Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown cause characterized by expansion of autoreactive lymphocytes. Regulatory T cells (Tregs) are a component of the normal immune system and contribute to the maintenance of peripheral tolerance. Treg abnormalities have been associated with several autoimmune diseases and there is interest in the role of Tregs in SLE. We previously demonstrated that transfer of expanded CD4+CD25+CD62LHI Tregs slows the development of lupus in (NZBxNZW)F1 (B/W) mice. However in the absence of Treg specific surface antigens, cell purification remains a compromise between the breadth and purity of the population isolated. Importantly, purified populations always contain Foxp3− effector T cells (Teffs) that theoretically could exacerbate autoimmunity in the recipient. Here we explore the impact of transferring the more comprehensive, but less pure Treg subset defined by CD4+CD25+ expression on development of murine lupus. All cells were FACS sorted and expanded prior to adoptive transfer. Development of proteinuria and survival were measured. We found that exogenous expansion of CD4+CD25+ cells produced a population containing 70–85% CD4+Foxp3+Tregs. Expanded Tregs had higher CTLA-4 and Foxp3 expression, increased in vitro suppression capacity, and prolonged in vivo survival as compared to freshly isolated cells. Adoptive transfer of expanded CD4+CD25+ Tregs inhibited the onset of glomerulonephritis and prolonged survival in mice. Importantly the population of Teff contained within the adoptively transferred cells had reduced survival and proliferation capacity as compared to either co-transferred Tregs or transferred Teffs expanded in the absence of Tregs. These studies demonstrate that adoptive transfer of expanded CD4+CD25+Foxp3+Tregs has the capacity to inhibit the onset of murine lupus and that this capacity is significant despite transfer of co-cultured Teff cells. These data indicate that when co-expanded with regulatory T cells, exogenously activated Teffs from autoimmune patients may not pose a significant risk of promoting disease.
Introduction
Systemic lupus erythematosus (SLE) is a prototypic autoimmune disease characterized by loss of tolerance to self-antigens, expansion of autoreactive lymphocytes, and immune mediated injury to multiple organ systems. The immunologic defects that permit development of SLE are incompletely understood, but as with other autoimmune diseases there is a failure to inhibit activation and expansion of autoreactive lymphocytes that escape central tolerance mechanisms. Natural regulatory T cells (Tregs) are a thymically derived subset of CD4+ lymphocytes present in both humans and mice that serve an essential role in modulating the function of the immune system and in maintaining peripheral tolerance[1]–[4]. In vitro, CD4+ Tregs have a strong capacity to inhibit activation and expansion of both alloreactive and autoreactive T cells[3], [5]. In vivo, the Treg T cell receptor (TCR) repertoire is biased towards recognition of self-antigens[6], [7] and upon activation, Tregs exert a potent capacity to inhibit autoreactive lymphocytes[6]–[11]. Recognition of the important role of Tregs in maintenance of peripheral tolerance has led to studies demonstrating an association between abnormal Treg function or prevalence and the development of autoimmune disease[8], [10], [12], [13]. In several mouse systems, studies have demonstrated that therapies that restore or supplement Treg function and numbers can inhibit the onset and progression of some autoimmune diseases[8], [12]–[16].
A number of studies have examined the relation between Tregs and SLE. In some but not all mouse models of lupus, defects in Treg prevalence or function have been reported [13], [17], [18]. Similarly, a growing number of reports have described defects in the number and function of Tregs isolated from peripheral blood of patients with active SLE[19]–[23]. These observations suggest that Treg dysfunction may be a contributing factor in the immune pathogenesis of SLE. We have previously demonstrated that supplementation of endogenous Tregs utilizing a highly purified, exogenously activated and expanded Treg subset characterized by CD4+CD25+CD62LHI expression can inhibit the onset and rate of progression of lupus in (NZBxNZW)F1 (B/W) mice[24]. This study suggested the possibility that augmentation of the regulatory T cell population and/or function in vivo might be beneficial. However, although these cells were able to inhibit disease, the population transferred reflected only a subset of natural Tregs in mice with active lupus. Some evidence suggests that different Treg subsets may have unique roles in the maintenance of peripheral tolerance and thus transfer of a more comprehensive Treg subset may have additional or unique therapeutic benefits[10], [25]–[27]. In addition, the intrinsic resistance of Tregs to exogenous expansion and the probability that a large number of Tregs will be required to enhance peripheral tolerance in humans makes it desirable to isolate a larger Tregs subset for exogenous expansion. Unfortunately, due to the lack of Treg specific surface antigens, purification of a more comprehensive Treg subset increases the contamination by the non-regulatory effector T cell (Teff) population. Despite a growing number of published purification protocols for isolating subsets of regulatory T cells, no approach to date has demonstrated the capacity to isolate the entire Treg population with 100 percent specificity. Recent studies demonstrate that even the intracellular transcription factor Foxp3, considered the gold standard for identification of regulatory cells, is expressed transiently in some activated non-regulatory human T cells[28] further highlighting the difficulty in both identifying and purifying a comprehensive regulatory T cell population. Importantly, in autoimmune prone mice or humans with SLE, contamination of therapeutic Tregs by non-regulatory effector cells can include pathologic autoreactive lymphocytes that may have potential to exacerbate autoimmune disease if expanded and transferred back into the donor.
In this study we isolate and expand CD4+CD25+ T cells from 29-week-old lupus prone B/W mice to assess the function of this more comprehensive, but less pure Treg population. We demonstrate that exogenously expanded CD4+CD25+ Tregs have a robust in vitro suppression capacity despite the inevitable presence of co-expanded non-regulatory Teffs, many of which are contained in the CD4+CD25+CD62LLO pool of recently activated T cells. Utilizing adoptive transfer studies, we demonstrate that CD4+CD25+Foxp3+ Tregs continue to divide in vivo and are long lived following adoptive transfer. Interestingly, while purified and in vitro expanded CD4+CD25−Foxp3− Teff cells also demonstrate the capacity to expand and survive following adoptive transfer, contaminating CD4+CD25+Foxp3− Teff cells expanded in co-culture with CD4+CD25+Foxp3+ Tregs cells exhibit poor survival and proliferation following adoptive transfer. Finally, we show that exogenously expanded, adoptively transferred CD4+CD25+ T cells containing a mixed population of Foxp3+Tregsand co-expanded Foxp3−Teffs, to lupus prone B/W mice can enhance suppression of autoreactive lymphocytes and delay the development of autoimmune disease.
Materials and Methods
Mice
Six-week-old B/W mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and housed in the AAALAC accredited San Francisco VAMC Animal Care Facility under the supervision of a licensed veterinarian. The VAMC Institutional Animal Care Use Committee reviewed and approved all protocols utilized in this study.
Antibodies and reagents
Monoclonal antibody (mAb) against CD4 (GK1.5), anti-Fc (2.4G2) and anti-CD3 (2C11), were purified in our lab. The GK1.5 mAb was FITC-conjugated. CD4 mAb (PerCp-Cy5.5 conjugated, RM4-5), CD62L (allophycocyanin(APC)-conjugated, MEL-14), and neutralizing antibodies to IL-10 (JES5-16E3) were purchased from BD Pharmingen (San Diego, CA). Pacific Blue-CD4 (RM4-5), Foxp3 mAb (FITC-conjugated and APC-conjugated, FJK-16s), isotype control (rat IgG2a), fixation and permeabilization buffers (catalog no. 00-5523) were purchased from eBioscience (San Diego, CA). Biotinylated goat anti-mouse IgG (catalog no. M30215), goat anti-mouse IgM (catalog no. M31515), and FITC-conjugated streptavidin (SA1001) were purchased from Caltag Laboratories (Burlingame, CA). Biotinylated rat anti-mouse C3 (11H9) was purchased from HyCult Biotechnology (The Netherlands). The anti-CD25 mAb (R-PE-conjugated, 7D4) and anti-CTLA4 mAb (R-PE-conjugated, 1B8) were purchased from Southern Biotechnology Associates, Inc (Birmingham, AL). Neutralizing antibodies to TGF-β (1D11) were purchased from R&D Systems (Minneapolis, MN). Carboxyfluoroscein succinimidyl ester (CFSE) and SNARF-1 carboxylic acid, acetate, succinimidyl ester (S22801, special packaging) was purchased from Molecular Probes/Invitrogen Life Technologies (Carlsbad, CA).
Lymphocyte isolation
Following animal sacrifice, the spleen and cervical, axillary, inguinal, and renal lymph nodes were harvested. Single cell suspensions were prepared in DMEM with 2% fetal calf serum (FCS) by passing tissue through nylon mesh. In experiments of CFSE or SNARF-1 labeled in vivo cell survival, salivary glands, kidneys, lung and liver were also mechanically dissociated and lymphocytes from these tissues were isolated by ficoll gradient.
FACS analysis and cell sorting
Cell suspensions were blocked with anti-Fc mAb prior to monoclonal antibody labeling. Lymphocytes were labeled with PerCp-Cy5.5-CD4 or FITC-CD4, PE-CD25, APC-CD62L. Foxp3 expression was measured utilizing an aliquot of cells stained with PerCp-Cy5.5-CD4 that was fixed and permeabilized, blocked with anti-Fc mAb, and stained with FITC-Foxp3 or FITC-IgG2a isotype control per manufacturers instructions (eBioscience, San Diego, CA). Combined expression of membrane bound and intracellular CTLA-4 expression was measured by first labeling surface antigen and then utilizing the fixation and permeabilization and blocking protocol defined for Foxp3 above to label intracellular antigen. All FACS analysis and sorting was performed on a FACSAria (Becton Dickinson, Mountain View, CA) instrument utilizing FACSDiva software and the purity prioritization algorithm. Purity checks of sorted cells routinely demonstrated greater than 98% purity.
Cell culture
Purified CD4+CD25+, CD4+CD25+CD62LHI, CD4+CD25+CD62LLO, and CD4+CD25− cells were isolated from 28–29 week old mice by FACS sorting. The frequency of Tregs in each sorted populations was measured by staining for Foxp3 expression in an aliquot of sorted cells. Cells were cultured utilizing the protocol previously described[29]. Briefly, purified cells were maintained at a concentration of 0.7−1×106 cells/ml over an 8-day culture period in DMEM (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Biosource International, Camarillo, CA). 2,000 IU/ml rhIL-2 (Hoffmann-LaRoche, Nutley, NJ generously provided by the National Cancer Institute), 5 mM Hepes (Sigma-Aldrich, St. Louis, MO), NEAA, 0.5 mM sodium pyruvate, 1 mM glutaMAX (all from Invitrogen) and 50 µM β-mercaptoethanol (Sigma-Aldrich). Cells were stimulated with bead coupled anti-CD3 and anti-CD28 antibodies (Xcyte beads, Xcyte Therapeutics Inc., Seattle, WA). Following expansion, cells were separated from the Xcyte beads by centrifugation in Lympholyte-M (Cedarlane Laboratories, Burlington, NC) at 230G for 25 minutes. Aliquots of expanded cells were routinely assessed for purity by measurement of CD4+ and Foxp3+ expression and for suppressive function in a mixed lymphocyte suppression assay.
Suppression assay
The CD4+CD25−CD62LHI T cells were purified by FACS and served as responder T cells (Tresp) in mixed lymphocyte suppression assays. CD4+ depleted splenocytes were irradiated at 2,000 rads and served as antigen presenting cells (APCs). Purified Tresp and APCs (75,000 each) were combined in 96 well U-bottom plates with soluble anti-CD3 at 4 µg/ml. Tregs were added to obtain a range of Tresp∶Treg ratios. Cells were cultured for 60 hours prior to addition of 1 µCi/well of [3H] thymidine and the assay was then harvested 12 hours later. All assays were performed in triplicate. Tritium incorporation was measured on a MicroBeta Scintillation Counter (Wallac Oy, Turku Finland). The tritium incorporation at each Tresp∶Treg was divided by incorporation in the Tresp only wells to calculate a suppression index (SI) for each experiment.
In vivo survival of adoptively transferred Tregs and Teffs
The in vivo survival of both freshly isolated and exogenously expanded Tregs and expanded Teffs was assessed in a series of experiments utilizing adoptively transferred CFSE or SNARF-1 labeled cells. All adoptive transfer studies reported in this paper utilized unmanipulated recipient mice (no irradiation or immune depletion prior to adoptive transfer). Lymphocytes from donor mice were FACS sorted and the frequency of Tregs and Teffs in freshly sorted populations was measured by FACS analysis of Foxp3 expression in an aliquot of sorted cells. Expanded cells obtained from the 8-day expansion protocol described above were also analyzed for Foxp3 expression to determine the ratio of Tregs to non-regulatory Teff post-expansion. Fresh or expanded cells were CFSE labeled, extensively washed in sterile saline, and concentrated in 200 µl of sterile PBS in preparation for tail-vein injection to each mouse. The survival of both freshly isolated and exogenously expanded cells was measured at set time intervals from 2 to 30 days following adoptive transfer. Mice were sacrificed and single cell suspensions of lymph node and spleen were prepared. In order to detect CFSE+ cells that trafficked outside of the lymphatic system, lymphocytes were also recovered from salivary glands, kidneys, lung and liver. Recovered cells were stained with Pacific Blue-CD4 and APC-Foxp3 mAbs prior to FACS analysis in order to differentiate the survival and proliferation of CFSE+CD4+Foxp3+Tregs from CFSE+CD4+Foxp3−Teffs. In these experiments, two types of CD4+Foxp3−Teffs were designated based on the culture conditions from which they were derived. The first Teff population was derived from the CD4+CD25+Foxp3−Teffs (5–10% of cells) co-isolated during sorting for CD4+CD25+Foxp3+Tregs. During cell culture, these contaminating Teffs are co-expanded in a Treg predominant environment and were therefore designated as “conditioned Teffs” (Teff-conditioned) to reflect potential conditioning of the Teff function by Treg co-culture. A second population of Teff cells was generated by first depleting the CD4+CD25+ T cells (the majority of Tregs) prior to the in vitro expansion of for 8-days. This expanded Teff population contained <2% CD4+Foxp3+ Treg cells and was therefore considered as “unconditioned” Teffs. In specified experiments designed to assess differential survival of “unconditioned” Teff cells from Teff-conditioned cells following co-injection with Tregs, mice received two tail-vein injections on the same day, one containing expanded, SNARF-1 labeled unconditioned Teffs and the second injection containing the expanded CFSE labeled Tregs/Teff-conditioned cells. Mice were sacrificed 4 or 5 days after injection and cell survival was assessed by FACS analysis of lymphocytes recovered from lymphatic and solid organ tissue of recipient mice (as defined above). SNARF-1+ and CFSE+ cells were then FACS sorted followed by labeling with FITC-Foxp3 or APC-Foxp3 mAbs respectively to permit differentiation of Tregs (CFSE+Foxp3+), Teff-conditioned (CFSE+Foxp3−) cells, and “unconditioned” Teff (SNARF-1+ Foxp3−) fractions. The presence of any SNARF-1+ Foxp3+ cells was also assessed.
Adoptive cell transfer to treatment study mice
All Treg and Teff cells were purified from donor B/W mice age-matched to recipient mice. Expanded CD4+CD25+ Tregs (containing 80–85% Foxp3+Tregs and 15–20%Foxp3−Teff-conditioned) and CD4+CD25−Teff controls (with <2% Tregs) were depleted of Xcyte beads as above and extensively washed in sterile saline. A total of 6×106 Tregs concentrated in 200 µl of sterile PBS were transferred by tail-vein injection to each mouse. Littermate mice were divided into two control groups and received either an equivalent volume of sterile PBS or 6×106 expanded CD4+CD25− T cells in 200 µl sterile PBS. A total of two separate cell sorts, exogenous expansion, and adoptive transfers were preformed in order to generate sufficient cells to treat the target of at least 20 mice per arm. The final treatment cohort contained 65 animals, comprised of 44 littermate mice divided into 15 Treg, 15 Teff, and 14 PBS recipients and 21 littermate mice divided into 7 mice per arm, for a total of 22 Treg treated mice, 22 Teff treated mice, and 21 PBS treated mice. All animals were 29-weeks-old at the time of adoptive transfer.
Assessment of lupus disease activity and survival
A total of 65 mice (21–22 mice per group) were followed for the development of renal disease as measured by proteinuria and for survival. Proteinuria was measured using Uristix (Bayer Corp, Elkhart, IN). Antibodies to double-stranded DNA (anti-dsDNA Ab) were measured utilizing an ELISA previously established in our lab[30]. In a separate experiment, cohorts of 29-week old mice (5 per arm) received either Tregs or PBS control (as per the above protocol) and these mice were then sacrificed 8-weeks following adoptive transfer to provide renal histology. Fixed kidney tissue was stained with hematoxylin and eosin for evaluation of glomerulosclerosis and tubular damage and cryopreserved sections of kidney tissue were stained with FITC conjugated antibodies to IgG, IgM, and C3 for assessment of immune complex deposition that was scored by a blinded reader using a scoring system previously established in our laboratory[30].
Statistical analysis
Suppression assays were performed in triplicate and the mean thymidine incorporation was assessed using the Mann-Whitney U test. Antigen expression by mean fluorescent intensity, anti-dsDNA Ab titers and renal histologic damage and immune complex deposition scores were compared by Mann-Whitney U test. Development of proteinuria, defined as ≥100 mg/dl on serial testing, and survival in treatment and control cohorts were compared by χ2 analysis using the Yates correction.
Results
Purification and expansion of CD4+CD25+T cells results in a mixed population of Tregs and Teffs
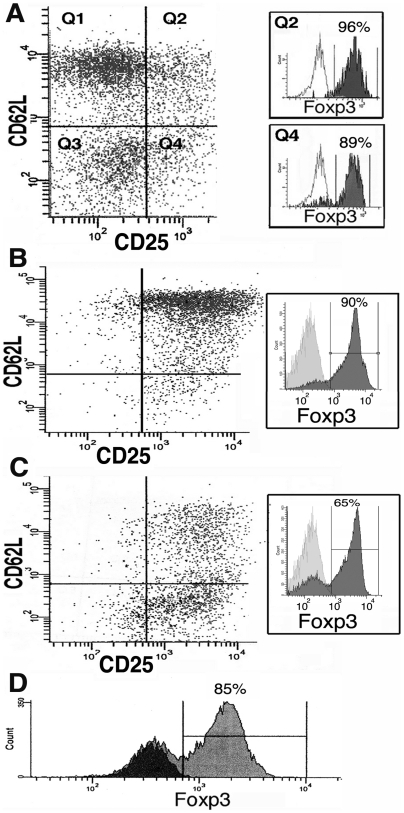
In B/W mice with active lupus, the frequency of natural Tregs, as measured by Foxp3+ expression, was 96±2% in the cells identified by CD4+CD25+CD62LHI expression (Figure 1A). The CD4+CD25+CD62LLO population contained both Foxp3+Tregs and a significant population of recently activated Foxp3−Teff, such that a typical sort of this quadrant produced a population containing 85–92% Foxp3+Tregs and 8–15% Foxp3− Teffs (Figure 1A, 36-week old B/W mouse with early proteinuria). In vitro expansion of cells over an 8-day period increased the relative frequency of Foxp3− cells due to the more rapid proliferation of Teffs as compared to Tregs during culture. The CD4+CD25+CD62LHI cells maintain this surface phenotype during expansion and produce an expanded population that is ∼85–90% Foxp3+ post-expansion (Figure 1B). Expansion of the CD62LLO subset produced cells with mixed surface phenotype (33% express CD62LHI) and a reduced Foxp3+ purity (65–85%) due to the higher initial Teff prevalence (Figure 1C). On average, culture of the entire CD4+CD25+ cell population produced a population that expanded 30 to 40-fold and was 70–85% CD4+Foxp3+ (Figure 1D).
Figure 1. Foxp3+ expression in freshly isolated and expanded CD4+ Treg cells.
A) Foxp3 expression in fresh CD25+CD62LHI cells (Q2, 96%) versus CD25+CD62LLO cells (Q4, 89%). Isotype control antibody shown for each histogram. B) Expanded Q2 cells demonstrating maintenance of CD62LHI and Foxp3+expression (90%). C) Expanded Q4 cells demonstrating up-regulation of CD62LHI on 33% of cells and a mixture of 65% Foxp3+Tregs and 35% Foxp3− Teff cells post-expansion. D) Foxp3+ expression (grey histogram, 85% of cells) following expansion of the entire CD4+CD25+ T cell subset. Isotype control shown in black histogram.
In vitro Treg suppressor function is similar between subsets of CD25+ Tregs and this function is enhanced by in vitro activation and expansion
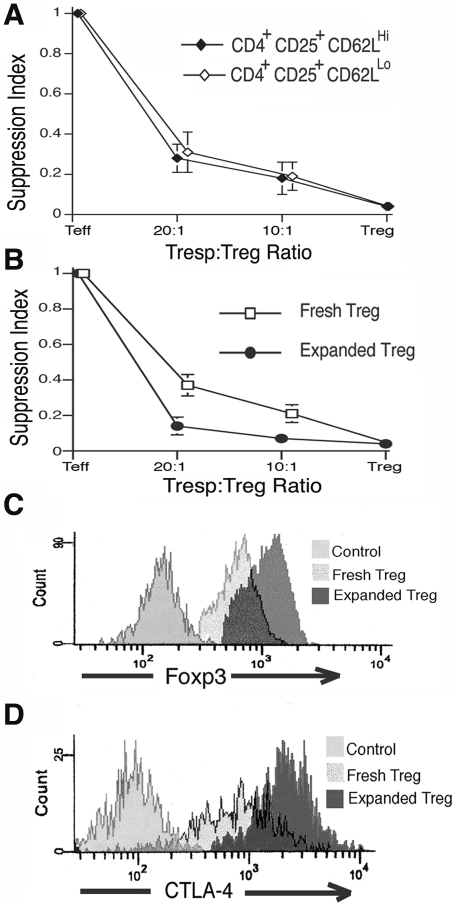
We previously demonstrated that the in vitro suppressive capacity of the CD4+CD25+CD62LHI Treg fraction is not affected by the age or disease state of donor B/W mice and is equivalent to that of similar cells from non-autoimmune prone BALB/c mice[24]. In this study, the CD4+CD25+CD62LLO Tregs from B/W mice with active disease were isolated by FACS sorting and the suppressor capacity was compared to the CD4+CD25+CD62LHI Treg fraction. Freshly isolated cells demonstrated equivalent suppression between these CD62LHI and CD62LLO Treg subsets (Figure 2A, p≥0.25 for each cell ratio, 3–6 mice per group).
Figure 2. CD4+CD25+Treg function and phenotype is enhanced by exogenous expansion.
A) Freshly isolated CD62LHI and CD62LLO fractions of CD4+CD25+Tregs demonstrate equivalent suppression capacity in vitro as measured by mixed lymphocyte suppression assay (p≥0.25 for each cell ratio, 3–6 mice per group). The Suppression Index measures the capacity of Tregs to suppress Tresp proliferation (as measured by decreased tritium-labeled thymidine incorporation) at different Tresp∶Treg ratios divided by the maximal Tresp proliferation in the absence of Tregs B) Ex vivo expansion of CD4+CD25+ Tregs significantly enhances the ability of these cells to suppress proliferation of Tresp cells derived from 29-week-old B/W mice (p≤0.04 for each cell ratio, 6 mice per group). C–D) Foxp3+ and CTLA-4 (surface and intracellular) expression in exogenously expanded Tregs were significantly greater than in freshly isolated Tregs as measured by mean-fluorescent intensity (p = 0.007, p<0.001 respectively, 3–6 mice per group).
To evaluate the effect of ex vivo expansion on suppressive function, we isolated the entire CD4+CD25+ T cell population and measured in vitro suppressive function before and after expansion. Despite the presence of 15–20% co-expanded Teffs, the expanded CD4+CD25+Tregs demonstrated significantly enhanced suppression capacity across the range of Tresp∶Treg ratios tested as compared to freshly isolated CD4+CD25+ cells (Figure 2B, p≤0.04 for each cell ratio, 6 mice per group). This enhanced Treg suppressor function following exogenous expansion is consistent with prior reports of Treg function in other murine models and with our previous findings with the CD4+CD25+CD62LHITreg fraction in B/W mice[24], [31]. Because levels of Foxp3+ and CTLA-4 expression in Tregs have been correlated with suppressor function of these cells and these antigens are also known to be up-regulated by Treg activation, we measured their expression in freshly isolated and exogenously expanded Tregs from young and old B/W mice by FACS. Levels of Foxp3 or CTLA-4 expression in freshly isolated Tregs as measured by mean fluorescent intensity (MFI) did not vary with mouse age or organ distribution (data not shown). However, consistent with prior reports in other mouse strains[29], [31] there was a marked increase in both Foxp3+ and CTLA-4 expression in B/W Tregs following exogenous expansion (Figure 2C–D, p<0.01 for both, 3–6 mice per group).
Exogenous expansion significantly enhances in vivo survival of Tregs, but not of co-cultured Teff
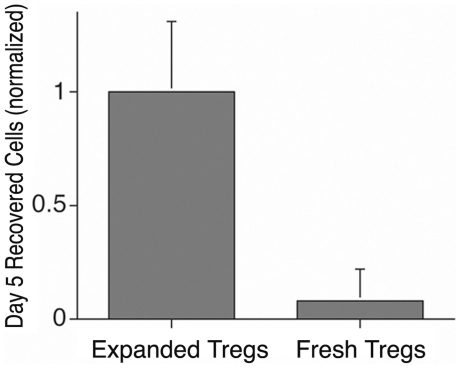
We assessed the in vivo survival of freshly isolated and exogenously expanded Tregs and expanded Teff cells in a series of transfer experiments utilizing mixed populations of expanded Tregs/Teff-conditioned, and Teffs. Prior studies have shown that Treg interactions with Teffs can induce long term Teff anergy[32], [33], but whether this in vitro interaction influences the in vivo survival and proliferation of contaminating Teffs within the expanded Treg population is unknown. The in vivo survival of freshly isolated, adoptively transferred CD4+CD25+Tregs was first compared to that of exogenously expanded Tregs. Cohorts of 3 mice each received aliquots of sorted fresh or expanded CFSE labeled cells, each containing 1.6×106 Foxp3+ Tregs. Five days following transfer, recipient mice were sacrificed and the lymphocyte recovery from lymph nodes, spleen and tissues (liver, kidney, lung, salivary glands) was measured. An aliquot of cells was then stained for Foxp3 prior to FACS analysis. The number of CFSE+Foxp3+ cells (as a percentage of all cells in the aliquot) and the total cell recovery from each mouse was then used to estimate the Treg survival in each mouse. As shown in Figure 3, in vivo survival of exogenously expanded, adoptively transferred Tregs at day 5 is significantly enhanced as compared to that of freshly isolated cells (p<0.01). Freshly isolated, adoptively transferred CFSE+Tregs could no longer be consistently detected by FACS when mice were examined 10 days post transfer.
Figure 3. Enhanced in vivo survival of expanded Tregs as compared to freshly isolated Tregs following adoptively transfer.
Bar graph demonstrates that the number of exogenously expanded CFSE+CD4+Foxp3+Tregs recovered from lymphatic and solid organs 5 days after adoptive transfer (adjusted for the variability in total cell recovery from each recipient mouse) is significantly greater than that for freshly isolated and adoptively transferred Tregs (p<0.01, 3 mice per group).
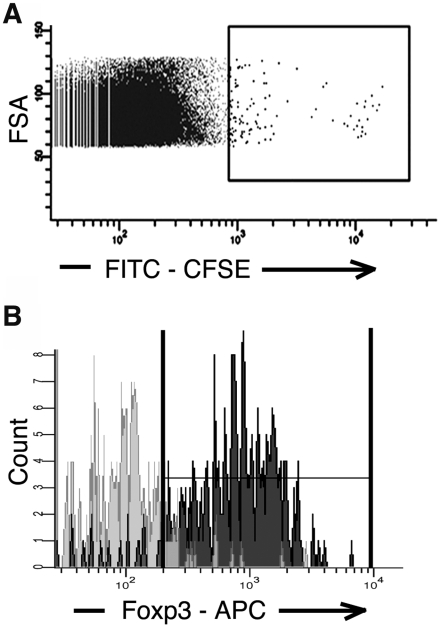
The long-term in vivo survival of exogenously expanded Tregs was then assessed by following 10×106 cells for up to 30 days following transfer (3 mouse cohort). As shown in Figure 4, a small population of CFSE+ positive cells could be detected at 30 days (Figure 4A, ∼0.2% of CD4+ T cells). To ensure these rare cells were actually the adoptively transferred CFSE labeled Tregs and not just very large cells or cellular doublets with bright autofluorescence, the CFSE bright population was FACS sorted and stained for Foxp3 expression. As shown in Figure 4B, Foxp3 was detected in >90% of CFSE+ cells consistent with long term survival and proliferation of exogenously expanded, adoptively transferred Treg.
Figure 4. In vivo survival and proliferation of expanded Tregs detected 30 days following adoptive transfer.
A) A small population of CFSE+ labeled cells could be detected and FACS sorted at 30 days from a pool of lymphocyte isolation from lymphatic, splenic, salivary gland, lung, liver and kidney tissue (FITC bright sort gate shown containing ∼0.2% of CD4+ T cells). B) Characteristic Foxp3 staining of sorted FITC bright cells demonstrating >90% of these cells are Foxp3+ (dark grey histogram), consistent with these cells being derived from the adoptively transferred CFSE labeled, Foxp3+Tregs and not FACS artifact (isotype control shown in light grey histogram, 3 mice cohort).
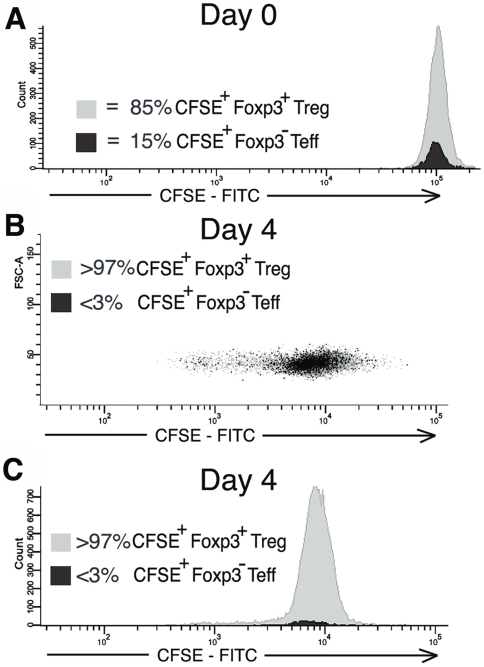
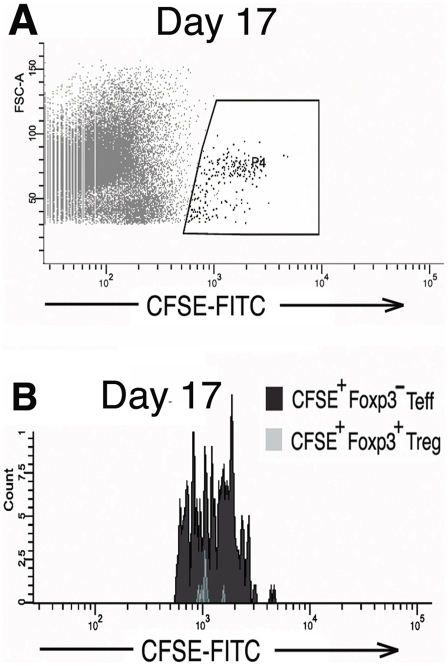
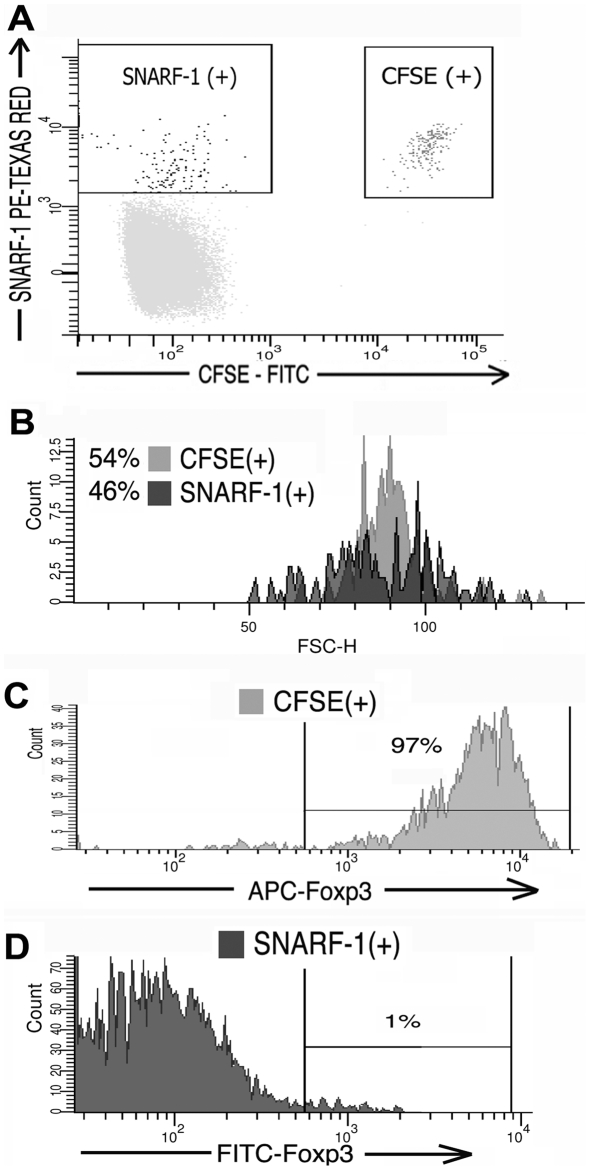
To assess the survival of contaminating, co-transferred Teff cells contained within the expanded Treg population, cohorts of 3 mice received expanded CD4+CD25+ cells derived from the high purity Treg culture that contained 85% CD4+Foxp3+Tregs and 15% contaminating CD4+Foxp3−Teff-conditioned (Figure 5A). Two to eight days following transfer mice were sacrificed and the lymph node, spleen, and tissue infiltrating lymphocytes were FACS analyzed for CFSE and Foxp3. As demonstrated in Figure 5B–C, the frequency of CFSE+Foxp3−Teff-conditioned relative to CFSE+Foxp3+Tregs rapidly declined such that by day 4 the Teff-conditioned comprised <3% of the transferred cells (an 80% decline) with no evidence of significant Teff-conditioned division by CFSE dilution. To determine if this decline in Teffs prevalence was a result of conditioning during co-culture with Tregs or an intrinsic property of all exogenously expanded, adoptively transferred Teffs, in vivo survival of Teffs expanded in the absence of Tregs was assessed (“unconditioned” Teffs generated from expansion of CD25-depleted CD4+ T cells. Expanded cells were 98% CD4+Foxp3−Teffs). In the first experiment, survival and division of 5×106 transferred CFSE labeled, Foxp3− unconditioned Teffs could be detected by FACS up to seventeen days post-transfer, at which time CFSE fluorescent intensity approached the autofluorescence baseline (Figure 6). The relative survival of unconditioned Teffs was then compared to survival of expanded Tregs following transfer of these two populations into the same mouse. Mice received injections of 3.45×106 expanded, CFSE labeled CD4+CD25+ cells (83% Tregs and 17% Teff-conditioned by Foxp3 stain) and a second injection of 2.86×106 expanded, SNARF-1 labeled CD4+CD25− Teffs (“unconditioned” Teffs). Initial ratio of injected cells was 45% Tregs, 10% Teff-conditioned, and 45% unconditioned Teffs. Four to five days following transfer, analysis of surviving CFSE+ and SNARF-1 cells demonstrated a persistence of Tregs and Teffs, but not Teff-conditioned. Figure 7A–B show a characteristic result at day 4 at which time approximately equal numbers of CSFE and SNARF-1 labeled cells could be recovered from the lymphatic system and organs (0.4% of total lymphocytes each, ratio of sorted CFSE to SNARF-1 is 54% to 46%). Foxp3 analysis of recovered CFSE+ cells demonstrates that these cells are comprised almost entirely of Tregs (Figure 7C, 97% of surviving CFSE+ cells are Foxp3+Tregs, 3% Foxp3−Teff-conditioned). Recovered SNARF-1+ were unconditioned Teffs as expected (Figure 7D, 99% Foxp3−) (characteristic findings from 5 mice). This data is consistent with evidence in Figures 5 and 6 demonstrating that impaired Teff-conditioned survival is a consequence of co-culture with Tregs and not an intrinsic characteristic of all adoptively transferred Teffs.
Figure 5. CD4+Foxp3−Teff-conditioned demonstrate impaired in vivo survival and proliferation following adoptive transfer.
At each time point, an aliquot of CFSE labeled cells was fixed and stained with Pacific-Blue Foxp3 to permit measurement of the ratio Tregs and Teff-conditioned. A) Baseline FITC-CFSE fluorescence of expanded CD4+CD25+ lymphocytes just prior to adoptive transfer. Foxp3 stain demonstrates this exogenous expanded population containing 85% Foxp3+Tregs and 15% Foxp3−Teff-conditioined (contaminating Teff cells contained within the expanded Tregs population) at the time of transfer. B–C) Dot plot and histogram of the FITC-CFSE fluorescent intensity of both Foxp3+ Tregs and Foxp3− Teff-conditioned cells recovered from lymphatic and solid organ tissue 4 days after adoptive transfer. Panels B–C demonstrate a significantly reduced survival and proliferation of Teff-conditioned as compared to Tregs such that Teff-conditioned comprise <3% of the recovered CFSE+ population. Data reflects typical finding from 6 separate experiments.
Figure 6. Persistent in vivo survival and proliferation of CD4+Foxp3− Teffs when these cells are first expanded in vitro under Treg depleted culture conditions (expanded, “unconditioned” Teffs).
A) Survival and division of CFSE labeled, unconditioned CD4+Foxp3− Teffs could be detected up to 17 days following adoptive transfer at which time the FITC intensity of CFSE labeled cells approached the auto-fluorescent baseline. Dot plot at day 17 demonstrating the sorting gate for FITC-bright, CFSE+ cells. B) Cells sorted from the gate in Panel A are >98% Foxp3− consistent with the adoptively transferred Teffs (characteristic findings from 3 mice).
Figure 7. In vivo survival and proliferation of expanded Tregs and Teffs (independently injected into the same mouse) indicating impaired survival of Teff-conditioned is not intrinsic to all transferred Teffs, but instead due to co-culture with Tregs.
A) CFSE+ and SNARF-1 cells recovered four days following independent injections of expanded CFSE+ CD4+CD25+ cells (containing Tregs and Teff-conditioned) and expanded SNARF-1+ CD4+CD25− Teffs (unconditioned Teffs) demonstrating approximately equal survival of both populations (∼0.4% of recovered lymphocytes each). The ratio of injected cells on Day 0 was 45% Tregs, 10% Teff-conditioned, 45% unconditioned Teffs. B) Histogram of recovered CFSE+ and SNARF-1+ cells at day 4 demonstrate persistence of “unconditioned” Teffs (SNARF-1+ cells) equivalent to survival of the CFSE+Treg/Teff-conditioned mix, a finding not observed when only Tregs and Teff-conditioned are co-transferred (as shown in Figure 5). C–D) Foxp3 analysis of sorted cells demonstrating that surviving CFSE+ cells are Tregs (97% Foxp3+) and SNARF-1+ cells are “unconditioned” Teffs (Foxp3−), indicating impaired survival and proliferation of transferred Teff-conditioned is a consequence of co-culture with Treg during exogenous expansion (characteristic findings from 5 mice).
Adoptive transfer of exogenously expanded CD4+CD25+ Tregs to pre-nephritis mice slows the progression to proteinuria and prolongs survival
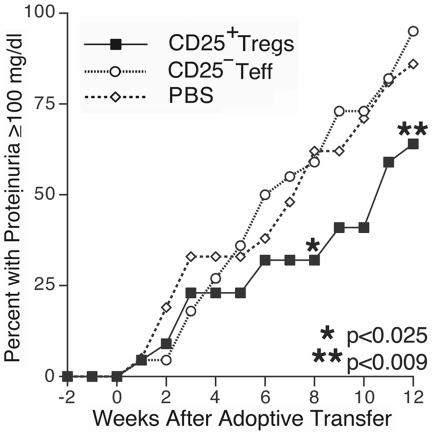
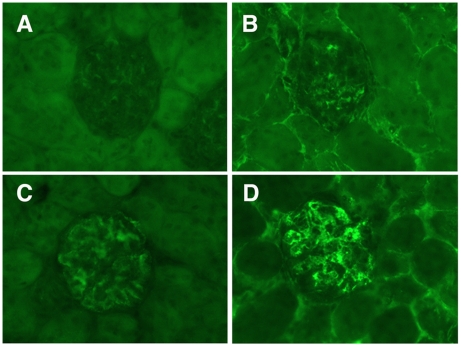
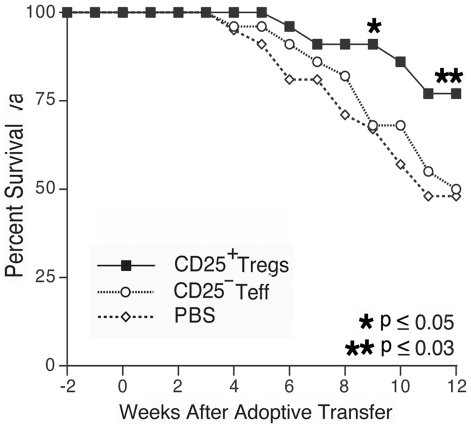
To determine if adoptive transfer of a mixed population of exogenously expanded CD4+CD25+ Tregs can suppress murine lupus, we transferred cells to a large cohort of 29 week-old B/W mice without clinical disease. Treatment mice received 6×106 Tregs via tail vein injection, based on our prior ability to delay disease onset utilizing this number of CD62LHI Tregs. Control mice received either an equivalent number of exogenous expanded CD4+CD25− cells or PBS. Following adoptive transfer, mice in the active treatment group had delayed progression to significant renal disease, such that eight weeks after cell transfer, only 32% of animals in the treatment group had developed proteinuria compared to 59% in the CD4+CD25− Teff mice and 62% in the PBS control group (Figure 8, p<0.025 for both comparisons. Dataset S1 contains proteinuria and survival data). Mice sacrificed for renal pathology and immunohistochemical evaluation at 8 weeks following the initiation of therapy exhibited diminished immune complex deposition among those that received Tregs (Dataset S2 contains proteinuria data). Indirect immunfluorescence demonstrated that kidneys from Treg treated mice (Figure 9, A–B) had less IgG and IgM immune complex deposition as compared to control treated mice (Figure 9, C–D, p≤0.05 for both comparisons. Differences in C3 deposition and renal damage pathologic scoring did not reach statistical significance). The prevalence of proteinuria in Treg treated mice remained significantly lower than either control group up to 12 weeks following the adoptive transfer (p≤0.008 for both comparisons). Inhibition of renal disease in Treg recipients correlated with an improved survival first evident 9 weeks following adoptive transfer (Figure 10), p<0.05 Treg vs. either control. This survival advantage in the Treg recipients remained significant during the 12 weeks following transfer (p≤0.026). In mice followed beyond the 12-week experiment (15 Treg, 15 Teff, 14 PBS), it was observed that the majority of mice in each treatment arm eventually developed proteinuria, but the delay in disease onset and prolonged survival of Treg recipient mice remained evident to 24 weeks following therapy (Dataset S1). At baseline, all mice in each treatment arm had anti-dsDNA antibody titers below the detection threshold. Significant variability in autoantibody titer was observed among mice in each group during follow-up and the difference between groups did not reach statistical significance.
Figure 8. Inhibition of proteinuria in Treg treated mice.
Mice receiving adoptively transferred Tregs had significantly reduced progression to proteinuria ≥100 mg/dl over the 12 weeks following transfer as compared to either PBS or CD4+CD25− T cell control groups (p≤0.025 starting week 8, n = 22 for Treg and Teff groups, n = 21 for PBS group).
Figure 9. Inhibition of immune complex renal damage in Treg treated mice.
Typical kidney sections demonstrating less severe IgG and IgM deposition in mice receiving adoptive Treg transfer (Figure 6A–B respectively) as compared to mice receiving either control therapy (Teff control mouse, Figure 6C–D).
Figure 10. Prolonged survival in Treg treated mice.
Mice receiving adoptive transfer of Tregs had significantly prolonged survival first evident 9 weeks after transfer (p<0.05 at week 9) as compared to either control group.
Discussion
In this report we expand on our prior work demonstrating the ability of exogenously expanded, adoptively transferred Tregs to inhibit the development of murine lupus. We demonstrate that exogenous expansion and transfer of Tregs contained within the population of CD4+CD25+ T cells suppresses the onset of glomerulonephritis and prolongs survival in a murine model of lupus. Importantly, the co-transfer of a population of co-purified/co-expanded Teffs did not negate this therapeutic benefit in mice. These results underscore the remarkable ability of Tregs to suppress autoreactive immune responses. They also address an important concern with respect to the therapeutic potential of this approach in terms of the risk of simultaneous transfer of activated, autoreactive and pathogenic Teffs to autoimmune recipients. In this study we found that co-expanded and transferred Teff, that are present as a consequence of sorting and expanding Tregs without the availability of Treg specific cell surface antigens, did not off-set the therapeutic benefit of exogenously expanded, adoptively transferred Tregs. In addition, we tracked the survival and proliferation of non-regulatory/Foxp3− cells co-transferred with the Treg population and found Teffs expanded in the presence of Tregs do not persist in vivo, particularly compared to transferred Foxp3+ Tregs. The poor survival and lack of division of the co-cultured Foxp3−Teffs produced under our in vitro expansion conditions indicates these cells are unlikely to have the capacity to contribute either to the disease suppression (as iTregs) or adversely to the pool of self-reactive Teffs. This observation is consistent with the evidence that activated Tregs produce a significant amount of TGF-β and that both human and mouse Teffs activated in the presence of TGF-β develop an anergic phenotype and under some conditions can be converted to an induced suppressor cell (iTreg)[32], [34], [35]. This finding is an important first step in considering the possibility of utilizing Treg as a therapeutic approach in humans.
The ability of adoptively transferred Tregs to suppress murine lupus also raises a number of questions about how exogenously expanded cells function to suppress disease that is not controlled by the endogenous Treg population. We previously demonstrated that B/W mice with active lupus have a significantly expanded Treg population that is equal to or exceeds the population found in non-autoimmune prone animals[24]. Yet it appears that this Treg expansion is insufficient to control the proliferation and function of autoreactive lymphocytes in lupus. One possible explanation for this failure is that the Treg expansion is simply insufficient to suppress the number of autoreactive lymphocytes that occur in lupus. Another possibility is that the endogenous Tregs do not have proper function in vivo. Our extensive in vitro evaluation of purified B/W Tregs suggest that these cells do not have a defect in their ability to suppress autologous T cell proliferation, however the true functional capacity of these cells in vivo is not known. Importantly, while the in vitro suppressive function of freshly isolated B/W Tregs is equivalent to Tregs isolated from non-autoimmune prone BALB/c mice, this function can nonetheless be significantly enhanced by in vitro activation. Consistent with observations in other murine models, expanded B/W Tregs also have higher Foxp3+ and CTLA-4 expression. In addition, the expanded Tregs demonstrate significantly enhanced in vivo survival as compared to freshly isolated cells. These data suggest a basis for the efficacy of ex-vivo expanded autologous Tregs in suppressing disease in lupus prone mice. In some autoimmunity models the Teffs are resistant to Treg suppression in vitro and/or in vivo[17], [36]. For this study we utilized Teff from 29-week-old mice that on average had developed antinuclear antibodies and low titers of dsDNA antibodies, but had not yet progressed to clinically active lupus. In this critical stage of disease evolution we did not observe resistance of Teff cells to CD4+CD25+ Treg mediated in vitro suppression. It is known that Tregs must undergo activation in the peripheral circulation in order to achieve a suppressor phenotype. It is thus possible that the in vitro suppression assay is insensitive to a heterogeneous Treg activation state due to the dominant suppression capacity of those Tregs that have already become activated. The enhanced in vitro and likely in vivo function of Tregs we observe following in vitro activation likely reflects at least in part the suppressive function of a more homogenously activated Treg population. Additional studies will be required to better characterize the factors responsible for the enhanced suppressor capacity of these expanded cells. In this study we did not attempt to compare disease suppression by supplementation of freshly isolated Tregs based on several observations. The protocol to isolate highly enriched Tregs results in a total recovery of fresh Tregs from each donor mouse of only 400,000 to 800,000 per mouse and it would therefore require an impractical average of 8 donor mice for each treatment mouse tested. In addition, the in vivo survival of freshly isolated, CFSE labeled Tregs demonstrated these cells had very poor survival relative to expanded cells decreasing the potential biologic impact of transferred freshly isolated cells.
Despite inhibition of lupus nephritis and prolonged survival, the majority of mice receiving Treg transfer progressed to active disease during long-term follow-up. This pattern of inhibiting disease onset, but not the final disease severity with a single adoptive transfer is similar to the benefit observed when a more restricted population of CD4+CD25+CD62LHI Tregs was adoptively transferred. While not compared directly in a single treatment study, no significant differences in either the size or duration of the treatment response is evident between mice receiving equal numbers of expanded CD4+CD25+CD62LHI or CD4+CD25+ Tregs. Future studies utilizing more sensitive biomarkers of disease activity than proteinuria, antibody formation, and survival will be required to clarify the role of different Treg subsets in disease suppression. Multiple factors may contribute to the duration of therapeutic benefit of a single adoptive transfer. Limitations on the ability to track adoptively transferred Tregs in our B/W mice leave unresolved the question of how long transferred cells survive and continue to exert a suppressor phenotype. Due to their wide distribution to both lymphatics and solid organ tissue, it is not possible to specify the exact number of surviving cells at a given time point following transfer. In the current study we detected transferred cells surviving and proliferating to 30 days, but the absolute number of recovered cells is small and may reflect a diminishing survival with time. From our sampling of major lymph nodes, spleen, and solid organs we estimate that ∼10% of cells persist 5–7 days post transfer, ∼1–3% persist 14 days post transfer, and <1% demonstrate long term survival to our detection threshold of 30 days. Whether the delay in disease onset is due to transient non-specific bystander suppression effect from the large number of activated Tregs transferred or from the smaller number of long lived, potentially antigen specific Tregs is unclear. While further studies will be required to expand the understanding of both the mechanisms of action and therapeutic potential of adoptively transferred Tregs in the treatment of SLE, this study supports the potential benefits of augmenting Treg number or function as a novel therapeutic approach to the treatment of this disease.
In conclusion, our results demonstrate that exogenous expansion and adoptive transfer of the regulatory T cell subset defined by CD4 and CD25 expression produces a population of highly suppressive, long-lived Tregs capable of delaying the onset of glomerulonephritis in a murine model of lupus. Co-culture and adoptive transfer of a small population of contaminating, potentially autoreactive Teffs does not exacerbate autoimmune disease or limit the potential of Tregs to suppress disease, in part due to the short in vivo survival time and anergic phenotype of these co-cultured cells.
Supporting Information
Proteinuria and Survival Data. Serial proteinuria and survival in treatment and control mice. Mice received CD4+CD25+Tregs, CD4+CD25−Teffectors control cells, or PBS. Proteinuria score is recorded as 0–4 as measured by urine dipstick. Mouse deaths noted by X marks.
(0.04 MB XLS)
Serial proteinuria in renal histology mice. Mice were sacrificed 8-weeks after adoptive transfer of CD4+CD25+Tregs or PBS. Proteinuria score is recorded as 0–4 as measured by urine dipstick.
(0.02 MB XLS)
Acknowledgments
We are grateful to Gail Cassafer for technical assistance with histology. Recombinant human IL-2 was produced by Hoffmann-LaRoche, Nutley, NJ and generously provided by the National Cancer Institute.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Dr. Scalapino's work was supported by a VA Career Development Award. This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. Additional support provided by University of California San Francisco Autoimmunity Center of Excellence and the Rosalind Russell Arthritis Research Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 3.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. Journal of Immunology. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 4.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 5.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buenafe AC, Tsaknaridis L, Spencer L, Hicks KS, McMahan RH, et al. Specificity of regulatory CD4+CD25+ T cells for self-T cell receptor determinants. J Neurosci Res. 2004;76:129–140. doi: 10.1002/jnr.20066. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, et al. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Bach JF, Chatenoud L. Tolerance to islet autoantigens in type 1 diabetes. Annu Rev Immunol. 2001;19:131–161. doi: 10.1146/annurev.immunol.19.1.131. [DOI] [PubMed] [Google Scholar]
- 9.La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black x New Zealand White)F1 mice suppress in vitro production of antibodies to DNA. J Immunol. 2004;173:3542–3548. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- 10.Lepault F, Gagnerault MC. Characterization of peripheral regulatory CD4+ T cells that prevent diabetes onset in nonobese diabetic mice. J Immunol. 2000;164:240–247. doi: 10.4049/jimmunol.164.1.240. [DOI] [PubMed] [Google Scholar]
- 11.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 12.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 13.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci U S A. 2002;99:12287–12292. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. Journal of Experimental Medicine. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mekala DJ, Geiger TL. Immunotherapy of autoimmune encephalomyelitis with redirected CD4+CD25+ T lymphocytes. Blood. 2005;105:2090–2092. doi: 10.1182/blood-2004-09-3579. [DOI] [PubMed] [Google Scholar]
- 16.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 17.Monk CR, Spachidou M, Rovis F, Leung E, Botto M, et al. MRL/Mp CD4+,CD25- T cells show reduced sensitivity to suppression by CD4+,CD25+ regulatory T cells in vitro: a novel defect of T cell regulation in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1180–1184. doi: 10.1002/art.20976. [DOI] [PubMed] [Google Scholar]
- 18.Wu HY, Staines NA. A deficiency of CD4+CD25+ T cells permits the development of spontaneous lupus-like disease in mice, and can be reversed by induction of mucosal tolerance to histone peptide autoantigen. Lupus. 2004;13:192–200. doi: 10.1191/0961203303lu1002oa. [DOI] [PubMed] [Google Scholar]
- 19.Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, Baranda L, Layseca-Espinosa E, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–118. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;21:273–276. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 21.Liu MF, Wang CR, Fung LL, Wu CR. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 22.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 23.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 24.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177:1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 25.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 27.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunnane G, Chan OT, Cassafer G, Brindis S, Kaufman E, et al. Prevention of renal damage in murine lupus nephritis by CTLA-4Ig and cyclophosphamide. Arthritis Rheum. 2004;50:1539–1548. doi: 10.1002/art.20147. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura E, Sakihama T, Setoguchi R, Tanaka K, Sakaguchi S. Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1189–1201. doi: 10.1093/intimm/dxh122. [DOI] [PubMed] [Google Scholar]
- 32.Ermann J, Szanya V, Ford GS, Paragas V, Fathman CG, et al. CD4(+)CD25(+) T cells facilitate the induction of T cell anergy. J Immunol. 2001;167:4271–4275. doi: 10.4049/jimmunol.167.8.4271. [DOI] [PubMed] [Google Scholar]
- 33.Qiao M, Thornton AM, Shevach EM. CD4+ CD25+ [corrected] regulatory T cells render naive CD4+ CD25- T cells anergic and suppressive. Immunology. 2007;120:447–455. doi: 10.1111/j.1365-2567.2007.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. Journal of Experimental Medicine. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, et al. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. Jouranl of Experimental Medicine. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proteinuria and Survival Data. Serial proteinuria and survival in treatment and control mice. Mice received CD4+CD25+Tregs, CD4+CD25−Teffectors control cells, or PBS. Proteinuria score is recorded as 0–4 as measured by urine dipstick. Mouse deaths noted by X marks.
(0.04 MB XLS)
Serial proteinuria in renal histology mice. Mice were sacrificed 8-weeks after adoptive transfer of CD4+CD25+Tregs or PBS. Proteinuria score is recorded as 0–4 as measured by urine dipstick.
(0.02 MB XLS)