Abstract
We have designed a device for long-term head fixation for use in behaving nonhuman primates that is robust yet minimally invasive and simple to use. This device is a modified version of the halo system that is used in humans for cervical traction and stabilization after spinal column injuries. This device consists of an aluminum halo with four titanium skull pins offset from the halo by aluminum posts. The titanium pins insert onto small segments of cranially reinforcing titanium plate, which are attached to the skull with titanium cortex screws. The surgery involves four scalp incisions, placement of the reinforcing plates, insertion of the pins for attachment of the halo, and incision closure. After the halo is attached, the animal’s head can be fixed to a primate chair using a custom-built attachment arm that provides three degrees of adjustability for proper positioning during behavioral tasks. We have installed this device on two Macaque monkeys weighing seven and ten kilograms. The halos have been in place on these animals for up to eight months without signs of discomfort or loss of fixation. Using this method of head fixation, we have been able to track the animals’ eye positions with an accuracy of less than two visual degrees while they perform behavioral tasks.
Introduction
Experiments in behaving nonhuman primates often require head fixation for accurate tracking of eye movements and stable neural recordings (Robinson, 1963; Evarts, 1968; Mountcastle et al., 1975). Many methods of head fixation have been developed, each with a particular set of advantages and disadvantages. Evarts (1968) and Mountcastle et al. (1975) were among the first to use head fixation in nonhuman primates to improve the outcome of behavioral experiments. Mountcastle et al. (1975) used a device that involved the placement of stainless steel bolts into the frontal and parietal regions of the animal’s skull. These bolts served as fixation points for an acrylic head cap that attached to a primate chair to provide immobilization of the animal’s head. This design has been extensively used for chronic head fixation in behaving primates, but is associated with several drawbacks. Attachment of this device requires a large incision to be made sagitally across the superior portion of the scalp and displacement of the skin in this area to make room for the acrylic cap. The invasiveness of this surgery can lead to chronic infections and prolonged discomfort for the animal. In addition, extended use of this device leads to bone necrosis around the implanted bolts and eventual failure. A failed head cap is traumatic to the animal, requires immediate surgical intervention, and results in a long-term interruption of behavioral experiments. Foeller et al. (2002) used this method of head fixation on 20 animals and observed an average time to failure of 11 months (range 5 months to 2 years) over the course of experimentation. Betelak et al. (2001) made improvements to this original design by using titanium endosseus implants for fixation, instead of stainless steel bolts, and allowing osteointegration to occur before the final attachment of the acrylic head cap. This method resulted in a more stable interface with the animal’s skull that nearly eliminated the occurrence of failure. The attachment procedure, however, took up to 6 months to complete and required the use of specialized dental equipment, making the overall implementation of this device more difficult.
Several researchers have proposed design alternatives to the acrylic head cap. These newer methods of head fixation have been created to address some of the problems that are associated with the original design, such as reducing the complexity and invasiveness of the attachment surgery. These methods, however, introduce new problems like device instability and device complexity. Pigarev et al. (1997) built a device consisting of a multifaceted aluminum frame that surrounds the head of the animal and holds 8 opposing posts. The posts are attached to the frame using dental cement and make contact with the skull surface through skin incisions. The posts, however, are not anchored directly to the bone. This device provided a stable interface for up to 2 years, and allowed for adjustability during growth. It was tested and used in a smaller species of Macaque monkey (Macaca fascicularis), but was never tested on larger monkeys like the Macaca mulatta. Since this device does not make a rigid connection with the bone, problems of strength and stability might arise when used with larger monkeys. Also, this device is composed of many angles and connections, making it more complex to build and implement.
In the early 1970’s, Friendlich (1973) developed a design for head fixation that was modeled after a system used in human orthopedics for cervical stabilization after spinal column injury (Vieweg et al., 2001). Later, Isoda et al. (2005) implemented a similar design with some minor modifications. Their design consisted of an aluminum ring or halo, four aluminum posts that attached to the halo at four fixed locations, and four stainless steel skull pins. The skull pins were threaded into the ends of each post, and the tips of the pins were held by pressure against the surface of the skull. This design was tested on two Macaca fuscata weighing approximately 5 kilograms and provided a stable interface for up to 18 months. Both Friendlich (1973) and Isoda et al. (2005), however, discovered problems with loosening over time due to bone necrosis at the skull/pin interface. Friendlich (1973) found that maintaining a stable attachment required regular tightening of the skull pins and eventual relocation of these pins to different areas of the skull.
We began the development of our head fixation device by replicating the design proposed by Isoda et al. (2005). We encountered similar problems with bone necrosis and loosening over time. As a result, we made several modifications to improve stability and minimize loosening. Our final design requires a minimally invasive surgery for attachment, is simple to construct, and has been stable for use in experiments lasting several months in larger Macaque monkeys. Since more mature and experienced monkeys are typically larger, this device will be useful for experiments that implement complex behavioral tasks requiring years of training. Because this device is minimally invasive, it is anticipated that the long-term risk of infection will be reduced. We have added several modifications to the design by Isoda et al. (2005). We have changed the main halo frame to include multiple holes for adjustability during the placement and alignment of the posts and skull pins. We have modified the posts to include a tab that adds a second point of attachment to the frame for increased strength against torsional forces. We have added reinforcing titanium plates, which attach to the skull with titanium cortex screws and strengthen the skull against penetrating and translational forces. By distributing the force of each skull pin over a larger surface area, the plates should minimize bone necrosis and erosion at the skull/pin interface, allowing for a more stable and long-term connection.
Materials and Methods
Overview
This device consists of an aluminum halo frame, four titanium skull pins offset from the halo by aluminum posts, and four pieces of cranially reinforcing titanium plate with titanium bone screws. The halo serves as a rigid support for the attachment of the posts and skull pins. The posts serve as an offset for the attachment of the skull pins to the frame, allowing the frame to rest above the ears and supraorbital ridge of the animal and out of its visual field. The reinforcing titanium plates and screws are attached to the surface of the skull at each pin location to add stability against translational and penetrating forces.
Halo
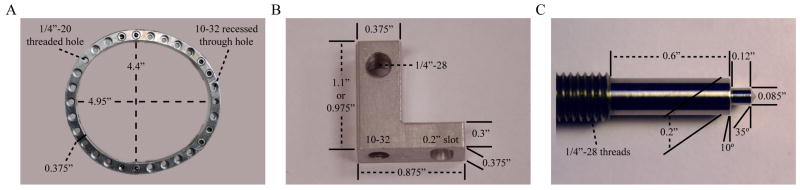
To build the halo frame, a three-dimensional model was created using AutoCAD software. This model was then used to cut the frame from 3/8 inch 6061 aluminum alloy plate using a computer numerical control (CNC) mill (Haas Automation, Inc., Oxnard, CA). The frame has a thickness of 3/8 inch, and the inner dimensions of the frame measure 4.95 inches/4.40 inches (major axis/minor axis). The frame was designed with 30 equally spaced holes at 12-degree increments around its perimeter. Twenty-seven of these 30 holes were made to accept #10–32 recessed machine bolts to allow for variability in post and pin placement. The remaining three holes were given standard 1/4″-20 threads to serve as attachment points to the attachment arm on the primate chair (Figure 1A).
Figure 1.
Device components with dimensions. A) Halo frame; B) Offset post; C) Modified titanium skull pin.
Offset Posts
Each post was manually cut from 3/8 inch 6061 aluminum alloy plate using a standard milling machine (Clausing Industrial, Inc., Kalamazoo, MI). At the base of each post, a tab was made that bolts to the halo frame to prevent rotation about its axis during pin insertion and tightening. At the end of the tab, a 0.2 inch diameter slot was created to allow for a small amount of rotation when aligning the skull pins with the skull surface. A 1/4″-28 tapped hole was placed at the end of each post to accept the corresponding skull pin. Each post has a thickness of 3/8 inch and comes in a variety of lengths ranging from 0.85 to 1.1 inches (Figure 1B).
Skull Pins
The 1/4″-28 × 2.5 inch long titanium skull pins were purchased from PMT Corporation (Chanhassen, MN), and the tips were modified on a metal lathe to provide a more stable interface with the skull and titanium reinforcement plates (Figure 1C). The small diameter portion of the tip matches the diameter of the holes in the reinforcement plates. The large diameter portion of the tip is made smooth to minimize the risk of infection at the device/skin interface.
Reinforcement Plates
Titanium MatrixMIDFACE adaption plates (0.8 mm thickness) and 1.5 × 4 mm long titanium self-tapping cortex screws were purchased from a medical device company (Synthes, Inc., Monument, CO). These plates and attachment screws were used to hold the tip of the skull pin to the skull surface and to reinforce the bone against penetrating and translational forces. The plates were cut to a length of 3 or 4 holes (~13–18 mm) during the surgery and attached by pre-drilling holes into the outer cortex of the skull and inserting the cortical screws at both ends of the plate. Holes were drilled using a Synthes 1.1 × 4 mm long drill bit.
Primate Chair
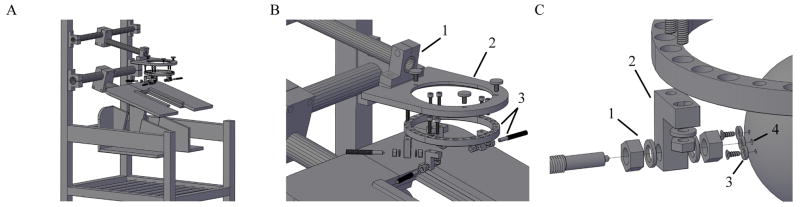
To complete the head fixation system, a Macaque chair (Primate Products, Inc., Woodside, CA) was modified to create an attachment arm to provide a connection between the chair and the halo frame during behavioral tasks (Figure 2). This attachment arm was created from pipes and connectors that were purchased from a modular framing company (80/20, Inc., Columbia City, IN). An attachment plate, which is bolted to this arm, was cut from 3/8 inch 6061 aluminum alloy plate using a CNC mill and was made to include a groove to match and accept the outer contour of the halo frame.
Figure 2.
A) Overall view of primate chair with halo device. B) Exploded view of the attachment arm (1), attachment plate (2), and halo frame with posts and skull pins (3). C) Enlarged view showing insertion path of skull pin. The pin passes through stainless steel nuts and washers (1), an aluminum offset post (2), a titanium plate that is attached to the skull with titanium cortex screws (3), and into a predrilled hole in the skull surface (4).
Device Alignment and Set-up
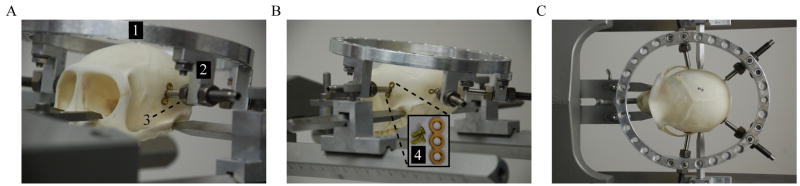
Before the surgical attachment procedure, the device is assembled and placed on a model Macaque skull to ensure the proper positioning of each skull pin. Each post is attached to the halo with two #10–32 machine bolts and rotated to provide a perpendicular interface between the pin and skull surface. The posts and pins are arranged so that the halo is centered above the skull (Figure 3C) and each pin tip contacts the correct skull location. The front pins should ideally be located on the temporal bone slightly anterior to the auditory canal and superior enough to minimize contact of the halo with the animal’s ears (Figure 3A). These pins should be placed close to the anterior portion of the ear because the bone in this area is thicker. The back pins should be placed slightly superior to the superior nuchal line on the occipital bone (Figure 3B). As much as possible, all pins should contact the skull at a perpendicular angle and be rotated to provide counter-balanced forces during tightening. Holes 8 and 13 for the front and the back pins, respectively (counting from the most anterior hole in a clockwise or counterclockwise direction), were found to provide a centered fit of the halo and correct positioning of each pin tip at the skull surface.
Figure 3.
Fixation device positioned on Macaque skull. A) Anterior view of device showing halo frame (1), offset post (2), and skull pin (3). The skull pin is placed in temporal bone superior and slightly anterior to the auditory canal. B) Posterior view of device showing post with skull pin and an enlarged image of the titanium reinforcement plate with cortex screws (4). The posterior pin is placed slightly superior to the superior nuchal line. C) Superior view of device showing pin position and angle with respect to cranial suture lines.
Attachment Procedure
All procedures were carried out following an IACUC approved protocol. The device is attached with the animal under general anesthesia using a sterile technique. To begin the procedure, anesthesia is induced with an injection of ketamine 10 mg/kg intramuscularly while the animal is in the primate chair. The animal is then carried to the surgery table, intubated, and placed under general anesthesia using 2–5% isoflurane, a tidal volume of 150 mL, and a respiratory rate of 10. Vitals are monitored continuously during the procedure to maintain an adequate heart rate, temperature, and oxygen saturation.
The animal is then placed in a stereotaxic frame. Stabilization bars are attached to the eye sockets and upper jaw to prevent rotation of the head around the ear bars during surgery. Ophthalmological salve is placed in the eyes to prevent drying, and intravenous fluids are initiated to maintain hydration. A prophylactic injection of the antibiotic enrofloxacin (5mg/kg i.m.) is given at this time. Clippers are used to trim the hair around the four pin sites, and the animal and the stereotaxic frame are prepped and draped in a sterile manner.
Before any incisions are made, the device is positioned on the animal’s head in the desired location. Each pin is hand tightened to mark the skin locations where incisions are to be made. The pins are then loosened, and the device is removed. A scalpel is used to make incisions in the skin at the marked locations. Blunt dissection is performed through subcutaneous tissue and muscle to expose the skull surface. A periosteal elevator is used to expose and clean enough bone surface to ensure proper positioning and attachment of the reinforcement plates.
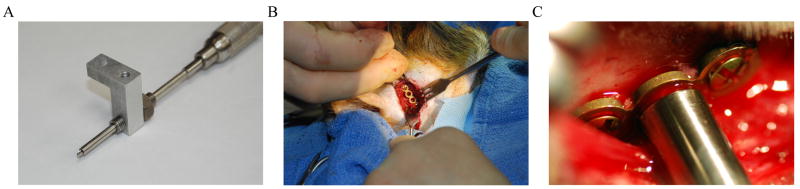
The device is repositioned on the animal’s head so that the halo rests out of the animal’s field of view and above the ears. Each pin location is verified to ensure proper positioning. The pins are hand tightened onto the skull surface to temporarily keep the device in place. Once the device is properly positioned, one pin is removed and replaced with the threaded drill guide. The drill guide is used to ensure correct alignment and stability during drilling. It also acts as a stop to provide safety by limiting drilling depth (Figure 4A). A 2 mm drill bit is used to drill a hole 3 mm deep into the skull surface where the tip of the skull pin will contact the surface of the skull. The drill and drill guide are then removed. A piece of titanium plate is cut to a length of 3 or 4 holes. The plate is bent to fit the curvature of the skull surface. The skull pin is then threaded through the offset post with nuts and lock washers on both sides. The pin is threaded so that the tip passes through the middle hole in the reinforcement plate and into the drilled hole in the surface of the skull. The pin is tightened by hand. With the plate now held in place by the pin, it is rotated about its axis to best fit the curvature of the skull surface and also to allow room for placement of the titanium screws. Pilot holes are drilled in the bone underlying the remaining holes in the plate using the Synthes 1.1 × 4 mm long drill bit. The two 4 mm long self-tapping titanium screws are placed into these holes to complete the attachment of the plate to the skull (Figure 4B,C). This process is then repeated for each of the remaining pin sites.
Figure 4.
A) Drill guide used to align the drill bit during the attachment procedure. B) Reinforcement plate for the temporal pin with titanium cortex screws at each end. C) Reinforcement plate for the occipital pin with the pin threaded through the plate and into the predrilled hole in the skull surface. The predrilled hole was made using the drill guide.
After all four plates and skull pins have been placed, the pins are tightened in an alternating pattern by hand using an Allen wrench. Prior to the addition of the titanium reinforcement plates in this procedure, we found that an applied torque of 2 inch-pounds, as measured using a torque screwdriver, was sufficient to cause fracturing of the underlying bone. Care must be taken to avoid over tightening. Each lock nut is then tightened on both sides of the pin to prevent loosening. Wounds are rinsed with sterile saline, infused with antibiotics, and closed using a nonabsorbable monofilament suture. The animal’s hair and skin are cleansed to remove the povidone-iodine solution, and neomycin ointment is applied to the wounds. Anesthesia is discontinued, and the animal is extubated. Postsurgical injections of ketoprofen (2 mg/kg i.m.) and buprenorphine (0.01 mg/kg i.m.) are administered at this time. Postoperative medications include enrofloxacin (5 mg/kg i.m. daily for 5 days), ketoprofen (2 mg/kg i.m. daily for 5 days), and buprenorphine (0.02 mg/kg i.m. twice a day for 2 days). Additionally, for the duration of the device attachment, pin sites are cleaned at least once a week with cotton swabs soaked in hydrogen peroxide.
Results
We have installed this device on two Macaque monkeys weighing 7 and 10 kilograms. Previously trained behavioral tasks were resumed three days after surgery. Tasks requiring eye tracking and head fixation were started two weeks after surgery. These animals have shown no signs of discomfort or pain while in their cages or during training. Shortly after attachment, these animals learned to include the device in the scheme of their body and were rarely found to catch the protruding edges on their cages or the primate chair. Because this device was designed to be lightweight (~0.5 pounds), it does not appear to be cumbersome to the animals or inhibit normal movement in their cages. Our animals are pair-housed and have access to an activity module to provide an enriched social environment. This device does not appear to alter the social or behavioral interactions of these monkeys with the other monkeys in the cage. The wounds at each pin site have healed with minimal scarring and show little or no edema or erythema (Figure 5A). One animal has been performing behavioral tasks requiring head fixation for 8 months and has experienced only a superficial infection at a temporal pin site. This infection was treated with daily cleansing using a combination of chlorhexidine solution and hydrogen peroxide. The other animal has been performing behavioral tasks for 5 months with no signs of infection or complications. The device has remained stable on both animals since the initial attachment procedure, and the skull pins have not required tightening during this time.
Figure 5.
A) Enlarged image of the right occipital pin site 5 months after surgery. B) Head-fixed animal performing a behavioral task requiring eye fixation. Eye positions were sampled at 1 kHz using the EyeLink 1000 infrared eye tracking system (SR Research, Ltd., Ontario, Canada). C) Graph of recorded eye positions in units of visual degrees. For each trial, the animal was required to fixate within an area of diameter 2.6° (1) while a stimulus (2) was presented and a response was acquired. If the animal looked outside of the defined area, the trial was aborted and no reward was received. A total of 118 trials were completed, each requiring a fixation duration of 1250 msec. Eye position data is plotted in red (3). Fixation positions were randomly alternated among five locations [(0,0); (5,0); (0,5); (−5,0); (0,−5)] during the task and are represented by the five clusters of eye position data. The central cluster of data follows a gaussian distribution with 2σ ≈ 0.8°.
We have tracked the eyes of each animal with an accuracy of less than 2 visual degrees during a behavioral task involving threshold detection of photic stimuli. Experiments were conducted in a dark chamber with a background luminance below 0.0001 cd/m2. The animal was dark adapted and then trained to place its hands on proximity sensors and to fixate within a central region (2.6° in diameter) of a CRT monitor. Fixation positions were randomly alternated among five locations during the progression of the task. A gaussian-shaped stimulus (1° in diameter) was then displayed on the screen for 500 msec at a fixed location with varying intensities. Following an auditory cue, the animal would indicate if it saw the stimulus by raising either its right or left hand (Figure 5B). Catch trials were inserted at random intervals, and the monkey was rewarded with a squirt of juice from a tube by answering correctly. Eye positions were sampled at 1 kHz using the EyeLink 1000 infrared eye tracking system (SR Research, Ltd., Ontario, Canada) and plotted in Figures 5C and 6 to demonstrate the accuracy and overall stability of the device.
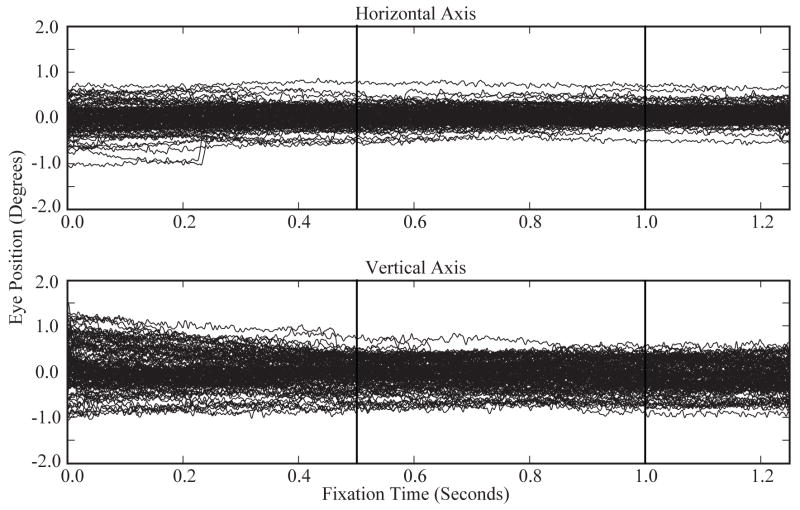
Figure 6.
Eye position data recorded at 1 kHz using the Cerebus data acquisition system (Blackrock Microsystems, Salt Lake City, UT). Plots show superimposed eye positions along the horizontal and vertical axis for 118 trials of the behavioral task described in Figure 5. Stimulus onset and offset are indicated by vertical lines. Individual tracings reveal small noise fluctuations on the order of minutes of visual angle. The smoothness of the tracings demonstrate the stability of this head fixation system while tracking eye movements with the EyeLink 1000 infrared eye tracking system (SR Research, Ltd., Ontario, Canada).
Discussion
This method of head fixation for nonhuman primate research offers a simple design that is easy to implement and less invasive than other methods of head fixation. This system is also very stable and appears to avoid the loosening and bone necrosis issues that have been experienced with other similar designs. The open structure of this device would allow room for recording chambers and external connectors with placements over many areas of the skull. The addition of reinforcing titanium plates to this design allows for easy removal for imaging purposes and later reattachment at the same location. Since these plates and the corresponding cortex screws are permanently attached to the skull, however, they may cause small distortion artifacts and be problematic for some studies. If imaging is required for a study, the reinforcement plates can be placed at specific locations to minimize distortion over the area of interest. If head fixation is necessary during the imaging process, this device can be constructed of magnetic resonant compatible materials. The halo frame and posts can easily be machined from high performance plastics like polyetherimide, and the titanium skull pins can be replaced with their ceramic counterparts (Dubowitz et al., 2001; Pinsk et al., 2005). This device performed well for behavioral tasks that require accurate tracking of the eyes. We expect that it would also provide sufficient stability to perform neural recordings from isolated neurons using single or multiple electrodes.
Acknowledgments
This work was funded by TATRC W81XWH-06-1-0497 and NIH R01EY019363-01 grants. The authors wish to thank the CMC veterinary staff, the University of Utah Physics Machine Shop, and Synthes, Inc. for their technical support.
Footnotes
Contributing authors: Kian Torab, B.S., Ph.D. Graduate Student, Department of Bioengineering, University of Utah
Paul House, M.D., Assistant Professor, Department of Neurosurgery, School of Medicine, University of Utah
Bradley Greger, Ph.D., Assistant Professor, Department of Bioengineering, University of Utah
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Betelak KF, Margiotti EA, et al. The use of titanium implants and prosthodontic techniques in the preparation of non-human primates for long-term neuronal recording studies. J Neurosci Methods. 2001;112(1):9–20. doi: 10.1016/s0165-0270(01)00442-3. [DOI] [PubMed] [Google Scholar]
- Dubowitz DJ, Chen DY, et al. Direct comparison of visual cortex activation in human and non-human primates using functional magnetic resonance imaging. J Neurosci Methods. 2001;107(1–2):71–80. doi: 10.1016/s0165-0270(01)00353-3. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968;31(1):14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Foeller P, Tychsen L. Eye movement training and recording in alert macaque monkeys: 1. Operant visual conditioning; 2. Magnetic search coil and head restraint surgical implantation; 3. Calibration and recording. Strabismus. 2002;10(1):5–22. doi: 10.1076/stra.10.1.5.8154. [DOI] [PubMed] [Google Scholar]
- Friendlich AR. Primate head restrainer using a nonsurgical technique. J Appl Physiol. 1973;35(6):934–5. doi: 10.1152/jappl.1973.35.6.934. [DOI] [PubMed] [Google Scholar]
- Isoda M, Tsutsui K, et al. Design of a head fixation device for experiments in behaving monkeys. J Neurosci Methods. 2005;141(2):277–82. doi: 10.1016/j.jneumeth.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, et al. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol. 1975;38(4):871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Pigarev IN, Nothdurft HC, et al. A reversible system for chronic recordings in macaque monkeys. J Neurosci Methods. 1997;77(2):157–62. doi: 10.1016/s0165-0270(97)00124-6. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Moore T, et al. Methods for functional magnetic resonance imaging in normal and lesioned behaving monkeys. J Neurosci Methods. 2005;143(2):179–95. doi: 10.1016/j.jneumeth.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A Method of Measuring Eye Movement Using a Scleral Search Coil in a Magnetic Field. IEEE Trans Biomed Eng. 1963;10:137–45. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Vieweg U, Schultheiss R. A review of halo vest treatment of upper cervical spine injuries. Arch Orthop Trauma Surg. 2001;121(1–2):50–5. doi: 10.1007/s004020000182. [DOI] [PubMed] [Google Scholar]