Abstract
In rats, whisking behavior is characterized by high-frequency synchronous movements and other stereotyped patterns of bilateral coordination that are rarely seen in the bilateral movements of the limbs. This suggests that the motor systems controlling whisker and limb movements must have qualitative or quantitative differences in their interhemispheric connections. To test this hypothesis, anterograde tracing methods were used to characterize the bilateral distribution of projections from the whisker and forepaw regions in the primary motor (MI) cortex. Unilateral tracer injections in the MI whisker or forepaw regions revealed robust projections to the corresponding MI cortical area in the contralateral hemisphere. Both MI regions project bilaterally to the neostriatum, but the corticostriatal projections from the whisker region are denser and more evenly distributed across both hemispheres than those from the MI forepaw region. The MI whisker region projects bilaterally to several nuclei in the thalamus, whereas the MI forepaw region projects almost exclusively to the ipsilateral thalamus. The MI whisker region sends dense projections to the contralateral claustrum, but those to the ipsilateral claustrum are less numerous. By contrast, the MI forepaw region sends few projections to the claustrum of either hemisphere. Bilateral deposits of different tracers in MI revealed overlapping projections to the neostriatum, thalamus, and claustrum when the whisker regions were injected, but not when the forepaw regions were injected. These results suggest that the bilateral coordination of the whiskers depends, in part, on MI projections to the contralateral neostriatum, thalamus, and claustrum.
Keywords: anterograde tracing, hemispheric coordination, corpus callosum, motor control
Exploratory whisking behavior in the rat is bilaterally coordinated during a variety of situations. During free-air whisking, for example, the whiskers on both sides of the head may move synchronously at similar amplitudes and frequencies (Gao et al., 2001; Sachdev et al., 2003; Sellien et al., 2005; Mitchinson et al., 2007). When whiskers on one side of the head contact a stimulus, however, the bilateral symmetry of the whisker movements is briefly decoupled. Thus, the stimulated whiskers immediately exhibit smaller sweeps while the contralateral whiskers exhibit larger sweeps, yet both sides continue to move in-phase at the same frequency (Sachdev et al., 2003; Mitchinson et al., 2007). Although bilateral sweeping movements of the whiskers are usually out-of-phase during horizontal head movements, the whiskers on both sides usually continue to move at similar frequencies (Towal and Hartmann, 2006). Hence, even though the whiskers may display asynchronous bilateral movements in response to tactile or vestibular inputs, their bilateral coordination is still apparent.
Bilateral coordination of whisking behavior suggests the presence of interhemispheric connections that coordinate the motor regions involved in controlling whisker movements. Several pieces of evidence indicate that MI cortex could play an important role in coordinating the bilateral movements of the whiskers. Electrical stimulation of MI cortex evokes abrupt twitches or sustained rhythmic movements of the contralateral whiskers (Hall and Lindholm, 1974; Gioanni and Lamarche, 1985; Brecht et al., 2004; Haiss and Schwarz, 2005), and these responses are consistent with MI projections to contralateral sites in the brainstem that control facial nerve activity (Hattox et al., 2002; Grinevich et al., 2005). Furthermore, unilateral ablation of MI cortex disrupts the bilateral symmetry of free-air whisking (Gao et al., 2003), and this suggests that some of the efferent projections from MI represent part of the interhemispheric pathways that coordinate bilateral whisker movements. In rodents, the MI whisker regions in each hemisphere are directly interconnected by short callosal pathways (Porter and White, 1983, 1984; Donoghue and Parham, 1983; Reep et al., 1987; Miyashita et al., 1994; Veinante and Deschenes, 2003), and such connections could synchronize these cortical regions (Sil’kis and Bogdanova, 1999). In addition, bilateral projections from MI cortex to other forebrain regions involved in motor control, such as the thalamus and neostriatum (Molinari et al., 1985; Wilson, 1986, 1987; Reiner et al., 2003; Leergaard et al., 2004; Alloway et al., 2008; Reep et al. 2008), could also facilitate interhemispheric synchronization of MI and other motor-related systems that control whisker movements.
While several tracing studies have reported bilateral projections from rat MI to the neostriatum and other brain regions, very few of them have characterized these pathways as a function of their origin in the whisker or limb representations of MI. Some studies have shown that the MI whisker region projects bilaterally to the thalamus, but corticothalamic projections from the MI forepaw region terminate almost exclusively on the ipsilateral side (Rouiller et al., 1991; Alloway et al., 2008). This functional distinction is significant because, when compared to whisking behavior, the forelimbs are less likely to exhibit high-frequency bilateral coordination that requires rapid interhemispheric communication.
Therefore, to characterize the interhemispheric connections of rat MI cortex with respect to its functional subdivisions, we injected anterograde tracers into the whisker or forepaw regions of MI and then reconstructed the bilateral distribution of the labeled terminals in forebrain regions that are involved in motor control. In some rats we injected a single tracer into only one hemisphere so that we could characterize labeling in the contralateral MI and determine how these callosal projections relate to bilateral labeling in other brain regions. In other rats, however, we made two separate tracer deposits, either bilaterally into both MI whisker regions or bilaterally into both MI forepaw regions. The present study characterizes and compares the MI projections to the neostriatum, thalamus, and claustrum because these forebrain regions receive bilateral projections from MI cortex, and these connections suggest that these neural structures have a role in the bilateral coordination of motor activity (Molinari et al., 1985; Wilson et al., 1987; Crescimanno et al., 1989; Rouiller et al., 1991; Reiner et al., 2003; Alloway et al., 2008).
MATERIALS AND METHODS
Neuronal tracing experiments were performed on adult male Sprague-Dawley rats (Charles River). All animal procedures followed NIH guidelines and were approved by the Penn State Institutional Animal Care and Use Committee.
Animal surgery
Prior to surgery, each rat was anesthetized with intramuscular (IM) injections of ketamine (20 mg/kg) and xylazine (6 mg/kg). Each rat received atropine methyl nitrate (0.05 mg/kg, IM), chloramphenicol (50 mg/kg, IM), and dexamethasone sodium phosphate (5 mg/kg, IM). Heart rate and end-tidal CO2 were monitored continuously, and body temperature was maintained at 37°C. After placing the rat in a stereotaxic instrument, the skin over the cranium was resected and the wound margins were infiltrated with 2% lidocaine. Craniotomies were made at stereotaxic coordinates consistent with previous MI maps (Hall and Lindholm, 1974; Neafsey et al., 1996; Hoffer et al., 2003).
Tracer injections
Intracranial microstimulation (ICMS) was used to locate the whisker and forepaw regions in MI cortex. Previously published maps and coordinates of MI cortex were used to guide the placement of electrodes in sites likely to evoke movements of the whiskers or forepaw (Hall and Lindholm, 1974; Gioanni and Lamarche, 1985; Neafsey et al., 1986). Cathodal pulse trains lasting 80 ms (0.7-ms pulses and 3.3-ms interpulse intervals) were administered by low impedance (0.3 to 1.8 MΩ) glass micropipettes that contained hypertonic (3M) saline. After using pulses of 100-150 μA to initiate twitches of the contralateral whiskers or forepaw, current was gradually reduced to threshold levels (< 50 μA) until twitching disappeared. After locating an appropriate tracer injection site, the stimulation electrode was removed and a tracer-filled pipette was inserted in its place. Injections into the whisker region varied from 1.6-3.1 mm rostral and 0.9 -2.1 mm lateral to bregma. Tracer injections into the MI forepaw region varied from 0.9-3.0 mm rostral and 1.9-4.2 mm lateral to bregma. This forepaw region corresponds to the caudal forelimb area identified previously (Rouiller et al., 1993).
The fluorescent anterograde tracers Fluoro-ruby (FR) and Alexa-fluoro (AF) (D-1817 and D-2290, Molecular Probes, Eugene, Oregon) were pressure-injected from small glass pipettes (40-120 μm tip) cemented to the needle of a Hamilton microsyringe. Ejection volumes were controlled by a calibrated injector-holder (model 5000, Kopf Instruments, Tujunga, CA) fastened to a micromanipulator on the stereotaxic frame. Injections were made at sites located 1.2-1.7 mm deep in MI cortex; the volume of pressure-injected tracers ranged from 100 to 170 nl.
In some rats, biotinylated dextran amine (BDA) (Molecular Probes, D-7135) was iontophoretically deposited into MI cortex. In these cases, a 15% solution of BDA in 0.01 M phosphate-buffered saline (pH 7.3) was placed in glass pipettes having an outer diameter of 40-80 μm. Positive current (5-6 μA) pulses were applied in alternating on-off intervals of 7 seconds for 6 to 8 minutes. Deposits of BDA were placed 1.2-1.7 mm below the pial surface. In a few cases, BDA was injected at 2 or 3 penetrations located within 200 μm of each other to increase the density of the BDA deposit.
Following tracer injections, the wound margin was sutured and the animal received an injection of atropine and dexamethasone. Each animal was returned to its home cage for 7-12 days.
Histology
Following induction of deep anesthesia with sodium pentobarbital (100 mg/kg, i.p.), each rat was transcardially perfused with physiological saline, 4% paraformaldehyde in 0.1 M phosphate buffer (pH 6.9), and 4% paraformaldehyde with 10% sucrose. The brain was removed and refrigerated in 4% paraformaldehyde containing 30% sucrose until it sank. Prior to sectioning the brain coronally at a thickness of 60 or 80 μm, a small slit was made in the ventral cortex and brainstem on the left side to distinguish the two sides of the brain. Sections were serially processed; one or two series were processed for tracer labeling and another series was either stained with thionin or processed for cytochrome oxidase to reveal cytoarchitectonic boundaries (Wong-Riley, 1979; Land and Simons, 1985).
Sections processed for BDA were handled as described previously (Kincaid and Wilson, 1996; Alloway et al., 1998). Sections were agitated in 0.3% H2O2 and then in 0.1 M PB with 0.3% Triton-X100 (pH 7.4) before being incubated in an activated avidin-biotinylated horseradish peroxidase solution (Vector Novocastra Laboratories, Burlingame, CA) for 2-4 hours. Following rinses in PB, sections were incubated in 0.05% diaminobenzidine (DAB), 0.005% H2O2, and 0.04% NiCl2 in 0.1M Tris buffer (pH 7.1) for 9-12 minutes, and then were briefly washed in a final rinse of PB. Processed sections were serially mounted on gel-coated slides, dried overnight, and dipped in alcohol and xylene before they were coverslipped with Cytoseal.
Sections processed for fluorescent labeling were mounted in serial order on gel-coated slides. After drying overnight, sections were dipped in alcohol and xylene, and then coverslipped with Cytoseal. A combined fluorescein isothiocyanate/tetrarhodamine isothiocyanate (FITC/TRITC) filter set (51004v2, Chroma Technology; Rockingham, VT) was used to visualize AF- or FR-labeled processes.
Anatomical Analysis
Labeled terminals were digitally reconstructed with respect to nearby nuclei and other anatomical landmarks as defined by Paxinos and Watson (1986). Cortical projections to the neostriatum, thalamus, and other regions have terminal arbors that contain beaded enlargements or varicosities along their axon terminals. Ultrastructural examination of corticostriatal terminals indicates that these varicosities contain vesicles and have other morphologic features that resemble en passant synapses (Kincaid and Wilson, 1996). Furthermore, beaded varicosities are associated with synaptic markers such as synaptophysin (Voigt et al., 1993; Meng et al., 2004). Labeled varicosities were plotted using an AccuStage reconstruction system (St. Paul, MN) in conjunction with an Olympus light microscope (BH-2). The reconstructions were stored on computer disk for subsequent analysis.
To compare the bilateral distribution patterns in each brain region, the plotted reconstructions of the labeled terminals were subdivided into a grid of square bins as described previously (Hoffer et al., 2005). Several bin sizes (50, 100, 150, and 200 μm2) were used to insure that the results did not depend on a specific bin size. For each tracer, we counted the number of bins in each brain region that contained terminals labeled by a specific tracer and then calculated the proportion of those bins that were located contralateral to the injection site (ie., ratio of contralateral-to-total labeling).
For rats that received bilateral tracer injections, we calculated the tracer overlap in each brain region. Bins that contained AF- or FR-labeled terminals were colored green or magenta, respectively, whereas those that contained both tracers were colored white. The green, magenta, and white bin counts in each brain region were summed across both sides of the brain, and the labeled overlap (ie., the number of white bins) was expressed as a proportion of the total number of bins that contained labeled terminals.
Digital photomicrographs of neuronal labeling were acquired by use of a Cool Snap HQ CCD digital camera (Roper Scientific, Tucson, AZ) mounted on the microscope, which was equipped with 1X, 2X, 4X, 10X, and 20X objectives. Images were stored as TIF files, which were imported into a software program (Deneba Systems, Inc., Canvas X; Miami, FL) in which image brightness and contrast were adjusted to portray labeling as it appeared in the microscope. In one image, some microscopic tears in a section of tissue resembled labeled fibers, and these artifacts were removed from the image.
RESULTS
Quantitative analysis was done only on cases that satisfied several criteria. The primary requirement was that labeled terminals were present in all brain regions known to receive projections from MI cortex (Molinari et al., 1985; Wilson, 1987; Miyashita et al. 1987; Hattox et al., 2002; Leergaard et al., 2004). Cases in which labeling was absent in the neostriatum, thalamus, or pons were discarded. We also discarded cases if part of the brain was damaged or if the brainstem had not been saved for sectioning. In addition, we discarded cases in which the tracer injections invaded the white matter or the histology was problematic. Based on these criteria, we analyzed 26 cases as shown in Table 1. In 14 of these cases, a single tracer was injected unilaterally into either the MI whisker (n = 7) or forepaw (n = 7) region. In the remaining 12 cases, two different tracers were separately injected into corresponding whisker (n = 6) or forepaw (n = 6) regions of each hemisphere. The corticothalamic projections in 14 of these 26 cases were described previously (Alloway et al., 2008), but some cases (n = 10) in that earlier report are not analyzed here because the brainstem had been discarded before the brain was sectioned.
Table 1. Summary of Tracer Injections in MI Cortex.
| Left Hemisphere | Right Hemisphere | |||
|---|---|---|---|---|
| Case | Tracer1 | Region2 | Tracer | Region |
| BN09 | AF - 1 | Agm - Wh | FR - 1 | Agm - Wh |
| BN11 | AF - 1 | Agm - Wh | FR - 1 | Agm - Wh |
| BN12 | AF - 1 | Agm - Wh | FR - 1 | Agm - Wh |
| BN15 | AF - 1 | Agl - Fp | FR - 1 | Agl - Fp |
| BN16 | AF - 1 | Agl - Fp | FR - 1 | Agl - Fp |
| BN19 | BDA - 2 | Agm - Wh | ||
| BN20 | BDA - 2 | Agm - Wh | ||
| BN21 | BDA - 2 | Agl - Fp | ||
| BN22 | FR - 1 | Agl - Fp | ||
| BN23 | FR - 1 | Agl - Fp | ||
| BN25 | FR - 1 | Agm - Wh | ||
| BN27 | BDA - 3 | Agm - Wh | ||
| BN28 | AF - 1 | Agl - Fp | FR - 1 | Agl - Fp |
| BN33 | AF - 1 | Agl - Fp | FR - 1 | Agl - Fp |
| BN37 | BDA - 2 | Agm - Wh | FR - 1 | Agm - Wh |
| BN38 | FR - 1 | Agm - Wh | ||
| BN39 | FR - 1 | Agm - Wh | ||
| BN40 | BDA - 2 | Agl - Fp | FR - 1 | Agl - Fp |
| BN41 | BDA - 2 | Agl - Fp | FR - 1 | Agl - Fp |
| BN45 | FR - 1 | Agm - Wh | ||
| BN49 | FR - 1 | Agm - Wh | BDA - 1 | Agm - Wh |
| BN51 | FR - 1 | Agl - Fp | ||
| BN52 | FR - 1 | Agl - Fp | ||
| BN53 | FR - 1 | Agm - Wh | BDA - 2 | Agm - Wh |
| BN54 | FR - 1 | Agl - Fp | ||
| BN56 | BDA - 2 | Agl - Fp | ||
AF, Alexa Fluoro; FR, Fluoro-Ruby; BDA, biotinylated dextran amine
Agm-Wh, medial agranular whisker region; Agl-Fp, lateral agranular forepaw region
Bilateral topography of projections from the MI whisker region
In 7 rats, a single tracer was deposited unilaterally at ICMS sites that evoked brief, caudally-directed deflections of one or more whiskers. As shown by Figure 1, histologic examination revealed that these whisker-related sites are associated with the medial agranular (Agm) zone of MI. This region is characterized by a narrow layer III and an upward expansion of layer V that adds to its overall thickness. By comparison, tracer injections at ICMS sites that evoked dorsiflexion of the forepaw were located in the lateral agranular (Agl) zone, which is characterized by a thinner layer V and a thicker layer III. These observations confirm a previous report that compared MI cytoarchitecture with detailed maps of the motor responses produced by ICMS (Brecht et al., 2004).
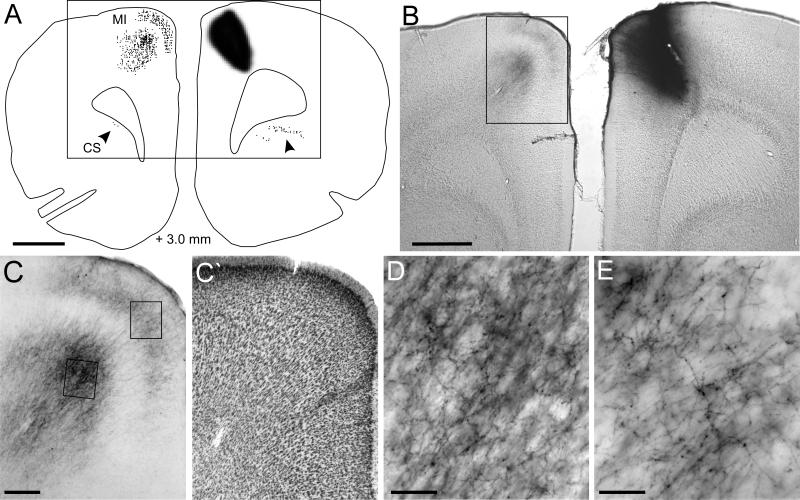
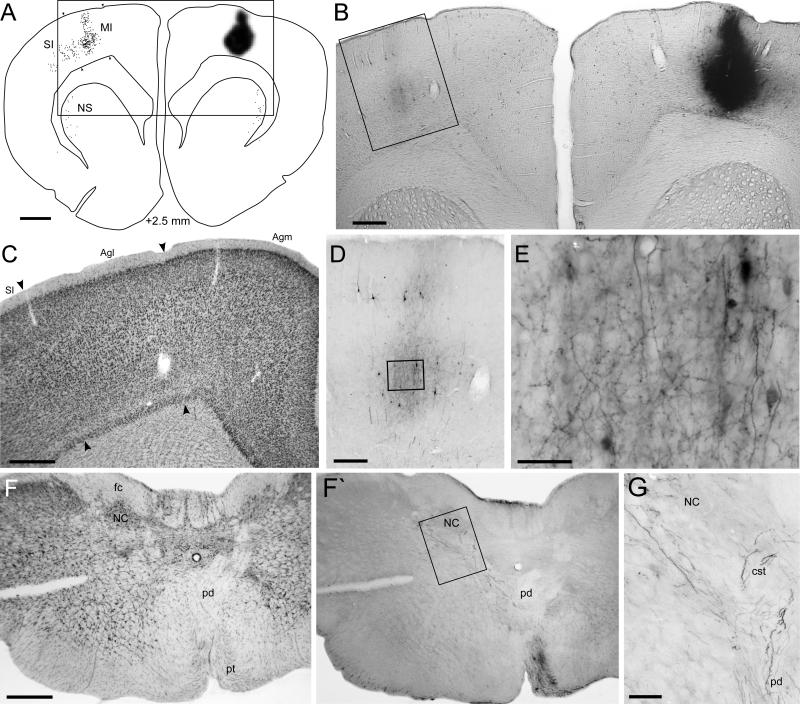
Fig. 1.
Callosal projections from the MI whisker region in BN27. A: Reconstruction of the BDA deposit and labeled terminals in the claustrum (arrowheads) and contralateral MI. Rectangle indicates panel B; distance from bregma indicated at bottom. B: Photomicrograph of the BDA deposit and labeled terminals in contralateral MI. Rectangle indicates panel C. C: Laminar pattern of BDA-labeling in MI. Rectangles indicate panels D and E. C’: Adjacent thionin-stained section showing the laminar cytoarchitecture of Agm. D: Labeled axons and varicosities in layer Vb. E: Labeled axons and varicosities in layers II. Scale bars: 1 mm (A, B), 250 μm (C, C’), and 50 μm (D, E).
Unilateral tracer deposits in the MI whisker region produced substantial amounts of terminal labeling in the contralateral MI cortex. This labeling was concentrated in two dense bands that were separated by a slender, unlabeled layer of cortex (see Fig. 1B, C). Previous reports showed similar features in the laminar patterns of the callosal projections from MI (Reep et al., 1987; Miyashita et al., 1994). Our comparison of these MI labeling patterns with adjacent thionin-stained sections indicates that the dense bands of labeled terminals were in layers III and V, and that the intervening unlabeled region represents the dorsal expansion of layer V that is characteristic of Agm cortex (compare Fig. 1C and 1C). Hence, the callosal projections from the MI whisker region are connected with the corresponding MI whisker region of the contralateral hemisphere. Furthermore, the labeled terminals in both bands of Agm are characterized by a dense plexus of arbors and beaded varicosities that signify presynaptic connections (Fig. 1D, E).
Many projections to the contralateral MI whisker region represent collateral fibers that diverged from the main callosal axon. As shown in Figure 2, the thickest part or apex of the external capsule contains many labeled fibers that divide into collateral axons (Fig. 2C, D), some of which ascended directly into the overlying MI cortex (Fig 2D, E). The other branches of these fibers proceeded laterally and ventrally in the external capsule, but their complete course and destination could not be followed to specific brain regions within a single coronal section. Nonetheless, many labeled fibers were observed that departed from the external capsule and entered the dorsolateral part of the neostriatum. As noted by other investigators (Veinante and Deschenes, 2003), some callosal fibers entered the contralateral parietal cortex, which contains primary somatosensory (SI) cortex. Because the labeled terminals in contralateral SI were relatively sparse and were widely separated from the MI whisker region, we usually did not reconstruct the projections to SI. Other labeled fibers continued further ventrolaterally in the external capsule and terminated in the contralateral claustrum, which is located near the rhinal fissure. Previous reports indicate that the claustrum receives dense projections from motor cortex (Minciacchi et al., 1985; Li et al., 1986; Crescimanno et al., 1989; Sadowski et al., 1997), and we always reconstructed the location of labeled varicosities that appeared in the claustrum.
Fig. 2.
Labeled collaterals in the contralateral external capsule. A: Reconstructed section for BN27 shows the caudal end of the BDA deposit and labeled varicosities in the neostriatum, claustrum, and contralateral MI. B: Photomicrograph of the section in panel A. Arrowhead indicates intense labeling in the contralateral claustrum; rectangle shows the region in panel C. C: Labeled axons in the external capsule; rectangle indicates panel D. D: Magnified view of BDA-labeled axons in the external capsule; rectangles indicate panels E and F. E,F: Labeled axons splitting into collaterals (arrowheads), some of which project to MI. Scale bars: 1 mm (A,B), 100 μm (C), 50 μm (D), and 25 μm (E,F).
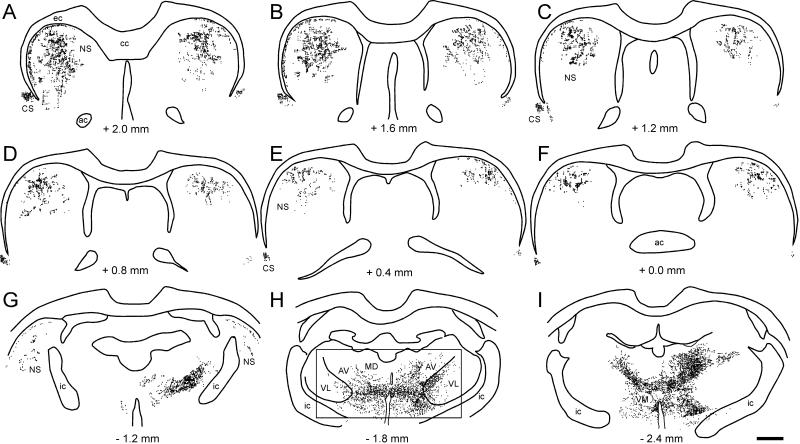
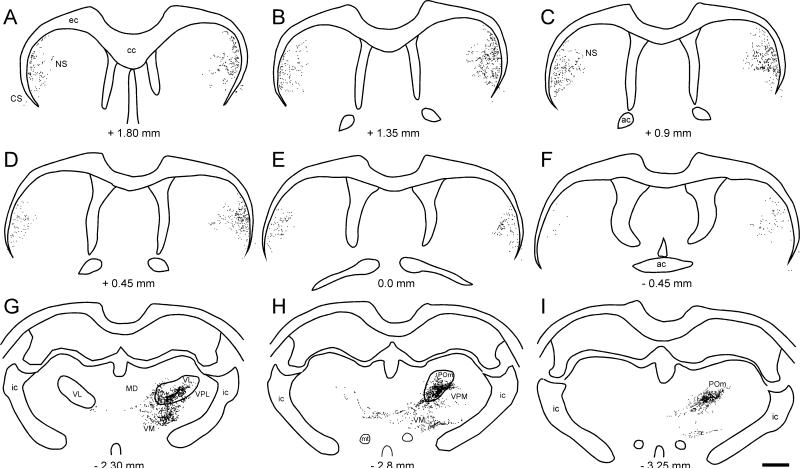
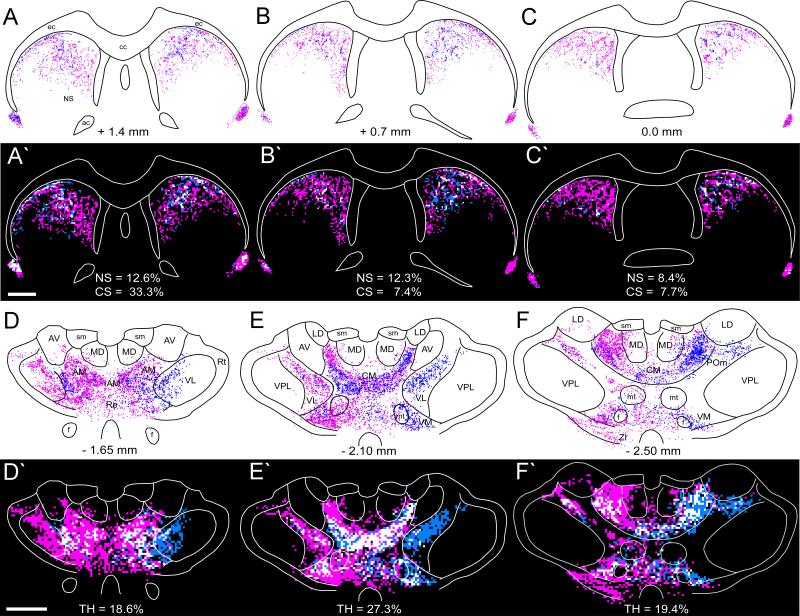
As shown by Figure 3, unilateral tracer deposits in the MI whisker region produced substantial amounts of bilateral labeling throughout the rostrocaudal extent of the neostriatum. Although a few faintly labeled neurons were occasionally observed in the neostriatum, virtually all of the neostriatal labeling was due to anterograde transport of the tracer. Corticostriatal projections from the MI whisker region were concentrated in the dorsolateral and central parts of the rostral neostriatum and, as shown in Figure 4, were dense enough to be visualized in a low power photomicrograph. Furthermore, the bilateral pattern of the corticostriatal projections from the MI whisker region was almost symmetrical across the two hemispheres. Nonetheless, even though neostriatal labeling was almost evenly distributed in both hemispheres of some cases, the majority of corticostriatal projections from the MI whisker region terminated on the ipsilateral side.
Fig. 3.
Bilateral distribution of BDA-labeled varicosities in the neostriatum, claustrum, and thalamus of BN27. Distance from bregma appears below each reconstruction; rectangles indicate regions depicted in Fig 4B and 4D. Scale, 1 mm.
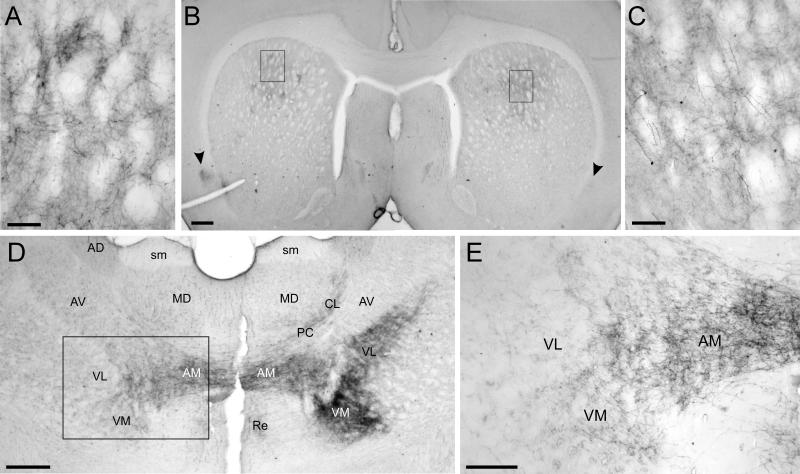
Fig. 4.
Photomicrographs of BDA labeling in the neostriatum, claustrum, and thalamus of BN27. A: Magnified view of BDA-labeled terminals in the contralateral neostriatum. B: Low power view of BDA labeling in the contralateral (left) and ipsilateral (right) neostriatum and claustrum (arrowheads). Rectangles indicate panels A and C. C: Magnified view of BDA-labeled terminals in the ipsilateral neostriatum. D: Low power view of BDA labeling in the thalamus. Rectangle indicates panel E. E: Labeled terminals in contralateral thalamus. Scale bars: 100 μm (A, C), 500 μm (B, D), and 250 μm (E).
Labeled terminals appeared bilaterally in the claustrum following tracer injections in the MI whisker region, but the amount of terminal labeling was noticeably higher on the contralateral side. As shown by photomicrographs and reconstructed sections in Figures 2, 3, and 4, both the density and spatial extent of labeled terminals in the claustrum are higher on the contralateral side. Some retrogradely-labeled neurons appeared in the claustrum as well, especially when the fluorescent tracers FR or AF were injected, but most of these labeled cells were on the ipsilateral side (data not shown).
As indicated by our recent report (Alloway et al., 2008), projections from the MI whisker region terminate bilaterally in the thalamus. As shown in Figures 3 and 4, the majority of the corticothalamic projections innervate several nuclei in the ipsilateral thalamus, including VL, VM, POm, AM, and parts of the intralaminar PC and CL. Other corticothalamic projections, however, crossed the midline and terminated in the contralateral thalamus, especially the AM and VM nuclei, and to a lesser extent in VL and some intralaminar nuclei (see Figs. 4D and 4E). Although injections of AF or FR into the MI whisker region produced many retrogradely-labeled neurons in the thalamus, very few labeled soma were seen in the thalamus when BDA was injected. Given that BDA produced dense terminal labeling in many parts of the contralateral thalamus (see Fig. 4D, E), the lack of BDA-labeled neurons in the ipsilateral thalamus indicates that the labeled terminals in the contralateral thalamus do not represent collaterals of thalamocortical projection neurons.
Bilateral topography of projections from the MI forepaw region
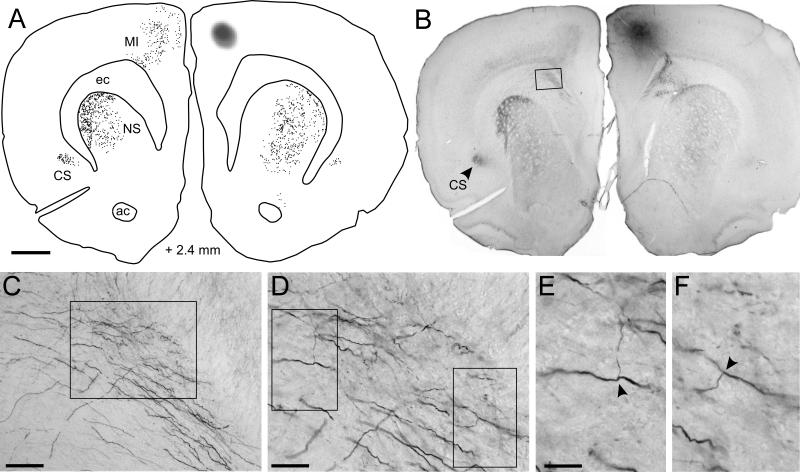
Tracer injections at ICMS sites that produced forepaw movements were always located in the Agl zone, which is located just lateral and dorsal to the apex of the external capsule (see Fig. 5). The location of tracer injections in the MI forepaw region was also verified by the presence of labeled fibers in the brainstem that crossed through the pyramidal decussation before entering the descending corticospinal tract (Fig. 5F’, G).
Fig. 5.
Projections from the MI forepaw regions in BN56. A: Reconstruction of BDA deposit and labeled terminals in the neostriatum and contralateral MI. Arrowheads indicate the boundaries of Agl. Rectangle indicates panel B; distance from bregma indicated at bottom. B: Photomicrograph of the BDA deposit and labeled terminals in contralateral MI. Rectangle indicates panel D. C: Adjacent thionin-stained section showing the boundaries (arrowheads) of Agl in the left hemisphere. D: Laminar distribution of BDA-labeled projections in the contralateral MI cortex. Rectangle indicates panel E. E: BDA-labeled terminals in contralateral MI. F, F’: Adjacent thionin and BDA-labeled sections through the pyramidal decussation. Rectangle indicates panel G. G: Labeled axons in the corticospinal tract. Scale bars: 1 mm (A), 500 μm (B, C, F, F’), 250 μm (D), 100 μm (G), and 50 μm (E).
Callosal projections from the MI forepaw region terminate in the corresponding part of the contralateral MI cortex. After crossing through the corpus callosum, many labeled axons in the external capsule could be seen splitting into collaterals, one branch of which proceeded into the overlying MI cortex (data not shown). Analysis of the adjacent thionin-stained section indicated that the labeled projections to the contralateral MI terminate in regions that correspond to Agl cortex, where layer III is noticeably thicker than in Agm (Fig. 5B, C). Hence, the MI forepaw region in one hemisphere is interconnected with the MI forepaw region of the contralateral hemisphere.
Like the callosal projections from the MI whisker region, the callosal projections from the MI forepaw region terminate in two layers that are separated by a sparsely-labeled region in the upper part of layer V. In contrast to the projections from the MI whisker region, however, the callosal projections from the MI forepaw region formed an extensive band of labeling within the coronal sections that stretched into the adjacent parietal region. Hence, when the MI forepaw region was injected, the boundary between MI and SI was not readily apparent from the labeling patterns and, therefore, we reconstructed terminal labeling in both the contralateral Agl and adjacent parts of the parietal cortex. The demarcation between these regions was subsequently determined by inspecting adjacent thionin-stained or CO-labeled sections. This contiguity in labeling across the borders of Agl and SI is consistent with the juxtaposition of the SI and MI forepaw regions in the rat brain (Sanderson et al., 1984; Chapin and Woodward, 1986; Li et al., 1990; Fabri and Burton, 1991).
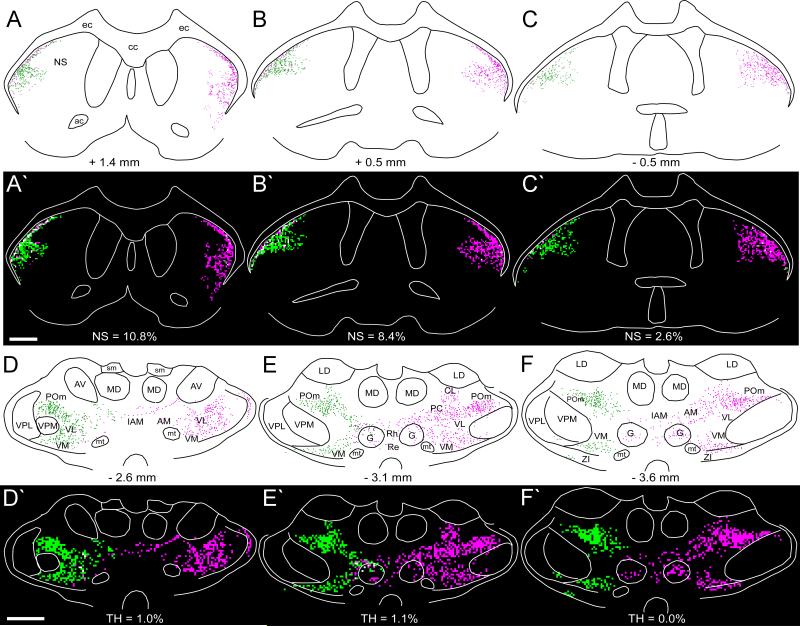
The bilateral forebrain projections from the MI forepaw region are noticeably different from the bilateral patterns produced by tracer deposits in the MI whisker region. As shown by Figure 6, tracer injections in the MI forepaw region produced little terminal labeling in the claustrum of either hemisphere. Retrogradely-labeled neurons in the claustrum were largely absent as well, although a few labeled neurons on the ipsilateral side were occasionally observed. Furthermore, in contrast to the dense labeling that appears in the contralateral thalamus after injecting the MI whisker region, very few projections from the MI forepaw region terminate in the contralateral thalamus. Virtually all corticothalamic projections from the MI forepaw region terminate on the ipsilateral side and are densest in VL, VM, POm, AM, and parts of the intralaminar nuclei.
Fig. 6.
Bilateral distribution of BDA-labeled varicosities in the neostriatum, claustrum, and thalamus of BN56. Distance from bregma appears below each diagram. Scale, 1 mm.
Both MI regions project bilaterally to the neostriatum, but the corticostriatal projection patterns from these regions are distinct. The corticostriatal projections from the MI forepaw region are sparser and less extensive than those from the MI forepaw region (compare Figures 3 and 6). Whereas the MI whisker region projects to the dorsolateral and central parts of the neostriatum, projections from the MI forepaw region are concentrated along its lateral and ventral edges. Furthermore, corticostriatal projections from the MI forepaw region terminate predominantly in the ipsilateral hemisphere, whereas those from the MI whisker region are more evenly balanced across both sides of the brain.
Relative distribution of MI projections
To assess these differences in the bilateral distribution of projections from the whisker and forepaw regions in MI, we compared the amount of terminal labeling in the forebrain regions of the 14 cases that received unilateral tracer injections in MI. Because tracer deposits varied in size and, presumably, in the number of efferent fibers that were labeled, the amount of labeling in each brain region was expressed as a proportion of the total labeling observed across all of the target regions that were analyzed. The relative distributions of these projections from the MI whisker (n = 7) and forepaw (n = 7) regions are shown in Figure 7.
Fig. 7.
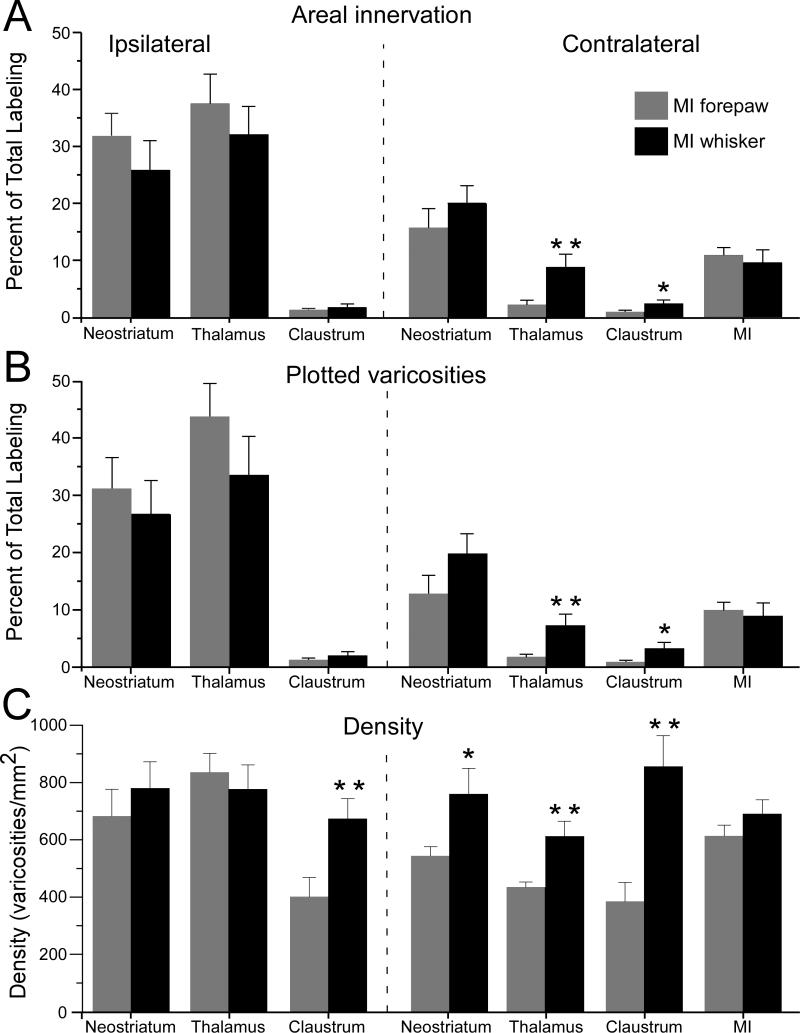
Bargraphs depicting the relative distribution of bilateral projections in cases that received a unilateral tracer injection in the MI whisker (n = 7) or forepaw (n = 7) regions. A: Mean proportion of labeled area in each of 7 brain regions. For each injected rat, the sum of the labeled bins (50 μm2) across the 7 brain regions equals 100%. B: Mean proportion of plotted varicosities in each of 7 brain regions. For each rat, the sum of the labeled varicosities across the 7 brain regions equals 100%. C: Density of plotted varicosities in each brain region. Brackets represent SEM; asterisks indicate significant differences between the MI whisker and MI forepaw groups (* p < 0.05, ** p < 0.01).
Consistent with differences in the size of the target brain regions, it was not surprising to find that most of the terminal labeling was in the thalamus and neostriatum, and that the claustrum contained the least amount of labeling. Furthermore, regardless of whether we injected the whisker or forepaw region in MI, the thalamus contained the most labeling on the ipsilateral side, and the neostriatum contained the most labeling on the contralateral side.
Despite these similarities, the placement of tracers in the whisker or forepaw regions of MI produced significant differences in the relative distribution of labeled terminals in some brain regions. Whether we analyzed the size of the innervated area or the number of labeled varicosities, the proportion of labeling in the contralateral thalamus was significantly larger when the MI whisker region was injected (t ≥ 2.71, p < 0.01). Measurements of these same parameters also showed that tracer deposits in the MI whisker region produced a larger proportion of labeling in the contralateral claustrum (t ≥ 2.10, p < 0.05). Although the proportion of labeling varied in the other brain regions, these differences were not significant.
We also analyzed the density of labeled terminals in each of the target brain regions in the ipsilateral and contralateral hemispheres. In contrast to measures that express terminal labeling as a fraction of total labeling (see Fig. 7A and 7B), labeling density is not biased by the relative size of the brain region. Furthermore, because postsynaptic targets are more likely to be activated by input projections that have a high density of terminal arbors, a density measurement provides an indication of whether MI cortex is likely to have greater or lesser impact on neurons in the target brain region.
Even though variations in tracer deposits could affect the relative density of projections from the MI whisker and forepaw regions, labeling density in the ipsilateral thalamus, ipsilateral neostriatum, and contralateral MI cortex was essentially the same for both groups of rats (see Fig. 7C). This suggests that the tracer deposits in the two groups of rats were probably equivalent in terms of their size and related factors. Nonetheless, when compared to the projections from the forepaw region, the projections from the MI whisker region had higher densities of labeled varicosities in the contralateral neostriatum (t = 2.24, p < 0.05), contralateral thalamus (t = 3.15, p < 0.01), contralateral claustrum (t = 3.70, p < 0.01), and ipsilateral claustrum (t = 2.77, p < 0.01).
Overlapping projections from MI
To assess whether the whisker and forepaw regions in MI project to overlapping parts of the forebrain, we injected different anterograde tracers into the corresponding MI regions of each hemisphere and then measured the amount of tracer overlap in the claustrum, neostriatum, and thalamus. Projections to contralateral MI were not analyzed because the tracer injections obscured the visualization of labeled terminals originating from the contralateral hemisphere. Figure 8 shows examples of bilateral tracer injections in the MI whisker and forepaw regions.
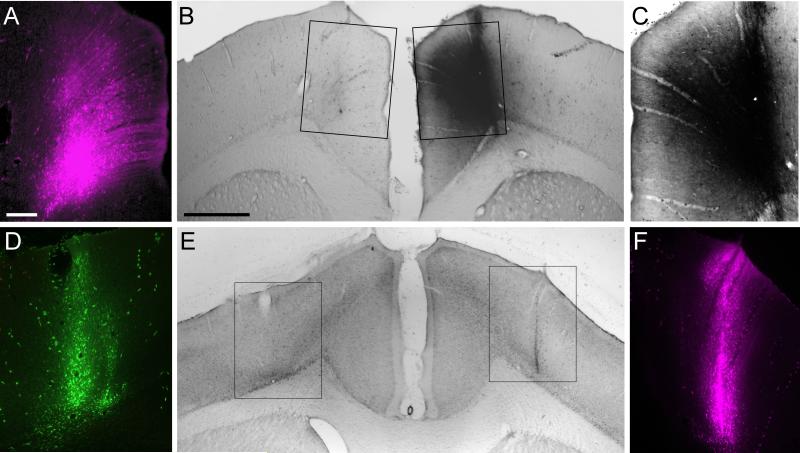
Fig. 8.
Bilateral tracer injections in the MI whisker (top panels) and forepaw (bottom panels) regions. A: FR injection in the left MI whisker area in BN53. B: Tracer injections in the left and right hemispheres of BN53 as seen in conventional light microscopy. Rectangles indicate panels A and C. C: BDA deposit in the right MI whisker area in BN53. D,E,F: AF (left) and FR (right) injections in the MI forepaw regions in BN16 shown as in the top panels. Scale bars: 250 μm (A, C, D, F) and 1.0 mm (B, E).
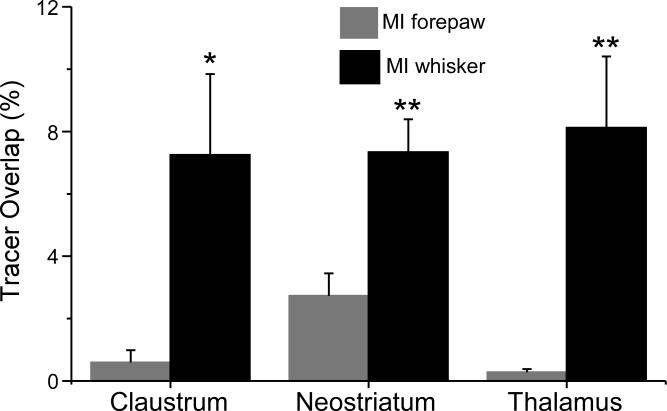
As indicated by Figure 9, deposits of different tracers into the whisker region of each hemisphere produced bilateral tracer overlap in the claustrum, neostriatum, and thalamus. The exact location and amount of tracer overlap in each brain region varied according to several factors. In the claustrum, for example, tracer overlap was greatest in rostral sections that contained a high density of labeled projections from both injection sites (see Fig. 9A, A’). More caudally, however, a rapid decline in the labeled projections to the ipsilateral claustrum produced a corresponding decrease in tracer overlap. The amount of tracer overlap in the claustrum was not bilaterally symmetrical because of slight differences in the location and volume of the tracer injections, as well as inherent differences in the transport efficiency of each tracer. The fluorescent tracer FR was generally the best in terms of transport efficiency, and FR usually produced more labeling in the ipsilateral claustrum than either AF or BDA. Consequently, after placing tracers bilaterally in the MI whisker region, the amount of tracer overlap in the claustrum was always greater on the side ipsilateral to the FR injection site.
Fig. 9.
Bilateral distribution of FR- and BDA-labeled projections from the MI whisker regions in BN53. A: Reconstructions of BDA- (blue dots) and FR- (magenta dots) labeled varicosities in the neostriatum and claustrum. The distance from bregma appears below each section. A’: Overlap analysis based on a grid of 50 μm2 bins. Bins that contained BDA- or FR-labeled varicosities are colored blue or magenta, respectively; those that contained projections from both injections are white. The proportion of white bins in the neostriatum (NS) and claustrum (CL) of each section are expressed as percentages. B, B’: Distribution of labeled terminals in the thalamus of BN53. Reconstructions and overlap analysis presented as in panels A and A’. Scale bars, 1 mm.
With respect to the neostriatum, overlapping projections from the MI whisker regions were highest in the rostral half of that structure. As seen in Figure 9, tracer overlap was most prevalent in the dorsolateral edge and central parts of the rostral neostriatum. The amount of corticostriatal labeling decreased at more caudal locations, and this produced a concomitant decrease in tracer overlap as well. As in the claustrum, tracer overlap in the neostriatum was not bilaterally symmetrical, largely because FR was more efficient for revealing projections to the contralateral neostriatum. Hence, corticostriatal overlap was usually higher on the side contralateral to the FR injection site.
Corticothalamic projections from the MI whisker regions produced tracer overlap in several thalamic nuclei, especially in those near the midline. As reported previously (Alloway et al., 2008), the IAM and AM nuclei received most of the overlapping projections from the MI whisker representations in each hemisphere. In addition, corticothalamic overlap was also observed in VM and, to a lesser extent, in VL and in the intralaminar nuclei, PC and CL.
Compared to the MI whisker regions, bilateral injections in the MI forepaw regions produced smaller amounts of tracer overlap. An example of the distribution of bilateral projections from both MI forepaw regions appears in Figure 10, which shows that most of the tracer overlap is in the neostriatum. Consistent with the relative lack of projections from the MI forepaw region to the claustrum, tracer overlap in the claustrum of BN16 was nonexistent, but was present to a small degree in some other cases. Very small amounts of tracer overlap appeared in the midline thalamic region or, to a lesser degree, in medial parts of the VM and VL nuclei. Overlapping projections were most prevalent in the neostriatum, and these were restricted almost entirely to a narrow strip along the lateral edge of the neostriatum, which is where most corticostriatal projections from the MI forepaw region are concentrated.
Fig.10.
Bilateral distribution of AF- and FR-labeled projections from the MI forepaw regions in BN16. AF and FR labeled varicosities shown as green and magenta, respectively. All other aspects of the labeling patterns (A,B) and overlap analysis (A’,B’) are shown as in Figure 9. Scale bars, 1 mm.
Quantitative analysis of the bilateral projections from MI
Bilateral deposits of different tracers into the whisker or forepaw regions of MI produced significant differences in the amount of tracer overlap in each of the analyzed brain regions. As shown by Figure 11, when 50 μm2 bins were used to quantify tracer overlap, injections in the MI whisker regions produced more overlap in the claustrum (t = 2.54, p < 0.05), neostriatum (t = 3.66, p < 0.01), and thalamus (t = 3.41, p < 0.01) than injections in the MI forepaw regions. The differences in the amount of overlapping projections from the whisker and forepaw regions were significant even when bin sizes of 100, 150, or 200 μm2 were used in the analysis (data not shown).
Fig. 11.
Mean amount of tracer overlap in the claustrum, neostriatum, and thalamus after depositing tracers bilaterally into the MI whisker (n = 6) or forepaw (n = 6) regions. Tracer overlap represents the proportion of 50 μm2 bins that contain labeled projections from both MI tracer injections. Brackets represent SEM; asterisks indicate significant differences between the MI whisker and MI forepaw groups (* p < 0.05, ** p < 0.01).
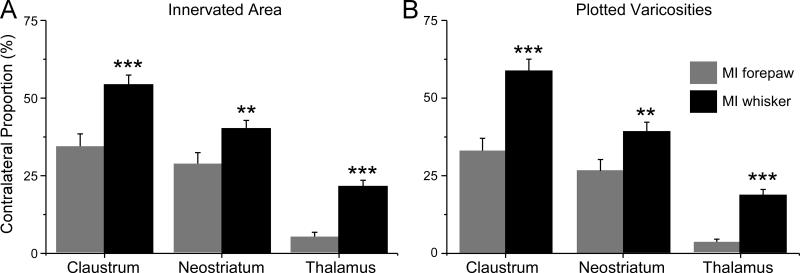
Data from rats receiving bilateral or unilateral injections were pooled together to analyze hemispheric imbalances in MI projections to the claustrum, neostriatum, and thalamus. For this analysis, we measured the total amount of tracer labeling in each brain region and then calculated the proportion that was located on the contralateral side. For rats that received bilateral tracer injections, we only analyzed the tracer that produced the most terminal labeling because this avoided any statistical bias associated with using multiple data points from the same subject. As shown by Figure 12, the mean proportion of contralateral labeling was always highest in the claustrum, lowest in the thalamus, and intermediate in the neostriatum. This rank-order relationship did not depend on which MI region was injected, nor did it depend on whether labeling was expressed by the size of the innervated area or by the total number of plotted varicosities.
Fig. 12.
Proportion of labeling in the claustrum, neostriatum, and thalamus that was located on the contralateral side after injecting the MI whisker (n = 13) or forepaw (n = 13) regions. The sum of contralateral and ipsilateral labeling equals 100% for each brain region. A: Mean proportion of labeled area on the contralateral side. B: Mean proportion of plotted varicosities on the contralateral side. Brackets represent SEM; asterisks indicate significant differences between the MI whisker and MI forepaw groups (** p < 0.01, *** p < 0.001).
For each forebrain region that was analyzed, tracer deposits in the whisker region produced a greater proportion of contralateral labeling than tracer deposits in the forepaw region. This result did not depend on whether we measured the areal extent of terminal labeling or the number of varicosities that were plotted. Using either method (compare panels A and B in Figure 12), the proportion of labeling in the contralateral thalamus was 4-5 times higher for projections from the whisker region than for projections from the forepaw region (t > 6.78, p < 0.001). Compared to the MI forepaw projections, the proportion of labeling in the contralateral neostriatum was 40% greater for projections from the MI whisker region (t > 2.62, p < 0.01). Similarly, the proportion of labeling in the contralateral claustrum was more than 50% greater when tracers were injected into the MI whisker region (t > 4.00, p < 0.001). Tracer injections in the MI forepaw region, however, produced very little terminal labeling in the claustrum, and the hemispheric differences in this group of rats is based on rather small tissue areas with few labeled varicosities.
DISCUSSION
Our results indicate that the MI whisker region has more interhemispheric forebrain connections than the MI forepaw region. While both of these functional subdivisions are connected with the corresponding MI region in the contralateral hemisphere, the MI whisker region has stronger interhemispheric connections with the thalamus, neostriatum, and claustrum. Indeed, the MI forepaw region has few connections with the contralateral thalamus and virtually none with the claustrum of either hemisphere. Consistent with these findings, bilateral tracer injections into corresponding MI regions indicate that the neostriatum, thalamus, and claustrum can integrate overlapping projections from the MI whisker regions.
Interhemispheric connections between corresponding regions in MI
Previous reports indicate that the MI cortical areas in each hemisphere of the rat are reciprocally connected by axonal projections through the corpus callosum (Donoghue and Parham, 1983; Reep et al., 1987; Miyashita et al., 1994; Veinante and Deschenes, 2003). Our results expand on these earlier findings by showing that the whisker (Agm) and forepaw (Agl) regions project to the corresponding cytoarchitectonic area in the contralateral hemisphere. Although the callosal routes that interconnect the Agm regions are slightly shorter than those interconnecting the Agl regions, other aspects of these pathways appear remarkably similar. This suggests that the callosal connections have the same computational functions for both MI regions.
Cross-correlation analysis of spontaneous activity recorded bilaterally in rat MI cortex has revealed neuronal synchronization in these regions (Sil’kis and Bogdanova, 1999). Reciprocal callosal connections in MI cortex could provide a substrate for synchronizing these cortical areas across the two hemispheres (Porter and White, 1984; Veinante and Deschenes, 2003). This view is supported by data showing that transections of the splenium block interhemispheric synchronization in the visual system (Nowak et al., 1995; Munk et al., 1995). If synchronous bilateral whisking behavior reflects interhemispheric synchronization in the MI whisker region, the whiskers may display more synchronous bilateral movements than the forelimbs because of stronger interconnections through the corpus callosum. Our unilateral tracer deposits in the whisker or forepaw regions of MI, however, did not reveal differences in the density or spatial extent of the callosal projections to Agm or Agl in the contralateral hemisphere.
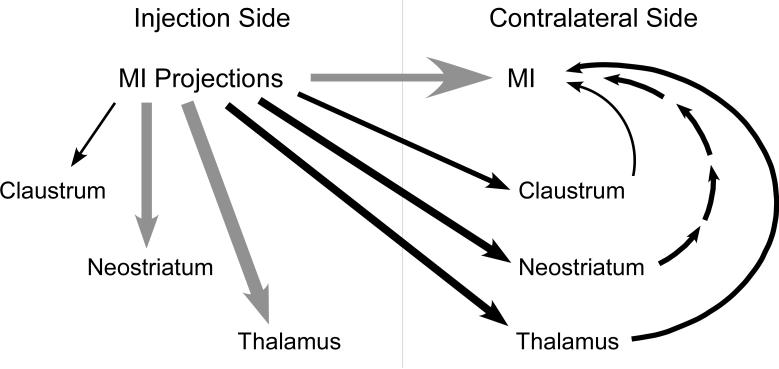
By contrast, the proportion of contralateral labeling in the neostriatum, thalamus, and claustrum depended on whether the tracers were injected in the whisker or forepaw regions of MI. Measuring the relative balance of labeling across the two hemispheres effectively normalizes variations in terminal labeling that might be due to differences in the size of the tracer deposits or other factors. Using this approach, we must conclude that bilaterally-coordinated whisker movements are modulated, at least in part, by the interhemispheric projections from the MI whisker region to the neostriatum, thalamus, and claustrum (Figure 13).
Fig. 13.
Hypothetical strength of projections from the MI whisker and forepaw regions. Line thickness is proportional to the relative extent and number of projections to the claustrum, neostriatum, thalamus and contralateral MI. Gray lines indicate projections from the whisker and forepaw regions that are equally strong; black lines indicate projections from the MI whisker region that are stronger than the corresponding projections from the MI forepaw region. Multisynaptic projections from neostriatum to MI cortex are indicated by a sequence of arrows on the contralateral side.
Bilateral forebrain projections from MI
Aside from the direct reciprocal connections that the MI forepaw region has with its counterpart in the contralateral hemisphere, most forebrain projections from this region terminate within the ipsilateral hemisphere. Tracer injections in the MI forepaw region revealed that virtually all of the labeled terminals in the thalamus were located in the ipsilateral side. The MI forepaw region projects to the contralateral neostriatum, but corticostriatal labeling was much denser and more extensive on the ipsilateral side. Other studies that characterized MI projections to the thalamus and neostriatum have reported similar findings (McGeorge and Faull, 1989; Rouiller et al., 1991, 1993, Alloway et al., 2006), but very few studies have analyzed these projections with respect to the functional subdivisions in MI cortex (Reep et al., 2008). Likewise, with the exception of the projections from the MI region in the contralateral hemisphere, the afferent projections to the Agl region originate almost exclusively from the thalamus and cortical sites in the ipsilateral hemisphere (Donoghue and Parham, 1983; Reep et al., 1990; Miyashita et al., 1994). These ipsilateral connection patterns are consistent with the well-established principle that the MI limb representations are concerned with processing information related to muscle control in the contralateral body.
By contrast, the MI whisker region sends a large portion of its forebrain projections to the contralateral hemisphere. Consistent with previous reports (Molinari et al., 1985; Rouiller et al., 1991; Shibata and Naito, 2005), tracer deposits in the MI whisker region revealed dense projections to the contralateral thalamus, especially in AM, VM, and parts of the intralaminar nuclei. Furthermore, when compared to projections from the MI forepaw region, the spatial extent and density of the projections from the MI whisker region were more evenly distributed across both sides of the neostriatum. In addition, MI whisker injections produced much more terminal labeling in the contralateral than in the ipsilateral claustrum. This is especially noteworthy because tracer injections in the MI forepaw region revealed few labeled terminals in the claustrum of either hemisphere.
These contralateral projections are consistent with many aspects of whisking behavior. Compared to the behavioral repertoire of the forelimbs, which exhibit many independent bilateral movements, whisking is a highly-constrained stereotyped behavior in which synchronous bilateral movements are tightly coordinated with sniffing and other head movements mediated by midline muscles (Welker, 1964; Kepecs et al., 2006). Whisking behavior is often characterized by rapid, bilateral whisker movements that are synchronized in amplitude and frequency (Gao et al., 2001; Sachdev et al., 2003; Sellien et al., 2005). Whisking frequency is relatively constant for brief epochs (ie., 1-4 seconds), but is marked by sequential bouts of whisking, which often shift to higher or lower frequencies as one epoch follows another (Berg and Kleinfeld, 2003). Unilateral contact with an external object immediately disrupts the bilateral symmetry of the whisker movements, but the decrease in whisking amplitude on the contacted side is coordinated with larger sweeping motions of the contralateral whiskers (Mitchinson et al., 2007). These behavioral characteristics suggest rapid interhemispheric communication between forebrain regions that modulate whisker movements.
Neural basis of bilateral whisker movements
The neural mechanisms that mediate bilateral whisker coordination have not been elucidated, and the precise role of the interhemispheric connections in the forebrain is speculative. Callosal projections are probably necessary for synchronizing the MI regions in each hemisphere, but these connections alone might not be sufficient for mediating synchronous bilateral whisker movements. This view is based on the fact that both MI forepaw regions are interconnected by commissural projections, but the forelimbs do not possess the same degree of bilateral coordination that is apparent in whisking behavior.
Several findings suggest that the bilateral projections from MI to the neostriatum play an important role in facilitating MI synchronization bilaterally. Neostriatal neurons have biophysical properties and other characteristics that enhance the detection of synchronous cortical inputs (Kawaguchi et al., 1989; Jiang and North, 1991). This suggests that overlapping corticostriatal projections should have a significant impact on the transmission of whisker-related information through the basal ganglia and subsequent thalamocortical circuits that project back to MI cortex.
We observed many callosal projections from the MI whisker region that split into collateral axons, one branch projecting to the overlying MI cortex while the other branch continued in the external capsule. Callosal projections from the MI forepaw region also split into collateral axons, but this subdivision of MI cortex did not project to the claustrum. Hence, the collateral branch that did not project to MI is probably more likely to terminate in the dorsolateral neostriatum. This suggests that some callosal projections from MI transmit the same information to both MI and the dorsolateral neostriatum in the contralateral hemisphere. This is supported by antidromic stimulation data indicating that many MI neurons have divergent commissural projections that terminate in MI and the neostriatum of the contralateral hemisphere (Wilson, 1987). In conjunction with overlapping bilateral corticostriatal projections from each MI whisker region, divergent callosal projections to MI and the neostriatum of the opposite hemisphere should facilitate synchronization in MI and connected regions of the neostriatum of both hemispheres. Tightly coordinated activity between MI and the neostriatum during whisking behavior is consistent with the view that the neostriatum plays a critical role in the production of stimulus-response learning and related motor behaviors that follow a stereotyped sequence of movements (Cromwell and Berridge, 1996; Packard and Knowlton, 2002; Doyon et al., 2003; Balleine et al., 2007).
Overlapping projections from the MI whisker region to the thalamus could facilitate bilateral synchronization among forebrain regions that modulate whisker movements. The MI whisker region projects bilaterally to the VM nucleus in the thalamus (Alloway et al., 2008), and this thalamic region receives inputs from the entopeduncular nucleus and the substantia nigra pars reticulata (Carter and Fibiger, 1978; Herkenham, 1979; Deniau et al., 1994), which represent the major outputs of the basal ganglia. Hence, some parts of the thalamus may receive convergent projections from two whisker-related pathways, a direct projection from the MI whisker region, and multisynaptic projections through the neostriatum and subsequent parts of the basal ganglia. The VM nucleus in each hemisphere projects to the ipsilateral MI whisker region, thereby completing an interhemispheric circuit that could reinforce the synchronization of the MI whisker regions in each hemisphere (Alloway et al., 2008).
Based on this line of reasoning, we hypothesize that the MI whisker regions and their bilateral projections to the neostriatum and thalamus may coordinate whisker movements bilaterally. The involvement of the basal ganglia is also compatible with the responses evoked by unilateral whisker contacts during exploratory stimulation. Thus, SI barrel cortex projects ipsilaterally to both the neostriatum and the MI whisker region (Brown et al., 1998; Alloway et al., 1999; Hoffer et al., 2003), but sends few projections to the contralateral neostriatum (McGeorge and Faull, 1989; Alloway et al., 2006). Furthermore, the dorsolateral neostriatum receives dense overlapping inputs from the ipsilateral SI and MI whisker representations (Hoffer and Alloway, 2001). Consequently, activation of SI cortex preferentially activates the ipsilateral neostriatum, thereby creating an imbalance in hemispheric processing that could disrupt synchronous bilateral whisker movements and initiate the transition to the asymmetric whisking patterns that occur in response to unilateral whisker contacts (Mitchinson et al., 2007).
Despite clear differences in the bilateral projections from the whisker and forepaw regions in MI, the abundance of bilateral projections from the MI whisker region might not be the critical factor that mediates the bilateral coordination of whisking behavior. It is conceivable that similar bilateral projection patterns are present in rodents that have whiskers but do not display whisking behaviors. Guinea pigs, muskrats, and chipmunks, for example, have whiskers and barrels in SI cortex, but do not display whisking behaviors (Rice, 1995). Therefore, characterizing the bilateral connections of the sensorimotor regions in non-whisking rodents would provide a valid test of our hypothesis.
MI whisker projections to the contralateral claustrum
The claustrum is present in a wide range of mammals, including rats (Kowianski et al., 1999), but its function is largely unknown. The elongated structure and widespread connectivity of the claustrum in primates suggests that it is involved in consciousness and multimodal integration (Crick and Koch, 2005). Examination of claustral connections in the rat has prompted the view that it is very important for interhemispheric communication. Thus, injections of tracers into large regions of frontal cortex revealed projections to both the contralateral and ipsilateral claustrum (Minciacchi et al., 1985). We also found that the MI whisker region projects to the claustrum in both hemispheres, but the projections to the contralateral claustrum were much stronger. Furthermore, our study demonstrates that the claustrum receives projections from the MI whisker region but not from the forepaw region, and this distinction indicates that the claustrum possesses a degree of functional specificity that was not previously recognized.
Additional pieces of evidence indicate that the claustrum sends a strong projection to the MI cortex in the ipsilateral hemisphere. Although we used tracers that were transported primarily in the anterograde direction, the few retrogradely-labeled neurons that we observed in the claustrum were located almost exclusively in the ipsilateral hemisphere. Consistent with this, other tracing studies in rats and cats have shown that claustrocortical projections terminate preferentially in the ipsilateral hemisphere (Norita 1977; Kowianski et al., 2000). In addition, the claustrum has much stronger connections with MI than with SI cortex (Kowianski et al., 2000). Physiology experiments in cats indicate that electrical stimulation of the claustrum exerts powerful synaptic inhibition of pyramidal tract neurons in MI cortex, and the incidence of this effect is much greater in the ipsilateral than in the contralateral hemisphere (Crescimanno et al., 1989). Collectively, these findings indicate that the claustrum receives afferent projections primarily from the contralateral MI whisker region and projects primarily to the ipsilateral MI whisker region.
The interhemispheric connections between the MI whisker region and the claustrum suggest that these interconnected structures may play a role in the bilateral coordination of whisker movements, but the nature of this role is unclear. Conceivably, these connections could reinforce the bilateral synchronization of symmetric whisker movements during exploratory whisking. Alternatively, if the claustrum inhibits MI outputs (Crescimanno et al., 1989), the claustrum may transiently decouple bilateral whisker movements in response to head movements or unilateral whisker contacts (Towal and Hartmann, 2006; Mitchison et al., 2007).
The precise role of the claustrum in whisking behavior must await further studies. Fortunately, because of its location near the cortical surface, lesions and other manipulations of the claustrum are feasible. Claustral lesions, for example, could be made unilaterally, either in isolation or in combination with lesions of the MI whisker region, to determine how claustral mechanisms are related to the bilateral kinematics of whisking behavior.
Technical considerations
Bulk tracer injections into the whisker and forepaw regions of MI cortex revealed differences in the projections from these functional subdivisions, but this technique has some limitations. Visualization of the labeled axons and their terminals enabled accurate reconstructions of the location of labeled varicosities, and this enabled quantification of the total area that was innervated on each side of the brain. The high density of labeling, however, often made it impossible to plot each varicosity. Therefore, in contrast to sampling techniques that are designed to measure the peak density of corticostriatal projections (Reep et al., 2008), our methodology does not achieve this level of precision. Nonetheless, our reconstructions depict topographic changes in labeling density that approximate the variations in density that we visualized in the microscope. Assuming that the labeled varicosities were plotted in densities that are proportional to their actual density in the tissue, our methods should reliably indicate hemispheric imbalances in the density of projections from the MI whisker and forepaw regions.
It is conceivable that labeled terminals represent the collateral projections of retrogradely-neurons. Thus, labeled terminals in the contralateral thalamus might represent collateral projections of thalamocortical neurons located ipsilateral to the injection site. Likewise, labeled terminals in the claustrum or neostriatum of the contralateral hemisphere could represent collateral projections from retrogradely-labeled neurons in the contralateral MI cortex. Several facts, however, suggest that these scenarios are unlikely. After injecting retrograde tracers into the thalamus, we observed many retrogradely-labeled neurons in the contralateral MI whisker region, but observed virtually no labeled neurons in the contralateral thalamus (Alloway et al., 2008). Hence, the labeled terminals observed in the contralateral thalamus after injecting the MI whisker region must represent corticothalamic projections. Furthermore, labeling of collateral projections does not explain why more anterograde labeling appears in the contralateral claustrum than in the ipsilateral claustrum. If terminal labeling in the contralateral claustrum is due to collateral transport from retrogradely-labeled MI neurons in the contralateral hemisphere, then MI neurons located at the injection site should also transport as much tracer to the ipsilateral claustrum. Finally, if terminal labeling in the contralateral neostriatum is due to collateral projections from retrogradely-labeled neurons in the contralateral MI cortex, then labeled collaterals should also appear in other MI projection targets. Tracer injections in MI cortex, however, did not produce terminal labeling in the POm nucleus of the contralateral thalamus. Hence, the terminal labeling observed in the contralateral hemisphere is due to projections from the MI injection site.
Supplementary Material
Acknowledgements
The authors wish to thank Damon Anderson for help with some of the anatomical reconstructions.
Grant sponsor: NIH grants NS37532 and NS052689
Abbreviations in Figures
- ac
anterior commissure
- AD
anterodorsal nucleus
- Agm
agranular cortex, medial
- Agl
agranular cortex, lateral
- AM
anteromedial nucleus
- AV
anteroventral nucleus
- cc
corpus callosum
- CL
centrolateral nucleus
- CS
claustrum
- cst
corticospinal tract
- ec
external capsule
- fc
fasciculus cuneatus
- G
gelatinosus nucleus
- GP
globus pallidus
- IAM
interanteromedial nucleus
- ic
internal capsule
- LD
laterodorsal nucleus
- MD
mediodorsal nucleus
- MI
motor cortex
- mt
mammillothalamic tract
- NC
nucleus cuneatus
- NS
neostriatum
- PC
paracentral nucleus
- pd
pyramidal decussation
- POm
posterior nucleus, medial
- pt
pyramidal tract
- Re
reuniens nucleus
- Rh
rhomboid nucleus
- SI
somatosensory cortex
- sm
stria medularis
- TH
thalamus
- VL
ventrolateral nucleus
- VM
ventromedial nucleus
- VPM
ventroposteromedial nucleus
- VPL
ventroposterolateral nucleus
- ZI
zona incerta
LITERATURE CITED
- Alloway KD, Crist J, Mutic JJ, Roy SA. Corticostriatal projections from rat barrel cortex have an anisotropic organization that correlates with vibrissal whisking behavior. J Neurosci. 1999;19:10908–10922. doi: 10.1523/JNEUROSCI.19-24-10908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway KD, Lou L, Nwabueze-Ogbo F, Chakrabarti S. Topography of cortical projections to the dorsolateral neostriatum in rats: multiple overlapping sensorimotor pathways. J Comp Neurol. 2006;499:33–48. doi: 10.1002/cne.21039. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Mutic JJ, Hoover JE. Divergent corticostriatal projections from a single cortical column in the somatosensory cortex of rats. Brain Res. 1998;785:341–346. doi: 10.1016/s0006-8993(97)01363-2. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Olson ML, Smith JB. Contralateral corticothalamic projections from MI whisker cortex: Potential route for modulating hemispheric interactions. J Comp Neurol. 2008;510:100–116. doi: 10.1002/cne.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol. 2003;89:104–117. doi: 10.1152/jn.00600.2002. [DOI] [PubMed] [Google Scholar]
- Brecht M, Krauss A, Muhammad S, Sinai-Esfahani L, Bellanca S, Margie TW. Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, micro-stimulation, and intracellular stimulation of identified cells. J Comp Neurol. 2004;479:360–373. doi: 10.1002/cne.20306. [DOI] [PubMed] [Google Scholar]
- Brown LL, Smith DM, Goldbloom LM. Organizing principles of cortical integration in the rat neostriatum: corticostriate map of the body surface is an ordered lattice of curved laminae and radial points. J Comp Neurol. 1998;392:468–488. [PubMed] [Google Scholar]
- Carter DA, Fibiger HC. The projections of the entopeduncular nucleus and globus pallidus in rat as demonstrated by autoradiography and horseradish peroxidase histochemistry. J Comp Neurol. 1978;177:113–123. doi: 10.1002/cne.901770108. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Woodward DJ. Distribution of somatic sensory and active-movement neuronal discharge properties in the MI-SI cortical border area in the rat. Exp Neurol. 1986;91:502–523. doi: 10.1016/0014-4886(86)90048-8. [DOI] [PubMed] [Google Scholar]
- Crescimanno G, Salerno MT, Cortimiglia R, Amato G. Claustral influence on ipsi- and contralateral motor cortical areas in the cat. Brain Res Bull. 1989;22:839–843. doi: 10.1016/0361-9230(89)90027-0. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C. What is the function of the claustrum? Phil Trans R Soc B. 2005;360:1271–1279. doi: 10.1098/rstb.2005.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci. 1996;16:3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniau JM, Menetrey A, Thieery AM. Indirect nucleus accumbens input to the prefrontal cortex via the substantia nigra pars reticulate: a combined anatomical and electrophysiological study in the rat. Neuroscience. 1994;61:533–545. doi: 10.1016/0306-4522(94)90432-4. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Parham C. Afferent connections of the lateral agranular field of the rat motor cortex. J Comp Neurol. 1983;217:390–404. doi: 10.1002/cne.902170404. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Fabri M, Burton H. Ipsilateral cortical connections of primary somatic sensory cortex in rats. J Comp Neurol. 1991;311:405–424. doi: 10.1002/cne.903110310. [DOI] [PubMed] [Google Scholar]
- Gao P, Bermejo R, Zeigler HP. Whisker deafferentation and rodent whisking patterns: behavioral evidence for a central pattern generator. J Neurosci. 2001;21:5374–5380. doi: 10.1523/JNEUROSCI.21-14-05374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Hattox AM, Jones LM, Keller A, Ziegler HP. Whisker motor cortex ablation and whisker movement patterns. Somatosen Mot Res. 2003;20:191–198. doi: 10.1080/08990220310001622924. [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Lamarche M. A reappraisal of rat motor cortex organization by intracortical microstimulation. Brain Res. 1985;344:49–61. doi: 10.1016/0006-8993(85)91188-6. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Brecht M, Osten P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. J Neurosci. 2005;25:8250–8258. doi: 10.1523/JNEUROSCI.2235-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiss F, Schwarz C. Spatial segregation of different modes of movement control in the whisker representation of rat primary motor cortex. J Neurosci. 2005;25:1579–1587. doi: 10.1523/JNEUROSCI.3760-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RD, Lindholm EP. Organization of motor and somatosensory neocortex in the albino rat. Brain Res. 1974;66:23–38. [Google Scholar]
- Hattox AM, Priest CA, Keller A. Functional circuitry involved in the regulation of whisker movements. J Comp Neurol. 2002;442:266–276. doi: 10.1002/cne.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J. Comp Neurol. 1979;183:487–517. doi: 10.1002/cne.901830304. [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Alloway KD. Organization of corticostriatal projections from the vibrissal representations in the primary motor and somatosensory cortical areas of rodents. J Comp Neurol. 2001;439:87–103. doi: 10.1002/cne.1337. [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Hoover JE, Alloway KD. Sensorimotor corticocortical projections from rat barrel cortex have an anisotropic organization that facilitates integration of inputs from whiskers in the same row. J Comp Neurol. 2003;466:525–544. doi: 10.1002/cne.10895. [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Arantes H, Roth R, Alloway KD. Functional circuits mediating sensorimotor integration: Quantitative comparisons of projections from rodent barrel cortex to MI cortex, neostriatum, superior colliculus, and pons. J Comp Neurol. 2005;488:82–100. doi: 10.1002/cne.20579. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurons in vitro. J Physiol. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Wilson CJ. Corticostriatal innervation of the patch and matrix in the rat neostriatum. J Comp Neurol. 1996;374:578–592. doi: 10.1002/(SICI)1096-9861(19961028)374:4<578::AID-CNE7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem Senses. 2005;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- Kowianski P, Dziewiatkowski J, Kowianski P. Comparative anatomy of the claustrum in selected species: A morphometric analysis. Brain Beh Evol. 1999;53:44–54. doi: 10.1159/000006581. [DOI] [PubMed] [Google Scholar]
- Kowianski P, Morys J, Sadowski M, Dziewiatkowski J. Qualitative and quantitative differences in the motor and somatosensory cortical projections of the rat claustrum - combined retrograde and stereological studies. Folia Morphol (Warsz) 2000;59:111–119. [PubMed] [Google Scholar]
- Land PW, Simons DJ. Cytochrome oxidase staining in the rat SmI barrel cortex. J Comp Neurol. 1985;238:225–235. doi: 10.1002/cne.902380209. [DOI] [PubMed] [Google Scholar]
- Leergaard TB, Alloway KD, Pham TA, Bolstead I, Hoffer Z, Pettersen C, Bjaalie JG. Three dimensional topography of corticopontine projections from rat sensorimotor cortex: comparisons with corticostriatal projections reveal diverse integrative organization. J Comp Neurol. 2004;478:306–322. doi: 10.1002/cne.20289. [DOI] [PubMed] [Google Scholar]
- Li X-G, Florence SL, Kaas JH. Areal distributions of cortical neurons projecting to different levels of the caudal brain stem spinal cord in rats. Somatosen Mot Res. 1990;7:315–335. doi: 10.3109/08990229009144711. [DOI] [PubMed] [Google Scholar]
- Li ZK, Takada M, Hattori T. Topographic organization and collateralization of claustrocortical projections in the rat. Brain Res Bull. 1986;17:529–532. doi: 10.1016/0361-9230(86)90220-0. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Meng Z, Li Q, Martin JH. The transition from development to motor control function in the corticospinal system. J Neurosci. 2004;24:605–614. doi: 10.1523/JNEUROSCI.4313-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minciacchi D, Molinari M, Bentivoglio M, Macchi G. The organization of the ipsi- and contralateral claustrocortical system in rat with notes on the bilateral claustrocortical projections in cat. Neuroscience. 1985;16:557–576. doi: 10.1016/0306-4522(85)90192-7. [DOI] [PubMed] [Google Scholar]
- Mitchinson B, Martin CJ, Grant RA, Prescott TJ. Feedback control in active sensing: Rat exploratory whisking is modulated by environmental contact. Proc Roy Soc B. 2007;274:1035–1041. doi: 10.1098/rspb.2006.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita E, Keller A, Asanuma H. Input-output organization of the rat vibrissal motor cortex. Exp Brain Res. 1994;99:223–232. doi: 10.1007/BF00239589. [DOI] [PubMed] [Google Scholar]
- Molinari M, Minciacchi D, Bentivoglio M, Macchi G. Efferent fibers from the motor cortex terminate bilaterally in the thalamus of rats and cats. Exp Brain Res. 1985;57:305–312. doi: 10.1007/BF00236536. [DOI] [PubMed] [Google Scholar]
- Munk MH, Nowak LG, Nelson JI, Bullier J. Structural basis of cortical synchronization. II. Effects of cortical lesions. J Neurophysiol. 1995;74:2401–2414. doi: 10.1152/jn.1995.74.6.2401. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Norita M. Demonstration of bilateral claustrro-cortical connections in the cat with the method of retrograde axonal transport of horseradish peroxidase. Arch Histol Jap. 1977;40:1–10. doi: 10.1679/aohc1950.40.1. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Munk MH, Nelson JI, James AC, Bullier J. Structural basis of cortical synchronization. I. Three types of hemispheric coupling. J Neurophysiol. 1995;74:2379–2400. doi: 10.1152/jn.1995.74.6.2379. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Ann Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. second edition Academic Press; New York: 1986. [Google Scholar]
- Porter LL, White EL. Afferent and efferent pathways of the vibrissal region of primary motor cortex in the mouse. J Comp Neurol. 1983;214:279–289. doi: 10.1002/cne.902140306. [DOI] [PubMed] [Google Scholar]
- Porter LL, White EL. Termination of callosal afferents onto identified callosal projection neurons in the primary motor cortex of the mouse. Neurosci Lett. 1984;47:37–40. doi: 10.1016/0304-3940(84)90382-3. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Hashimoto A, Watson RT. Efferent connections of the rostral portion of medial agranular cortex in rats. Brain Res Bull. 1987;19:203–221. doi: 10.1016/0361-9230(87)90086-4. [DOI] [PubMed] [Google Scholar]
- Reep RL, Goodwin GS, Corwin JV. Topographic organization in the corticocortical conections of medial agranular cortex in rats. J Comp Neurol. 1990;294:262–280. doi: 10.1002/cne.902940210. [DOI] [PubMed] [Google Scholar]
- Reep RL, Wu JH, Cheatwood JL, Corwin JV, Kartje GL, Mir A. Quantification of synaptic density in corticostriatal projections from rat medial agranular cortex. Brain Res. 2008;1233:27–34. doi: 10.1016/j.brainres.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei WL. Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intra striatal terminals in rats. J Comp Neurol. 2003;457:420–440. doi: 10.1002/cne.10541. [DOI] [PubMed] [Google Scholar]
- Rice FL. Comparative aspects of barrel structure and development. In: Jones EG, Diamond IT, editors. Cerebral Cortex. Vol. 11. The barrel cortex of rodents. Plenum Press; New York: 1995. pp. 1–75. [Google Scholar]
- Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10:269–289. doi: 10.3109/08990229309028837. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Moret V, Liang F, Wiesendanger M. Patterns of corticothalamic terminations following injection of Phaseolus vulgaris leucoagglutinin (PHA-L) in the sensorimotor cortex of the rat. Neurosci Lett. 1991;125:93–97. doi: 10.1016/0304-3940(91)90139-k. [DOI] [PubMed] [Google Scholar]
- Sachdev RN, Berg RW, Champney G, Kleinfeld D, Ebner FF. Unilateral vibrissa contact: changes in amplitude but not timing of rhythmic whisking. Somatoses Mot Res. 2003;20:163–169. doi: 10.1080/08990220311000405208. [DOI] [PubMed] [Google Scholar]
- Sadowski M, Morys J, Jakubowska-Sadowska K, Narkiewicz O. Rat’s claustrum shows two main cortico-related zones. Brain Res. 1997;756:147–152. doi: 10.1016/s0006-8993(97)00135-2. [DOI] [PubMed] [Google Scholar]
- Sanderson KJ, Welker W, Shambes GM. Reevaluation of motor cortex and of sensorimotor overlap in cerebral cortex of albino rats. Brain Res. 1984;292:251–260. doi: 10.1016/0006-8993(84)90761-3. [DOI] [PubMed] [Google Scholar]
- Sellien H, Eshenroder DS, Ebner FF. Comparison of bilateral whisker movement in freely exploring and head-fixed adult rats. Somatosens Mot Res. 2005;22:97–114. doi: 10.1080/08990220400015375. [DOI] [PubMed] [Google Scholar]
- Shibata H, Naito J. Organization of anterior cingulate and frontal cortical projections to the anterior and laterodorsal thalamic nuclei in the rat. Brain Res. 2005;1059:93–103. doi: 10.1016/j.brainres.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Sil’kis IG, Bogdanova OG. The properties and possible mechanisms of interhemispheric synchronoization in the motor cortex of the rat. Neurosci Beh Physiol. 1999;29:523–530. doi: 10.1007/BF02461144. [DOI] [PubMed] [Google Scholar]
- Towal RB, Hartmann MJ. Right-left asymmetries in the whisking behavior of rats anticipate head movements. J Neurosci. 2006;26:8838–8846. doi: 10.1523/JNEUROSCI.0581-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Dechenes M. Single-cell study of motor cortex projections to the barrel field in rats. J Neurosci. 2003;464:98–103. doi: 10.1002/cne.10769. [DOI] [PubMed] [Google Scholar]
- Voigt T, De Lima AD, Beckmann M. Synaptophysin immunohistochemiostry reveals inside-out pattern of early synaptogenesis in ferret cerebral cortex. J Comp Neurol. 1993;330:48–64. doi: 10.1002/cne.903300105. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing in the albino rat. Behaviour. 1964;12:223–244. [Google Scholar]
- Wilson CJ. Postsynaptic potentials evoked in spiny neostriatal projection neurons by stimulation of ipsilateral and contralateral neocortex. Brain Res. 1986;367:201–213. doi: 10.1016/0006-8993(86)91593-3. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J Comp Neurol. 1987;263:567–580. doi: 10.1002/cne.902630408. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.