Abstract
Growth hormone (GH) and insulin-like growth factor-I (IGF-I) provide trophic support during development and also appear to influence cell structure, function and replacement in the adult brain. Recent studies demonstrated effects of the GH/IGF-I axis on adult neurogenesis, but it is unclear whether the GH/IGF-I axis influences glial turnover in the normal adult brain. In the current study we used a selective model of adult-onset GH and IGF-I deficiency to evaluate the role of GH and IGF-I in regulating glial proliferation and survival in the adult corpus callosum. GH/IGF-I-deficient dwarf rats of the Lewis strain were made GH/IGF-I replete via twice daily injections of GH starting at postnatal day 28 (P28), approximately the age at which GH pulse amplitude increases in developing rodents. GH/IGF-I deficiency was initiated in adulthood by removing animals from GH treatment. Quantitative analyses revealed that adult-onset GH/IGF-I deficiency decreased cell proliferation in the white matter and decreased the survival of newborn oligodendrocytes. These findings are consistent with the hypothesis that aging-related changes in the GH/IGF-I axis produce deficits in ongoing turnover of oligodendrocytes, which may contribute to aging-related cognitive changes and deficits in remyelination after injury.
Keywords: white matter, trophic factor, oligodendrocytes, demyelination, aging
INTRODUCTION
Declines in circulating levels of GH and IGF-I are robust hallmarks of aging in mammals (e.g., Sonntag et al., 2005; Sherlock and Toogood, 2007). Recent studies indicate that the neurobiological consequences of the decline in GH/IGF-I include decreased neurogenesis in the dentate gyrus of the hippocampus (Lichtenwalner et al., 2001; 2006), where IGF-I appears to affect primarily the survival of newborn neurons, but also may influence the maturation and differentiation of newborn cells (Aberg et al., 2000; Darnaudéry et al., 2006). The effects of the GH/IGF-I axis on cell turnover in the adult brain probably are not limited to neuronal progenitors, since IGF-I can promote proliferation of oligodendrocyte progenitor cells (OPCs) and differentiation and survival of oligodendrocytes (e.g., McMorris and McKinnon, 1996; Mason et al., 2000; Aberg et al., 2007; Pang et al., 2007). It is reasonable to hypothesize that the aging-related decline in GH/IGF-I and dependent changes in oligodendrocyte genesis and/or maturation may contribute to impaired remyelination in the central nervous system of aged individuals (Gilson and Blakemore, 1993; Shields et al., 1999; Franklin et al., 2002; Sim et al., 2002) and to a decline in normal cognitive function.
Evaluating the impact of circulating growth factors like GH and IGF-I on the brain during aging is complicated by the many neurobiological changes that occur with aging. Among studies of the effects of IGF-I and regulatory members of the IGF system on oligodendrocytes and OPCs, most have examined effects in vitro (e.g., McMorris et al., 1986; Kühl et al., 2002; Mason and Goldman, 2002; Cui and Almazan, 2007), during development (e.g., Goddard et al., 1999; Ye et al., 2004; Zeger et al., 2007), following neural injury (e.g., Mason et al., 2000; Genoud et al., 2005; Kumar et al., 2007; Pang et al., 2007; Wood et al., 2007; Ye et al. 2007; Chesik et al., 2008), and/or in transgenic models in which IGF-dependent effects on brain development limit assessment of effects in adult animals (Ye and D’Ercole, 1999; Ye et al., 2000; 2007; Genoud et al., 2005). To evaluate specifically the role of the GH/IGF-I axis in the regulation of ongoing OPC proliferation and oligodendrocyte survival and differentiation in the white matter of adult rats, we used a model of selective, adult-onset GH/IGF-I deficiency in dwarf (dw/dw) rats derived from the Lewis strain (Charlton et al., 1988; Carter et al., 2002). A spontaneous mutation in these rats causes a selective, 90% reduction in pituitary production of GH and a reduction in plasma IGF-I (Charlton et al., 1988), comparable in magnitude to the decline seen in aging (e.g., Breese et al., 1991; Sonntag et al., 1992). The specificity of this model and its similarity to the aging-related changes in pituitary hormones stand in contrast to other dwarf rodents (Cheng et al., 1983) and to hypophysectomized animals (Schoenle et al., 1982; Aberg et al., 2000; 2007), in which multiple pituitary hormones are depleted. Thus, dw/dw rats provide an excellent model for testing whether normal GH and IGF-I levels in the plasma are required to sustain normal glial genesis in adults.
This study assessed the effects of GH and IGF-I deficiency on several aspects of glial turnover. To evaluate proliferation of progenitors, dividing cells were labeled with bromodeoxyuridine (BrdU) after two weeks of GH/IGF-I deficiency and then the rats were perfused 18 hours later. In other animals a cohort of newborn cells was labeled with BrdU immediately prior to initiation of GH/IGF-I deficiency. We then analyzed the survival and maturation of those cells after a four week period of GH/IGF-I deficiency, using antibodies to BrdU and markers of oligodendrocytes, in order to test whether GH/IGF-I deficiency reduced the survival of BrdU-labeled cells and/or altered their differentiation and maturation.
MATERIALS AND METHODS
Animals and treatment with growth hormone
The breeding and treatment of dw/dw rats has been described previously (Lichtenwalner et al., 2006). In brief, male dw/dw pups were bred from dw/+ females and dw/dw males, identified by significantly reduced body weight at 28 days of age, and injected twice daily (subcutaneously, 8:00, 16:00) with porcine GH (200 µg; provided by Dr. A.F. Parlow, UCLA Harbor Medical Center) starting at P28 (approximately 3 days after pulsatile secretion of GH normally begins [see Edén, 1979; Gabriel et al., 1992; LeRoith et al., 2001] and the youngest age at which dwarf animals can be reliably identified). At P98 one half of the rats were switched to saline injections (GH/IGF-I deficient) while the remaining animals continued to receive GH twice daily (GH/IGF-I replete). Plasma samples were taken at the time of perfusion and analyzed by radioimmunoassay to confirm the deficiency-induced reduction in circulating IGF-I levels. The body weight of each rat was recorded weekly. Two groups of male dw/+ heterozygous litter mates (n = 8 Proliferation group, n=8 Survival group) were monitored for parameters supporting model validation, body weight gain and plasma IGF-I levels (Lichtenwalner et al., 2006).
S-phase labeling
To label a cohort of newborn cells, rats received three subcutaneous injections of BrdU (50 mg/kg body weight, in sterile 0.9% NaCl plus 0.007 N NaOH) administered two hours apart. Animals in the Proliferation group received BrdU injections on P111, two weeks after the onset of GH/IGF-I deficiency on P98, and were perfused 18 hours after the last BrdU injection to assess whether two weeks of GH/IGF-I deficiency affected the baseline rate of proliferation in the corpus callosum (CC). Rats in the Survival group received BrdU injections on P98 and then were perfused four weeks later on P126. Importantly, all animals in the Survival group were GH/IGF-I replete at the time of labeling of newborn cells but the subsequent survival and differentiation of newly generated cells occurred under conditions of either GH/IGF-I deficiency or repletion.
Tissue preparation and Immunolabeling
Each animal was sacrificed by anesthetic overdose (sodium pentobarbital, 150 mg/kg body weight) and perfused with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4). A complete series of coronal cryostat sections of the brain (40 µm) from bregma −1.50 to −7.5 (Paxinos and Watson, 1998) were collected in cryoprotectant (25% glycerol, 25% ethylene glycol in 0.1M phosphate buffer, pH 7.4) and stored at −20°C until processed for immunohistochemistry (IHC) or immunofluorescence (IF). The density of BrdU-labeled cells was analyzed in sections labeled by IHC using rat monoclonal anti-BrdU (2.5 µg/ml; Accurate Chemical & Scientific Corp., see Lichtenwalner et al., 2006).
For analysis of OPCs, sections from rats in the Proliferation group were labeled by IF using rat anti-BrdU and rabbit anti-platelet-derived growth factor receptor-α (PDGFR-α; Santa Cruz (C-20); 0.2 µg/ml). For analysis of oligodendrocyte turnover, sections from rats in the Survival group were labeled with rat anti-BrdU and two oligodendrocyte markers: rabbit anti-glutathione S-transferase pi (anti-GSTpi, Novocastra; 1:150; Gotts and Chesselet, 2005) and mouse anti-adenomatous polyposis coli (anti-APC, EMD Biosciences Calbiochem®; 1 µg/ml; Bhat et al., 1996). GSTpi and APC have been used widely as markers of mature oliogodendrocytes and also appear to recognize oligodendrocytes early in their differentiation (e.g., Bhat et al., 1996; Kumar et al., 2007; Tamura et al. 2007). Immunolabeling for BrdU and cell type specific antibodies was visualized using secondary antibodies conjugated to Alexa 488, Alexa 633 (Invitrogen Corp., Molecular Probes®) and Cy3 (Jackson ImmunoResearch Laboratories, Inc.).
Quantification
All analyses were performed blindly using coded sections. A modification of the optical disector method (Gundersen et al., 1988; Kempermann et al., 1998) was used for quantification of cell proliferation and survival. Since BrdU labeled cells were not distributed homogenously, often occurring in clusters, the material in this study was not well suited for stereological determination of the total number of BrdU labeled cells in each region of interest (ROI). We counted all of the BrdU-labeled cells in the CC in series of systematically randomly selected sections through a portion of the anterior-posterior extent of the hippocampus (bregma −1.6 to −5.3, Paxinos and Watson, 1998), analyzing three sections from each rat in the Proliferation group and five sections from each rat in the Survival group. Using the Neurolucida system for quantitative morphometry (Microbrightfield, Inc., Colchester, VT), BrdU-labeled cells were counted within the CC in each section, excluding cells in the top focal plane to avoid overestimation. To estimate the total density of BrdU-labeled cells in the CC, the sum of cell counts for all sections was divided by the total volume of the CC analyzed in each animal and counts of BrdU+ cells presented as number per unit volume. Mean values were calculated for each group.
In order to test for effects of GH/IGF-I deficiency on glial turnover in gray matter, BrdU-labeled cells were analyzed in pyriform cortex. Although the borders of most cortical regions are indistinct and difficult to identify consistently, it was possible to reproducibly define a region comprising primarily pyriform cortex using only three points: 1) the cortical surface radially above the ventral-medial margin of the layer of superficial pyramidal neurons that characterizes pyriform cortex (easily visualized using differential-interference contrast optics), 2) the center of the rhinal fissure, and 3) the superficial border of the subcortical white matter radially beneath the center of the rhinal fissure. Points 1 and 2 were connected along the pial surface and points 2 and 3 were connected by a straight line. For rats in both the Proliferation and Survival groups, BrdU-labeled cells were counted in three sections representing the same anterior-posterior extent defined for the analysis of the CC (N=5 rats per condition).
For analysis of the differentiation of BrdU-labeled cells in the CC, sections labeled for BrdU and PDGFR-α or BrdU, GSTpi and APC were examined using a Leica SP2 laser scanning confocal microscope and 63x oil-immersion apochromatic objective. To avoid misinterpretation of multiple labeling due to potential overlap in excitation and emission spectra, sections were imaged first at 488 nm and 633 nm to visualize Alexa 488- and Alexa 633-conjugated secondary antibodies and then at 543 nm to visualize Cy3. In each section analyzed every BrdU-labeled cell within the CC was scored for co-expression of the other marker(s); a range of 150–250 cells were analyzed per animal. Analysis of co-expression was completed on-line with no processing or enhancement of the confocal images. In addition to analyses in the CC, the phenotypes of BrdU-labeled cells in the pyriform cortex (as defined above) were analyzed in three replete and three deficient rats in the Proliferation (double-labeling for BrdU and PDGFR-α) and Survival groups (double-labeling for BrdU and APC).
Statistical analyses
Except where noted, seven replete and seven deficient dw/dw rats were analyzed in the Proliferation group and nine replete and nine deficient animals were analyzed in the Survival group. Statistical analyses were performed using SigmaStat 3.0 statistical system software (Systat Software Inc., San Jose CA). The experimental variables related to glial turnover were compared between GH/IGF-I replete and deficient dw/dw rats for each sacrifice group using two-tailed t-tests. The dependent variables included: number of BrdU-labeled cells, percentage of BrdU-labeled cells co-labeled with PDGFR-α or oligodendrocyte markers, and estimated number of cells co-labeled for BrdU and PDGFR-α or oligodendrocyte markers. We made no correction for multiple t-tests since the number of comparisons was small and all were planned a priori; the threshold of significance was set at p ≤ 0.05. All data are presented as mean ± SEM.
RESULTS
Regulation of GH/IGF-I in dw/dw rats
The regulation of IGF-I levels and of brain and body weight in the dw/dw rats used in this study have been described previously (Lichtenwalner et al., 2006). In brief, IGF-I levels in plasma and cerebrospinal fluid (CSF) did not differ between dw/+ control rats and dw/dw rats treated twice daily with GH (GH/IGF-I replete) but were reduced by approximately 50% in dw/dw rats following cessation of GH treatment. GH injections increased the growth rate of dwarf rats to match that of heterozygous controls, whereas the weight gain of untreated dwarfs was significantly lower. After GH-treated dw/dw rats were switched from GH to saline injections (on P98) their weight gain ceased and within three days they weighed less than their replete littermates.
The primary measure of proliferation and cell survival was the density of BrdU-labeled cells in the CC. It was necessary, therefore, to establish that there was no change in brain size or the volume of the CC analyzed that might produce a change in density that did not reflect a change in cell number. Brain weight did not differ between replete and deficient rats (1.63±0.03 vs 1.61±0.01 g, respectively) and previous analyses revealed no effect of GH/IGF deficiency on the volume of the granule cell layer or hilus in the dentate gyrus of the hippocampus (Lichtenwalner et al., 2006). In the present study, there was no effect of GH/IGF deficiency on the average area of the CC in the sections analyzed (1.55±0.04 mm2 in replete vs 1.59±0.04 mm2 in deficient, p>0.5), indicating that the size of the CC in the region selected for analysis was unchanged.
GH/IGF-I deficiency decreases proliferation of OPCs in the cerebral white matter
Counts of BrdU-labeled cells in the CC in rats in the Proliferation and Survival groups served as measures of overall cell proliferation and of survival of the newborn cohort, respectively (Figure 1 and Figure 2). Testing for effects of GH/IGF status on proliferation by comparing the density of BrdU-labeled cells in replete and deficient rats in the Proliferation group revealed that GH/IGF deficiency decreased proliferation in the CC by approximately 25% (Figure 2A, p<0.005, two-tailed t-test).
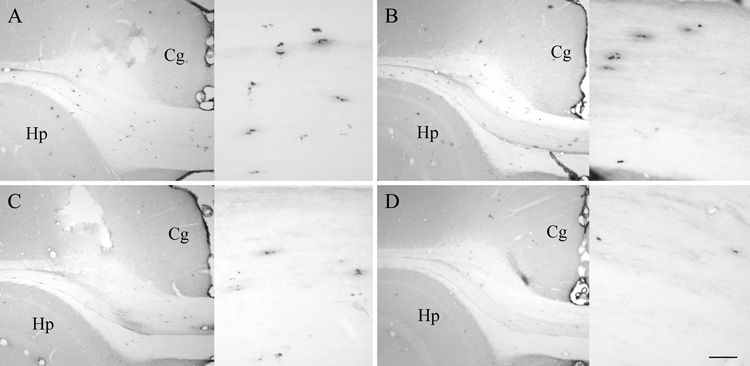
Figure 1. S-phase labeling with BrdU.
IHC labeling for BrdU identified newborn cells in the subcortical white matter at 18 hours (Proliferation group, A and B) and four weeks (Survival group, C and D) after BrdU injection in GH/IGF-I replete (A and C) and deficient (B and D) rats. The cingulate cortex (Cg) and hippocampus (Hp) are identified in each section for orientation. Inset: medial CC at higher magnification. Scale bar = 250 µm, 50 µm (inset).
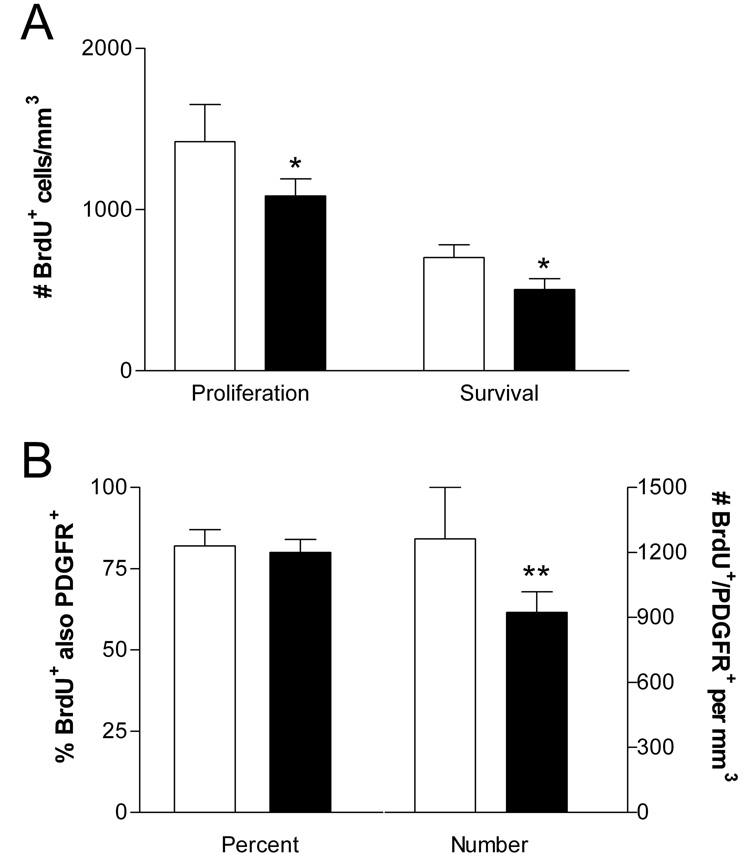
Figure 2. Counts of proliferating cells and OPCs.
The density of BrdU+ cells (A) is shown for GH/IGF-I replete (open bars) and deficient rats (filled bars) in the Proliferation and Survival groups. BrdU+ cells were reduced in deficient compared to replete rats in both groups, indicating decreased proliferation of progenitor cells and decreased survival of newborn cells. In the Proliferation group (B) the percentage of BrdU+ cells that were PDGFR-α+ was not affected by GH/IGF-I deficiency, but the estimated density of BrdU+/ PDGFR-α+ cells (#/mm3) was reduced. All values mean±sd; *p<0.005 and **p<0.02 versus GH/IGF-I replete littermates.
Counting BrdU-labeled cells that were co-labeled for PDGFR-α assessed specifically the proliferation of presumptive OPCs. The percentage of BrdU-positive cells that were PDGFR-α+ was the same in GH/IGF-I replete and deficient rats in the Proliferation group (82±5% vs 80±4%, respectively), but the estimated number of BrdU+/ PDGFR-α+ cells in the CC (calculated from the density of BrdU+ cells and percentage that were PDGFR-α+) was reduced approximately 25% in deficient rats (Figure 2B, C; Figure 3A–L; p<0.02 by two-tailed t-test).
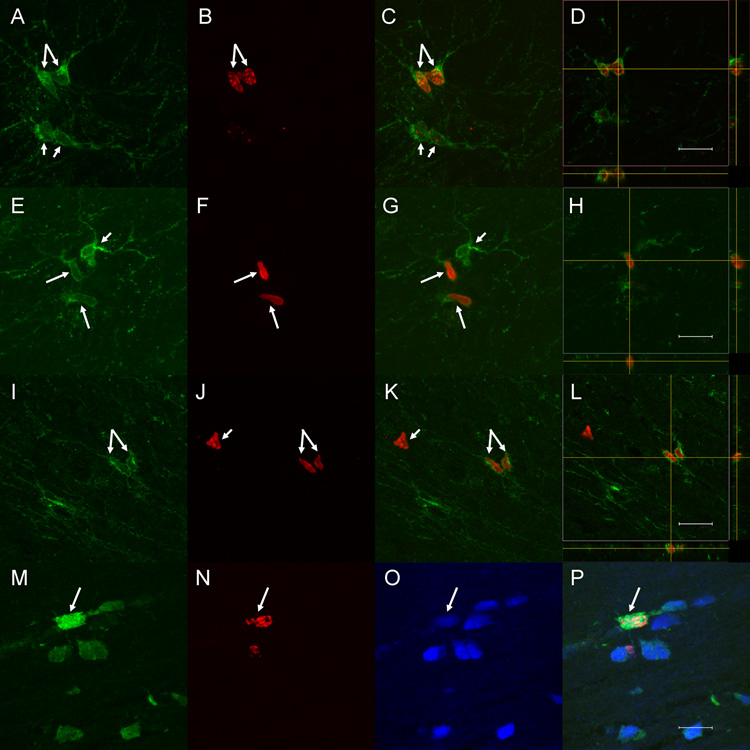
Figure 3. Immunofluorescent labeling for BrdU, PDGFR-α, and oligodendrocyte markers.
Double labeling for BrdU (red) and PDGFR-α (green) in Proliferation group rats (A–L) revealed numerous double labeled cells (long arrows), some adjacent pairs that suggested recent division (e.g., C and K). PDGFR-α+ cells that were not BrdU+ (short arrows in A–G) and BrdU+ cells that were not PDGFR-α+ (short arrow in J–K) also were evident. Three-dimensional imaging and orthogonal views (D, H and L) confirmed that BrdU+ nuclei were located within PDGFR-α+ cells, not adjacent to them. Triple labeling for BrdU (red), APC (green) and GSTpi (blue) in sections from rats in the Survival group (M–P) demonstrated triple labeled cells (long arrow, merged image in P), evidence of ongoing addition of oligodendrocytes in adult rats. Scale bars = 12 µm (A–H; M–P), 16 µm (I–L).
GH/IGF-I modulates the survival of adult born oligodendrocytes in the CC
In Survival group animals the twice daily injections of GH were continued in all animals through the day of BrdU injections and then deficient animals were switched to saline injections for the remainder of the experiment. Thus, all animals in the Survival group had the same GH/IGF status at the time of BrdU labeling and BrdU labeling of all animals in the Survival group was similar to that in GH/IGF-I replete animals in the Proliferation group (but cannot be equated exactly; rats in the Proliferation group were injected with BrdU at P111 versus P98 in Survival group animals). The density of BrdU labeled cells in all rats in the Survival group was substantially lower than that in replete rats in the Proliferation group, indicating that only a fraction of adult born cells in the CC survived for four weeks. Significantly, the density of BrdU-labeled cells was almost 30% lower in GH/IGF deficient animals than in replete littermates in the Survival group (p<0.0001, Figure 2A, two-tailed t-test), indicating that GH/IGF deficiency decreased the survival of adult born cells in the CC.
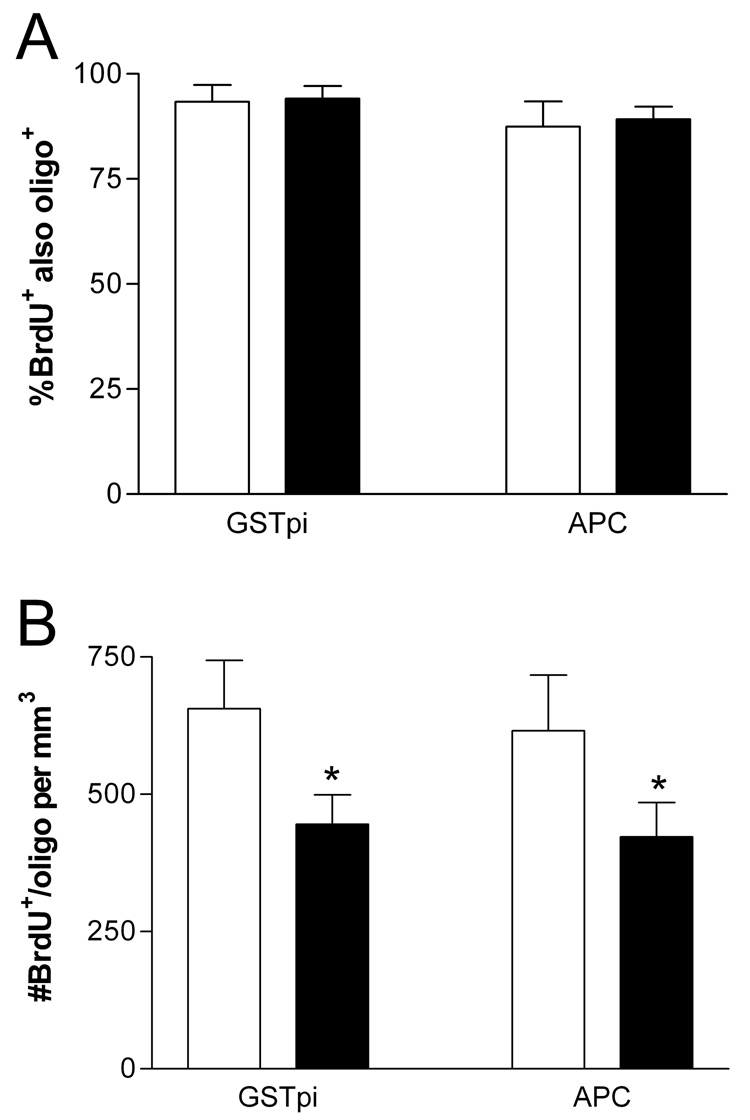
To assess specifically the differentiation and survival of oligodendrocytes, sections from GH/IGF replete and deficient rats in the Survival group were double-labeled for BrdU and two markers of oligodendrocytes, APC and GSTpi (Figure 3M–P and Figure 4). APC and GSTpi labeled essentially the same population of oligodendrocytes, since virtually all cells labeled for APC also were positive for GSTpi. APC+ and/or GSTpi+ cells accounted for approximately 90% of the BrdU labeled cells in Survival group animals (Figure 4A). The percentage of BrdU+ cells that expressed oligodendrocyte markers did not differ between GH/IGF-I deficient and replete rats (p>0.5), but estimation of the number of BrdU+/APC+ and BrdU+/GST+ cells (from the counts of BrdU+ cells and percentages of double-labeled cells) indicated that the number of oligodendrocytes in the BrdU+ population was 30% lower in GH/IGF deficient rats than in replete littermates (p<0.0005 for each marker, two-tailed t-test, Figure 4B).
Figure 4. Counts of newborn oligodendrocytes.
The percentage of BrdU+ cells that were labeled with oligodendrocyte markers (A) and the estimated density of BrdU+ cells that were GSTpi+ and/or APC+ (B) are shown for GH/IGF-I replete (open bars) and deficient (filled bars) rats in the Survival group. GH/IGF-I deficiency significantly reduced the number of newborn (BrdU+) cells that were identified as oligodendrocytes. All values mean±sd; *p<0.0005 versus GH/IGF-I replete littermates.
Oligodendrocyte turnover in gray matter
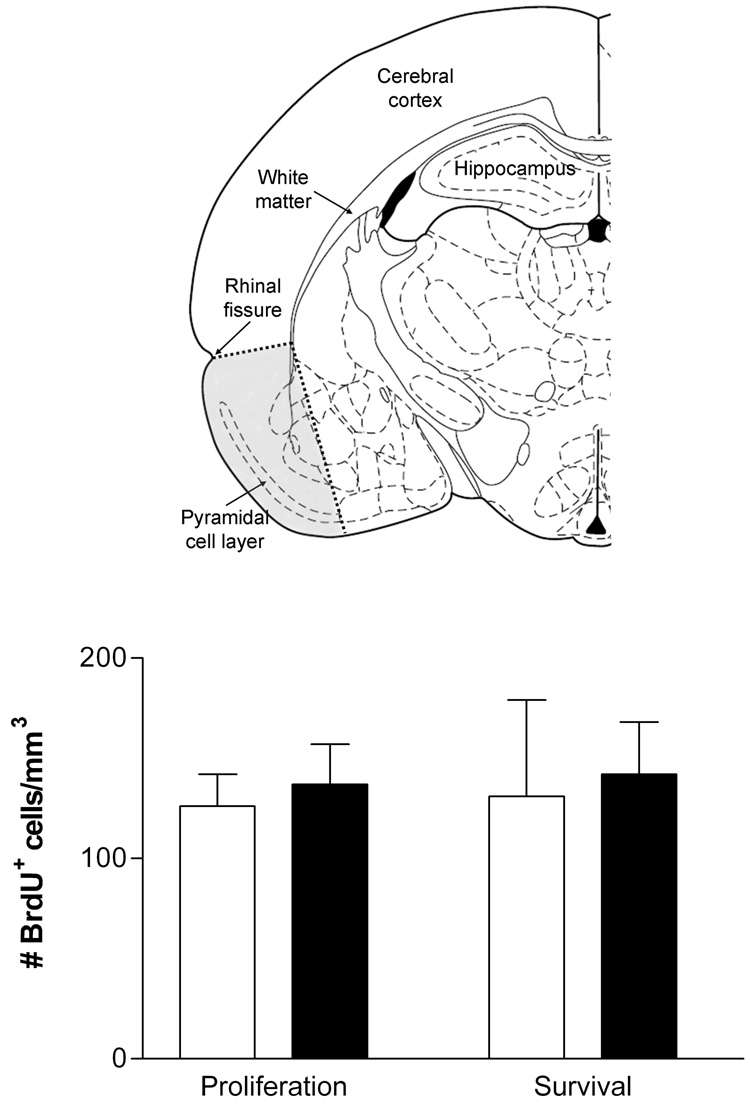
In rats of both the Proliferation and Survival groups BrdU-labeled cells were distributed throughout the brain. Counts of BrdU-labeled cells in the pyriform cortex revealed that i) the density of BrdU-labeled cells was approximately five-fold lower than in the CC, ii) the density was not appreciably lower in the Survival group than in the Proliferation group, and iii) there was no detectable difference in density between GH/IGF-I deficient and replete rats in the Proliferation or Survival group (Figure 5). Regardless of GH/IGF-I status, approximately 80% of BrdU+ cells in the pyriform cortex of Proliferation group rats expressed PDGFR-α and approximately 95% of BrdU+ cells in Survival group rats expressed APC. Qualitative assessment indicated that similar majorities of BrdU-labeled cells in other regions of gray matter were oligodendrocyte precursors (Proliferation group) or oligodendrocytes (Survival group).
Figure 5. Counts of proliferating and newborn cells in the pyriform cortex.
Oligodendrocyte turnover in the cerebral cortex was quantified in pyriform cortex (shaded area above) as defined in the text. The density of BrdU+ cells, shown for GH/IGF-I replete (open bars) and deficient rats (filled bars) in the Proliferation and Survival groups, was similar at the two time points and did not differ between GH/IGF-I deficient and replete rats. All values mean±sd.
DISCUSSION
Accumulating evidence indicates IGF-I is an important regulator of adult neurogenesis and that decreased GH/IGF-I signaling contributes to the aging-related decline in neurogenesis (Åberg et al., 2000; Lichtenwalner et al., 2001; 2006; Trejo et al., 2001; Shetty et al., 2005). This study demonstrates that circulating GH and/or IGF-I dynamically influence oligodendroglial turnover in the white matter. Two to four weeks of adult-onset deficiency of GH/IGF-I decreased proliferation of dividing OPCs in the subcortical white matter and decreased survival of newborn oligodendrocytes. Since both GH and IGF-I levels are reduced in this model it is not possible to establish the critical mediator(s) of the observed effects on glial genesis. IGF-I is viewed as the effector for GH and several studies implicate IGF-I as a regulator of remyelination in multiple sclerosis (reviewed in Chesik et al., 2007), but GH also may have direct effects in the brain. GH and IGF-I in circulation can cross the blood brain barrier (Coculescu, 1999; Armstrong et al., 2000; Pan et al., 2005); in addition, GH and IGF-I are produced (Niblock et al., 1998; Donahue et al., 2002; Sun et al., 2005) and their receptors are abundant in numerous brain areas (e.g., Bohannon et al., 1988; Lesniak et al., 1988; Lobie et al., 1993; Zhai et al., 1994; Nyberg and Burman, 1996; Guan et al., 1997). Thus, there is the potential for both endocrine and paracrine/autocrine actions of both GH and IGF-I. Analysis of a separate cohort of GH/IGF-I replete and deficient rats revealed no change in IGF-I levels in the brain, despite the significant decrease in plasma (Linville et al., 2008), suggesting that direct effects of GH and/or plasma levels of GH and/or IGF-I were responsible for the observed effects on oligodendrocyte turnover.
Regulation of Proliferation by GH/IGF-I
The BrdU injection protocol used in the present study provided a reasonable index of cell proliferation, since animals were administered BrdU over six hours and perfused 18 hours later. Among limited estimates of cell cycle kinetics for glial precursors in the adult brain, Watanabe et al. (2002) estimated a cell cycle time for oligodendrocyte precursors greater than 24 hours following a demyelinating lesion and turnover appears to be much slower in the intact adult brain (see, for example, McCarthy and Leblond, 1988; Shi et al., 1998). The 18 hour survival period following BrdU labeling in the present study likely was insufficient for precursors to re-enter the cell cycle and give rise to another generation of daughter cells. Thus, our protocol provided a reasonable assessment of proliferation of progenitor cells in the CC.
The present results demonstrate that two weeks of GH/IGF-I deficiency is sufficient to reduce proliferation of dividing cells in the CC by 25%, suggesting dynamic regulation of progenitors in the white matter. Moreover, analysis of double labeling for BrdU and PDGFR-α revealed a GH/IGF-dependent reduction in the number of OPCs within the BrdU+ population that was similar in magnitude to the decline in BrdU+ cells. Thus, it appears that the deleterious effects on proliferation are specific for OPCs.
Regulation of Survival and Maturation
Our results reveal a robust effect of GH/IGF-I on survival of adult-born oligodendrocytes. Within the BrdU+ cohort of newborn cells, the number that expressed markers of mature oligodendrocytes was reduced 30% by GH/IGF-I deficiency. In principle, GH/IGF deficiency could have affected only survival or also commitment of cells to the oligodendrocyte lineage, but the most parsimonious explanation for the current findings is that GH/IGF-I deficiency primarily decreased survival. If GH/IGF-I deficiency reduced commitment to the oligodendrocyte lineage, one would expect in deficient animals an increase in the percentage of BrdU+ cells that did not express markers of oligodendrocytes, which was not the case. The reduction in the total number of cells in the BrdU+ cohort in GH/IGF-I deficient rats was accounted for by the reduction in APC+/GSTpi+ cells. Regulation of survival of adult-born oligodendrocytes is consistent with previous evidence that IGF-I affects survival of both mature oligodendrocytes and differentiating oligodendrocytes following demyelinating lesions or other manipulations that deplete oligodendrocytes (e.g., Liu et al., 1995; Ye and D’Ercole, 1999; Mason et al., 2000; Guan et al., 2001; Kumar et al., 2007).
An effect of GH/IGF-I on migration of newborn oligodendrocytes also could have contributed to decreased turnover of oligodendrocytes in the CC of deficient rats. Regulation of oligodendrocyte migration by the GH/IGF-I system has not been directly investigated, but there is evidence that IGF-I promotes motility and migration of Schwann cells and glioblastoma cells (Guvakova, 2006; Schlenska-Lange et al., 2008). Moreover, combinations of growth factors that include IGF-I have been shown to increase oligodendrocyte migration following experimental demyelination (Espinosa-Jeffrey et al., 2006; Kumar et al., 2007).
Significance of GH/IGF-I-dependent regulation of oligodendrocyte turnover
We have demonstrated that plasma levels of GH/IGF-I influence adult neurogenesis (Lichtenwalner et al., 2001; 2006) and glial turnover in the white matter (current study). It is reasonable to suspect that such modulatory influences of the GH/IGF-I axis on cell turnover contribute to age-related changes in cognitive ability (e.g., Barnes, 1979; Churchill et al., 2002) and the ability of GH and IGF-I to reverse cognitive impairment associated with aging or disease (e.g., Markowska et al., 1998; Thornton et al., 2000; Lupien et al., 2003; Ramsey et al., 2004). The functional impact of changes in neuronal turnover likely is limited, since adult neurogenesis is restricted to the dentate gyrus of the hippocampus and the subventricular zone/rostral migratory stream/olfactory bulb system. In contrast, influences on the regulation of glial genesis may be widespread, since there is ongoing turnover of oligodendrocytes throughout the brain, with actively dividing OPCs in both white- and gray matter (Reynolds and Hardy, 1997; Levison et al., 1999; Gensert and Goldman, 2001; Levine et al., 2001; Liu and Rao, 2004; Polito and Reynolds, 2005). Consistent with those previous reports, qualitative assessment of brain sections from rats in the Survival group in this study revealed BrdU+/APC+/GSTpi+ cells in the cerebral cortex. In the present study GH/IGF-I deficiency did not have a demonstrable effect on oligodendrocyte turnover in pyriform cortex, suggesting that regulation by the GH/IGF-I axis may be specific to white matter, but the approximately five-fold lower density of proliferating cells in gray matter compared to white matter limits the ability to detect changes in the former.
There has been little assessment of the significance of ongoing oligodendrocyte replacement for normal brain function and little analysis of aging-related changes in basal turnover of oligodendrocytes. Information on oligodendrocyte turnover in normal adults comes primarily from control animals in studies of remyelination in models of demyelinating disorders. Significantly, many manipulations used to reduce adult neurogenesis and probe its role in cognitive function (e.g., irradiation - Meshi et al., 2006; Saxe et al., 2006, or mitotic inhibitors - Shors et al., 2001; 2002) also affect glial proliferation. Thus, the suppression of glial turnover could contribute to cognitive changes interpreted primarily in the context of changes in neurogenesis.
It is not clear whether the maintenance of OPCs and oligodendrocyte turnover in the adult brain serves normal function or only provides a rapidly recruitable population of cells for myelin repair following damage. Proliferation of oligodendrocyte precursors and recruitment of new, myelinating oligodendrocytes from immature precursors contribute to myelin repair following demyelinating lesions. Following demyelination, proliferation of OPCs and commitment, differentiation and survival of adult-born oligodendrocytes all appear to be targets of regulation by inflammatory cytokines and growth factors (see Armstrong, 2007 for recent review). The GH/IGF-I system appears to play a particularly critical role in myelin repair. IGF expression is induced in multiple models of demyelination and is increased during remyelination (e.g., Liu et al., 1994; Hinks and Franklin, 1999; Fushimi and Sharabe, 2004). Treatment with IGF-I or overexpression of IGF-I in transgenic mice inhibits oligodendrocyte death during demyelination and/or enhances remyelination following demyelinating lesions (e.g., McMorris and McKinnon, 1996; Mason et al., 2000; Kumar et al., 2007). Studies of lysolecithin-induced demyelination in the spinal cord of young adult and old adult rats suggest that slower and less effective increases in IGF-I expression contribute to an aging-related decrease in the efficacy of remyelination (Hinks and Franklin, 2000), which appears to be attributable to impairments in both OPC recruitment and differentiation of newborn oligodendrocytes (Sim et al, 2002; Chari et al., 2003).
Although IGF-I may have potential in the treatment of demyelinating disorders, IGF-I has not been protective in all experimental studies of demyelination (Canella et al., 2000; O’Leary et al., 2002; Genoud et al., 2005) and a pilot study of recombinant IGF-I in multiple sclerosis patients failed to show benefits (Frank et al., 2002). Given the potential of IGF to promote tumorigenesis and the complexity of the IGF system, with multiple binding proteins regulating activity (discussed in Chesik et al., 2007; 2008), translating evidence from experimental studies that IGF influences remyelination to clinical practice is a significant challenge. Evidence from assays of plasma and cerebrospinal fluid (CSF) from MS patients that revealed no change in IGF-I levels but reduced GH in CSF (Poljakovic et al., 2006; Hosback et al., 2007) may support more investigation of direct effects of GH on oligodendrocytes and myelination, independent of any actions mediated by IGF-I.
Conclusion
There is accumulating evidence that the GH/IGF-I system is a critical regulator of the genesis and turnover of oligodendrocytes. Effects of the GH/IGF-I axis are not limited to development, but rather may influence replacement of oligodendrocytes in the normal adult brain and following demyelinating lesions. Aging-related changes in GH/IGF-I signaling may contribute to aging-related changes in the ability of the CNS to repair damage and to cognitive changes associated with normal aging. The relatively rapid and dynamic regulation of OPCs and developing oligodendrocytes demonstrated in the present study suggests that short-term manipulation of GH and/or IGF-I, if appropriately timed, might have benefits in the treatment of demyelinating disorders.
Acknowledgments
This work was supported by National Institutes of Health Grant PO1 AG11370 (D.R.R., W.E.S.) and by WFUSM Venture Funds (D.R.R.). The authors wish to thank Rhonda Ingram for technical assistance.
REFERENCES
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20(8):2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg ND, Johansson UE, Aberg MA, Hellstrom NA, Lind J, Bull C, Isgaard J, Anderson MF, Oscarsson J, Eriksson PS. Peripheral infusion of insulin-like growth factor-I increases the number of newborn oligodendrocytes in the cerebral cortex of adult hypophysectomized rats. Endocrinology. 2007;148(8):3765–3772. doi: 10.1210/en.2006-1556. [DOI] [PubMed] [Google Scholar]
- Armstrong CS, Wuarin L, Ishii DN. Uptake of circulating insulin-like growth factor-I into the cerebrospinal fluid of normal and diabetic rats and normalization of IGF-II mRNA content in diabetic rat brain. J Neurosci Res. 2000;59(5):649–660. doi: 10.1002/(SICI)1097-4547(20000301)59:5<649::AID-JNR8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Armstrong RC. Growth factor regulation of remyelination: behind the growing interest in endogenous cell repair of the CNS. Future Neurol. 2007;2(6):689–697. doi: 10.2217/14796708.2.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Neurophysiological comparison of dendritic cable properties in adolescent, middle-aged, and senescent rats. Exp Aging Res. 1979;5(3):195–206. doi: 10.1080/03610737908257198. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17(2):169–174. doi: 10.1002/(SICI)1098-1136(199606)17:2<169::AID-GLIA8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bohannon NJ, Corp ES, Wilcox BJ, Figlewicz DP, Dorsa DM, Baskin DG. Localization of binding sites for insulin-like growth factor-I (IGF-I) in the rat brain by quantitative autoradiography. Brain Res. 1988;444(2):205–213. doi: 10.1016/0006-8993(88)90931-6. [DOI] [PubMed] [Google Scholar]
- Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol. 1991;46(5):B180–B187. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- Canella KA, Seidman MM. Mutation spectra in supF: approaches to elucidating sequence context effects. Mutat Res. 2000;450(1–2):61–73. doi: 10.1016/s0027-5107(00)00016-6. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002;18(6):295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- Chari DM, Crang AJ, Blakemore WF. Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age. J Neuropathol Exp Neurol. 2003;62(9):908–916. doi: 10.1093/jnen/62.9.908. [DOI] [PubMed] [Google Scholar]
- Charlton HM, Clark RG, Robinson IC, Goff AE, Cox BS, Bugnon C, Bloch BA. Growth hormone-deficient dwarfism in the rat: a new mutation. J Endocrinol. 1988;119(1):51–58. doi: 10.1677/joe.0.1190051. [DOI] [PubMed] [Google Scholar]
- Cheng TC, Beamer WG, Phillips JA, 3rd, Bartke A, Mallonee RL, Dowling C. Etiology of growth hormone deficiency in little, Ames, and Snell dwarf mice. Endocrinology. 1983;113(5):1669–1678. doi: 10.1210/endo-113-5-1669. [DOI] [PubMed] [Google Scholar]
- Chesik D, Wilczak N, De Keyser J. The insulin-like growth factor system in multiple sclerosis. Int Rev Neurobiol. 2007;79:203–226. doi: 10.1016/S0074-7742(07)79009-8. [DOI] [PubMed] [Google Scholar]
- Chesik D, De Keyser J, Wilczak N. Insulin-like Growth Factor System Regulates Oligodendroglial Cell Behavior: Therapeutic Potential in CNS. J Mol Neurosci. 2008;35(1):81–90. doi: 10.1007/s12031-008-9041-2. Epub 2008 Feb 26. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23(5):941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Coculescu M. Blood-brain barrier for human growth hormone and insulin-like growth factor-I. J Pediatr Endocrinol Metab. 1999;12(2):113–124. doi: 10.1515/jpem.1999.12.2.113. [DOI] [PubMed] [Google Scholar]
- Cui QL, Almazan G. IGF-I-induced oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK, and Src-like tyrosine kinases. J Neurochem. 2007;100(6):1480–1493. doi: 10.1111/j.1471-4159.2006.04329.x. [DOI] [PubMed] [Google Scholar]
- Darnaudery M, Perez-Martin M, Belizaire G, Maccari S, Garcia-Segura LM. Insulin-like growth factor 1 reduces age-related disorders induced by prenatal stress in female rats. Neurobiol Aging. 2006;27(1):119–127. doi: 10.1016/j.neurobiolaging.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Donahue CP, Jensen RV, Ochiishi T, Eisenstein I, Zhao M, Shors T, Kosik KS. Transcriptional profiling reveals regulated genes in the hippocampus during memory formation. Hippocampus. 2002;12(6):821–833. doi: 10.1002/hipo.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden S. Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology. 1979;105(2):555–560. doi: 10.1210/endo-105-2-555. [DOI] [PubMed] [Google Scholar]
- Frank JA, Richert N, Lewis B, Bash C, Howard T, Civil R, Stone R, Eaton J, McFarland H, Leist T. A pilot study of recombinant insulin-like growth factor-1 in seven multiple sclerosis patients. Mult Scler. 2002 Feb;8(1):24–29. doi: 10.1191/1352458502ms768oa. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Zhao C, Sim FJ. Ageing and CNS remyelination. Neuroreport. 2002;13(7):923–928. doi: 10.1097/00001756-200205240-00001. [DOI] [PubMed] [Google Scholar]
- Fushimi S, Shirabe T. Expression of insulin-like growth factors in remyelination following ethidium bromide-induced demyelination in the mouse spinal cord. Neuropathology. 2004;24(3):208–218. doi: 10.1111/j.1440-1789.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- Gabriel SM, Roncancio JR, Ruiz NS. Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology. 1992;56(5):619–625. doi: 10.1159/000126284. [DOI] [PubMed] [Google Scholar]
- Genoud S, Maricic I, Kumar V, Gage FH. Targeted expression of IGF-1 in the central nervous system fails to protect mice from experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;168(1–2):40–45. doi: 10.1016/j.jneuroim.2005.06.033. Epub 2005 Aug 24. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol. 2001;48(2):75–86. [PubMed] [Google Scholar]
- Gilson J, Blakemore WF. Failure of remyelination in areas of demyelination produced in the spinal cord of old rats. Neuropathol Appl Neurobiol. 1993;19(2):173–181. doi: 10.1111/j.1365-2990.1993.tb00424.x. [DOI] [PubMed] [Google Scholar]
- Goddard DR, Berry M, Butt AM. In vivo actions of fibroblast growth factor-2 and insulin-like growth factor-I on oligodendrocyte development and myelination in the central nervous system. J Neurosci Res. 1999;57(1):74–85. doi: 10.1002/(SICI)1097-4547(19990701)57:1<74::AID-JNR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Gotts JE, Chesselet MF. Migration and fate of newly born cells after focal cortical ischemia in adult rats. J Neurosci Res. 2005;80(2):160–171. doi: 10.1002/jnr.20434. [DOI] [PubMed] [Google Scholar]
- Guan J, Bennet L, George S, Wu D, Waldvogel HJ, Gluckman PD, Faull RL, Crosier PS, Gunn AJ. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow Metab. 2001;21(5):493–502. doi: 10.1097/00004647-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48(1):23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96(10):857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJ. Distinctive patterns of PDGF-A, FGF-2, IGF-I, and TGF-beta1 gene expression during remyelination of experimentally-induced spinal cord demyelination. Mol Cell Neurosci. 1999;14(2):153–168. doi: 10.1006/mcne.1999.0771. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJ. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol Cell Neurosci. 2000;16(5):542–556. doi: 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- Hosback S, Hardiman O, Nolan CM, Doyle MA, Gorman G, Lynch C, O'Toole O, Jakeman P. Circulating insulin-like growth factors and related binding proteins are selectively altered in amyotrophic lateral sclerosis and multiple sclerosis. Growth Horm IGF Res. 2007;17(6):472–479. doi: 10.1016/j.ghir.2007.06.002. Epub 2007 Aug 13. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18(9):3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl NM, De Keyser J, De Vries H, Hoekstra D. Insulin-like growth factor binding proteins-1 and -2 differentially inhibit rat oligodendrocyte precursor cell survival and differentiation in vitro. J Neurosci Res. 2002;69(2):207–216. doi: 10.1002/jnr.10293. [DOI] [PubMed] [Google Scholar]
- Kumar S, Biancotti JC, Yamaguchi M, de Vellis J. Combination of growth factors enhances remyelination in a cuprizone-induced demyelination mouse model. Neurochem Res. 2007;32(4–5):783–797. doi: 10.1007/s11064-006-9208-6. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- Lesniak MA, Hill JM, Kiess W, Rojeski M, Pert CB, Roth J. Receptors for insulin-like growth factors I and II: autoradiographic localization in rat brain and comparison to receptors for insulin. Endocrinology. 1988;123(4):2089–2099. doi: 10.1210/endo-123-4-2089. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24(1):39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57(4):435–446. [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107(4):603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I decreases survival of dentate granule neurons: insights into the regulation of adult hippocampal neurogenesis. J Neurosci Res. 2006;83(2):199–210. doi: 10.1002/jnr.20719. [DOI] [PubMed] [Google Scholar]
- Liu X, Yao DL, Bondy CA, Brenner M, Hudson LD, Zhou J, Webster HD. Astrocytes express insulin-like growth factor-I (IGF-I) and its binding protein, IGFBP-2, during demyelination induced by experimental autoimmune encephalomyelitis. Mol Cell Neurosci. 1994;5(5):418–430. doi: 10.1006/mcne.1994.1052. [DOI] [PubMed] [Google Scholar]
- Liu X, Yao DL, Webster H. Insulin-like growth factor I treatment reduces clinical deficits and lesion severity in acute demyelinating experimental autoimmune encephalomyelitis. Mult Scler. 1995;1(1):2–9. doi: 10.1177/135245859500100102. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rao MS. Glial progenitors in the CNS and possible lineage relationships among them. Biol Cell. 2004;96(4):279–290. doi: 10.1016/j.biolcel.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Lobie PE, Garcia-Aragon J, Lincoln DT, Barnard R, Wilcox JN, Waters MJ. Localization and ontogeny of growth hormone receptor gene expression in the central nervous system. Brain Res Dev Brain Res. 1993;74(2):225–233. doi: 10.1016/0165-3806(93)90008-x. [DOI] [PubMed] [Google Scholar]
- Lupien SB, Bluhm EJ, Ishii DN. Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. J Neurosci Res. 2003;74(4):512–523. doi: 10.1002/jnr.10791. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87(3):559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Mason JL, Ye P, Suzuki K, D'Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J Neurosci. 2000;20(15):5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Goldman JE. A2B5+ and O4+ Cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci. 2002;20(1):30–42. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- McCarthy GF, Leblond CP. Radioautographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. J Comp Neurol. 1988;271(4):589–603. doi: 10.1002/cne.902710409. [DOI] [PubMed] [Google Scholar]
- McMorris FA, Smith TM, DeSalvo S, Furlanetto RW. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci U S A. 1986;83(3):822–826. doi: 10.1073/pnas.83.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris FA, McKinnon RD. Regulation of oligodendrocyte development and CNS myelination by growth factors: prospects for therapy of demyelinating disease. Brain Pathol. 1996;6(3):313–329. doi: 10.1111/j.1750-3639.1996.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9(6):729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Niblock MM, Brunso-Bechtold JK, Lynch CD, Ingram RL, McShane T, Sonntag WE. Distribution and levels of insulin-like growth factor I mRNA across the life span in the Brown Norway x Fischer 344 rat brain. Brain Res. 1998;804(1):79–86. doi: 10.1016/s0006-8993(98)00645-3. [DOI] [PubMed] [Google Scholar]
- Nyberg F, Burman P. Growth hormone and its receptors in the central nervous system--location and functional significance. Horm Res. 1996;45(1–2):18–22. doi: 10.1159/000184753. [DOI] [PubMed] [Google Scholar]
- O'Leary MT, Hinks GL, Charlton HM, Franklin RJ. Increasing local levels of IGF-I mRNA expression using adenoviral vectors does not alter oligodendrocyte remyelination in the CNS of aged rats. Mol Cell Neurosci. 2002;19(1):32–42. doi: 10.1006/mcne.2001.1062. [DOI] [PubMed] [Google Scholar]
- Pan W, Yu Y, Cain CM, Nyberg F, Couraud PO, Kastin AJ. Permeation of growth hormone across the blood-brain barrier. Endocrinology. 2005;146(1):4898–4904. doi: 10.1210/en.2005-0587. [DOI] [PubMed] [Google Scholar]
- Pang Y, Zheng B, Fan LW, Rhodes PG, Cai Z. IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia. 2007;55(11):1099–1107. doi: 10.1002/glia.20530. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207(6):707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljakovic Z, Zurak N, Brinar V, Korsic M, Basic S, Hajnsek S. Growth hormone and insulin growth factor-I levels in plasma and cerebrospinal fluid of patients with multiple sclerosis. Clin Neurol Neurosurg. 2006;108(3):255–258. doi: 10.1016/j.clineuro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129(1):119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47(5):455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenle E, Zapf J, Humbel RE, Froesch ER. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982;296(5854):252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- Sherlock M, Toogood AA. Aging and the growth hormone/insulin like growth factor-I axis. Pituitary. 2007;10(2):189–203. doi: 10.1007/s11102-007-0039-5. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51(3):173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18(12):4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SA, Gilson JM, Blakemore WF, Franklin RJ. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia. 1999;28(1):77–83. doi: 10.1002/(sici)1098-1136(199910)28:1<77::aid-glia9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22(7):2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lenham JE, Ingram RL. Effects of aging and dietary restriction on tissue protein synthesis: relationship to plasma insulin-like growth factor-1. J Gerontol. 1992;47(5):B159–B163. doi: 10.1093/geronj/47.5.b159. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4(2):195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26(6):929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Intracellular translocation of glutathione S-transferase pi during oligodendrocyte differentiation in adult rat cerebral cortex in vivo. Neuroscience. 2007;148(2):535–540. doi: 10.1016/j.neuroscience.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Thornton PL, Ingram RL, Sonntag WE. Chronic [D-Ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J Gerontol A Biol Sci Med Sci. 2000;55(2):B106–B112. doi: 10.1093/gerona/55.2.b106. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21(5):1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69(6):826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Wood TL, Loladze V, Altieri S, Gangoli N, Levison SW, Brywe KG, Mallard C, Hagberg H. Delayed IGF-1 administration rescues oligodendrocyte progenitors from glutamate-induced cell death and hypoxic-ischemic brain damage. Dev Neurosci. 2007;29(4–5):302–310. doi: 10.1159/000105471. [DOI] [PubMed] [Google Scholar]
- Ye P, D'Ercole AJ. Insulin-like growth factor I protects oligodendrocytes from tumor necrosis factor-alpha-induced injury. Endocrinology. 1999;140(7):3063–3072. doi: 10.1210/endo.140.7.6754. [DOI] [PubMed] [Google Scholar]
- Ye P, Lee KH, D'Ercole AJ. Insulin-like growth factor-I (IGF-I) protects myelination from undernutritional insult: studies of transgenic mice overexpressing IGF-I in brain. J Neurosci Res. 2000;62(5):700–708. doi: 10.1002/1097-4547(20001201)62:5<700::AID-JNR9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ye P, Popken GJ, Kemper A, McCarthy K, Popko B, D'Ercole AJ. Astrocyte-specific overexpression of insulin-like growth factor-I promotes brain overgrowth and glial fibrillary acidic protein expression. J Neurosci Res. 2004;78(4):472–484. doi: 10.1002/jnr.20288. [DOI] [PubMed] [Google Scholar]
- Ye P, Kollias G, D'Ercole AJ. Insulin-like growth factor-I ameliorates demyelination induced by tumor necrosis factor-alpha in transgenic mice. J Neurosci Res. 2007;85(4):712–722. doi: 10.1002/jnr.21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, Nave KA, Rowitch D, D'Ercole AJ, Ye P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55(4):400–411. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q, Lai Z, Roos P, Nyberg F. Characterization of growth hormone binding sites in rat brain. Acta Paediatr Suppl. 1994;406:92–95. doi: 10.1111/j.1651-2227.1994.tb13433.x. [DOI] [PubMed] [Google Scholar]