Abstract
Background
The dexamethasone/corticotropin releasing hormone (Dex/CRH) test has been proposed as a potential tool for identifying endophenotypes relevant to mood disorders. Several studies have shown abnormal cortisol reactivity in phenotypically healthy adults without psychiatric disorders as a function of exposure to adverse early environments.
Methods
Following a battery of self-report and interview assessments, 230 adults without major Axis I Disorders completed the Dex/CRH test. Childhood maltreatment was evaluated with the Childhood Trauma Questionnaire (CTQ). Effect of childhood emotional abuse (EA) on cortisol responses to the Dex/CRH test was examined with repeated measures general linear models including age, sex and other types of maltreatment. Post-hoc models examined the significant interaction between EA and age, and tested the stability of the main findings with selected covariates.
Results
A history of self-reported childhood EA independently and significantly diminished cortisol response. This effect was amplified with advancing subject age, and was independent of the effects of other types of childhood maltreatment, lifetime diagnoses, and symptom scores.
Conclusions
Dampened cortisol reactivity may be a consequence of childhood emotional abuse that is cumulative over time. Prospective longitudinal investigation is needed to evaluate the potential of this proposed endophenotype.
Keywords: childhood abuse, mood disorders, depression, cortisol, HPA axis
Introduction
Reactivity of the hypothalamus-pituitary-adrenal (HPA) axis represents a potential marker of vulnerability for a variety of stress-related diseases and neuropsychiatric disorders. Both excessive and dampened cortisol responses to acute psychosocial stress or neuroendocrine challenge have been associated with disease states in humans. For example, recurrent depression (1) and type 2 diabetes mellitus (2) are associated with cortisol hyperresponsivity, while atypical depression (3; 4), chronic fatigue syndrome (5), chronic stress/burnout (6; 7), fibromyalgia (8), and brain lesions in multiple sclerosis (9) are associated with cortisol hyporesponsivity. Many of the HPA neuroendocrinopathies associated with these disorders are identified in acutely ill patient groups, but persist after the clinical syndrome is remitted (10), raising the possibility that the endophenotype reflects a “scar” or consequence of the disease state rather than a risk marker. Studies of HPA axis responsivity in healthy subjects who have not yet manifested the illness may prove useful in detecting viable endophenotypes for the prediction of vulnerability to develop the disorder.
In the present study, we used the combined dexamethasone/corticotropin releasing hormone (Dex/CRH) laboratory test, which is considered to be a highly sensitive probe of HPA axis activity (11). This test allows examination of an individual's physiological HPA response to neuroendocrine provocation (i.e., intravenous CRH bolus) in a psychologically neutral setting, largely bypassing limbic and higher cortical inputs engaged by a physically or emotionally threatening stressor. This test can be easily administered in a standard laboratory setting. As such, the test may prove to be a useful assessment tool for identification of endophenotypes relevant to risk for neuropsychiatric disorders (12).
Quality of the relationship between infants and caregivers during stages of early brain development appears to be a critical determinant of life-long biobehavioral regulation, including HPA axis reactivity, in the offspring. An abundant preclinical literature describes enduring effects on HPA system regulation as a consequence of frequent exposure to stress during early life (reviewed in 13). In studies of rodents and non-human primates, adverse rearing environments and chronic stress exposure have been linked to extreme patterns of corticosterone reactivity, both exaggerated and blunted relative to those observed in non-stressed controls (14).
Clinical studies of adults with a history of adverse parenting experiences or other forms of adversity also show alterations of HPA axis reactivity. Recent reports by Heim and colleagues (15) have described lower basal and stimulated plasma cortisol concentrations in response to intravenous CRH and adrenocorticotropic hormone (ACTH) challenge tests in twenty nondepressed women reporting childhood physical or sexual abuse, relative to twenty adults without a psychiatric history of childhood abuse. However, in a recent study of adult men, fourteen of whom did not have current depression and reported a history of childhood sexual or physical abuse, and fourteen with no abuse or psychiatric history, these investigators found increased plasma ACTH and cortisol responses to the Dex/CRH test in the group with abuse (16). Our group recently studied healthy adults (n=50) without posttraumatic stress disorder (PTSD) or major depressive disorder (MDD) and found that moderate-to-severe self-reported early life maltreatment (of any type, n=23) was associated with significantly diminished total plasma cortisol and ACTH responsivity in a standardized psychosocial stress test (17). Yet when subtypes of abuse were examined separately, we discovered that childhood sexual abuse and was a significant predictor of higher cortisol curves, while emotional neglect and physical abuse were associated with lower/flatter curves. These findings raised the possibility that different types of childhood abuse may portend differential consequences with regard to HPA axis responsivity in adulthood.
A meta-analysis exploring the variability in seemingly contradictory findings in the field of chronic stress and HPA function revealed that net activity of the system is shaped by the nature of the threat, emotions elicited by the stress, controllability of the stress, and individual response to the situation (18). Emotional, physical, and sexual types of childhood abuse and neglect often occur together, but may generate different emotional responses in the young victims, perhaps translating into unique biological perturbations. In addition, variability in previous findings may be due to the modest size of these studies as well as differences in sample characteristics including sex, history of psychiatric disorder, and type of neuroendocrine challenge paradigm studied.
In the present study, we investigated the impact of self-reported childhood adversity on total plasma cortisol responsivity in a large sample of healthy adults without current psychopathology, testing for independent effects of each of five subtypes of systematically-assessed maltreatment (physical abuse, sexual abuse, emotional abuse, emotional neglect, and physical neglect). Based on our past results with the psychosocial stress test in a similar sample, we hypothesized that early life adversity, and in particular childhood emotional neglect, would predict blunted cortisol responsivity on the Dex/CRH test.
Methods and Materials
Two hundred thirty adults (100 Men and 130 women, ages 18-61) participated in this Providence, Rhode Island based study between 2002 and 2008. Subjects were recruited from the community via flyers as well as through Internet and newspaper advertisements for “healthy adults” and “healthy adults with a history of early-life stress.” Phone screens and diagnostic interviews at the first visit were used to exclude participants meeting diagnostic criteria for current major depressive disorder, bipolar disorder, posttraumatic stress disorder, generalized anxiety disorder, obsessive-compulsive disorder, social anxiety disorder, panic disorder, substance use disorders, and all disorders characterized by psychosis. Subjects underwent physical and neurological examinations. Electrocardiograms and laboratory studies for complete blood count, serum electrolytes, thyroid stimulating hormone, urine toxicology, and urinalysis were conducted. Subjects were excluded from the present study if they worked night shifts, or if they had one or more of the following conditions: acute or unstable medical illness, a history of brain injury, seizure disorder, endocrine disease, or recreational use of illicit substances. Also excluded were individuals undergoing treatment with drugs which might influence HPA axis function, including psychotropic medications, beta blockers, angiotensin-converting enzyme inhibitors, ketoconazole, metyrapone, and corticosteroids. Subjects were free of these medications for at least two weeks (or five half-lives, if appropriate) prior to participation. Oral contraceptives and estrogen replacement therapies were allowed, and 46 of the 130 women were currently taking such medication. There were ten subjects who smoked cigarettes included in the sample. The following item was used as a measure of socioeconomic adversity during childhood “My family was generally financially stable when I was growing up, and all of my basic needs (food, shelter, and clothing) were met during my childhood.” Twenty-four subjects did not endorse this in the affirmative and were thus considered to have had socioeconomic adversity. All subjects gave voluntary written informed consent to participate in this study, which was approved by the Butler Hospital Institutional Review Board.
Subjects seeking treatment for psychiatric symptoms or disorders were excluded from this study. The presence of current or lifetime history of Axis I psychiatric diagnoses was assessed using the Structured Clinical Interview for DSM-IV (SCID;(19)). The 28-item version of the Childhood Trauma Questionnaire (CTQ; (20)) was used to assess childhood maltreatment. The CTQ (28-item version) is a self-report measure that encompasses experiences during the age period 0-17 and generates a total score and subscale scores for five types of maltreatment (emotional abuse, EA; physical abuse, PA; sexual abuse, SA; emotional neglect, EN; and physical neglect, PN). Cronbach's alpha for these subscales in this sample was .88, .92, .71, .93, and .77, respectively. CTQ subscale scores reaching the threshold for the “moderate to severe” range were considered positive for maltreatment. Subjects scoring “none or minimal” for each subscale were considered controls with regard to that particular type of maltreatment. The following additional self-report measures were included to further characterize subjects: (1) Inventory of Depressive Symptomatology-Self Report (IDS-SR; (21)), (2) State-Trait Anxiety Questionnaire (STAI; (22)), (3) Perceived Stress Scale (PSS (23)).
On a subsequent visit subjects completed the Dex/CRH test as follows. The night before the test, a single oral dose of dexamethasone 1.5 mg was self-administered at 11:00 p.m. The following day, participants arrived at 12:00 p.m., were given lunch, and were queried about whether they had experienced any somatic symptoms, stressors or changes in usual habits over the preceding week. Participants who reported any significant aberrations had the visit rescheduled. Participants completed the IDS-SR and PSS measures again at this visit, and the scores from this visit were used in subsequent analyses. Participants who were not able to complete the Dex/CRH test within 60 days of the assessment visit completed updated assessments which were used in the analyses. Topical anesthetic cream containing lidocaine 2.5% and prilocaine 2.5% was applied to the subject's forearm between 12:30 and 12:45 p.m. At 1:00 p.m., an indwelling intravenous (IV) catheter was inserted in the forearm by a research nurse with extensive experience with IV catheter placement. Subjects then remained in a semi-recumbent position throughout the procedure except to use the bathroom. They were permitted to read or watch pre-selected movies that did not contain emotionally-charged material. Vital signs were monitored throughout the test. At 3 p.m., CRH (corticorelin ovine triflutate, Acthrel®, Ferring Pharmaceuticals, Inc.) 100 μg reconstituted in 2 ml 0.9% sodium chloride was infused intravenously over 30 seconds. Blood samples were drawn at 2:59 p.m. (baseline) and every 15 minutes thereafter until 5:00 p.m. Samples were immediately stored on ice, centrifuged within 45 minutes, and then stored at -80° C. Cortisol assays were performed on plasma samples from 2:59 p.m., 3:30 p.m., 3:45 p.m., 4:00 p.m., 4:15 p.m., and 5:00 p.m. The GammaCoat cortisol I-125 coated-tube radioimmunoassay (RIA) kit (INCSTAR Corp., Stillwater, Minn.) was used to measure total cortisol (free and bound) at each time point. The intra-assay and inter-assay CVs observed for quality assessment samples (5 and 20 μg/dl) were less than 5% and 10%, respectively. The term cortisol is used to refer to total cortisol concentrations throughout the remainder of the manuscript.
Statistical analyses were conducted using SPSS 16.0 for Windows. All analyses were two-tailed with alpha set to p <.05. Cortisol values were positively skewed and were therefore log-transformed to meet normal distribution requirements for statistical tests; raw data are displayed in graphs. Repeated measures general linear models (GLMs) were used to determine (1) whether potential covariates were associated with cortisol responses to the Dex/CRH test, and (2) the effects of different types of maltreatment on cortisol responsivity in a model that included all five CTQ subscales, age and sex. Additional models tested for interactions of EA with sex and with age. A series of models examined a significant interaction between EA and subject age. Age was used as a continuous variable in the statistical models. The cohort was also dichotomized with respect to age in order to form two sub-cohorts (younger and older) for illustration of the EA × age effect. In order to ensure that subjects with EA would be represented in both groups, the age cohorts were formed using the median age for the EA group and the sample was split at 35 and younger versus 36 and older. Huynh-Feldt adjustment was made for violations of sphericity assumptions. Independent t-tests and chi-square tests were performed to compare baseline subject characteristics of groups with (n=41) and without (n=189) EA in the total population and within the two age cohorts. Additional post-hoc analyses were conducted to examine whether lifetime diagnoses could account for effects of childhood maltreatment with respect to cortisol responses to the Dex/CRH test.
Results
Cortisol Response to the Dex/CRH Test
All models revealed significant changes in plasma cortisol concentrations over time during the Dex/CRH test, as reflected in multivariate tests. Given space limitations, detailed statistical results for significant GLM effects are limited to within-subjects and between-groups analyses below.
Analysis of Potential Covariates
There was a significant within-subjects effect of age (F[2.1, 43.8]=3.4, p=.03). There was also a significant between-groups effect of sex (F[1, 228] =8.8, p=.003), reflecting higher cortisol concentrations in women than men. There were no significant effects of cigarette smoking, education level, socioeconomic adversity in childhood, depressive symptoms (IDS-SR) or anxiety symptoms (STAI). Estrogen or combination estrogen/progesterone use, present for 46 of 130 women, produced a highly significant within-subjects effect (F[2, 257] =12.0, p<.001), which reflected significantly higher cortisol values among hormone users at the first (pre-CRH infusion) timepoint in the curve compared to women not taking exogenous hormones. Remaining analyses therefore controlled for the significant effects of age and sex, and results of an analysis of the influence of hormone use on the effects of childhood maltreatment in women are presented below.
Effect of Childhood Maltreatment Subtypes
In this sample, 41 participants were positive for a history of emotional abuse, 13 were positive for sexual abuse, 18 for physical abuse, 27 for emotional neglect, and 18 were positive for physical neglect. Of the five CTQ maltreatment subtypes, only emotional abuse (EA) produced significant effects on Dex/CRH test cortisol response curves when all five subtypes were considered together in the same model (n=230) with age and sex (see below). Items on the CTQ used for assessment of childhood EA included “people in my family called me things like ‘stupid,’ ‘lazy,’ or ‘ugly’,” “people in my family said hurtful or insulting things to me,” “the punishments I received seemed cruel,” “I believe I was emotionally abused,” and “I thought that my parent wished I had never been born.”
Effect of Emotional Abuse and Age
The model (n=230) revealed significant within-subjects (F[2.1, 468]=3.7, p=.02) and between-groups (F[1, 221]=4.8, p=.03) effects of EA, reflecting a flatter slope in cortisol rise following CRH bolus and lower cortisol concentrations throughout the Dex/CRH test in those who reported having been emotionally abused in childhood (Figure 1, panel A). A significant between-groups effect of sex (F[1, 221]=8.8, p=.003) was also present, however an additional model showed that there was no significant interaction of EA by sex in the prediction of cortisol response. There was also a significant within-subjects effect of age (F[2.1, 468]=3.4, p=.03), and a model constructed for further exploration of this effect revealed a highly significant interaction between EA and age (F[2.1, 472]=6.2, p=.002), such that the impact of EA on cortisol responsivity appeared to be greatest in older subjects. When the interaction term (EA × age) was present in the model, the independent effect of age on cortisol was reduced to trend level. To better illustrate this interaction effect, the analysis was run separately in the “younger” (n=174) and “older” (n=56) cohorts. There were no significant cortisol curve effects of EA within subjects or between groups in the younger cohort of subjects (Figure 1, panel B). However, the within-groups effect of EA was highly significant in the older cohort (F[2.3, 106]=6.9, p=.001), as reflected by a flattened rise in cortisol among those subjects aged 36+ years with EA (Figure 1, panel C). The between-groups effects of EA remained significant in the older subject cohort (F[1, 47]=4.8, p=.03), reflecting lower overall output of cortisol throughout the test among those with EA.
Figure 1.
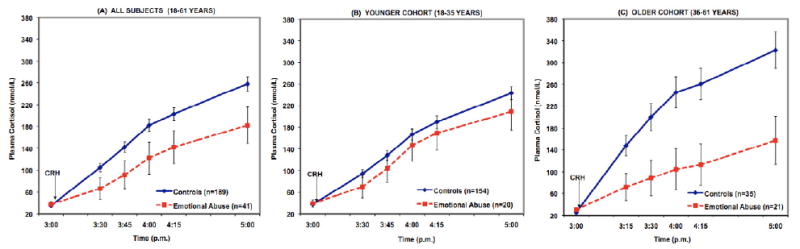
Cortisol response to the Dex/CRH test in healthy medication-free adults without current psychopathology, grouped according to presence or absence of childhood emotional abuse (EA; moderate or severe on CTQ subscale). Values are adjusted for age, sex, and for effects of four other maltreatment subtypes included in the model. The left panel (A) depicts the entire sample (n=230) where we found significant within-subjects effects of time (p<.001), time × EA (p<.05), and time × age (p<.05), as well as significant between-groups effects of EA (p<.05), and sex (p<.01). Panels (B) and (C) show the same sample split into two age cohorts for better illustration of the significant EA × age interaction effect (p<.01). In the younger cohort (B, middle), only a within-subjects effect of time (p<.001) is significant. In the older cohort (C, right), the within-subjects effect of sex (p<.05) and EA (p=.001) are significant, along with between-groups effects of sex (p<.05) and EA (p<.05).
Effect of Exogenous Hormone Use
Because exogenous hormone use in women was associated with greater cortisol responses to the Dex/CRH test, we repeated the final model which examined all subtypes of maltreatment in just women without hormone use (n=83), and the results described above remained significant.
Comparison of Subject Groups Defined by EA
Table 1 compares the clinical features of subject groups defined by the presence of EA (n=41 with EA, n=189 without EA). Those with EA were significantly older and had more sub-threshold lifetime Axis I disorders. EA subjects were also significantly more likely to report having experienced all other subtypes of childhood maltreatment on CTQ subscales (Table 1). The EA and control groups were somewhat more closely matched within the younger/older age cohorts. Variables showing significant group differences were entered in post-hoc GLM models.
Table 1.
| ALL 230 Subjects (18-61 yrs) | YOUNGER Cohort (18-35 yrs) | OLDER Cohort (36-61 yrs) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Emotional Abuse (n=41) |
Control (n=189) |
p-value | Emotional Abuse (n=20) |
Control (n=154) |
p-value | Emotional Abuse (n=21) |
Control (n=35) |
p-value | |
| Age (y), mean (SD) | 35.90 (11.60) | 27.78 (9.52) | <.001 | 25.60 (5.46) | 23.94 (4.58) | n.s. | 45.71 (5.77) | 44.69 (6.79) | n.s. |
| Range | 19-54 | 18-61 | 19-35 | 18-35 | 36-54 | 36-61 | |||
| Sex, n (%) | |||||||||
| Male | 14 (34%) | 86 (46%) | n.s. | 8 (40%) | 69 (45%) | n.s. | 6 (29%) | 17 (49%) | n.s. |
| Female | 27 (66%) | 103 (54%) | 12 (60%) | 85 (55%) | 15 (71%) | 18 (51%) | |||
| Highest Level of Education Completed, n (%) | |||||||||
| Partial High School | 0 | 3 (2%) | 0 | 1 (1%) | 0 | 2 (6%) | |||
| High School Graduate | 3 (%) | 14 (8%) | 1 (5%) | 13 (9%) | 2 (10%) | 1 (3%) | |||
| Technical Degree | 0 | 1 (1%) | n.s. | 0 | 1 (1%) | n.s. | 0 | 0 | n.s. |
| Partial College | 14 (34%) | 82 (44%) | 9 (45%) | 76 (50%) | 5 (24%) | 6 (18%) | |||
| College Graduate | 18 (44%) | 56 (30%) | 8 (40%) | 45 (30%) | 10 (48%) | 11 (32%) | |||
| Professional Degree | 6 (15%) | 29 (16%) | 2 (10%) | 15 (10%) | 4 (19%) | 14 (41%) | |||
| Perceived Stress Scale | |||||||||
| Mean (SD) | 18.37 (6.61) | 17.05 (6.13) | n.s. | 18.65 (6.45) | 16.83 (5.96) | n.s. | 18.10 (6.91) | 18.03 (6.87) | n.s. |
| Inventory of Depressive Symptomatology - Self Report (IDS-SR) | |||||||||
| Total Score, Mean (SD) | 11.68 (7.84) | 8.05 (5.97) | .001 | 8.90 (5.73) | 7.79 (5.49) | n.s. | 14.33 (8.75) | 9.23 (7.74) | <.05 |
| State-Trait Anxiety Inventory (STAI) | |||||||||
| Mean (SD) | |||||||||
| State Anxiety | 2.83 (8.51) | 29.66 (8.71) | <.05 | 31.35 (8.24) | 29.41 (8.25) | n.s. | 34.24 (8.72) | 30.74 (10.60) | n.s. |
| Trait Anxiety | 35.71 (8.63) | 31.65 (8.82) | <.01 | 36.70 (8.64) | 31.34 (8.28) | <.01 | 34.76 (8.73) | 32.97 (10.93) | n.s. |
| Childhood Trauma Questionnaire (CTQ) | |||||||||
| Total Score, mean (SD) | 12.17 (2.59) | 6.45 (1.65) | <.001 | 11.49 (2.12) | 6.31 (1.54) | <.001 | 12.85 (2.88) | 7.09 (1.96) | <.001 |
| CTQ Subscales, n (%) meeting threshold for moderate-severe | |||||||||
| Emotional Abuse | 41 (100%) | 0 | n/a | 20 (100%) | 0 | n/a | 21 (100%) | 0 | n/a |
| Physical Abuse | 18 (45%) | 6 (3%) | <.001 | 9 (45%) | 4 (3%) | <.001 | 9 (45%) | 2 (6%) | .001 |
| Sexual Abuse | 13 (32%) | 13 (7%) | <.001 | 3 (15%) | 8 (5%) | n.s. | 10 (48%) | 5 (14%) | <.01 |
| Physical Neglect | 18 (44%) | 12 (6%) | <.001 | 7 (35%) | 10 (6%) | .001 | 11 (52%) | 2 (6%) | <.001 |
| Emotional Neglect | 27 (66%) | 18 (10%) | <.001 | 12 (60%) | 12 (8%) | <.001 | 15 (71%) | 6 (17%) | <.001 |
| History of Past Psychiatric Disorder on SCID | |||||||||
| n (%) meeting criteria for | |||||||||
| Lifetime Diagnosis* | 24 (59%) | 58 (31%) | .001 | 7 (35%) | 42 (27%) | n.s. | 17 (81%) | 16 (46%) | <.01 |
| Oral Contraceptive or Estrogen Replacement | |||||||||
| n (% of women per group) | 5 (19%) | 41 (40%) | <.05 | 3 (25%) | 37 (44%) | n.s. | 2 (13%) | 4 (22%) | n.s. |
Lifetime SCID diagnoses included: Major depressive disorder, n=32; dysthymic disorder, n=11; depressive disorder NOS, n=10; adjustment disorder, n=8; posttraumatic stress disorder, n=6; social anxiety disorder, n=3; obsessive-compulsive disorder=1; panic disorder, n=2; anxiety disorder NOS, n=4; alcohol use disorders (abuse or dependence), n=24; substance use disorders (abuse or dependence), n=19. Subject may have met SCID criteria for more than one lifetime disorder. “n.s.” in p-value column denotes test comparing Emotional Abuse and Control Group was not significant at p<.05
Post-hoc Testing for Extraneous Effects
Additional post-hoc tests were conducted to ensure that lifetime psychiatric disorders, which are associated with maltreatment, were not responsible for the association between EA and cortisol response. A positive history of lifetime Axis I diagnoses on SCID interview did not produce any significant effects on cortisol response, and did not diminish the significance of EA effects. Further, the main findings remained unchanged when subcategories of lifetime diagnosis were examined independently as covariates (i.e., major depressive disorder, any mood disorder, any anxiety disorder, posttraumatic stress disorder, substance use disorders) and when the analyses were re-run, excluding the subjects who endorsed SCID criteria for lifetime major depressive episode (n=32) or lifetime posttraumatic stress disorder (n=6).
Discussion
The main finding of this investigation was a significant dampening of cortisol reactivity in adulthood associated with self-reported emotional abuse in childhood. This effect was consistently observed in both men and women, regardless of current level of subthreshold depressive/anxiety symptoms, and regardless of reported history of past symptoms meeting diagnostic criteria for major depression or other Axis I psychiatric disorder. Of particular note, the effect appeared to increase with advancing age. Subjects in the older cohort (i.e., 36 – 61 years, mean age= 46) reporting childhood EA showed a markedly decreased cortisol response to the Dex/CRH test, while the effect was not present at a statistically significant level in a younger cohort (age 18-35 years, mean age= 26). Several research groups (24-27) have demonstrated an increase in cortisol responsivity as a function of increasing age, rather than the decrease we observed in our older subjects reporting EA. The significant interaction of exposure to childhood EA and increasing age on HPA axis response to a standardized challenge may reflect cumulative or progressive effects of early environment across the life span. Such an interpretation would be consistent with the concept of allostatic load (28) or long-term wear and tear on physiological systems as a result of chronic stress or demand on stress-adaptive biological mechanisms.
Other investigators have hypothesized a trajectory of initial hyper-activation of the HPA system progressing to a state of chronic adrenal stress hyporeactivity (13; 29) as a type of counter-regulatory adaptation following acute or sustained exposure to excessive ACTH during stressful early development (18). This proposed mechanism is consistent with available longitudinal data on cortisol levels and the development of posttraumatic stress disorder (30; 31), and mirrors findings from preclinical laboratories of blunted adrenal stress responsiveness in primates (32) and rodents (33; 34) exposed to chronic social stress models. Over time, experimentally stressed animals persistently show lower levels of hippocampal mineralocorticoid receptor expression, suggesting long-term and perhaps epigenetic alterations in gene expression regulation. In addition to receptor down-regulation, other mechanisms of counter-regulatory adaptation to excessive stimulation of the HPA system could include increased negative feedback sensitivity, and reduced biosynthesis or depletion of hormones at various levels of the axis (35; 36). Particularly relevant to risk for psychiatric disorders in mid-life, McEwen and colleagues have noted that the aging brain appears more vulnerable to hippocampal damage from prolonged psychosocial stress (37). Our results in humans may lend additional support to the hypothesis that stress exposure, in the form of perceived EA, during a crucial developmental time period results in lasting effects on physiological and behavioral parameters throughout life, which may in turn contribute to an enhanced vulnerability to stress-induced diseases. We can speculate that EA represents a chronic stressor over a decade or more of a child's life, or that emotionally abused children develop difficulties with attachment and interpersonal interaction that perpetuate a pattern of chronic stress in subsequent relationships across the life span.
However, since we did not measure duration or chronicity of stress exposure, we cannot conclude that our findings are specifically related to adverse social or interpersonal conditions that were repeated or sustained for any particular period of time during childhood development or into adulthood. Moreover, we did not assess other possible contributing factors such as genetic effects or the influence of parental factors such as mental illness on the quality of parental care.
As reviewed above, published findings describing the direction of observed abnormalities in basal cortisol levels or dynamic cortisol responsivity among subjects with childhood adversity relative to controls have not been consistent. Discrepancies in the literature are likely attributable to multiple effects impacting HPA axis function. The nature and timing of the abuse or trauma during development, the degree and chronicity of contemporary stressors in adulthood, concurrent psychiatric and medical disorders, and age or other individual qualities of the subjects being studied may all contribute significantly (38-40), particularly if the trajectory from HPA axis hyper-responsivity to hypo-responsivity occurs over several decades of stress exposure. Despite using different neuroendocrine probes, the results we report here with the Dex/CRH test in healthy adults are similar to findings of blunted cortisol response to a standardized laboratory stress test in adults reporting childhood maltreatment reported by our group (17) and by others (41). Recently, investigators of cortisol responsivity have utilized designs intended to diminish potential confounds and better isolate specific effects, as we have in this study. We did not detect effects of other types of self-reported maltreatment, perhaps owing to the relatively small proportions of subjects endorsing criteria for physical abuse (10%), sexual abuse (11%), and physical neglect (13%) in our sample.
Emotional neglect (20%) and emotional abuse (EA; 18%) were the two subtypes of childhood maltreatment most commonly reported by our subjects, perhaps reflecting some selection bias introduced by exclusion of individuals with more significant mental health histories. The CTQ is a self-report measure that relies on retrospective recall and self-ratings of early environment, and it is important to acknowledge the inherent subjectivity of this data as well as the potential for recall bias. The perception of life events and the experience of stress or trauma are shaped by a variety of influences, including perceptual factors, personality traits, coping skills, and social supports. In addition to altering the appraisal of a potentially stressful event, these factors may themselves influence neuroendocrine activity. The EA subscale may detect a subset of participants that are more sensitive to interpersonal rejection or have higher levels of attachment anxiety. If that were the case, one could speculate that EA subjects may be more likely to experience inadequate social supports and fear of negative social evaluation as a stressor persisting into adulthood (3; 42; 43). It is possible that subjective individual differences such as sensitivity to interpersonal stressors account for our finding of dampened cortisol response to Dex/CRH, but some evidence against this comes from our measure of perceived stress level in the past month before the Dex/CRH test. EA and non-EA groups did not differ with regard to the degree they reported feeling challenged by stressors in the weeks leading up to their participation in our research protocol on the Perceived Stress Scale. Genetic factors, such as polymorphisms in the serotonin reuptake transporter gene, have been found to modify cortisol responses to psychosocial stressors in young girls (44), reflecting a possible intermediate mechanism by which some individuals are more vulnerable to negative interpersonal environments. Future work exploring genotypes, childhood maltreatment, and cortisol reactivity may further our understanding of how differential responses to stress challenge are related to an individual's tendency to interpret an interpersonal experience as emotionally abusive, and how these factors confer risk for psychopathology.
Significantly more of our subjects reporting childhood EA endorsed criteria for past Axis I psychiatric disorders. The EA group also had consistently higher levels of sub-threshold mood and anxiety symptoms at the time of testing. While these factors did not statistically account for the association between EA and dampened cortisol responsivity, they support the notion that this particular pattern of response may signal a subtype of subthreshold mood/anxiety spectrum disorders akin to that observed in patients with secondary adrenal insufficiency (45).
In summary, we found that of the five childhood maltreatment subtypes evaluated, emotional abuse emerged as the only significant predictor of cortisol response to the Dex/CRH test in healthy adults. Emotional abuse was associated with a blunted cortisol response, and this effect appeared more robust with increasing age. The association of emotional abuse with cortisol response was independent of several demographic factors, the other maltreatment subtypes, lifetime diagnoses, and current depressive or anxious symptomatology. Longitudinal studies will be needed to determine if the low cortisol reactivity endophenotype we identified is a meaningful premorbid marker signaling risk for development of medical or mental health disorders.
Acknowledgments
The authors thank Kelly Colombo, B.A. for her assistance with data management. This work was supported by R01 MH068767 (LLC).
Footnotes
Financial Disclosures: The authors disclose the following biomedical financial interests over the past two years and the foreseeable future. Drs. Tyrka, Price, and Carpenter have received grant/research support from the National Institutes of Health, the US Department of the Interior, the US Department of Defense, Sepracor, Pfizer, Cyberonics, UCB Pharma, and Medtronic. Dr. Price has received speakers' bureau honoraria from Jazz Pharmaceuticals and has served as a consultant for Gerson Lehrman, Wiley, Springer, and Lundbeck. Dr. Carpenter has served as a consultant or on the advisory board for Cyberonics, Neuronetics, Novartis and Wyeth, and has received honoraria for continuing medical education from AstraZeneca and speakers' bureau honoraria for Cyberonics. All other authors report no biomedical financial interests or potential conflicts of interest. No therapeutic pharmaceutical or device products were utilized in this research protocol. Acthrel™ was provided at a discounted price by Ferring Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gervasoni N, Bertschy G, Osiek C, Perret G, Denis R, Golaz J, et al. Cortisol responses to combined dexamethasone/CRH test in outpatients with a major depressive episode. Journal of Psychiatric Research. 2004;38:553–557. doi: 10.1016/j.jpsychires.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, et al. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2007;92:2439–2445. doi: 10.1210/jc.2006-2540. [DOI] [PubMed] [Google Scholar]
- 3.Tops M, Riese H, Oldehinkel AJ, Rijsdijk FV, Ormel J. Rejection sensitivity relates to hypocortisolism and depressed mood state in young women. Psychoneuroendocrinology. 2008;33:551–559. doi: 10.1016/j.psyneuen.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Levitan RD, Vaccarino FJ, Brown GM, Kennedy SH. Low-dose dexamethasone challenge in women with atypical major depression: pilot study. Journal of Psychiatry and Neuroscience. 2002;27:47–51. [PMC free article] [PubMed] [Google Scholar]
- 5.Van Den Eede F, Moorkens G, Hulstijn W, Van Houdenhove B, Cosyns P, Sabbe BG, et al. Combined dexamethasone/corticotropin-releasing factor test in chronic fatigue syndrome. Psychological Medicine. 2008;38:963–973. doi: 10.1017/S0033291707001444. [DOI] [PubMed] [Google Scholar]
- 6.Rydmark I, Wahlberg K, Ghatan PH, Modell S, Nygren A, Ingvar M, et al. Neuroendocrine, cognitive and structural imaging characteristics of women on longterm sickleave with job stress-induced depression. Biological Psychiatry. 2006;60:867–873. doi: 10.1016/j.biopsych.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Bellingrath S, Weigl T, Kudielka BM. Cortisol dysregulation in school teachers in relation to burnout, vital exhaustion, and effort-reward-imbalance. Biological Psychology. 2008;78:104–113. doi: 10.1016/j.biopsycho.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Wingenfeld K, Heim C, Schmidt I, Wagner D, Meinlschmidt G, Hellhammer DH. HPA axis reactivity and lymphocyte glucocorticoid sensitivity in fibromyalgia syndrome and chronic pelvic pain. Psychosomatic Medicine. 2008;70:65–72. doi: 10.1097/PSY.0b013e31815ff3ce. [DOI] [PubMed] [Google Scholar]
- 9.Schumann EM, Kumpfel T, Then Bergh F, Trenkwalder C, Holsboer F, Auer DP. Activity of the hypothalamic-pituitary-adrenal axis in multiple sclerosis: correlations with gadolinium-enhancing lesions and ventricular volume. Annals of Neurology. 2002;51:763–767. doi: 10.1002/ana.10187. [DOI] [PubMed] [Google Scholar]
- 10.Bhagwagar Z, Cowen PJ. ‘It's not over when it's over’: persistent neurobiological abnormalities in recovered depressed patients. Psychological Medicine. 2008;38:307–313. doi: 10.1017/s0033291707001250. [DOI] [PubMed] [Google Scholar]
- 11.Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. Journal of Psychiatric Research. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 12.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 13.Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, et al. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neuroscience and Biobehavioral Reviews. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 15.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. The American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 16.Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biological Psychiatry. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P) New York, NY: New York State Psychiatric Institute, Biometrics Research; 1996. [Google Scholar]
- 20.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 21.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD. State-Trait Anxiety Inventory (Form Y) Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 23.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 24.Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26:225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 25.Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Kirschbaum C. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology. 1999;70:422–430. doi: 10.1159/000054504. [DOI] [PubMed] [Google Scholar]
- 26.Kunugi H, Ida I, Owashi T, Kimura M, Inoue Y, Nakagawa S, et al. Assessment of the dexamethasone/CRH test as a state-dependent marker for hypothalamic-pituitary-adrenal (HPA) axis abnormalities in major depressive episode: a Multicenter Study. Neuropsychopharmacology. 2006;31:212–220. doi: 10.1038/sj.npp.1300868. [DOI] [PubMed] [Google Scholar]
- 27.Goncharova ND, Lapin BA. Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mechanisms of Ageing and Development. 2002;123:1191–1201. doi: 10.1016/s0047-6374(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 28.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 29.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Pervanidou P. Biology of post-traumatic stress disorder in childhood and adolescence. Journal of Neuroendocrinology. 2008;20:632–638. doi: 10.1111/j.1365-2826.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 31.Delahanty DL, Nugent NR. Predicting PTSD prospectively based on prior trauma history and immediate biological responses. Annals of the New York Academy of Sciences. 2006;1071:27–40. doi: 10.1196/annals.1364.003. [DOI] [PubMed] [Google Scholar]
- 32.Saltzman W, Hogan BK, Abbott DH. Diminished cortisol levels in subordinate female marmosets are associated with altered central drive to the hypothalamic-pituitary-adrenal axis. Biological Psychiatry. 2006;60:843–849. doi: 10.1016/j.biopsych.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Pohorecky LA, Baumann MH, Benjamin D. Effects of chronic social stress on neuroendocrine responsiveness to challenge with ethanol, dexamethasone and corticotropin-releasing hormone. Neuroendocrinology. 2004;80:332–342. doi: 10.1159/000083682. [DOI] [PubMed] [Google Scholar]
- 34.Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Hormones and Behavior. 2008;53:386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Fries E, Hellhammer DH, Hellhammer J. Attenuation of the hypothalamic-pituitary-adrenal axis responsivity to the Trier Social Stress Test by the benzodiazepine alprazolam. Psychoneuroendocrinology. 2006;31:1278–1288. doi: 10.1016/j.psyneuen.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 37.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiology of Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 38.Weems CF, Carrion VG. The association between PTSD symptoms and salivary cortisol in youth: the role of time since the trauma. Journal of Traumatic Stress. 2007;20:903–907. doi: 10.1002/jts.20251. [DOI] [PubMed] [Google Scholar]
- 39.Michaud K, Matheson K, Kelly O, Anisman H. Impact of stressors in a natural context on release of cortisol in healthy adult humans: a meta-analysis. Stress (Amsterdam, Netherlands) 2008;11:177–197. doi: 10.1080/10253890701727874. [DOI] [PubMed] [Google Scholar]
- 40.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biological Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Ditzen B, Schmidt S, Strauss B, Nater UM, Ehlert U, Heinrichs M. Adult attachment and social support interact to reduce psychological but not cortisol responses to stress. Journal of Psychosomatic Research. 2008;64:479–486. doi: 10.1016/j.jpsychores.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Quirin M, Pruessner JC, Kuhl J. HPA system regulation and adult attachment anxiety: individual differences in reactive and awakening cortisol. Psychoneuroendocrinology. 2008;33:581–590. doi: 10.1016/j.psyneuen.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hahner S, Loeffler M, Fassnacht M, Weismann D, Koschker AC, Quinkler M, et al. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. The Journal of Clinical Endocrinology and Metabolism. 2007;92:3912–3922. doi: 10.1210/jc.2007-0685. [DOI] [PubMed] [Google Scholar]


