Abstract
Phospholipase C-γ1 (PLC-γ1) mediates cell adhesion and migration through an undefined mechanism. Here, we examine the role of PLC-γ1 in cell-matrix adhesion in a hanging drop assay of cell aggregation. Plcg1 Null (−/−) mouse embryonic fibroblasts formed aggregates that were larger and significantly more resistant to dissociation than cells in which PLC-γ1 is re-expressed (Null + cells). Aggregate formation could be disrupted by inhibition of fibronectin interaction with integrins, indicating that fibronectin assembly may mediate aggregate formation. Fibronectin assembly was mediated by integrin α5β1 in both cell lines, while assays measuring fibronectin assembly revealed increased assembly in the Null cells. Null and Null + cells exhibited equivalent fibronectin mRNA levels and equivalent levels of fibronectin protein in pulse-labeling experiments. However, levels of secreted fibronectin in the conditioned medium were increased in Null cells. The data implicates a negative regulatory role for PLC-γ1 in cell aggregation by controlling the secretion of fibronectin into the media and its assembly into fibrils.
Keywords: Signal Transduction, Extracellular Matrix, Fibronectin Assembly
Introduction
Phospholipase C γ1 (PLC-γ1) is a tyrosine kinase activated enzyme that hydrolyzes phosphotidylinositol 4,5 bisphosphate to form the second messengers inositol 1,4,5 trisphosphate and diacylglycerol. The former stimulates the release of intracellular calcium from the endoplasmic reticulum, while the latter activates protein kinase C. Activation of PLC-γ1 requires tyrosine phosphorylation by receptor or non-receptor tyrosine kinases. PLC-γ1 can be phosphorylated downstream of growth factor stimulation or integrin activation[1–5]. Adhesion to fibronectin results in Src-mediated phosphorylation and activation of PLC-γ1[1, 6]. Biochemical data and mutagenesis experiments have shown that phosphorylation of Y783 is required for enhancement of PLC-γ1 enzymatic activity [1, 5, 7].
PLC-γ isozymes are required for adhesion, spreading and migration in a variety of cell systems; however, the mechanistic basis of this is unknown [1, 8–12]. PLC-γ1 (−/−) or Null cells exhibit impaired adhesion to fibronectin and this impairment is attenuated by increased concentrations of fibronectin [1]. PLC-γ1 mediated adhesion to fibronectin requires phosphorylation of PLC-γ1 on Y783. While integrin levels and clustering are not affected by the absence of PLC-γ1, Null fibroblasts exhibit reduced migration towards fibronectin [1]. PLC-γ1 is known to localize to the leading edge of migrating cells and to cell-matrix adhesions [8–10].
To further investigate role of PLC-γ1 in adhesion to the extracellular matrix, we have examined fibronectin matrix assembly in Plcg1 Null and Null + cell lines. The results indicate that PLC-γ1 negatively regulates fibronectin secretion and assembly into fibrils.
Materials and Methods
Materials
Antibodies for mouse anti-actin, mouse anti-vimentin, and mouse anti-fibronectin were purchased from Sigma Aldrich, while those to integrins α5 (5H10-27), β1 (9EG7), β1 (HA2/5), β3 (2C9.G2) and αV (RMV-7) were purchased from BD Biosciences. The rabbit anti-fibronectin used for immunoprecipitation was purchased from Santa Cruz Biotechnology. Cy3 conjugated goat anti-mouse was purchased from Jackson Immunoresearch and Alexa 647-conjugated goat anti-mouse was a product of Molecular Probes. Mouse and rabbit secondary antibodies used in western blotting were purchased from Licor Biosciences. CycloRGD (RGDfV), and cycloRAD (RADfV) were obtained from Biomol International. Peptides were dissolved at a concentration of 5mg/ml in 25% DMSO/PBS and used at a concentration of 250 μg/ml. The 70 kDa fibronectin fragment [11] was purchased from Sigma and dissolved in cell culture medium.
Cell Culture and Staining
Plcg1 Null and Null + immortalized mouse embryonic fibroblasts have been described previously [12]. Cells were cultured in DMEM supplemented with 10% FBS. In experiments using fibronectin-free FBS, fibronectin was removed by passing FBS through a gelatin-Sepharose column (GE Health Sciences). For cell staining, cells were fixed in 4% PFA and stained for fibronectin. Stained cells or hanging drop aggregates were visualized using a Zeiss LSM 510 confocal microscope.
Hanging Drop Assay
Null and Null + cells were detached using Accutase (Innovative Cell Technologies) and re-suspended at 500,000 cells/ml in cell culture medium. The 70 kDa fragment, cyclic RGD or RAD peptides, or integrin β1 or β3 blocking antibodies were added at indicated concentrations. Drops (30 μl) were placed on the lid of a 24 well plate and the lid was inverted over the cell culture wells, which contained PBS to avoid evaporation of the hanging drop. Cells were cultured in the hanging drop overnight. Subsequently, hanging drops were photographed, pipetted 20 times to disrupt cell aggregates and then photographed again using a Leica inverted microscope with 10× objective. Aggregates were measured using Metamorph software. Aggregates were traced and the area measured.
Deoxycholic Acid (DOC) Solubility Assays
The DOC solubility assays have been previously described [13]. In brief, 12 × 105 cells were plated in 100 mm dishes in DMEM supplemented with 10% fibronectin-free FBS. After 4–24 hrs, the cells were lysed in 2% DOC lysis buffer (2% DOC, 20mM tris-CL pH 8.8, 2mM PMSF, 2mM EDTA, 2mM iodoacetic acid, and 2 mM N-ethylmaleimide) and lysates were passed through a 25 gauge needle and centrifuged (16,000 × g, 20 min) at 4°C. The supernatant was removed and saved as the DOC soluble fraction, while the pellet was washed in DOC lysis buffer and then re-suspended in 2X LDS reducing sample buffer (Invitrogen). Protein levels were determined for DOC soluble fractions by BCA assay (Pierce). Equal amounts of DOC-soluble and -insoluble protein were resolved on a 4–12% SDS-PAGE gel.
Metabolic Labeling
Null and Null + cells were placed in labeling medium (methionine and cysteine-free DMEM, 10% FN free FBS, 20 μM unlabeled methionine, and 50 uCi/ml 35S in vitro cell labeling mix (GE Healthsciences)). Cells were then harvested as described above and fibronectin was precipitated using mouse anti-human fibronectin (BD Biosciences). To measure levels of secreted fibronectin, conditioned medium was collected and phenylmethylsulfonyl fluoride was added to a final concentration of 2mM. Gelatin-Sepharose beads were added to adsorb fibronectin and the samples were incubated overnight. Beads were washed and 2X reducing sample buffer was added. DOC-soluble, DOC-insoluble, and conditioned medium samples were resolved on a 4–20% SDS-PAGE. The gel was dried and exposed to a Phosphorimager screen. Bands were quantified using Image J software.
For pulse experiments, cells were pulsed for 10 min with 35S-methionine prior to harvesting the cells in TGH lysis buffer (1% Triton X 100, 10% glycerol, 50mM Hepes pH 7.2, 100mM NaCl). Lysates were sonicated and fibronectin was precipitated using rabbit anti-fibronectin. For pulse-chase experiments, cells were labeled for one hr with 35S-methionine prior to a 2-hr chase. Conditioned medium was collected and radioactivity analyzed by scintillation counting. Radioactivity was normalized to protein levels.
Exogenous Assembly Assays
Bovine plasma fibronectin (Sigma) was biotinylated using the manufacturer’s protocol (Pierce). Null and Null + cells were re-suspended at a concentration of 1.2 × 105 cells/ml in DMEM supplemented with 10% FN-free FBS and biotinylated fibronectin (20μg/ml) and then plated in 60 mm dishes. After 4 hrs, the cells were lysed and DOC-soluble and -insoluble fractions were isolated. Fractions were resolved on 4–12% SDS-PAGE gels and transferred to a PVDF membrane. Membranes were blotted with NeutrAvidin® HRP (Pierce) or Streptavidin IR680 (Licor inc) and visualized by chemiluminescence or an Oddysey fluorescence imager (Licor inc).
RNA Analysis
Cells were plated at equal density and cultured overnight. The next day, RNA was isolated using the Qiagen RNeasy mini kit. Total RNA was subjected to northern blot and QRT-PCR. For northern blots, the 683 bp human fibronectin probe was cut from plasmid pSP73 RFN 2375-6090 (provided by Dr. Jean Schwarzbauer, Princeton University) using EcoRV and BamHI. Blots were stripped and re-probed for cyclophilin as a loading control. Fibronectin QRT-PCR primers were purchased from Applied Biosystems. cDNA was generated using the iscript cDNA Synthesis Kit (BioRad). QRT-PCR was performed using IQ Supermix (BioRad) and the Biorad ICycler. Values were normalized to actin using the ΔΔcT method.
Results
PLC-γ1 Deficiency Increases Cell Aggregation
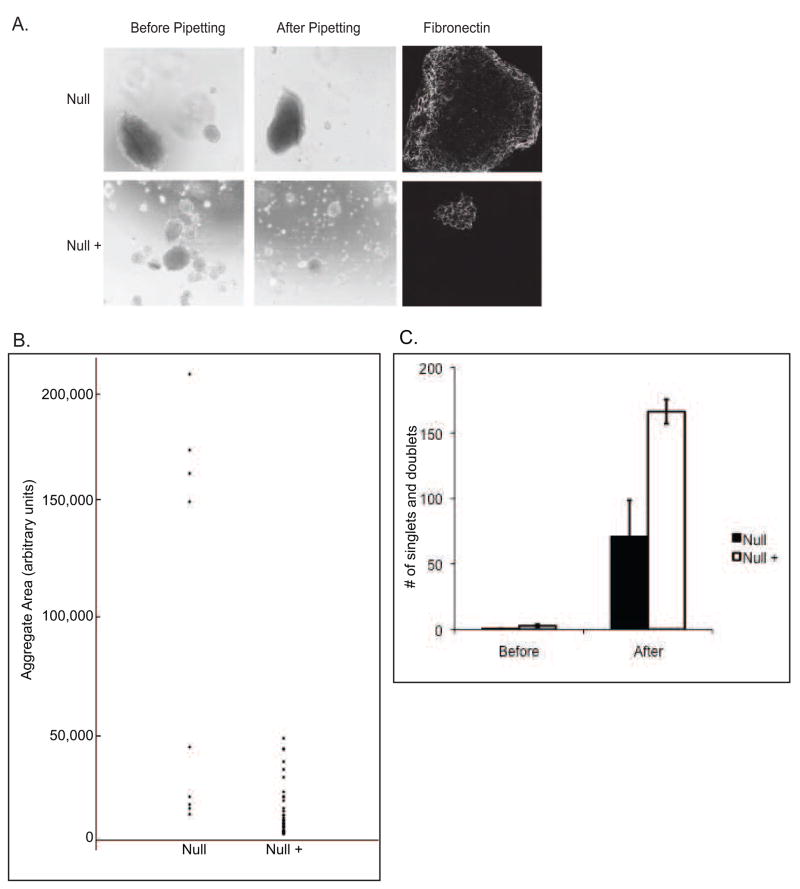
Plcg1 Null and Null + cells were cultured in hanging drops overnight to examine cell aggregation. The data in Figure 1A demonstrate that in any single hanging drop the Null cells form one large aggregate and a few smaller aggregates, whereas aggregates formed by Null + cells were all of the smaller size. The total number of aggregates formed by the Null cells was fewer than the Null + cells but the size of many of the aggregates was much larger in the Null cells (Figure 1B). Attempts to disperse Null cell aggregates by pipetting were unsuccessful, while similar attempts with Null + cells yielded a significant number of single cells or doublets (Figure 1C). These results show that cells deficient in PLC-γ1 display increased aggregation under these culture conditions.
Figure 1.
Assessment of cell aggregation by Null and Null + cells: A) Cells were detached and 5 × 105 cells were re-suspended in cell culture medium. 30 μ1 drops were added to the lid of a 24 well dish and hanging drops were cultured overnight. The next morning, drops were photographed (left panels), pipetted 20 times to disperse, and photographed again (middle panels) using a Leica conventional inverted microscope. All pictures were taken at the same magification. Pictures are representative images of multiple hanging drops. Cellular aggregates were stained for fibronectin and then visualized using a Zeiss confocal microscope (right panels). B) Quantification of aggregate area. Aggregate area was measured from four experiments as described in Materials and Methods. Each dot represents one aggregate. C) Quantification of the number of singlet’s and doublets in photographs before and after pipetting. Values are the average of five different hanging drops. Error bars indicate standard deviation.
Cell aggregation is mediated by either cadherin -dependent cell:cell interactions and/or fibronectin-dependent cell:matrix interactions [14, 15]. In cell-fibronectin interactions, integrin α5β1 has been shown to mediate the formation of strong fibronectin-mediated cell aggregates[16]. Fibronectin is the major matrix protein secreted by fibroblasts and following secretion, fibronectin is assembled into fibrils by integrins α5β1 or αVβ3 [17]. To determine whether the cell aggregates observed in Figure 1 contain assembled fibronectin, aggregates were fixed and stained for fibronectin. Fibronectin was detected in the aggregates of both Null and Null + cells (Figure 1A). Western blotting showed that Null and Null + cells express equivalent levels of N-cadherin (data not shown).
To determine whether fibronectin assembly contributes to the cell aggregation shown in the hanging drops of Figure 1, cells were treated with cyclic RGD or inactive cyclic RAD to block fibronectin interaction with the integrins. Treatment with RGD peptides has been shown to inhibit fibronectin assembly, as the RGD sequence of fibronectin is a critical motif for recognition by integrins [14, 18–22]. However, treatment with cyclic RGD had no effect on the increased cell aggregation exhibited by Null cells (data not shown). Cyclic RGD treated aggregates stained positive for fibronectin, indicating that the peptide was ineffective at blocking fibronectin interaction with the cell.
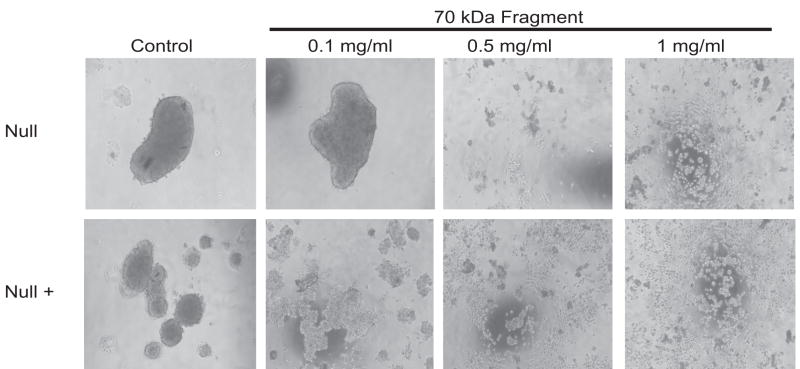
Since previous reports have indicated that RGD peptides are not stable during incubations longer than 6–8 hrs and that cyclic RGD may be more specific for integrins αVβ3 than α5β1[21, 23], a 70 kDa N-terminal fibronectin fragment, generated from proteolytic digestion of fibronectin, was used [11, 21, 23, 24]. The region of fibronectin encompassed by this fragment has been shown to block initiation of fibronectin assembly and also to bind integrin α5β1 [11, 25]. Treatment of hanging drops with the 70 kDa fragment completely abolished cell aggregation at concentrations at or above 500 μg/ml. At a concentration of 100 μg/ml, cell aggregation was decreased by this fragment in the Null + cells, but had no effect on the Null cell aggregation (Figure 2).
Figure 2.
Treatment of Hanging Drops with 70 kDa fragment: Null and Null + hanging drops were treated as in Figure 1 except for the addition of 1000 μg/ml, 500 μg/ml, or 100 ug/ml 70 kDa fibronectin fragment.
Influence of PLC-γ1 on Fibronectin Assembly
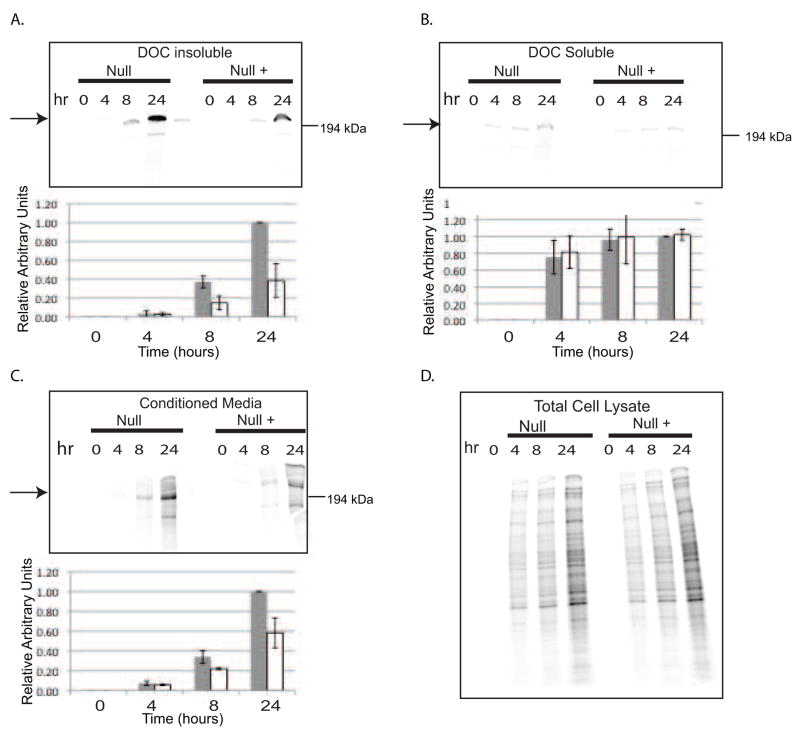
To determine whether fibronectin assembly is increased in Null cells, deoxycholic acid (DOC) assembly assays were performed. Null and Null + cells were labeled with 35S methionine to compare the assembly of endogenous fibronectin. The results, shown in Figure 3, demonstrate that the Null cells assemble more fibronectin than Null + cells. An increase in the level of labeled fibronectin in the Null cell conditioned medium was also detected. Increased fibronectin assembly was also observed in the Null cells when the assembly of exogenous fibronectin was measured (Figure 4). When the assembly assay was performed using the Null and Null + cells in the hanging drop cell aggregate culture conditions, The Null cells demonstrated a 1.6- fold increase in the level of fibronectin assembly (Supplementary Figure 1). These results show that the increased cell aggregation observed with Null cells is correlated with the presence of fibronectin fibrils in the aggregates and increased fibronectin assembly.
Figure 3.
Assessment of fibronectin assembly in Null and Null + cells: Null and Null + cells were metabolically labeled with 35S methionine for the times indicated. Parts A, B, and C: Top panels - phosphorimager image. Arrows indicate 220 kDa fibronectin; Bottom panels-densitometric analysis. Values are relative to Null cells at 24 hours. Gray bars represent Null cells and white bars represent Null + cells. A) DOC-insoluble fraction; B) DOC-soluble fraction; C) Conditioned media; D) Total cell lysate.
Figure 4.
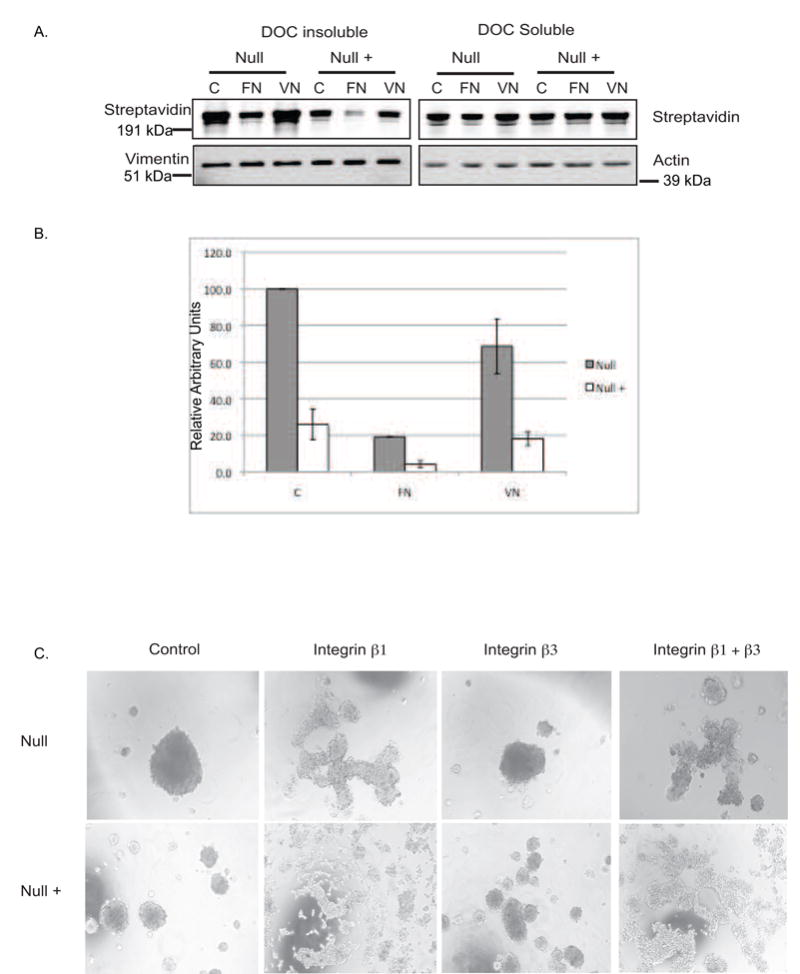
Fibronectin assembly is mediated by Integrin α5β1 in Null and Null + cells: A) Exogenous assembly assay; Cells were plated on 10ug/ml fibronectin or 5 ug/ml vitronectin in fibronectin free medium prior to the addition of biotinylated fibronectin. Cells were incubated overnight followed by collection of lysates and separation into DOC-soluble and insoluble fractions. Left panels: DOC-insoluble fractions. Biotinylated fibronectin is detected by blotting with IR Dye 680 Streptavidin. Vimentin serves as a loading control. Right Panels: DOC-soluble fraction: Actin serves as a loading control. B) Densitometric analysis of DOC insoluble fraction in panel A. Gray bars represent Null cells. White bars represent Null + cells. C) Hanging drop assay: Cells were treated as in figures 1 and 2 except for treatment with 100 μg/ml inhibitory antibodies against integrins β1 (HA 2/5), β3 (2C9.G2) or a combination of both.
Increased fibronectin assembly can reflect increased integrin or fibronectin expression. Previous work with these cells revealed similar integrin expression in the two cell lines [1]. To confirm this, we compared the expression of integrins α5, αV, and β1 in Null and Null + cells by FACS analysis. Null cells express approximately 1.25-fold more integrin α5, 1.2-fold more β1, and 1.4-fold more αV as compared to Null + cells (Supplementary Figure 2). To determine which integrin pair is responsible for fibronectin assembly in these cells, exogenous assembly assays were performed with cells plated on either fibronectin or vitronectin. If the cells utilize integrin α5β1 to assemble fibronectin, then plating cells on fibronectin would reduce the assembly of exogenous biotinylated fibronectin. However, if cells use integrin αVβ3, also known as the vitronectin receptor, for assembly, then plating cells on vitronectin would also reduce fibronectin assembly. When the Null and Null + cells were plated on fibronectin, there was a significant decrease in fibronectin assembly in both cell lines, while plating on vitronectin had no effect on assembly (Figure 4). This indicates that integrin α5β1 is responsible for fibronectin assembly in Null and Null + cells.
To test whether the α5β1 integrin is required for the cell aggregation observed in Figure 1, blocking antibodies to either integrin β1 or β3 (as a control) were incorporated into the hanging drop assay (Figure 4B). Integrin β1 blocking antibodies effectively reduced cell aggregation, while integrin β3 antibodies had no effect. Treatment with both antibodies yielded similar results to integrin β1 antibody treatment, indicating that the β1, but not β3 integrin is required for the formation of tight aggregates in Null and Null + cells.
PLC-γ1 Negatively Regulates the Levels of Secreted Fibronectin
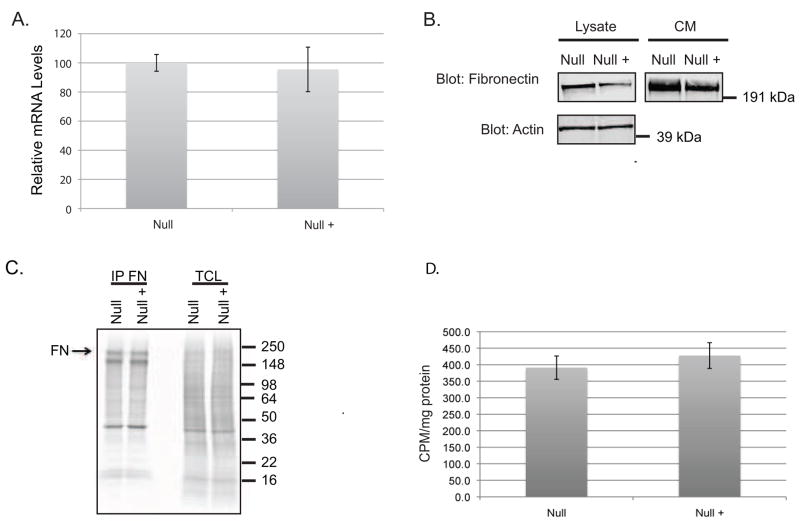
As PLC-γ1 Null cells display an increased capacity to assemble fibronectin into fibrils, it is possible that this reflects increased levels of fibronectin mRNA and/or protein. To determine whether steady-state fibronectin mRNA levels differ in the two cell lines, RNA was extracted from Null and Null + cells and mRNA levels were compared using qRT-PCR. The data show that steady state fibronectin mRNA levels are equivalent in both cell lines using both qRT-PCR (Figure 5A) and northern blotting (Supplementary Figure 3).
Figure 5.
Comparison of fibronectin mRNA and protein levels: A) QRT-PCR of RNA extracted from Null and Null + cells. B) Null and Null + cells were plated and cultured overnight. Cell lystates were harvested in RIPA buffer and conditioned medium (CM) was collected. Equal amounts of protein were resolved by SDS-PAGE followed by detection with rabbit FN and mouse actin antibodies. C) Cells were pulsed for 10 minutes with 35S methionine followed by lysis and Immunoprecipitation for fibronectin. Abbreviations FN Fibronectin; TCL total cell lysate D) Null and Null + were pulsed for 35S methionine followed by a two hour chase. The levels of radioactivity were determined using scintillation counter and normalized to cell lysate protein levels.
DOC assembly assays indicate that there may be an increase in fibronectin protein expression in the conditioned medium of Null cells (Figure 3). Therefore, fibronectin levels in the conditioned medium of Null and Null + cells were compared. Consistent with previous data, these results show that the Null cells secrete two-fold more fibronectin into the medium than Null + cells (Figure 5B). To determine whether fibronectin translation is increased in Null cells, Null and Null + cells were pulsed with 35S-methionine for 10 min prior to lysis and fibronectin immunoprecipitated. The results, shown in Figure 5, show that fibronectin protein production is equivalent in Null and Null + cells.
To determine whether PLC-γ1 exerts a global effect on protein secretion, a pulse chase experiment was performed. Cells were labeled with 35S-methionine for 90 min followed by a 90 min chase. Conditioned medium was collected and the radioactivity quantitated by scintillation counting. Null and Null + cells secreted equivalent levels of radiolabeled protein, indicating that PLC-γ1 selectively regulates the secretion of fibronectin (Figure 5D).
Discussion
While numerous reports have demonstrated a requirement for PLC-γ1 in cell adhesion and/or migration in various cell types [1, 26–29], a mechanistic understanding of this requirement has not been elucidated. The data in this manuscript show that PLC-γ1 has a regulatory role in controlling the amount of fibronectin produced and assembled into fibronectin fibrils. As fibronectin is a major component of the fibroblast extracellular matrix, which mediates cell adhesion and migration, the level of fibronectin production needs to be tightly controlled to avoid an abnormal composition of extracellular matrix. There are several examples of aberrant matrix compositions that affect cell function [30–32]. For example, when cell migration is measured on increasing concentrations of assembled fibronectin, migration levels exhibit a biphasic effect. Migration rates increase as fibril concentrations increase until an optimal concentration for maximum migration and above this increased concentrations of assembled fibronectin reduce migration [33–36].
The data in this manuscript show that while PLC-γ1 does not influence mRNA levels nor production of fibronectin protein within the cell, it does regulate the levels of fibronectin protein that is secreted. This increase in fibronectin secretion is not part of a global effect on secretion as both Null and Null + cell lines secrete equivalent levels of protein. The data also show that the increase in secreted fibronectin in cells genetically deficient in PLC-γ1 is accompanied by an increase in fibronectin assembly into fibrils. The increase in fibronectin assembly is observed in assays that rely on both the assembly of endogenous fibronectin as well as exogenous fibronectin. While the latter might indicate a separate role of PLC-γ1 in assembly, there is published data indicating that an increase in the levels of endogenous fibronectin does in fact result in an increase in the assembly of exogenous fibronectin into fibrils [37, 38]. The data would indicate that PLC-γ1 functions to set the limit for the maximal level of fibronectin secretion, and in the absence of this protein is oversecreted.
The fact that the absence of PLC-γ1 increases the level of secreted fibronectin protein, but not mRNA or protein production is novel. The second messengers formed by PIP2 hydrolysis are well described for their capacity to effect signaling that impinges on gene expression. However, how these second messengers may affect post-translational processes is less well known.
Supplementary Material
Supplementary Figure 1. Fibronectin assembly in hanging drop cell aggregates. Null and Null + cells were cultured in hanging drops overnight followed by lysis and isolation of the DOC-insoluble fraction. DOC-insoluble lysates were blotted for fibronectin. Vimentin was used as a loading control.
Supplementary Figure 2. Integrin surface expression in Null and Null + cells: Null and Null + cells were stained for mouse integrins α5, β1, and αV and subjected to FACs analysis.
Supplementary Figure 3. Northern hybridization using a fibronectin probe. RNA isolated from Null and Null + cells subjected to northern hybridization using a probe for fibronectin. The membrane was stripped and re-blotted for cyclophilin as a loading control.
Acknowledgments
We gratefully acknowledge the financial support provided by NIH grant 75195 and a predoctoral fellowship (BC050705) from the Department of Defense. In addition, we thank the Vanderbilt Cell Imaging Shared Resource and the Vanderbilt Skin Diseases Research Core.
Abbreviations
- PLC-γ1
phospholipase C γ1
- DOC
deoxycholic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tvorogov D, Wang XJ, Zent R, Carpenter G. Integrin-dependent PLC-gamma1 phosphorylation mediates fibronectin-dependent adhesion. Journal of cell science. 2005;118:601–610. doi: 10.1242/jcs.01643. [DOI] [PubMed] [Google Scholar]
- 2.Wahl MI, Nishibe S, Suh PG, Rhee SG, Carpenter G. Epidermal growth factor stimulates tyrosine phosphorylation of phospholipase C-II independently of receptor internalization and extracellular calcium. Proc Natl Acad Sci. 1989;86:1568–1572. doi: 10.1073/pnas.86.5.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JW, Sim SS, Kim UH, Nishibe S, Wahl MI, Carpenter G, Rhee SG. Tyrosine residues in bovine phospholipase C-gamma phosphorylated by the epidermal growth factor receptor in vitro. J Biol Chem. 1990;265:3940–3943. [PubMed] [Google Scholar]
- 4.Wahl MI, Olashaw NE, Nishibe S, Rhee SG, Pledger WJ, Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989;9:2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HK, Kim JW, Zilberstein A, Margolis B, Kim JG, Schlessinger J, Rhee SG. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Chattopadhyay A, Ji Q-s, Owen JD, Ruest PJ, Carpenter G, Hanks SK. Focal adhesion kinase promotes phospholipase C-gamma 1 activity. Proc Natl Acad Sci. 1999;96:9021–9026. doi: 10.1073/pnas.96.16.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekiya F, Poulin B, Kim YJ, Rhee SG. Mechanism of tyrosine phosphorylation and activation of phospholipase C-gamma 1. Tyrosine 783 phosphorylation is not sufficient for lipase activation. J Biol Chem. 2004;279:32181–32190. doi: 10.1074/jbc.M405116200. [DOI] [PubMed] [Google Scholar]
- 8.Todderud G, Wahl MI, Rhee SG, Carpenter G. Stimulation of phospholipase C-gamma 1 membrane association by epidermal growth factor. Science. 1990;249:296–298. doi: 10.1126/science.2374928. [DOI] [PubMed] [Google Scholar]
- 9.Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBride K, Rhee SG, Jaken S. Immunocytochemical localization of phospholipase C-gamma in rat embryo fibroblasts. Proc Natl Acad Sci. 1991;88:7111–7115. doi: 10.1073/pnas.88.16.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji Q-s, Chattopadhyay A, Vecchi M, Carpenter G. Physiological Requirement for Both SH2 Domains for Phospholipase C-gamma 1 Function and Interaction with Platelet-Derived Growth Factor Receptors. Mol Cell Biol. 1999;19:4961–4970. doi: 10.1128/mcb.19.7.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wierzbickka-Patynowski I, Mao Y, Schwarzbauer JE. Analysis of Fibronectin Matrix Assembly. In: Morgan KS, editor. Current Protocols in Cell Biology. Vol. 2. John Wiley and Sons; 2004. pp. 10.12.11–10.12.10. [DOI] [PubMed] [Google Scholar]
- 14.Robinson EE, Foty RA, Corbett SA. Fibronectin Matrix Assembly Regulates alpha5 beta1-mediated Cell Cohesion. Mol Biol Cell. 2004;15:973–981. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lash JW, Seitz AW, Cheney CM, Ostrovsky D. On the role of fibronectin during the compaction stage of somitogenesis in the chick embryo. J Exp Zool. 1984;232:197–206. doi: 10.1002/jez.1402320207. [DOI] [PubMed] [Google Scholar]
- 16.Robinson EE, Zazzali KM, Corbett SA, Foty RA. {alpha}5{beta}1 integrin mediates strong tissue cohesion. J Cell Sci. 2003;116:377–386. doi: 10.1242/jcs.00231. [DOI] [PubMed] [Google Scholar]
- 17.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biology. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 19.Nagai T, Yamakawa N, Aota S, Yamada SS, Akiyama SK, Olden K, Yamada KM. Monoclonal antibody characterization of two distant sites required for function of the central cell-binding domain of fibronectin in cell adhesion, cell migration, and matrix assembly. J Cell Biol. 1991;114:1295–1305. doi: 10.1083/jcb.114.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sechler JL, Corbett SA, Schwarzbauer JE. Modulatory roles for integrin activation and the synergy site of fibronectin during matrix assembly. Mol Biol Cell. 1997;8:2563–2573. doi: 10.1091/mbc.8.12.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lash JW, Linask KK, Yamada KM. Synthetic peptides that mimic the adhesive recognition signal of fibronectin: differential effects on cell-cell and cell-substratum adhesion in embryonic chick cells. Dev Biol. 1987;123:411–420. doi: 10.1016/0012-1606(87)90399-x. [DOI] [PubMed] [Google Scholar]
- 22.Feral CC, Zijlstra A, Tkachenko E, Prager G, Gardel ML, Slepak M, Ginsberg MH. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J Cell Biol. 2007;178:701–711. doi: 10.1083/jcb.200705090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaff M, Tangemann K, Muller B, Gurrath M, Muller G, Kessler H, Timpl R, Engel J. Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. J Biol Chem. 1994;269:20233–20238. [PubMed] [Google Scholar]
- 24.McDonald JA, Quade BJ, Broekelmann TJ, LaChance R, Forsman K, Hasegawa E, Akiyama S. Fibronectin’s cell-adhesive domain and an amino-terminal matrix assembly domain participate in its assembly into fibroblast pericellular matrix. J Biol Chem. 1987;262:2957–2967. [PubMed] [Google Scholar]
- 25.Takahashi S, Leiss M, Moser M, Ohashi T, Kitao T, Heckmann D, Pfeifer A, Kessler H, Takagi J, Erickson HP, Fassler R. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J Cell Biol. 2007;178:167–178. doi: 10.1083/jcb.200703021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepard CR, Kassis J, Whaley DL, Kim HG, Wells A. PLC gamma contributes to metastasis of in situ-occurring mammary and prostate tumors. Oncogene. 2006;26:3020–3026. doi: 10.1038/sj.onc.1210115. [DOI] [PubMed] [Google Scholar]
- 27.Kundra V, Escobedo JA, Kazlauskas A, Kim HK, Rhee SG, Williams LT, Zetter BR. Regulation of chemotaxis by the platelet-derived growth factor receptor-beta. Nature. 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Tomar A, George SP, Khurana S. Obligatory role for Phospholipase C-gamma1 in villin-induced epithelial cell migration. American journal of physiology. 2007 doi: 10.1152/ajpcell.00420.2006. [DOI] [PubMed] [Google Scholar]
- 29.Wells A, Grandis JR. Phospholipase C-gamma1 in tumor progression. Clin Exp Metastasis. 2003;20:285–290. doi: 10.1023/a:1024088922957. [DOI] [PubMed] [Google Scholar]
- 30.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development (Cambridge, England) 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 31.Sottile J, Hocking DC, Swiatek PJ. Fibronectin matrix assembly enhances adhesion-dependent cell growth. Journal of cell science. 1998;111(Pt 19):2933–2943. doi: 10.1242/jcs.111.19.2933. [DOI] [PubMed] [Google Scholar]
- 32.Sottile J, Hocking DC. Fibronectin Polymerization Regulates the Composition and Stability of Extracellular Matrix Fibrils and Cell-Matrix Adhesions. Mol Biol Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hocking DC, Chang CH. Fibronectin matrix polymerization regulates small airway epithelial cell migration. Am J Physiol Lung Cell Mol Physiol. 2003;285:L169–179. doi: 10.1152/ajplung.00371.2002. [DOI] [PubMed] [Google Scholar]
- 34.Smith JT, Elkin JT, Reichert WM. Directed cell migration on fibronectin gradients: effect of gradient slope. Experimental cell research. 2006;312:2424–2432. doi: 10.1016/j.yexcr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Morla A, Zhang Z, Ruoslahti E. Superfibronectin is a functionally distinct form of fibronectin. Nature. 1994;367:193–196. doi: 10.1038/367193a0. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Guan JL, Chien S. Biochemistry and biomechanics of cell motility. Annu Rev Biomed Eng. 2005;7:105–150. doi: 10.1146/annurev.bioeng.7.060804.100340. [DOI] [PubMed] [Google Scholar]
- 37.Bae E, Sakai T, Mosher DF. Assembly of exogenous fibronectin by fibronectin-null cells Is dependent on the adhesive substrate. J Biol Chem. 2004;279:35749–35759. doi: 10.1074/jbc.M406283200. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Cheng HC, Isom R, Chen CS, Levine RA, Pauli BU. Protein kinase C epsilon mediates polymeric fibronectin assembly on the surface of blood-borne rat breast cancer cells to promote pulmonary metastasis. J Biol Chem. 2008;283:7616–7627. doi: 10.1074/jbc.M705839200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Fibronectin assembly in hanging drop cell aggregates. Null and Null + cells were cultured in hanging drops overnight followed by lysis and isolation of the DOC-insoluble fraction. DOC-insoluble lysates were blotted for fibronectin. Vimentin was used as a loading control.
Supplementary Figure 2. Integrin surface expression in Null and Null + cells: Null and Null + cells were stained for mouse integrins α5, β1, and αV and subjected to FACs analysis.
Supplementary Figure 3. Northern hybridization using a fibronectin probe. RNA isolated from Null and Null + cells subjected to northern hybridization using a probe for fibronectin. The membrane was stripped and re-blotted for cyclophilin as a loading control.