Abstract
We review the cellular mechanisms implicated in cholesterol trafficking and distribution. Recent studies have provided new information about the distribution of sterols within cells, including analysis of its transbilayer distribution. The cholesterol interaction with other lipids and its engagement in various trafficking processes will determine its proper level in a specific membrane; making the cholesterol distribution uneven among the various intracellular organelles. The cholesterol content is important since cholesterol plays an essential role in membranes by controlling their physicochemical properties as well as key cellular events such as signal transduction and protein trafficking. Cholesterol movement between cellular organelles is highly dynamic, and can be achieved by vesicular and non-vesicular processes. Various studies have analyzed the proteins that play a significant role in these processes, giving us new information about the relative importance of these two trafficking pathways in cholesterol transport. Although still poorly characterized in many trafficking routes, several potential sterol transport proteins have been described in detail; as a result, molecular mechanisms for sterol transport among membranes start to be appreciated.
Keywords: cholesterol, lipids, membrane, bilayer, transport
Introduction
Understanding intracellular sterol dynamics is very important because the proper abundance of sterol in the plasma membrane (PM) and organelle membranes is critical for many cellular functions. Sterol is carried between membrane organelles as a component of lipid bilayers in transport vesicles, and it is also moved between membranes by non-vesicular processes using poorly characterized mechanisms involving carrier proteins. The overall rates of sterol transport among organelles can be very rapid (i.e., re-equilibration between two organelles within a few minutes).
Among the major lipids found in membranes of eukaryotic cells, sterols have the most atypical chemistry, containing a single hydroxyl as the only polar component, a nearly planar assembly of four rings, and a short alkyl chain [1]. This structure contrasts with most glycerophospholipids and sphingolipids, with their large polar headgroups and long hydrocarbon tails. These molecular characteristics give sterols an influential role in the physicochemical properties of the membrane as well as the ability to move rapidly between the two membrane leaflets (flip-flop). Compared to other lipids, sterols have a lower free energy barrier to escape from the lipid bilayer [2]. Sterols, like other lipids, can be shuttled by soluble carrier proteins from one membrane to another, and this can allow rapid transport among the membranes in a cell. These transport properties may allow sterols to approach a state of chemical equilibrium among some cellular membranes (i.e., the chemical activity of cholesterol, the thermodynamic measure of availability for a chemical or physical transition, may be nearly equal among these cellular membranes). Nevertheless, the concentration of cholesterol could still vary greatly among these membranes as a consequence of the relative stabilization of sterol in the various membranes by other constituents. That is, the chemical activity coefficient of cholesterol may be lowered by favorable interactions. (a = γc ; where a is chemical activity, c is concentration, and γ is the chemical activity coefficient. At equilibrium, the chemical activity of cholesterol in various membranes would be equal, but if the membranes had different activity coefficients, the concentrations could be unequal.)
In studies of model membrane systems, the biophysical basis for the relative stabilization of sterols in various membranes, based on sterol-lipid interactions, has been described using various models, including the “umbrella model” [3] that underlines the necessity for a sterol molecule to be protected from the water by other lipids for its stabilization, and the “condensed complex model” [4], which describes the formation of stoichiometric complexes of low free-energy between cholesterol and lipids. Recent studies in cells have provided new information about the distribution of sterols within cells, including analysis of its transbilayer distribution. At the same time, genetic and biochemical studies have analyzed the proteins that play an important role in sterol transport, and structural studies of sterol transport proteins are beginning to demonstrate the molecular mechanisms for sterol transport among membranes.
In this review, we will first focus on recent work on cholesterol-lipids interactions and try to reconcile these studies with latest findings in cellular sterol distribution. New findings on sterol transbilayer distribution will be discussed as well. Then, we will focus on the sterol transport between the different organelle membranes.
Cholesterol-phospholipid interactions
Biophysical concepts and sterol chemical activity
The umbrella model [3] and the condensed complex model [4] take different approaches to analyze sterol stability in various lipid membrane environments based on interactions with neighboring phospholipids. The umbrella model is based on the amphipathic structural mismatch of the cholesterol molecule with other lipids in the bilayer: its small hydroxyl head facing the aqueous milieu only partially protects the hydrophobic ring system from water. Since this water exposure is very unfavorable, the sterol associates with neighboring phospholipids with larger polar headgroups in order to shield its hydrophobic rings from water (Figure 1). As a result, phospholipids like phosphatidylcholine (PC) or sphingomyelin (SM) bearing relatively large headgroups (~70 Å3) [5] would be preferred partners for cholesterol as compared to phosphatidylethanolamine (PE), which possesses a smaller polar head (~40 Å3). Indeed, a PC “umbrella” can shield two cholesterol molecules whereas only one cholesterol molecule can take cover under a PE headgroup [3]. In addition to the size of the headgroup, the level of acyl chain unsaturation is important for understanding sterol stabilization within the framework of the umbrella model because of effects on lipid geometry within the bilayer [6]. As depicted in Figure 1, greater unsaturation leads to a conical shape because of the relative large cross section of the acyl chains as compared to the headgroup, whereas saturated lipids tend to be more cylindrical. The headgroup/body size ratio of conical lipids, like dioleoyl-PC (DOPC), is less well suited to shield neighboring sterol molecules than dipalmitoyl-PC (DPPC), with its two saturated acyl chains [6].
Figure 1. Structural interactions between cholesterol and other lipids.
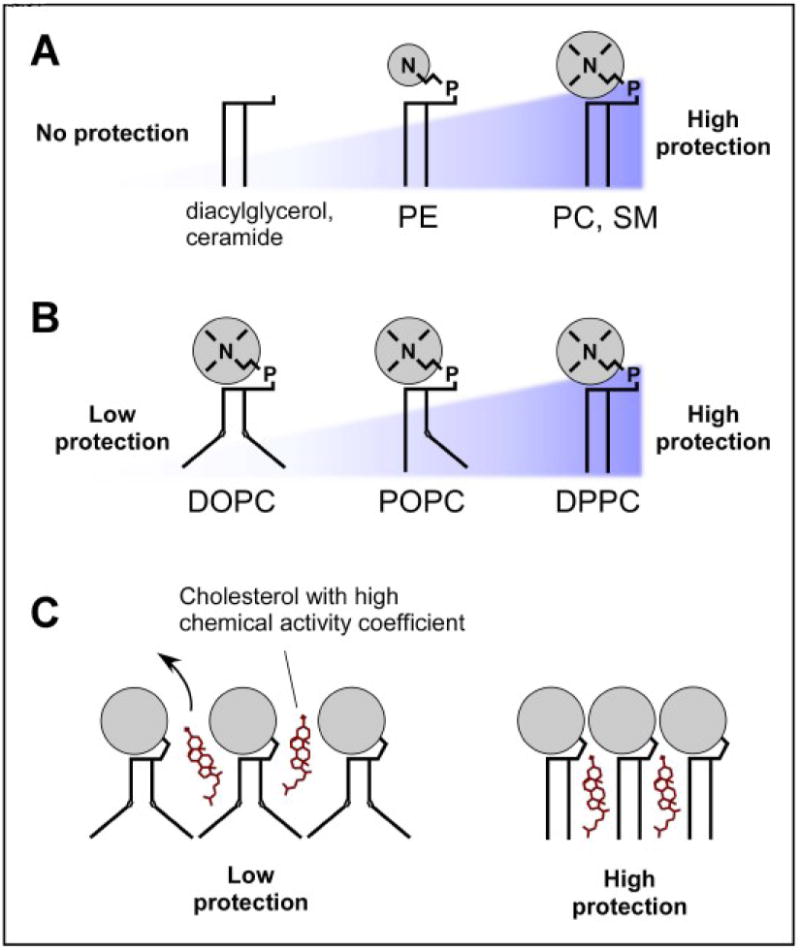
The sterol stability in a membrane depends on its interaction with neighboring lipids. A. Lipids bearing large polar head groups are preferred partners for cholesterol because they provide better protection from water. B. The level of acyl chain saturation also influences the sterol stability because it is directly related to the lipid shape. Lipids with unsaturated acyl chains containing one double bond are more bulky than lipids with saturated chains; these unsaturated lipids are less suited to afford protection from water to the neighboring cholesterol. C. Poorly protected sterols (e.g., in a DOPC-rich bilayer) have a high chemical activity coefficient; they can leave the membrane readily. In contrast, well protected sterols form with their associated lipids a structure of low chemical activity coefficient. DOPC, dioleoyl-phosphatidylcholine (PC); POPC, palmitoyl-oleoyl-PC; DPPC, dipalmitoyl-PC.
In the umbrella model, cholesterol-cholesterol interactions are unfavorable because sterol shielding of sterol clusters by adjacent phospholipids would cost much more free energy than shielding a single sterol. Unshielded or poorly shielded sterols have a higher chemical activity coefficient (i.e., a higher propensity to leave the membrane) as a consequence of the free energy penalty associated with exposure of hydrophobic surfaces to water. In the umbrella model, the sterol chemical activity coefficient is directly related to the geometric aspect (i.e., cones versus cylinders) of the neighboring lipids, so at the same sterol concentration, the sterol chemical activity is higher in a DOPC bilayer than in a DPPC bilayer [6].
The condensed complex model analyzes the effects of stoichiometric associations between cholesterol and phospholipids with long saturated acyl chains (e.g., SM or DPPC). These lipids tend to associate with sterols in a reversible manner to form compact complexes of low free energy and small molecular lateral area [7, 8]. Other “unreactive” phospholipids (e.g., DOPC) have reduced tendency to form such condensed complexes. The closer packing of cholesterols and phospholipids in these condensed structures should order the lipid acyl chains in the trans conformation [9, 10]. According to the condensed complex model, the chemical activity coefficient of sterol in a membrane depends on the membrane composition. Sterols in condensed complexes have lower chemical activity coefficients than those outside. As a result, the sterol chemical activity can increase sharply when its concentration exceed the stoichiometric capacity [4, 8].
Despite their different frameworks for analysis, these models are not necessarily mutually exclusive. Both models invoke an attractive interaction between cholesterol and saturated phospholipids. In either model the sterol chemical activity is expected to increase abruptly when the amount of sterol goes beyond the holding capacity of phospholipids in the membrane. Experimental evidence for this behavior in cells was provided by quantifying the extractability of cholesterol from the PM by extracellular cyclodextrin acceptors, or by measuring the ability of cholesterol oxidase to modify cholesterol, which both directly reflect the cholesterol chemical activity [11]. Interestingly, it was shown that a small increase in PM cholesterol content in fibroblasts leads to a large jump in the cholesterol chemical activity. A similar rise in the cholesterol activity was shown when membrane intercalating molecules with small polar heads (e.g., octanol, ceramide, diacylglyceride) were introduced in the PM of human red blood cells [12]. These agents can competitively displace cholesterol from association with phospholipids in the bilayer.
Since the phospholipid composition varies among organelles, the sterol-phospholipid saturation threshold will also vary. Consequently, this simple physicochemical effect would play a major role in the regulation of cellular cholesterol levels. In addition to phospholipids, cytosolic and membrane proteins can interact with cholesterol [13, 14], and they may also influence the cholesterol distribution. It has been proposed that some membrane proteins attract “shells” of lipids, including cholesterol, to their neighborhood [15]. Additionally, it has been reported that peripheral membrane proteins harboring clusters of basic and hydrophobic amino acids (e.g., MARCKS) can laterally sequester cholesterol when they adsorb to the PM cytosolic leaflet [16]. At present the exact contribution of those interactions in the overall cholesterol stabilization in membranes is unknown.
Do sterols interact preferentially with sphingolipids?
Preferential interaction of SM with cholesterol was originally suggested by the condensation of SM in presence of cholesterol within a lipid monolayer at the air-water interface [17]. This and other observations led to the proposal that SM and cholesterol form condensed complexes of high affinity. Evidence in support of such behavior in cells includes the observation that cholesterol efflux from a lipid monolayer to a cholesterol-binding cyclodextrin in the aqueous milieu decreases when the SM/PC ratio is increased [18]. In another experiment, reduction in the amount of SM by sphingomyelinase treatment of cells leads to release of cholesterol from the PM [19, 20] (but see discussion below). It has also been suggested that preferential van der Waals interactions occur between the hydrocarbon tail of the sphingoid base of SM and cholesterol, indicating that these interactions might promote SM-cholesterol complex formation [21-23]. Hydrogen bonding between the cholesterol OH group and the amide of the SM was also reported [24]. Taken together, these findings indicated that there might be a higher affinity of cholesterol for SM than for PCs.
However, a lack of specific interaction between cholesterol and SM has been suggested recently. NMR studies do not detect significant hydrogen bonding between SM and cholesterol [25], and a detailed fluorescence study suggests that pyrene-labeled SM and PC derivatives associate similarly with cholesterol [26]. Treatment of cells with sphingomyelinase, which creates ceramide, may have complex effects. Ceramide has a relatively high flip-flop rate in membranes [27, 28]. Like cholesterol, ceramide has a small polar headgroup, and it has been shown to compete with cholesterol for sites in ordered domains of membranes [12, 29]. Thus, sphingomyelinase treatment of cells could displace cholesterol from the cytoplasmic leaflet of the PM by increasing the ceramide content in the cytoplasmic leaflet.
Biophysical analyses do show that SM and cholesterol can form condensed complexes [30]; however, such an interaction is not unique to sphingolipids. Similar cholesterol-dependant condensations have been reported for lipids present in the inner leaflet of the PM, such as PC, PS and PE, and the condensation was found to be dependent on the lipid acyl chain chemistry [23, 31, 32]. Thus, if any preferential interaction between SM and cholesterol exists, it is quantitative rather than qualitative as compared to favorable cholesterol interactions with other lipids that have saturated acyl chains and large head groups.
Sterol distribution
Distribution of sterol among organelles
Cholesterol and phospholipids are distributed heterogeneously among the membranes of the cellular compartments (for review: [33]). In the PM of mammalian cells, cholesterol is approximately 30 mole% of the lipids [34]. There are varying estimates of the fraction of cellular cholesterol that is in the PM, but it is approximately 60% of the total cellular cholesterol [35]. In contrast, the endoplasmic reticulum (ER) has about 5 mole% cholesterol [36] and accounts for about 5% of the total cholesterol in the cell [37]. The cholesterol/phospholipid ratio in the Golgi is intermediate between the PM and the ER [38]. It has also been suggested that the cholesterol concentration increases gradually from the cis-Golgi to the trans-Golgi, based on electron microscopy of filipin-labeled cells [39]. In the endocytic pathway, cholesterol is highly enriched in the endocytic recycling compartment (ERC), which constitutes the major intracellular pool of sterol in Chinese Hamster Ovary (CHO) cells and macrophages [40, 41]. In CHO cells, the ERC holds about 35% of the total cellular cholesterol [42]. A fraction of the cholesterol endosomal pool is also thought to be carried by internal vesicles of multivesicular bodies, while the overall amount in lysosomes is low [43]. These great variations in the intracellular cholesterol distribution lead us to ask the following question: How is this heterogeneous distribution maintained in the cell?
The differences in concentration could be due to specific trafficking of cholesterol to or from various organelles either by vesicle traffic or by non-vesicular, carrier-mediated transport. These transport mechanisms will be discussed later. At least part of the uneven distribution is due to differences in the cholesterol-phospholipid interactions that result from differences in other lipid components in the various organelles. Several studies in model membranes have shown that lipids differ in their ability to stabilize cholesterol in the bilayer [44]. If cholesterol content of membranes is driven toward equilibrium by non-targeting transport processes, differences in cholesterol content would follow from the different lipid compositions of the membranes [37]. Several studies have shown that cholesterol preferentially associates with lipids containing mainly saturated hydrocarbons [6, 8]. As discussed earlier, this preference can be described in terms of detailed analysis of van der Waal’s, hydrogen bonding, and packing. In the umbrella model, the capacity of cholesterol stabilization by a membrane depends on the geometry of the neighboring phospholipids that directly results from the saturation level of their acyl chains [6]. The PM contains a much higher level of saturated and mono-unsaturated lipids than the total cell lipids, which would be dominated by the ER membranes [45]. Only 25% of the PS acyl chains are saturated in the ER, as compared to 89% in the PM [38]. These differences in acyl chain saturation, and the resulting relative changes in cholesterol stabilization, could account for a large part of the differences in cholesterol content of organelles. It should be noted that the mechanisms for maintaining these differences in phospholipid content in the presence of a large amount of membrane traffic are not well understood.
The sterol transbilayer distribution
While the lipid distribution between the luminal and the cytosolic leaflets in the ER membrane is nearly symmetrical [46], this is not the case for most other organelle membranes, including Golgi, endosomes and the PM. The PM concentrates as much as 75% of the PC species and more than 85% of the SM in its outer leaflet, while 80% of the PE and more than 96% of the PS face the cytosolic milieu [47]. Additionally, phosphoinositides are almost entirely in the inner leaflet of the PM. The asymmetric aspect of the membrane is established during lipid synthesis, but it is also maintained by transporters that promote translocation, such as energy-dependent ATP-binding cassette (ABC) transporters [48]. The asymmetric distribution of lipids has important biophysical consequences. The PM’s charge, provided primarily by the PS and phosphoinositides, is completely displayed on its cytosolic leaflet. This plays an important role in the cellular localization of several peripheral polycationic proteins [49], including several signaling molecules.
Although cholesterol is one of the most abundant lipid molecules in the PM, its transbilayer distribution has remained uncertain [50]. Several studies in model membranes have shown that favorable interactions between cholesterol and sphingolipids can exist (as discussed earlier), which might suggest that cholesterol would be preferentially in the exofacial leaflet along with sphingolipids. However, other studies in model membranes have also shown that cholesterol can have favorable interactions with glycero-phospholipid molecules that have saturated acyl chains, and the enrichment of saturated acyl chains on PC, PE and PS in the PM indicate that these lipids, which are abundant in the cytosolic leaflet could also stabilize cholesterol.
While the transbilayer distribution of some phospholipids can be determined using enzymatic modifications of the head groups [51-53] or molecules that bind to head groups, the rapid inter-leaflet flipping of cholesterol [27, 54] makes it very difficult to apply these methods for sterols. For example, analysis methods such as filipin binding, which sequesters sterol in a given leaflet [55, 56], or reaction with cholesterol oxidase [57, 58] are difficult to interpret since the sterol could flip many times during the assay, but even a transient appearance in the outer leaflet would be recorded in these assays as outer leaflet sterol.
Schroeder and coworkers examined the sterol transbilayer distribution in PM of several cell lines, including erythrocytes and fibroblasts, using dehydroergosterol (DHE), an intrinsically fluorescent sterol, and trinitrobenzenesulfonate (TNBS) a fluorescence quencher [59, 60]. The DHE serves as excellent cholesterol mimetic in both biophysical and cell biological studies, and is also naturally occurring in several yeasts [61]. TNBS was incubated with the cells at pH 8.5 for 45-80 minutes at 4°C to allow covalent crosslinking of the TNBS to glycoproteins and glycolipids on the exofacial leaflet of the PM. After cell lysis, isolated PM preparations from the different cell lines were used to measure DHE fluorescence. Schroeder et al. found that only a small fraction of DHE was quenched by the attached TNBS in fibroblast and in human erythrocyte membranes, whereas the majority of DHE was quenched in rat and mouse erythrocytes [59, 60]. While the fluorescence approach to measure the transbilayer distribution of sterol in the PM as described in these papers was a major advance, several steps in the method raise concerns. The effects of the lengthy low temperature TNBS pre-incubations on sterol distribution are not known, and it seems likely that the PM purification that was required for nucleated cells could perturb the transbilayer distribution of cholesterol. Other studies using cholestatrienol (CTL), another fluorescent sterol demonstrated to be a close analog of cholesterol, attempted to measure the sterol transbilayer distribution in human platelets [62, 63]. TNBS was added to suspensions of CTL-labeled cells, and it was reported that 65% of the CTL fluorescence was quenched. However, TNBS causes significant absorption of the excitation light used for CTL fluorescence in a cuvette, requiring a large “inner-filter” correction [64], but this correction was not discussed in these reports.
Our laboratory has developed fluorescence imaging techniques to observe the subcellular distribution of DHE and CTL in living cells [42]. This has several important advantages for studying the transbilayer distribution of sterols [64]. Because the sterol fluorescence is imaged, the incorporation and the distribution of sterol in living cells can be directly evaluated. Moreover, since the path length of illumination is very short, there is negligible absorption of light by the quencher, and this allows measurement of instantaneous quenching by molecules such as TNBS, which avoids lengthy labeling procedures.
We examined the susceptibility of DHE and CTL in CHO cells to quenching by different quenchers, such as TNBS and a spin-labeled phosphatidylcholine. We showed that sterol fluorescence in the PM is reduced only 15-30% by quenchers that have access solely to the outer leaflet of the bilayer. In contrast, fluorescence of DHE and CTL is quenched by ~60% in the PM when quenchers have access to the cytoplasmic leaflet through microinjection, and by 70-80% when both leaflets are accessible (i.e., in permeabilized cells). The transbilayer distribution of sterol in the ERC was also investigated since it is the major intracellular pool of sterol. We observed about 65% quenching of the DHE or CTL fluorescence of this organelle upon microinjection of quenchers that would only have access to the cytoplasmic leaflet. Taken together, these results indicate that sterols are mainly in the cytoplasmic leaflet of the PM and the ERC in CHO cells [64]. If we consider that cholesterol is approximately 30 mole% of the lipids in the PM, then about 45 mole% of the lipids in the cytosolic leaflets are sterol.
While association with glycero-phospholipids with saturated acyl chains is favorable, there is no evidence that association with these lipids is thermodynamically preferred to associations with sphingolipids. Thus, the asymmetric distribution of the phospholipids in the PM cannot explain the sterol preference for the inner leaflet of the PM, so other forces must play a role to maintain this sterol asymmetry. Dynamic processes such as lipid metabolism, lipid flipping, or membrane trafficking could possibly reduce the relative amount of phospholipids in the cytoplasmic leaflet, and sterols could flip spontaneously as required to compensate for this lipid deficit. Further studies will be required to understand the mechanistic basis for the asymmetric transbilayer distribution of cholesterol in the PM and in the ERC, where the lipid composition resembles that of PM.
Sterol transport
Mammalian cells acquire cholesterol by endogenous synthesis and by uptake of lipoproteins. Low-density lipoprotein (LDL)-receptor bound LDL is internalized via clathrin-coated pits and transported to late endosomes and lysosomes (LE/LY), where it is digested by enzymes, including lysosomal acid lipase, the enzyme responsible for cholesteryl ester hydrolysis. Cellular cholesterol biosynthesis occurs in the ER, which contains key metabolic enzymes such as HMG-CoA reductase [65]. These two mechanisms take place in organelles that are relatively low in cholesterol content, indicating that cholesterol escapes rapidly from these organelles to maintain cellular sterol homeostasis.
Intracellular transport of cholesterol from one organelle to another can be achieved by vesicular and non-vesicular trafficking processes [66]. At least some of these transport processes must have a high capacity; in studies with DHE, equilibration between the plasma membrane and the ERC occurred within 2-3 minutes, requiring transport of about 106 sterol molecules per second [42, 67]. The role of vesicular transport can be evaluated, in part, using chemical inhibitors or altered expression of specific proteins that are required for vesicle transport [68, 69]. Non-vesicular transport could occur, in principle, by spontaneous desorption and free diffusion of cholesterol through the cytoplasm, but the rates of cholesterol desorption from bilayers and its solubility in water are too low to support any significant amount of transport. However, there are several proteins that are in the general class of soluble lipid transfer proteins (LTPs), which have been shown to be capable of facilitating transfer of sterols between membranes. LTPs can interact directly with lipid membranes to extract sterol, and then carry the sterol to acceptor membranes, which may or may not be specifically targeted by the LTP. The LTPs could also function to shuttle sterol between closely apposed membranes, which would provide a mechanism for targeted delivery and would presumably increase the rate of transport. X-ray crystallography studies of some sterol-binding LTPs (NPC2 [70, 71], Osh4p [72], StarD4 [73], MLN64-START [74]) show that they contain a hydrophobic pocket that could bind a single sterol molecule. In several cases, these pockets have a “lid” that could be opened and closed upon interaction with the bilayer as suggested in molecular dynamics simulations of sterol entry or release from such proteins [75]. Sterol-binding LTPs can dramatically increase the rates of transport between liposomes [76]. These findings would be consistent with a role for these LTPs in sterol transport. However, in many cases the precise role of these proteins in cellular sterol transport remains uncertain, and overall the relative importance of vesicular versus non-vesicular transport processes is not established.
Trafficking between the ER and the PM
Although the PM is the largest pool of cellular sterol, the most important regulators of cholesterol levels are in the ER. Thus, it is important to have transport pathways between these two cellular compartments so that newly synthesized sterol can be delivered to the PM and so that the ER can respond to changes in the sterol level in the PM. The rates of transport between the organelles can be substantial. It has been estimated that newly made ergosterol in S. cerevisiae equilibrates between the ER and the PM with t1/2 of about 10 min [77], which would imply that about 105 sterol molecules are transported per second [78]. Mammalian cell studies indicate that newly synthesized cholesterol in the ER is rapidly transported to the PM with a half-time of 10-20 minutes [35, 79].
Oxysterol-binding protein (OSBP)-related protein 2 (ORP2) was suggested as an ER-PM sterol carrier candidate since its overexpression in mammalian cell lines enhanced efflux of newly synthesized cholesterol from ER to extracellular cyclodextrin, without perturbing the PM cholesterol content [80]. The well-conserved protein SCP-2 (sterol carrier protein-2) was also proposed to enhance newly synthesized cholesterol trafficking to the PM in human fibroblasts and rat hepatoma cells [81, 82]. However, it appears that SCP-2 lacks lipid specificity since this protein can receive various lipids including sterols, phospholipids and fatty acids [83, 84]. Additionally, this protein harbors a peroxisomal targeting signal and is involved in other sterol trafficking routes as well as in lipid metabolism events [85, 86], making it difficult to ascertain SCP-2’s primary function.
In S. cerevisiae transport of ergosterol from the ER to the PM requires energy, but conditional defects in Sec18p/NSF, a protein involved in the secretory pathway and essential for vesicle transport, do not block sterol transport or reduce its rate (t1/2=10 min) at the restrictive temperature [77]. These results suggest that the sterol trafficking pathway between the ER and PM is mostly non-vesicular, although the basis for the ATP requirement in this transport is not understood [37]. In mammalian cells, cholesterol is transported from the ER to the PM by an ATP-dependent process, which is unaltered by microtubule disruption or disassembly of the Golgi complex by brefeldin A treatment [68, 87], again pointing to non-vesicular transport as the most likely mechanism.
Esterification of radiolabeled sterol, which was initially delivered to the PM, by the ER resident enzyme acyl-CoA cholesterol acyl transferase (ACAT) has been used as an assay for PM to ER transport [88]. Studies performed in yeast showed that sterol movement from the PM to the ER, or other cell compartments, was not affected in mutants bearing conditional defects in key proteins required for vesicular trafficking [89]. Treatment of mammalian cells with sphingomyelinase releases sterol from the PM, and this cholesterol is delivered to the ER even in ATP-depleted conditions or in presence of vesicular traffic inhibitors, as judged by increased cholesterol esterification [90, 91]. These studies of transport from the PM again point mainly to non-vesicular transport processes.
While several candidates have been proposed, the identity of proteins responsible for non-vesicular transport of sterols between organelles remains unclear. Because of their advantages in genetic analyses [92-94], many studies to identify sterol transport mechanisms have been carried out in S. cerevisiae. Sterol binding proteins like OSBP-related proteins (ORPs) are one set of cytoplasmic proteins that can play a role in transport between the ER and the PM [95, 96]. The seven S. cerevisiae-encoded OSBP homologues (Osh1p-7p) (reviewed in [97]), were tested for their ability to transfer newly synthesized ergosterol from ER to PM [78]. Using a strain missing 6 of the Osh genes and bearing a conditional defect in the seventh (Osh4p/Kes1p), as required for yeast viability, it was found that ER to PM ergosterol transport was reduced approximately 5-fold at the restrictive temperature in comparison to wild-type cells [78, 98]. Cholesterol uptake and esterification assays performed on different Osh-deficient yeast strains suggested that Osh3p and Osh5p may play a greater role in transferring sterol from PM to ER than the others [98]. It seems unlikely that these proteins could serve as diffusional carriers capable of transporting 105 molecules of sterol per second between well-separated organelles because there are only about 2500 of these proteins per cell [99], but a small number of transport molecules could function in this way if they operated in contact sites between organelles. Structural studies show that Osh4p/Kes1p can accommodate a single sterol in its binding pocket and that it can facilitate ATP-independent transfer of sterol between membranes [72, 100]. However, deletion of Osh4p/Kes1p did not reduce the rate of esterification of exogenously added sterol [98]. Osh4p/Kes1p also binds preferentially to phosphoinositides (PIPs) such as PI(4,5)P2 [101], and it recognizes membrane curvature through an ALPS (amphipathic lipid packing sensor) motif included in its N-terminal “lid” α-helix [102]. Osh4p/Kes1p is mostly observed in the Golgi apparatus, where it regulates some aspects of vesicular transport, potentially by acting locally or sensing the lipid organization [99, 101].
It has been suggested that Osh proteins, as well as mammalian OSBP, could affect non-vesicular transport by acting as sterol sensors [37]. In this perspective, membranes somewhat defective in sterol would be replenished in the presence of sterol-bound Osh protein, while an elevated sterol chemical activity in membrane would be “silenced” by a pool of empty Osh proteins. Therefore, instead of being a sterol transfer protein per se, which regulates a vectorial transport of sterol from one membrane to another, Osh proteins would modulate dynamically the sterol content of membranes according to their physicochemical properties and their ability to attract Osh proteins (e.g., by the abundance of PIPs or membrane curvature in the case of Osh4p).
Overall, it seems that a clear mechanistic understanding of the trafficking between the ER and the PM is still lacking, and more work will be needed to determine the relative importance of non-vesicular transport processes in this route.
Sterol endosomal trafficking
Over the last 10 years, several insights into endosomal sterol trafficking have been obtained by imaging the fluorescent sterol DHE in living cells [40, 103]. DHE, which was transferred into the PM from an extracellular cyclodextrin donor, became equilibrated with the ERC within minutes, even in energy-depleted CHO cells in which endocytic delivery of transferrin to the ERC was blocked completely [42]. Interestingly, the DHE was delivered to ERC in fixed and permeabilized cells as well, indicating that this organelle behaves as a “sterol sink” just by its intrinsic properties. In contrast, the recycling of the sterol from the ERC to the PM seemed to follow a vesicular membrane pathway, at least in part. It has been estimated that 106 molecules of sterol enter and leave the ERC per second [67]; however the machinery involved in the PM to ERC non-vesicular transport is still unidentified.
Cholesterol efflux from late endosomes and lysosomes
Receptor-mediated endocytosis of lipoproteins, such as LDL, and hydrolysis of their cholesteryl ester cores in LE/LY constitute a major source of cellular cholesterol. Since sterols are not particularly enriched in LE/LY, the processed cholesterol must escape rapidly from these compartments. How LDL-derived sterols are trafficked out of LE/LY to reach other organelles is not completely understood, except that the mechanism involves at least two key proteins that reside in LE/LY: NPC1 (Niemann-Pick Type C-1 protein) and NPC2 [104]. Indeed, mutations of either one of these proteins lead to the retention of cholesterol and other lipids in these organelles, and such defects are the basis of the inherited autosomal recessive Niemann-Pick Type C (NPC) disease [105-107]. The NPC cell phenotype involves the formation of LE/LY-like storage organelles (LSOs), which are sterol “sinks” not only for LDL-derived sterol, but also for sterols coming from the PM and other membranes [107]. Accordingly, it has been shown that DHE introduced in the PM of NPC cells is progressively amassed in the LSOs, which is consistent with earlier findings suggesting that the PM is sterol-depleted in NPC cells as compared to normal cells [108, 109]. Interestingly, the NPC cell phenotype can be mimicked by treating normal cells by various amphiphile compounds, like the steroid U18666A, which was shown to inhibit PM cholesterol esterification and to induce cholesterol biosynthesis [110, 111]. The mechanism of action of U18666A is not known; it has been suggested that this compound may bind directly to the NPC1 protein, possibly as a sterol competitor [112, 113]. Alternatively, the positive charge of U18666A could associate with the negative headgroups of bis-(monoacylglycerol)-phosphate (BMP), a lipid that is abundant in late endosomes [107, 114, 115].
NPC1 is a multi-spanning membrane protein that is presumed to contain 13 transmembrane helices encompassing a putative sterol-sensing domain (SSD) [116]. The N-terminal domain (NTD) of NPC1 (a.a. 25-264), which is one of the three large luminal domains of the protein, is highly conserved and binds sterols, including the oxysterols 24, 25 or 27-hydroxycholesterol as well as cholesterol [117, 118]. However, whether the NTD facilitates cholesterol egress from the LE/LY is not yet determined. Indeed, an NPC1 mutant protein (Q79A) that has strong defects in sterol binding still rescues the cholesterol transport in NPC1-deficient CHO cells [118]. Thus, it seems likely that sterol binding to the NPC1 NTD is not critical for NPC1 transport function, but it may contribute to another regulatory process. The role of the NPC1’s putative SSD in the cholesterol trafficking was also investigated [119, 120]. NPC1 mutants with single mutation in the SSD (Y635C or P692S) were unable to restore the cholesterol transport in NPC1-deficient cells. Interestingly, in a cell study using a photoactivable cholesterol analog, the authors reported that NPC1 WT, but not NPC1 carrying either one of the two loss-of-function single mutations cited above, was able to be photolabeled (i.e., cross-linked with the sterol analog) [121]. These results suggest that a functional SSD is required for the sterol binding in NPC1. However, since this study did not provide evidence of direct sterol binding to the SSD, the role of this domain in the NPC1 function remains uncertain. Indeed, it remains unclear precisely what NPC1 does to facilitate sterol exit from LE/LY.
Approximately 5% of the patients affected by the NPC disease have a mutation in NPC2 [106]. In contrast to NPC1, NPC2 is a small soluble protein that is delivered to the lumen of the LE/LY as a consequence of its mannose-6-phosphate modification [122]. This protein was shown to bind sterol and to increase the sterol transfer rate between liposomes by two orders of magnitude, as compared to spontaneous sterol transfer [123]. Structural characterization of NPC2 with or without its ligand indicates that this protein must experience a change in conformation as it binds the sterol [70, 71]. Indeed, a hydrophobic tunnel on the protein surface, initially too tight to accommodate a sterol molecule, is proposed to expand while the sterol goes through. This flexibility is consistent with the reported wide sterol specificity of NPC2 [124]. NPC2 interacts directly with the membrane to promote the sterol transfer, and this transfer is highly enhanced when donor liposome membranes contain BMP [123]. This unusual negatively charged lipid is highly enriched in LE/LY, and it is a major structural element of the internal vesicles in these compartments [125, 126]. Interestingly, uptake of anti-BMP antibody reduced the sterol transfer rate out of LE/LY [115]. These data suggest that NPC2 facilitates the transport of LDL-derived cholesterol from the interior of LE/LY to the limiting membrane of this compartment.
The possibility of a functional interplay between NPC2 and the NTD of NPC1 in sterol transport was investigated in detail [127]. Three points are noteworthy in this study. First, NPC2 can accept and release cholesterol much more rapidly than NPC1(NTD), which is consistent with a role for NPC2 in sterol transfer. Second, NPC2 can rapidly remove the cholesterol from NPC1(NTD), or directly deliver cholesterol to NPC1(NTD). Similarly, a cholesterol molecule can be exchanged between two NPC2 proteins. Third, there is no noticeable effect of the NTD of NPC1 in the transfer of cholesterol from a membrane to NPC2, or from NPC2 to a membrane. The mechanistic ordering of the role of NPC1 and NPC2 in sterol efflux remains unclear.
Cholesterol transport to mitochondria
Mitochondria are particularly poor in cholesterol [33]; however, trafficking of cholesterol into this organelle is important since several metabolic processes, such as cholesterol conversion into 27-hydroxycholesterol [128] and biogenesis of steroid hormones from cholesterol in steroidogenic tissues, occur in mitochondria [129]. Various studies have examined the transport pathways of cholesterol from other cellular organelles into the inner mitochondrial membrane (IMM), where the conversion enzymes reside [130]. The StAR (steroidogenic acute regulatory) protein, synthesized in response to hormonal stimuli [131], is required for transferring cholesterol to the IMM [132]. This protein binds a single molecule of cholesterol and behaves as an LTP in vitro [133]; however, its role in vivo seems to be confined to the cytosolic face of the outer mitochondrial membrane (OMM) [134, 135]. Recent studies suggest that StAR may cooperate with other proteins on the OMM surface in order to facilitate the cholesterol transfer to the IMM [136]. In this view, it has been reported that a functional interplay may occur between StAR, the OMM translocator protein (also known as peripheral benzodiazepine receptor), and other regulatory proteins [137-139]; however a clear mechanistic understanding for the cholesterol transport from the OMM to the IMM, including the action of the StAR protein, is still lacking.
The late endosomal membrane protein MLN64 (also known as STARD3) has been shown to promote the mobilization of LE/LY cholesterol toward mitochondria [140, 141]. This protein is firmly attached to the LE/LY limiting membrane through its N-terminal domain, whereas its C-terminal region, which consists in a START (StAR-related lipid transfer) domain, is situated in the cytoplasm [142]. The MLN64 START domain is a hydrophobic pocket that binds a single molecule of cholesterol [74, 75]. A proposed mechanism is that MLN64 may operate in inter-organelle sterol transfer, implying tight contact between membranes of LE/LY and mitochondria [143].
Cellular cholesterol efflux
Cholesterol release to extracellular acceptors such as apolipoproteins is another important cellular homeostatic mechanism implicated in the removal of excess free sterol by export to high-density lipoprotein (HDL) and its apo-lipoprotein, apoA-I [144]. This process is facilitated by transmembrane proteins that are members of the ABC superfamily of transporters, such as the ubiquitously expressed ABCA1 protein [145]. In a process called reverse cholesterol transport, circulating lipid-poor forms of apolipoprotein (mostly apoA-I) recognize ABCA1 proteins at the surface of extra-hepatic cells. This binding apparently initiates a multi-stage process, including enhancement of ABCA1 activity, which leads to the transfer of phospholipids and cholesterol to the apoA-I associated HDL [146-148]. The newly formed HDL particle then separates from the cell, and the HDL-associated cholesterol is transferred to the liver. While there is evidence that ABCA1 plays a key role in the transfer of cholesterol and phospholipids to apoA-I and HDL , the molecular mechanisms for this are still obscure [145, 149]. One significant question is whether the cholesterol is a direct substrate (or co-substrate, together with a phospholipid molecule) of ABCA1 or if it “flops” toward the PM outer leaflet because of a local change in the membrane physicochemical properties induced by ABCA1 and/or apoA-I [144, 150]. Indeed, one could ask why a spontaneously-flipping lipid such as a sterol would need an energy-consuming mechanism to cross the bilayer [151].
Some studies indicated that ABCA1 may also operate in intracellular compartments, as judged by its colocalization with apoA-I in endosomes [152]. Additionally, LDL-derived cholesterol in LE/LY has been suggested to be a preferential source for ABCA1-mediated cholesterol efflux in macrophages [153]. Another ABC transporter, ABCG1, which is highly expressed in macrophages, has been proposed to facilitate the HDL cholesterol-loading through a sequential cooperation with ABCA1 [154, 155]. Recent double knock-out (Abca1-/-, Abcg1-/-) studies performed on mice indicate that the combined effect of those ABC transporters is essential for macrophage cholesterol efflux, considering the severe defects generated by this deficiency in the reverse cholesterol transport [156, 157]. While there is very strong evidence for the role of the ABC transporters in the cellular efflux of cholesterol , the molecular mechanisms involved in this process are still unclear.
Conclusion
Figure 2 shows a schematic illustration of putative pathways on intracellular sterol transport. There is increasing evidence that the transport mechanisms implicated in sterol trafficking and contributing to the cellular sterol distribution are mostly not vesicular [158]. There has been good progress using biochemical and cell biological methods to describe the basic properties of this transport, such as its rates of transport and the relative abundance of sterols in various organelles. Unfortunately, at this time there is little solid understanding of the molecular mechanisms of this transport. Several proteins have been identified as candidate transporters for movement of sterol through the cytoplasm, but none of these have been fully validated by genetic and biochemical methods. It remains unclear if most non-vesicular transport occurs by diffusion through the cytoplasm or if inter-organelle transport occurs mainly at contact sites between membranes. Similarly, it is unknown if transport carriers mediate vectorial transport between specific organelles or if much of the transport is simply driving sterol toward its lowest free energy distribution by random exchange between organelles. The mechanisms for maintaining sterol asymmetry across bilayers remain speculative, but this may have important implications in the dynamics of non-vesicular cholesterol transport. Membrane protein and lipid composition create a diversity of physicochemical environments in the cell. The general sterol trafficking routes between them are starting to be appreciated, but more work needs to be done to further characterize these movements and to discover the proteins implicated in those processes.
Figure 2. Intracellular cholesterol movements.
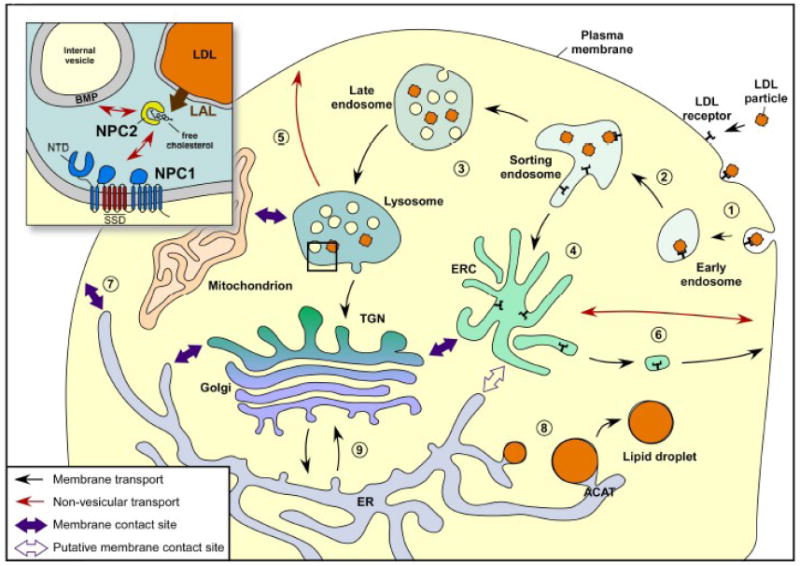
The circulating LDL particles carrying cholesterol and cholesteryl-ester are internalized through LDL receptor and transported to sorting endosomes (1, 2). LDL particles are subsequently transported to LE and LY (3), while LDL receptors are recycled via the ERC to the PM (4). Cholesteryl-ester hydrolysis by specific lipases such as LAL in LE/LY produces free cholesterol that can efflux from these compartments to other intracellular membranes, such as PM, mostly by non-vesicular transport (5). A precise mechanism for free cholesterol egress from LE/LY is still lacking; however, the membrane-embedded NPC1 and the soluble luminal NPC2 proteins are both required in this process (inset). Cholesterol in the PM can traffic to the ERC by a non-vesicular mechanism; whereas recycling of cholesterol from this compartment back to the PM occurs partly by vesicular and non-vesicular processes (6). Cholesterol translocation from PM to ER allows the homeostatic machinery to be informed about the free cholesterol levels in the cell (7). When it is in excess, the free cholesterol is esterified by ACAT, and fatty acid sterol esters are packed into lipid droplets (8). Newly synthesized cholesterol in the ER is transported mostly to the PM by a non-vesicular process bypassing the Golgi, although some of it would follow the secretory pathway, passing through the Golgi (9).
Acknowledgments
We thank Drs. Mousumi Mondal, Arun Radhakrishnan and Anant Menon (Weill Cornell Medical College, NY) for comments on the manuscript. Supported by grants from the NIH (R37-DK27083) and the Ara Parseghian Medical Research Foundation.
Abbreviations
- PM
plasma membrane
- ER
endoplasmic reticulum
- ERC
endosomal recycling compartment
- LE
late endosome
- LY
lysosome
- LSO
LE/LY-like storage organelle
- PC
phosphatidylcholine
- SM
sphingomyelin
- PS
phosphatidylserine
- PE
phosphatidylethanolamine
- DO
dioleoyl
- DP
dipalmitoyl
- BMP
bis-(monoacylglycerol)-phosphate
- DHE
dehydroergosterol
- CTL
cholestatrienol
- TNBS
trinitrobenzenesulfonate
- CHO
Chinese hamster ovary
- ATP
adenosine triphosphate
- ABC
ATP-binding cassette
- NPC
Niemann-Pick type C
- SSD
sterol-sensing domain
- LTP
lipid transfer protein
- OSBP
oxysterol-binding protein
- StAR
steroidogenic acute regulatory protein
- ACAT
acyl-CoA cholesterol acyl transferase
- LAL
lysosomal acid lipase
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Meer G. Cellular lipidomics. EMBO J. 2005;24:3159–3165. doi: 10.1038/sj.emboj.7600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips MC, Johnson WJ, Rothblat GH. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim Biophys Acta. 1987;906:223–276. doi: 10.1016/0304-4157(87)90013-x. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Feigenson GW. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys J. 1999;76:2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radhakrishnan A, McConnell HM. Condensed complexes of cholesterol and phospholipids. Biophys J. 1999;77:1507–1517. doi: 10.1016/S0006-3495(99)76998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiNitto JP, Cronin TC, Lambright DG. Membrane recognition and targeting by lipid-binding domains. Sci STKE. 2003;2003:re16. doi: 10.1126/stke.2132003re16. [DOI] [PubMed] [Google Scholar]
- 6.Ali MR, Cheng KH, Huang J. Assess the nature of cholesterol-lipid interactions through the chemical potential of cholesterol in phosphatidylcholine bilayers. Proc Natl Acad Sci U S A. 2007;104:5372–5377. doi: 10.1073/pnas.0611450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McConnell HM, Radhakrishnan A. Condensed complexes of cholesterol and phospholipids. Biochim Biophys Acta. 2003;1610:159–173. doi: 10.1016/s0005-2736(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 8.Radhakrishnan A, McConnell H. Condensed complexes in vesicles containing cholesterol and phospholipids. Proc Natl Acad Sci U S A. 2005;102:12662–12666. doi: 10.1073/pnas.0506043102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindblom G, Oradd G. Lipid lateral diffusion and membrane heterogeneity. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamem.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Rog T, Pasenkiewicz-Gierula M, Vattulainen I, Karttunen M. Ordering effects of cholesterol and its analogues. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamem.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Lange Y, Ye J, Steck TL. How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc Natl Acad Sci U S A. 2004;101:11664–11667. doi: 10.1073/pnas.0404766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange Y, Ye J, Steck TL. Activation of membrane cholesterol by displacement from phospholipids. J Biol Chem. 2005;280:36126–36131. doi: 10.1074/jbc.M507149200. [DOI] [PubMed] [Google Scholar]
- 13.Lee AG. Lipid-protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta. 2003;1612:1–40. doi: 10.1016/s0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin S. How clusters of basic/hydrophobic residues on proteins interact with lipids in membranes. Biophys J. 2004;SP23 [Google Scholar]
- 17.Demel RA, Jansen JW, van Dijck PW, van Deenen LL. The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta. 1977;465:1–10. doi: 10.1016/0005-2736(77)90350-9. [DOI] [PubMed] [Google Scholar]
- 18.Slotte JP. Sphingomyelin-cholesterol interactions in biological and model membranes. Chem Phys Lipids. 1999;102:13–27. doi: 10.1016/s0009-3084(99)00071-7. [DOI] [PubMed] [Google Scholar]
- 19.Slotte JP, Bierman EL. Depletion of plasma-membrane sphingomyelin rapidly alters the distribution of cholesterol between plasma membranes and intracellular cholesterol pools in cultured fibroblasts. Biochem J. 1988;250:653–658. doi: 10.1042/bj2500653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta AK, Rudney H. Plasma membrane sphingomyelin and the regulation of HMG-CoA reductase activity and cholesterol biosynthesis in cell cultures. J Lipid Res. 1991;32:125–136. [PubMed] [Google Scholar]
- 21.McIntosh TJ, Simon SA, Needham D, Huang CH. Interbilayer interactions between sphingomyelin and sphingomyelin/cholesterol bilayers. Biochemistry. 1992;31:2020–2024. doi: 10.1021/bi00122a018. [DOI] [PubMed] [Google Scholar]
- 22.Radhakrishnan A, McConnell H. Composition fluctuations, chemical exchange, and nuclear relaxation in membranes containing cholesterol. J Chem Phys. 2007;126:185101. doi: 10.1063/1.2730805. [DOI] [PubMed] [Google Scholar]
- 23.Keller SL, Anderson TG, McConnell HM. Miscibility critical pressures in monolayers of ternary lipid mixtures. Biophys J. 2000;79:2033–2042. doi: 10.1016/S0006-3495(00)76451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sankaram MB, Thompson TE. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 1990;29:10670–10675. doi: 10.1021/bi00499a014. [DOI] [PubMed] [Google Scholar]
- 25.Guo W, Kurze V, Huber T, Afdhal NH, Beyer K, Hamilton JA. A solid-state NMR study of phospholipid-cholesterol interactions: sphingomyelin-cholesterol binary systems. Biophys J. 2002;83:1465–1478. doi: 10.1016/S0006-3495(02)73917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holopainen JM, Metso AJ, Mattila JP, Jutila A, Kinnunen PK. Evidence for the lack of a specific interaction between cholesterol and sphingomyelin. Biophys J. 2004;86:1510–1520. doi: 10.1016/S0006-3495(04)74219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai J, Pagano RE. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry. 1997;36:8840–8848. doi: 10.1021/bi970145r. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Montero I, Rodriguez N, Cribier S, Pohl A, Velez M, Devaux PF. Rapid transbilayer movement of ceramides in phospholipid vesicles and in human erythrocytes. J Biol Chem. 2005;280:25811–25819. doi: 10.1074/jbc.M412052200. [DOI] [PubMed] [Google Scholar]
- 29.Megha E. London, Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 30.McConnell HM, Vrljic M. Liquid-liquid immiscibility in membranes. Annu Rev Biophys Biomol Struct. 2003;32:469–492. doi: 10.1146/annurev.biophys.32.110601.141704. [DOI] [PubMed] [Google Scholar]
- 31.Zheng L, McQuaw CM, Ewing AG, Winograd N. Sphingomyelin/phosphatidylcholine and cholesterol interactions studied by imaging mass spectrometry. J Am Chem Soc. 2007;129:15730–15731. doi: 10.1021/ja0741675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smaby JM, Brockman HL, Brown RE. Cholesterol’s interfacial interactions with sphingomyelins and phosphatidylcholines: hydrocarbon chain structure determines the magnitude of condensation. Biochemistry. 1994;33:9135–9142. doi: 10.1021/bi00197a016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeagle PL. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985;822:267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 35.Liscum L, Munn NJ. Intracellular cholesterol transport. Biochim Biophys Acta. 1999;1438:19–37. doi: 10.1016/s1388-1981(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 36.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maxfield FR, Menon AK. Intracellular sterol transport and distribution. Curr Opin Cell Biol. 2006;18:379–385. doi: 10.1016/j.ceb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 38.van Meer G. Lipids of the Golgi membrane. Trends Cell Biol. 1998;8:29–33. doi: 10.1016/s0962-8924(97)01196-3. [DOI] [PubMed] [Google Scholar]
- 39.Blanchette-Mackie EJ. Intracellular cholesterol trafficking: role of the NPC1 protein. Biochim Biophys Acta. 2000;1486:171–183. doi: 10.1016/s1388-1981(00)00055-x. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee S, Zha X, Tabas I, Maxfield FR. Cholesterol distribution in living cells: fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys J. 1998;75:1915–1925. doi: 10.1016/S0006-3495(98)77632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wustner D, Mondal M, Tabas I, Maxfield FR. Direct observation of rapid internalization and intracellular transport of sterol by macrophage foam cells. Traffic. 2005;6:396–412. doi: 10.1111/j.1600-0854.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 42.Hao M, Lin SX, Karylowski OJ, Wustner D, McGraw TE, Maxfield FR. Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. J Biol Chem. 2002;277:609–617. doi: 10.1074/jbc.M108861200. [DOI] [PubMed] [Google Scholar]
- 43.Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, Slot JW, Geuze HJ. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 44.Silvius JR. Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim Biophys Acta. 2003;1610:174–183. doi: 10.1016/s0005-2736(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 45.Fridriksson EK, Shipkova PA, Sheets ED, Holowka D, Baird B, McLafferty FW. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- 46.Devaux PF, Morris R. Transmembrane asymmetry and lateral domains in biological membranes. Traffic. 2004;5:241–246. doi: 10.1111/j.1600-0854.2004.0170.x. [DOI] [PubMed] [Google Scholar]
- 47.Zachowski A. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem J. 1993;294(Pt 1):1–14. doi: 10.1042/bj2940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprong H, van der Sluijs P, van Meer G. How proteins move lipids and lipids move proteins. Nat Rev Mol Cell Biol. 2001;2:504–513. doi: 10.1038/35080071. [DOI] [PubMed] [Google Scholar]
- 49.Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 50.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 51.Dolis D, de Kroon AI, de Kruijff B. Transmembrane movement of phosphatidylcholine in mitochondrial outer membrane vesicles. J Biol Chem. 1996;271:11879–11883. doi: 10.1074/jbc.271.20.11879. [DOI] [PubMed] [Google Scholar]
- 52.Bretscher MS. Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol. 1972;236:11–12. doi: 10.1038/newbio236011a0. [DOI] [PubMed] [Google Scholar]
- 53.Op den Kamp JA. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- 54.Steck TL, Ye J, Lange Y. Probing red cell membrane cholesterol movement with cyclodextrin. Biophys J. 2002;83:2118–2125. doi: 10.1016/S0006-3495(02)73972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blau L, Bittman R. Cholesterol distribution between the two halves of the lipid bilayer of human erythrocyte ghost membranes. J Biol Chem. 1978;253:8366–8368. [PubMed] [Google Scholar]
- 56.Fisher KA. Analysis of membrane halves: cholesterol. Proc Natl Acad Sci U S A. 1976;73:173–177. doi: 10.1073/pnas.73.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gottlieb MH. The reactivity of human erythrocyte membrane cholesterol with a cholesterol oxidase. Biochim Biophys Acta. 1977;466:422–428. doi: 10.1016/0005-2736(77)90335-2. [DOI] [PubMed] [Google Scholar]
- 58.Brasaemle DL, Robertson AD, Attie AD. Transbilayer movement of cholesterol in the human erythrocyte membrane. J Lipid Res. 1988;29:481–489. [PubMed] [Google Scholar]
- 59.Hale JE, Schroeder F. Asymmetric transbilayer distribution of sterol across plasma membranes determined by fluorescence quenching of dehydroergosterol. Eur J Biochem. 1982;122:649–661. doi: 10.1111/j.1432-1033.1982.tb06488.x. [DOI] [PubMed] [Google Scholar]
- 60.Schroeder F, Nemecz G, Wood WG, Joiner C, Morrot G, Ayraut-Jarrier M, Devaux PF. Transmembrane distribution of sterol in the human erythrocyte. Biochim Biophys Acta. 1991;1066:183–192. doi: 10.1016/0005-2736(91)90185-b. [DOI] [PubMed] [Google Scholar]
- 61.Wustner D. Fluorescent sterols as tools in membrane biophysics and cell biology. Chem Phys Lipids. 2007;146:1–25. doi: 10.1016/j.chemphyslip.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Boesze-Battaglia K, Clayton ST, Schimmel RJ. Cholesterol redistribution within human platelet plasma membrane: evidence for a stimulus-dependent event. Biochemistry. 1996;35:6664–6673. doi: 10.1021/bi951846w. [DOI] [PubMed] [Google Scholar]
- 63.Boesze-Battaglia K, Schimmel RJ. Collagen-stimulated unidirectional translocation of cholesterol in human platelet membranes. J Exp Biol. 1999;202:453–460. doi: 10.1242/jeb.202.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mondal M, Mesmin B, Mukherjee S, Maxfield FR. Sterols Are Mainly in the Cytoplasmic Leaflet of the Plasma Membrane and the Endocytic Recycling Compartment in CHO Cells. Mol Biol Cell. 2008 doi: 10.1091/mbc.E08-07-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 66.Maxfield FR, Wustner D. Intracellular cholesterol transport. J Clin Invest. 2002;110:891–898. doi: 10.1172/JCI16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maxfield FR, Mondal M. Sterol and lipid trafficking in mammalian cells. Biochem Soc Trans. 2006;34:335–339. doi: 10.1042/BST0340335. [DOI] [PubMed] [Google Scholar]
- 68.Heino S, Lusa S, Somerharju P, Ehnholm C, Olkkonen VM, Ikonen E. Dissecting the role of the golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc Natl Acad Sci U S A. 2000;97:8375–8380. doi: 10.1073/pnas.140218797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hinrichsen L, Harborth J, Andrees L, Weber K, Ungewickell EJ. Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J Biol Chem. 2003;278:45160–45170. doi: 10.1074/jbc.M307290200. [DOI] [PubMed] [Google Scholar]
- 70.Friedland N, Liou HL, Lobel P, Stock AM. Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc Natl Acad Sci U S A. 2003;100:2512–2517. doi: 10.1073/pnas.0437840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J Biol Chem. 2007;282:23525–23531. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Im YJ, Raychaudhuri S, Prinz WA, Hurley JH. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 2005;437:154–158. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romanowski MJ, Soccio RE, Breslow JL, Burley SK. Crystal structure of the Mus musculus cholesterol-regulated START protein 4 (StarD4) containing a StAR-related lipid transfer domain. Proc Natl Acad Sci U S A. 2002;99:6949–6954. doi: 10.1073/pnas.052140699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsujishita Y, Hurley JH. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol. 2000;7:408–414. doi: 10.1038/75192. [DOI] [PubMed] [Google Scholar]
- 75.Murcia M, Faraldo-Gomez JD, Maxfield FR, Roux B. Modeling the structure of the StART domains of MLN64 and StAR proteins in complex with cholesterol. J Lipid Res. 2006;47:2614–2630. doi: 10.1194/jlr.M600232-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Prinz WA. Non-vesicular sterol transport in cells. Prog Lipid Res. 2007;46:297–314. doi: 10.1016/j.plipres.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baumann NA, Sullivan DP, Ohvo-Rekila H, Simonot C, Pottekat A, Klaassen Z, Beh CT, Menon AK. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry. 2005;44:5816–5826. doi: 10.1021/bi048296z. [DOI] [PubMed] [Google Scholar]
- 78.Sullivan DP, Ohvo-Rekila H, Baumann NA, Beh CT, Menon AK. Sterol trafficking between the endoplasmic reticulum and plasma membrane in yeast. Biochem Soc Trans. 2006;34:356–358. doi: 10.1042/BST0340356. [DOI] [PubMed] [Google Scholar]
- 79.Kaplan MR, Simoni RD. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J Cell Biol. 1985;101:446–453. doi: 10.1083/jcb.101.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hynynen R, Laitinen S, Kakela R, Tanhuanpaa K, Lusa S, Ehnholm C, Somerharju P, Ikonen E, Olkkonen VM. Overexpression of OSBP-related protein 2 (ORP2) induces changes in cellular cholesterol metabolism and enhances endocytosis. Biochem J. 2005;390:273–283. doi: 10.1042/BJ20042082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puglielli L, Rigotti A, Greco AV, Santos MJ, Nervi F. Sterol carrier protein-2 is involved in cholesterol transfer from the endoplasmic reticulum to the plasma membrane in human fibroblasts. J Biol Chem. 1995;270:18723–18726. doi: 10.1074/jbc.270.32.18723. [DOI] [PubMed] [Google Scholar]
- 82.Baum CL, Reschly EJ, Gayen AK, Groh ME, Schadick K. Sterol carrier protein-2 overexpression enhances sterol cycling and inhibits cholesterol ester synthesis and high density lipoprotein cholesterol secretion. J Biol Chem. 1997;272:6490–6498. doi: 10.1074/jbc.272.10.6490. [DOI] [PubMed] [Google Scholar]
- 83.Lige B, Jayabalasingham B, Zhang H, Pypaert M, Coppens I. Role of an Ancestral D-bifunctional Protein Containing Two Sterol-carrier Protein-2 Domains in Lipid Uptake and Trafficking in Toxoplasma. Mol Biol Cell. 2008 doi: 10.1091/mbc.E08-05-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dyer DH, Lovell S, Thoden JB, Holden HM, Rayment I, Lan Q. The structural determination of an insect sterol carrier protein-2 with a ligand-bound C16 fatty acid at 1.35-A resolution. J Biol Chem. 2003;278:39085–39091. doi: 10.1074/jbc.M306214200. [DOI] [PubMed] [Google Scholar]
- 85.Gallegos AM, Atshaves BP, Storey SM, McIntosh AL, Petrescu AD, Schroeder F. Sterol carrier protein-2 expression alters plasma membrane lipid distribution and cholesterol dynamics. Biochemistry. 2001;40:6493–6506. doi: 10.1021/bi010217l. [DOI] [PubMed] [Google Scholar]
- 86.Mukherji M, Kershaw NJ, Schofield CJ, Wierzbicki AS, Lloyd MD. Utilization of sterol carrier protein-2 by phytanoyl-CoA 2-hydroxylase in the peroxisomal alpha oxidation of phytanic acid. Chem Biol. 2002;9:597–605. doi: 10.1016/s1074-5521(02)00139-4. [DOI] [PubMed] [Google Scholar]
- 87.Urbani L, Simoni RD. Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J Biol Chem. 1990;265:1919–1923. [PubMed] [Google Scholar]
- 88.Tabas I, Rosoff WJ, Boykow GC. Acyl coenzyme A:cholesterol acyl transferase in macrophages utilizes a cellular pool of cholesterol oxidase-accessible cholesterol as substrate. J Biol Chem. 1988;263:1266–1272. [PubMed] [Google Scholar]
- 89.Li Y, Prinz WA. ATP-binding cassette (ABC) transporters mediate nonvesicular, raft-modulated sterol movement from the plasma membrane to the endoplasmic reticulum. J Biol Chem. 2004;279:45226–45234. doi: 10.1074/jbc.M407600200. [DOI] [PubMed] [Google Scholar]
- 90.Zha X, Pierini LM, Leopold PL, Skiba PJ, Tabas I, Maxfield FR. Sphingomyelinase treatment induces ATP-independent endocytosis. J Cell Biol. 1998;140:39–47. doi: 10.1083/jcb.140.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skiba PJ, Zha X, Maxfield FR, Schissel SL, Tabas I. The distal pathway of lipoprotein-induced cholesterol esterification, but not sphingomyelinase-induced cholesterol esterification, is energy-dependent. J Biol Chem. 1996;271:13392–13400. doi: 10.1074/jbc.271.23.13392. [DOI] [PubMed] [Google Scholar]
- 92.Sullivan DP, Georgiev A, Menon AK. Tritium suicide selection identifies proteins involved in the uptake and intracellular transport of sterols in Saccharomyces cerevisiae. Eukaryot Cell. 2008 doi: 10.1128/EC.00135-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fei W, Alfaro G, Muthusamy BP, Klaassen Z, Graham TR, Yang H, Beh CT. Genome-wide analysis of sterol-lipid storage and trafficking in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:401–414. doi: 10.1128/EC.00386-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reiner S, Micolod D, Zellnig G, Schneiter R. A genomewide screen reveals a role of mitochondria in anaerobic uptake of sterols in yeast. Mol Biol Cell. 2006;17:90–103. doi: 10.1091/mbc.E05-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beh CT, Cool L, Phillips J, Rine J. Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics. 2001;157:1117–1140. doi: 10.1093/genetics/157.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beh CT, Rine J. A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J Cell Sci. 2004;117:2983–2996. doi: 10.1242/jcs.01157. [DOI] [PubMed] [Google Scholar]
- 97.Schulz TA, Prinz WA. Sterol transport in yeast and the oxysterol binding protein homologue (OSH) family. Biochim Biophys Acta. 2007;1771:769–780. doi: 10.1016/j.bbalip.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raychaudhuri S, Im YJ, Hurley JH, Prinz WA. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J Cell Biol. 2006;173:107–119. doi: 10.1083/jcb.200510084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fairn GD, McMaster CR. Emerging roles of the oxysterol-binding protein family in metabolism, transport, and signaling. Cell Mol Life Sci. 2008;65:228–236. doi: 10.1007/s00018-007-7325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Canagarajah BJ, Hummer G, Prinz WA, Hurley JH. Dynamics of cholesterol exchange in the oxysterol binding protein family. J Mol Biol. 2008;378:737–748. doi: 10.1016/j.jmb.2008.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–146. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- 103.Wustner D. Plasma membrane sterol distribution resembles the surface topography of living cells. Mol Biol Cell. 2007;18:211–228. doi: 10.1091/mbc.E06-05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liscum L, Sturley SL. Intracellular trafficking of Niemann-Pick C proteins 1 and 2: obligate components of subcellular lipid transport. Biochim Biophys Acta. 2004;1685:22–27. doi: 10.1016/j.bbalip.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 105.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 106.Vance JE. Lipid imbalance in the neurological disorder, Niemann-Pick C disease. FEBS Lett. 2006;580:5518–5524. doi: 10.1016/j.febslet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 107.Mukherjee S, Maxfield FR. Lipid and cholesterol trafficking in NPC. Biochim Biophys Acta. 2004;1685:28–37. doi: 10.1016/j.bbalip.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 108.Pipalia NH, Hao M, Mukherjee S, Maxfield FR. Sterol, protein and lipid trafficking in Chinese hamster ovary cells with Niemann-Pick type C1 defect. Traffic. 2007;8:130–141. doi: 10.1111/j.1600-0854.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- 109.Karten B, Vance DE, Campenot RB, Vance JE. Cholesterol accumulates in cell bodies, but is decreased in distal axons, of Niemann-Pick C1-deficient neurons. J Neurochem. 2002;83:1154–1163. doi: 10.1046/j.1471-4159.2002.01220.x. [DOI] [PubMed] [Google Scholar]
- 110.Lange Y, Steck TL. Cholesterol homeostasis. Modulation by amphiphiles. J Biol Chem. 1994;269:29371–29374. [PubMed] [Google Scholar]
- 111.Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem. 2006;281:31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- 112.Lange Y, Ye J, Rigney M, Steck T. Cholesterol movement in Niemann-Pick type C cells and in cells treated with amphiphiles. J Biol Chem. 2000;275:17468–17475. doi: 10.1074/jbc.M000875200. [DOI] [PubMed] [Google Scholar]
- 113.Liu R, Lu P, Chu JW, Sharom FJ. Characterization of fluorescent sterol binding to purified human NPC1. J Biol Chem. 2008 doi: 10.1074/jbc.M803741200. [DOI] [PubMed] [Google Scholar]
- 114.Liscum L. Niemann-Pick type C mutations cause lipid traffic jam. Traffic. 2000;1:218–225. doi: 10.1034/j.1600-0854.2000.010304.x. [DOI] [PubMed] [Google Scholar]
- 115.Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 116.Davies JP, Ioannou YA. Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J Biol Chem. 2000;275:24367–24374. doi: 10.1074/jbc.M002184200. [DOI] [PubMed] [Google Scholar]
- 117.Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J Biol Chem. 2008;283:1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- 118.Infante RE, Radhakrishnan A, Abi-Mosleh L, Kinch LN, Wang ML, Grishin NV, Goldstein JL, Brown MS. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283:1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 119.Watari H, Blanchette-Mackie EJ, Dwyer NK, Watari M, Neufeld EB, Patel S, Pentchev PG, Strauss JF., 3rd Mutations in the leucine zipper motif and sterol-sensing domain inactivate the Niemann-Pick C1 glycoprotein. J Biol Chem. 1999;274:21861–21866. doi: 10.1074/jbc.274.31.21861. [DOI] [PubMed] [Google Scholar]
- 120.Ko DC, Gordon MD, Jin JY, Scott MP. Dynamic movements of organelles containing Niemann-Pick C1 protein: NPC1 involvement in late endocytic events. Mol Biol Cell. 2001;12:601–614. doi: 10.1091/mbc.12.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ohgami N, Ko DC, Thomas M, Scott MP, Chang CC, Chang TY. Binding between the Niemann-Pick C1 protein and a photoactivatable cholesterol analog requires a functional sterol-sensing domain. Proc Natl Acad Sci U S A. 2004;101:12473–12478. doi: 10.1073/pnas.0405255101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- 123.Xu Z, Farver W, Kodukula S, Storch J. Regulation of sterol transport between membranes and NPC2. Biochemistry. 2008;47:11134–11143. doi: 10.1021/bi801328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liou HL, Dixit SS, Xu S, Tint GS, Stock AM, Lobel P. NPC2, the protein deficient in Niemann-Pick C2 disease, consists of multiple glycoforms that bind a variety of sterols. J Biol Chem. 2006;281:36710–36723. doi: 10.1074/jbc.M608743200. [DOI] [PubMed] [Google Scholar]
- 125.Chevallier J, Chamoun Z, Jiang G, Prestwich G, Sakai N, Matile S, Parton RG, Gruenberg J. Lysobisphosphatidic acid controls endosomal cholesterol levels. J Biol Chem. 2008;283:27871–27880. doi: 10.1074/jbc.M801463200. [DOI] [PubMed] [Google Scholar]
- 126.Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- 127.Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A. 2008;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Okuda KI. Liver mitochondrial P450 involved in cholesterol catabolism and vitamin D activation. J Lipid Res. 1994;35:361–372. [PubMed] [Google Scholar]
- 129.Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- 130.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta. 2007;1771:663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 131.Jefcoate CR. Liver X receptor opens a new gateway to StAR and to steroid hormones. J Clin Invest. 2006;116:1832–1835. doi: 10.1172/JCI29160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clark BJ, Stocco DM. Expression of the steroidogenic acute regulatory (StAR) protein: a novel LH-induced mitochondrial protein required for the acute regulation of steroidogenesis in mouse Leydig tumor cells. Endocr Res. 1995;21:243–257. doi: 10.3109/07435809509030440. [DOI] [PubMed] [Google Scholar]
- 133.Kallen CB, Billheimer JT, Summers SA, Stayrook SE, Lewis M, Strauss JF., 3rd Steroidogenic acute regulatory protein (StAR) is a sterol transfer protein. J Biol Chem. 1998;273:26285–26288. doi: 10.1074/jbc.273.41.26285. [DOI] [PubMed] [Google Scholar]
- 134.Arakane F, Sugawara T, Nishino H, Liu Z, Holt JA, Pain D, Stocco DM, Miller WL, Strauss JF., 3rd Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action. Proc Natl Acad Sci U S A. 1996;93:13731–13736. doi: 10.1073/pnas.93.24.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bose H, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- 136.Papadopoulos V, Liu J, Culty M. Is there a mitochondrial signaling complex facilitating cholesterol import? Mol Cell Endocrinol. 2007;265(266):59–64. doi: 10.1016/j.mce.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 137.Lacapere JJ, Papadopoulos V. Peripheral-type benzodiazepine receptor: structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids. 2003;68:569–585. doi: 10.1016/s0039-128x(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 138.Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulos V. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into leydig cell mitochondria. Mol Endocrinol. 2005;19:540–554. doi: 10.1210/me.2004-0307. [DOI] [PubMed] [Google Scholar]
- 139.Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem. 2006;281:38879–38893. doi: 10.1074/jbc.M608820200. [DOI] [PubMed] [Google Scholar]
- 140.Alpy F, Stoeckel ME, Dierich A, Escola JM, Wendling C, Chenard MP, Vanier MT, Gruenberg J, Tomasetto C, Rio MC. The steroidogenic acute regulatory protein homolog MLN64, a late endosomal cholesterol-binding protein. J Biol Chem. 2001;276:4261–4269. doi: 10.1074/jbc.M006279200. [DOI] [PubMed] [Google Scholar]
- 141.Zhang M, Liu P, Dwyer NK, Christenson LK, Fujimoto T, Martinez F, Comly M, Hanover JA, Blanchette-Mackie EJ, Strauss JF., 3rd MLN64 mediates mobilization of lysosomal cholesterol to steroidogenic mitochondria. J Biol Chem. 2002;277:33300–33310. doi: 10.1074/jbc.M200003200. [DOI] [PubMed] [Google Scholar]
- 142.Alpy F, Tomasetto C. MLN64 and MENTHO, two mediators of endosomal cholesterol transport. Biochem Soc Trans. 2006;34:343–345. doi: 10.1042/BST0340343. [DOI] [PubMed] [Google Scholar]
- 143.Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- 144.Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 145.Baldan A, Bojanic DD, Edwards PA. The ABCs of sterol transport. J Lipid Res. 2008 doi: 10.1194/jlr.R800044-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang C, Vaughan AM, Anantharamaiah GM, Oram JF. Janus kinase 2 modulates the lipid-removing but not protein-stabilizing interactions of amphipathic helices with ABCA1. J Lipid Res. 2006;47:107–114. doi: 10.1194/jlr.M500240-JLR200. [DOI] [PubMed] [Google Scholar]
- 147.Arakawa R, Yokoyama S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J Biol Chem. 2002;277:22426–22429. doi: 10.1074/jbc.M202996200. [DOI] [PubMed] [Google Scholar]
- 148.Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J Clin Invest. 2003;111:99–107. doi: 10.1172/JCI16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alder-Baerens N, Muller P, Pohl A, Korte T, Hamon Y, Chimini G, Pomorski T, Herrmann A. Headgroup-specific exposure of phospholipids in ABCA1-expressing cells. J Biol Chem. 2005;280:26321–26329. doi: 10.1074/jbc.M413993200. [DOI] [PubMed] [Google Scholar]
- 150.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 151.Small DM. Role of ABC transporters in secretion of cholesterol from liver into bile. Proc Natl Acad Sci U S A. 2003;100:4–6. doi: 10.1073/pnas.0237205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Neufeld EB, Stonik JA, Demosky SJ, Jr, Knapper CL, Combs CA, Cooney A, Comly M, Dwyer N, Blanchette-Mackie J, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr The ABCA1 transporter modulates late endocytic trafficking: insights from the correction of the genetic defect in Tangier disease. J Biol Chem. 2004;279:15571–15578. doi: 10.1074/jbc.M314160200. [DOI] [PubMed] [Google Scholar]
- 153.Chen W, Sun Y, Welch C, Gorelik A, Leventhal AR, Tabas I, Tall AR. Preferential ATP-binding cassette transporter A1-mediated cholesterol efflux from late endosomes/lysosomes. J Biol Chem. 2001;276:43564–43569. doi: 10.1074/jbc.M107938200. [DOI] [PubMed] [Google Scholar]
- 154.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 155.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 156.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Van Eck M. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28:258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 158.Lange Y, Steck TL. Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog Lipid Res. 2008;47:319–332. doi: 10.1016/j.plipres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


