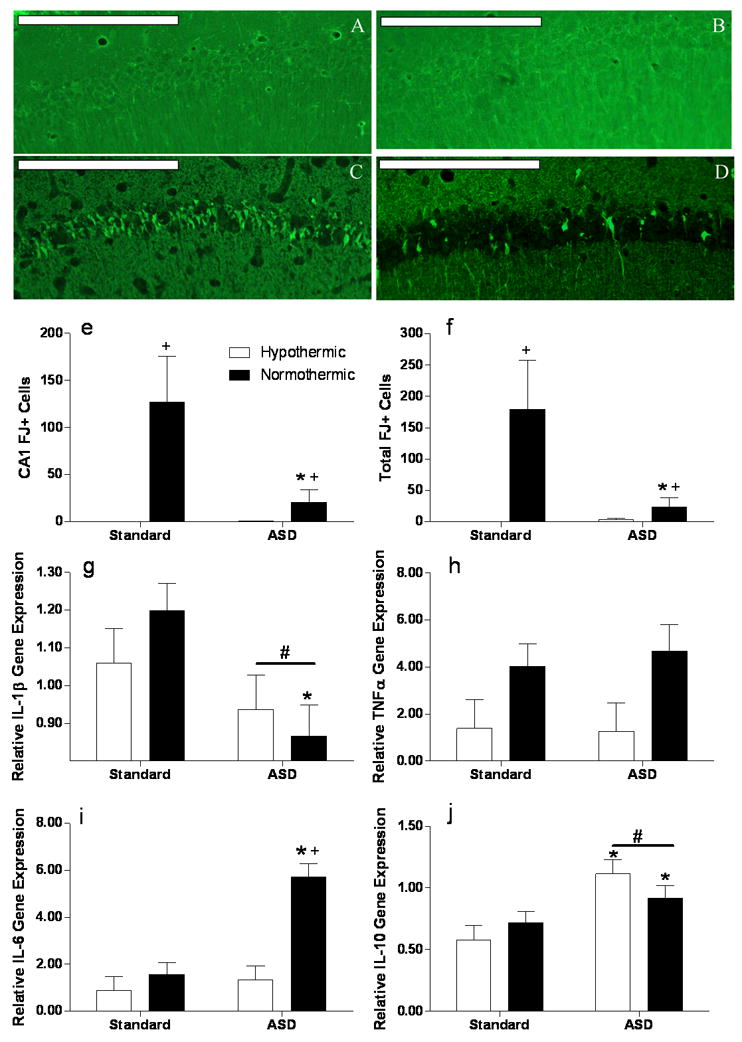
Representative Fluoro-Jade C stained sections of the CA1 field of the hippocampus following CA/CPR in A) standard housed hypothermic mice, B) ASD hypothermic mice, C) standard housed normothermic animals and D) ASD normothermic animals; scale bar = 200μm. E) Fluoro-Jade positive cells in the CA1 field and F) summed across the whole hippocampal formation. The cell death data are displayed in the figure are untransformed but statistical testing was conducted on log-transformed data. RT-PCR analyses of hippocampal mRNA 24 hours post reperfusion of the cytokines G) interleukin-1β (IL-1β), H )tumor necrosis factor alpha (TNFα), I) interleukin-6 (IL-6) and J) interleukin 10 (IL-10). + Significantly different from hypothermic mice in the same sleep condition. * Significantly different from standard housed mice. Differences were considered significant if p<0.05. ASD, acute sleep deprivation. All data are presented as means ( ± SEM). N=6–7 animal/group for histology and 5–6/group for mRNA analysis.
At 24 h post-ischemia, mRNA for the proinflammatory cytokine IL-1β was significantly lower in the sleep deprived mice than the controls maintained in standard housing (F1,23=7.09, p<0.05; see Fig 3g). Planned comparisons between normothermic groups indicated that sleep deprivation reduced IL-1β gene expression (p<0.05). Sleep deprivation had no effect on IL-1β expression among the hypothermic ischemic controls. In contrast, TNFα gene expression was not modulated by sleep deprivation (F1,23=0.06, p>0.05; see Fig 3h), but was significantly lower in hypothermic mice (F1,23=7.31, p<0.05) regardless of sleep status (F1,23=0.13, p>0.05).
Interestingly, sleep deprivation significantly increased the gene expression of two cytokines, IL-6 and IL-10, that play a causal role in neuronal protection following cerebral ischemia (Liesz, et al., 2009). Sleep deprivation increased IL-6 gene expression only among mice that underwent normothermic ischemia (F1,23=20.54, p<0.001; F1,23=10.75, p>0.01; see Fig 3i), while IL-10 gene expression was significantly increased in sleep deprived mice (F1,20=16.30, p<0.001; see Fig 3j) regardless of the ischemic condition (F1,20=16.27, p<0.05). Preplanned comparisons indicated that sleep deprivation reduced Fluoro-Jade staining in the CA1 and whole hippocampus compared to non-sleep deprived mice (p<0.05 in all cases). Thus, the decrease in IL-1β expression induced by sleep deprivation is associated with a decrease in neuronal damage. Circulating corticosterone was higher in sleep deprived mice prior to cardiac arrest (F1,47=4.34, p<0.0001; Standard housing = 28.01 ± 9.12, ASD = 112.9 ± 19.07).
Treatment with the glucocorticoid receptor antagonist RU486 during the sleep deprivation period did not alter sleep deprivation effects. To determine the role of corticosterone in the ischemic neuroprotection afforded by sleep deprivation, we treated mice with the glucocorticoid receptor antagonist, RU486, twice daily beginning at initiation of the sleep deprivation procedure. Qualitatively, RU486 attenuated Fluoro-Jade staining in the CA1 subfield and hippocampus (relative to untreated animals). While there were no detectable ASD-induced differences in RU486 treated mice, this may represent a floor effect or a possible role for corticosterone in ASD-induced neuroprotection
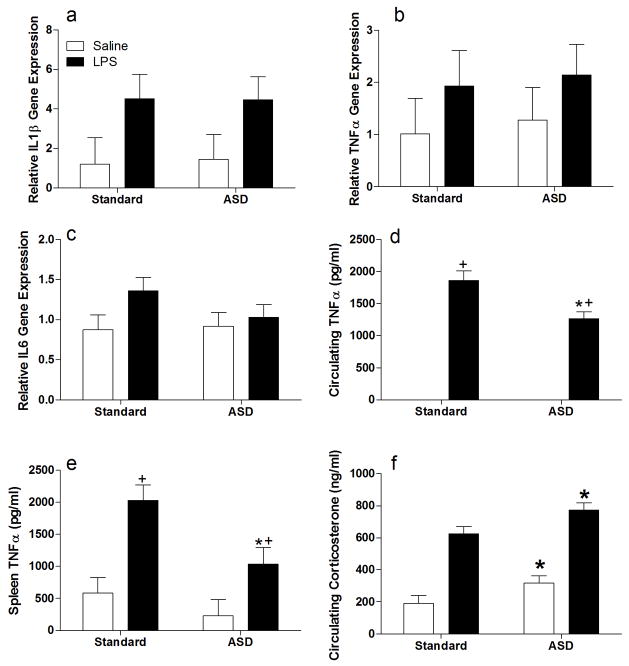
To determine whether suppression of inflammatory responses via sleep deprivation was specific to cerebral ischemia, sleep deprived and non-sleep deprived male mice were treated systemically with 400 μg/kg of LPS dissolved in pyrogen-free saline or the saline vehicle (Fig 1B for experimental timeline), Four hours after the injection, forebrain TNFα gene expression was significantly increased by LPS treatment (F1,25=15.49, p<0.001; see Fig 4a) and attenuated by prior sleep deprivation (F1,25= 4.83, p<0.05); the effect of sleep deprivation was mediated by a reduction in TNFα in LPS, but not saline treated mice (F1,25=6.34, p<0.05). Forebrain IL-1β gene expression was significantly elevated by LPS treatment (F1,25=6.64, p<0.05), but was not significantly altered by sleep deprivation (F1,25=3.22, p>0.05; see Fig 4b). However, an interaction between sleep status and LPS treatment was evident (F1,25=5.07, p<0.05). Forebrain IL-6 gene expression was not altered by LPS treatment (F1,29=0.13, p>0.05; see Fig 4c), sleep deprivation (F1,29=0.01, p>0.05), or the interaction between the two variables (F1,29=0.30, p>0.05). In contrast, forebrain IL-10 gene expression was induced by LPS treatment (F1,23=48.18, p<0.001) and by sleep deprivation (F1,23=11.39, p<0.01), although there was not a significant interaction between the variables.
Figure 4. Acute sleep deprivation antagonizes LPS induced cytokine expression in the CNS.
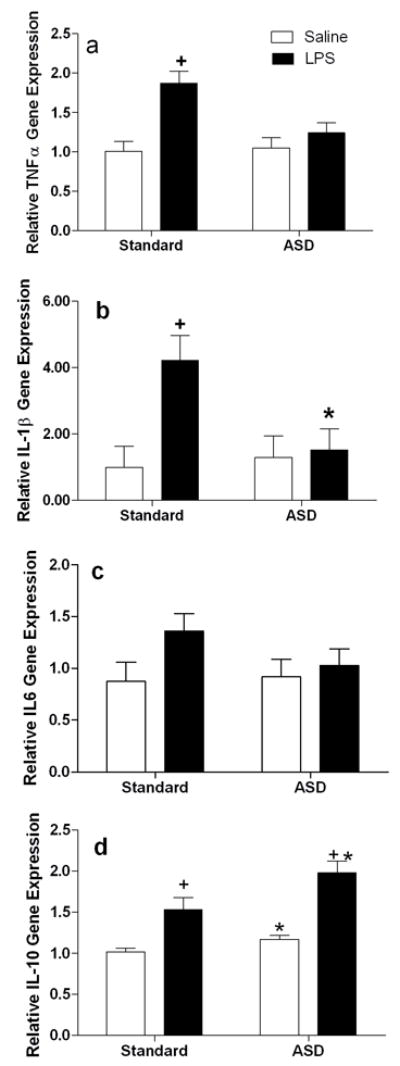
RT-PCR analysis of forebrain homogenates 4 hours after lipopolysaccharide (LPS; 400μg/kg) or vehicle treatment administration A) interleukin-1β (IL-1β), B) tumor necrosis factor alpha (TNFα), C) interleukin-6 (I-6) and D) interleukin 10 (IL-10). All gene expression data are presented after relativization to 18s rRNA expression. Differences were considered significant if p<0.05. ASD, acute sleep deprivation. All data are presented as means (± SEM). N=5–6/group.
Peripheral inflammatory responses were attenuated by sleep deprivation as well. The mRNA expression of the cytokine IL-1β in the spleen was significantly elevated 4 h after LPS treatment relative to saline injected animals (F1,27 = 6.3, p<0.05; see Fig 5a). However, IL-1β expression was not altered by sleep deprivation (F1,27 = 0.01, p>0.05) or the interaction between sleep status and LPS treatment (F1,27=0.13, p>0.05). TNFα gene expression was not significantly elevated in the spleen 4 h following injection with LPS (F1,26 = 1.92, p>0.05; see Fig 5b), sleep deprivation (F1,26=0.14, p>0.05), or the interaction between these two variables (F1,26 = 0.002, p>0.05). Similarly, IL-6 gene expression was not significantly elevated in the spleen by LPS treatment (F1,27=3.03, p>0.05; see Fig 5c) and there was no effect of sleep deprivation (F1,27 =0.71, p>0.05) or an interaction between sleep status and LPS treatment (F1,27 = 1.20, p>0.05). However, TNFα protein concentrations measured by ELISA were altered by both LPS and sleep deprivation. In the spleen, TNFα protein concentrations were significantly elevated in LPS treated mice (F1,25 = 20.93, p<0.0005; see Fig 5d). Further, ASD reduced TNFα concentrations (F1,25 = 7.55, p<0.05) and the reduction in TNFα protein by sleep deprivation was similar in all treatments (F1,25=1.68, p>0.05). In the peripheral blood, LPS also greatly increased TNFα protein (F1,23=278.76, p<0.001; see (Fig 5E), an effect that was attenuated by prior sleep deprivation (F1,23=10.21, p<0.005), but only in the LPS-treated animals (F1,23=10.21, p<0.005), as circulating TNFα was undetectable in saline-treated mice. LPS treatment activated the HPA axis as indicated by increased circulating corticosterone (F1,28=94.80, p<0.001). Circulating glucocorticoids also were elevated by sleep deprivation (F1,28=8.97, p<0.01; see Fig 5f), but sleep deprivation-induced increases in circulating corticosterone were similar in LPS- and saline-treated mice (F1,28=0.05, p>0.05).
Figure 5. Acute sleep deprivation abrogates LPS induced cytokine production in the periphery.
RT-PCR analysis of spleen homogenates four hours after LPS (400μg/kg) or vehicle administration on A) interleukin-1β (IL-1β), B) tumor necrosis factor α (TNFα) and C) interleukin-6 (IL-6). TNFα protein concentrations measured by ELISA in D) splenic lysates and E) peripheral blood. F) circulating corticosterone concentrations at the four hour post-LPS time point + Significantly different from hypothermic mice in the same sleep condition. * Significantly different from standard housed mice. Differences were considered significant if p<0.05. ASD, acute sleep deprivation. All data are presented as means (± SEM). N=5–6/group.
Although corticosterone concentrations are elevated during sleep deprivation, this hormonedoes not appear to be the mechanism underlying the effects on neuroinflammation, at least not by acting through RU486-sensitive glucocorticoid receptors. Treatment with RU486 during the sleep deprivation period did not abrogate the effects of sleep deprivation on proinflammatory cytokine signaling at the protein or mRNA levels. In the spleen, TNFα protein was induced by LPS atreatment (F1,31=34.68, p<0.00001; see Fig 6a) and suppressed by prior sleep deprivation (F1,31=6.65, p<0.05), an effect largely mediated by a reduction in TNFα protein concentrations in sleep deprived mice treated with LPS (F1,31=6.08, p<0.05). Similarly, TNFα gene expression in the CNS was elevated in LPS treated animals (F1,26=12.075, p<0.01; see Fig 6b). There was not an overall decrease in TNFα in sleep-deprived mice (F1,26=2.94 p>0.05) or an interaction between the two variables (F1,26=2.32, p>0.05). Planned comparisons indicated a trend towards reduced TNF-α in LPS treated mice that had been previously sleep deprived (p=0.10). Interestingly, the pattern of circulating corticosterone concentrations was significantly altered by treating mice with RU486 during the sleep deprivation period. Circulating corticosterone was significantly elevated by LPS treatment (F1,28=28.19, p<0.0001; see Fig 6c), but there was no overall sleep deprivation effect (F1,29=2.92, p=0.1). Mice that had been sleep deprived and treated with mifepristone, had higher circulating corticosterone than did saline-treated mice that had not been sleep- deprived, yielding a significant interaction (F1,28=11.10, p<0.01)
Figure 6. Glucocorticoid receptor antagonism during sleep deprivation does not alter ASD-induced derangement of central and peripheral inflammatory responses.
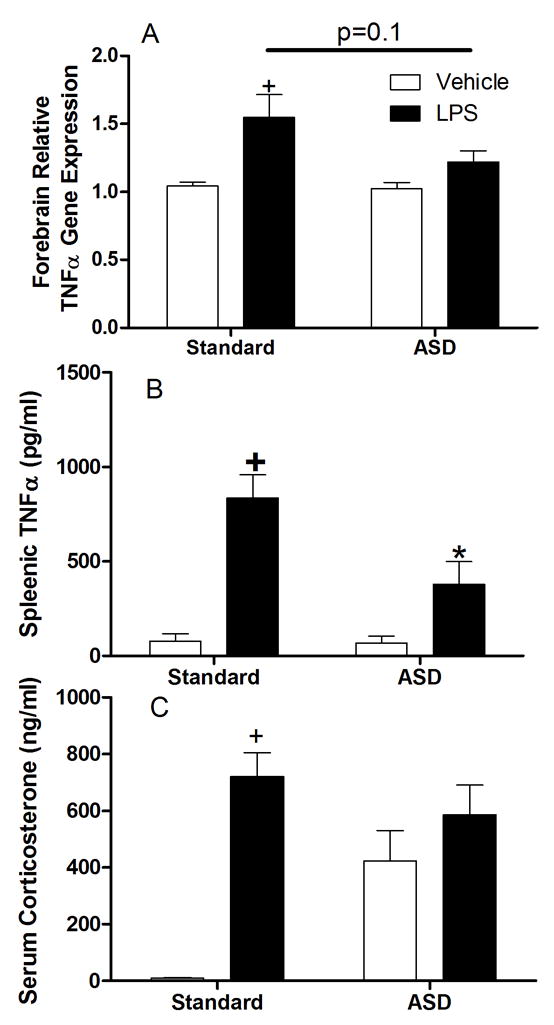
Mice treated with RU-486 every 12 hours during the sleep deprivation period and then injected with either LPS (400μg/kg) or vehicle and tissue collected A) forebrain tumor necrosis factor α gene expression, B) splenic tumor necrosis factor α protein concentrations measured by ELISA and C) circulating corticosterone concentrations. N=5–6/group.
Discussion
Acute sleep deprivation alters inflammatory responses in both the periphery and central nervous system and provides significant protection from ischemic cell death. Together the ischemia and LPS studies indicate that acute sleep deprivation increases the expression of IL-10 mRNA in the brain, while sleep deprivation increases IL-6 mRNA expression only after cerebral ischemia. Sleep deprivation also was associated with a concomitant reduction in pro-inflammatory cytokine expression in the hippocampus after both ischemia and LPS treatment.
The neuroprotective effects of acute sleep deprivation, although contrary to our hypothesis, are in agreement with previous reports of neuroprotection and attenuation of microglial responses in rats subjected to the four vessel occlusion model of global ischemia (Chee and Choo, 2004, Hsu, et al., 2003). Elevated serum corticosterone has been identified as the mechanism underlying suppressed neurogenesis in sleep deprived rats (Mirescu, et al., 2006) and given the influence of corticosteroids on cognitive processes and affective behaviors, it is possible that cortisol may underlie some of the deficits identified in sleep deprived humans as well.
The possibility exists that 48-h of sleep deprivation served as an ischemic preconditioning stimulus. Preconditioning is the phenomenon whereby small ischemic events (or a variety of mildly noxious non-ischemic stimuli) can abrogate damage from more severe subsequent attacks (Nawashiro, et al., 1997, Stagliano, et al., 1999) a process similar to the toxicological phenomenon hormesis, wherein small quantities of a toxin can have beneficial effects (Arumugam, et al., 2006). In ischemia, preconditioning fundamentally alters the responses of specific tissues to subsequent injury and in effect produces a state of relative ischemic tolerance (Dhodda, et al., 2004, Gidday, 2006). Ischemic preconditioning has been reported after treatment with a variety of stimuli including neurotransmitters, oxidative agents, and components of the inflammatory response (Pera, et al., 2004). Importantly, many of the preconditioning effects require inflammatory responses (Tang, et al., 2006). Our data suggest that using sleep deprivation as a preconditioning stimulus could provide neuroprotection during medical procedures associated with a risk of ischemia-reperfusion injury, such as cardiac surgery (Barber, et al., 2008).
Together, this study provides important evidence that acute sleep deprivation can reduce susceptibility to cerebral ischemia damage, and central and peripheral inflammatory responses to a bacterial inflammogen. These data suggest that further research should be conducted to determine whether acute sleep deprivation can improve outcome from planned surgical procedures that sometimes result in ischemia or other forms of central or peripheral inflammation. Another possibility that deserves consideration is whether intermittent sleep deprivation could have a beneficial effect for individuals with chronic inflammatory diseases.
Supplementary Material
Acknowledgments
This research was supported by NSF grant IOS 04-16897 (RJN), NIH grants MH57535 (RJN), NS40267, and HL080249 (ACD), The American Heart Association (EIA award to ACD) and The Ohio State University Presidential Fellowship (ZMW). Additional support was provided by NIH P30NS045758. The authors thank Sarah Bhagat and Ilia Karatsoreos for helpful comments and discussion of the data and manuscript. Figure Legends.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arumugam TV, Gleichmann M, Tang SC, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Research Reviews. 2006;5:165–178. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427–1433. doi: 10.1161/STROKEAHA.107.502989. [DOI] [PubMed] [Google Scholar]
- 3.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 4.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. Journal of Neuroscience. 2004;24:4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeVries AC, Craft TK, Glasper ER, Neigh GN, Alexander JK. 2006 Curt P. Richter award winner: Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. Journal of Neurochemistry. 2004;89:73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- 7.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nature Reviews: Neuroscience. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 8.Hsu JC, Lee YS, Chang CN, Ling EA, Lan CT. Sleep deprivation prior to transient global cerebral ischemia attenuates glial reaction in the rat hippocampal formation. Brain Research. 2003;984:170–181. doi: 10.1016/s0006-8993(03)03128-7. [DOI] [PubMed] [Google Scholar]
- 9.Katz DA, McHorney CA. Clinical Correlates of Insomnia in Patients With Chronic Illness. Am Med Assoc. 1998:1099–1107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 10.Leung RST, Douglas Bradley T. Sleep Apnea and Cardiovascular Disease. Am Thoracic Soc. 2001:2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 11.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature Medicine. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 12.Lorton D, Lubahn CL, Estus C, Millar BA, Carter JL, Wood CA, Bellinger DL. Bidirectional Communication between the Brain and the Immune System: Implications for Physiological Sleep and Disorders with Disrupted Sleep. Neuroimmunomodulation. 2006;13:357–374. doi: 10.1159/000104864. [DOI] [PubMed] [Google Scholar]
- 13.Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci U S A. 2006;103:19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navara KJ, Nelson RJ. The dark side of light at night: physiological, epidemiological, and ecological consequences. Journal of Pineal Research. 2007;43:215–224. doi: 10.1111/j.1600-079X.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- 15.Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. Journal of Cerebral Blood Flow & Metabolism. 1997;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Neigh GN, Kofler J, Meyers JL, Bergdall V, La Perle KM, Traystman RJ, DeVries AC. Cardiac arrest/cardiopulmonary resuscitation increases anxiety-like behavior and decreases social interaction. Journal of Cerebral Blood Flow & Metabolism. 2004;24:372–382. doi: 10.1097/01.WCB.0000112323.75217.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunes JGP, Tufik S. Validation of the modified multiple platform method, MPM, of paradoxical sleep deprivation in rats. Sleep Research. 1994;23:419. [Google Scholar]
- 18.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Medicine Reviews. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 19.Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 20.Pera J, Zawadzka M, Kaminska B, Szczudlik A. Influence of chemical and ischemic preconditioning on cytokine expression after focal brain ischemia. J Neurosci Res. 2004;78:132–140. doi: 10.1002/jnr.20232. [DOI] [PubMed] [Google Scholar]
- 21.Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001;358:999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 22.Silva RH, Abílio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, Medrano WA, Calzavara MB, Registro S, Andersen ML. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46:895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Stagliano NE, Perez-Pinzon MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. Journal of Cerebral Blood Flow & Metabolism. 1999;19:757–761. doi: 10.1097/00004647-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, Pacary E, Fréret T, Divoux D, Petit E, Schumann-Bard P, Bernaudin M. Effect of hypoxic preconditioning on brain genomic response before and following ischemia in the adult mouse: Identification of potential neuroprotective candidates for stroke. Neurobiology of Disease. 2006;21:18–28. doi: 10.1016/j.nbd.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Toth LA. Sleep, sleep deprivation and infectious disease: Studies in animals. Advances in Neuroimmunology. 1995;5:79–92. doi: 10.1016/0960-5428(94)00045-p. [DOI] [PubMed] [Google Scholar]
- 26.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Archives of Internal Medicine. 1994;154:1705–1711. [PubMed] [Google Scholar]
- 27.Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Current Problems in Cardiology. 2005;30:625–662. doi: 10.1016/j.cpcardiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.