Abstract
Transplants from α1,3-galactosyltransferase (Gal) gene-knockout pigs to nonhuman primates are largely protected from hyper-acute but not acute humoral xenograft rejection. The present study investigates the role of Gal in cytokine responses using a novel pig-to-human whole blood in vitro model, developed for species-specific analysis of porcine and human cytokines. Porcine (n = 7) and human (n = 27) cytokines were measured using ELISA or multiplex technology, respectively. Porcine aortic endothelial cells from control (Gal+/+) and Gal-deficient (Gal−/−) pigs were incubated with human lepirudin anticoagulated whole blood from healthy donors. E-selectin expression was measured by flow cytometry. The C3 inhibitor compstatin and a C5aR antagonist were used to study the role of complement. Cytokine species specificity was documented, enabling detection of 2 of 7 porcine cytokines and 13 of 27 human cytokines in one single sample. Gal+/+ porcine aortic endothelial cells incubated with human whole blood showed a marked complement C5b-9 dependent up-regulation of E-selectin and secretion of porcine IL-6 and IL-8. In contrast, Gal−/− cells responded with E-selectin and cytokine expression which was so weak that the role of complement could not be determined. Human IL-6, IL-8, IFN-γ, MIP-1α, MIP-1β, eotaxin, and RANTES were detected in the Gal+/+ system, but virtually no responses were seen in the Gal−/− system (p = 0.03). The increase in human cytokine release was largely complement dependent and, in contrast to the porcine response, mediated through C5a. Species-specific analysis of cytokine release revealed a marked, complement-dependent response when Gal+/+ pig cells were incubated with human whole blood, compared with Gal−/− cells which induced virtually no cytokine release.
Xenotransplantation holds promise for unrestricted supply of organs, but several immunological hurdles must be overcome before xenotransplantation can become a clinical reality. Pigs are currently considered the donor of choice for human recipients. Nevertheless, discordant xenotransplantation, e.g., pig to primate, induces strong immune responses that lead to rapid loss of the graft. The primary hyperacute rejection (HAR)3 in human and nonhuman primates is caused by naturally occurring Abs that bind to the carbohydrate epitope Galα1,3Gal (Gal) on pig vascular endothelium and subsequently activate the complement system (1–4). Accordingly, HAR can be prevented by using pig organs transgenic for complement regulators (5, 6) or deficient for Gal by targeted deletion of the α1,3-galactosyltransferase (GalT) gene (7, 8). If HAR is inhibited, organs may subsequently be rejected in a process called acute humoral xenograft rejection (AHXR), also termed acute vascular rejection (9, 10). The pathogenesis of AHXR is not fully understood and organs from Gal-deficient pigs are still rejected by AHXR when transplanted into nonhuman primates (11, 12), and Abs against non-Gal epitopes may partly mediate this rejection (13–15).
Currently, a lot of attention is paid to the mechanism of rejection in the absence of Gal. In the present study, we have extended our in vitro model to include incubation of porcine aortic endothelial cells (PAEC) with human whole blood instead of serum (16). Thus, the effects of such coculture on both the human blood and the PAECs were explored. To this end, we have focused on establishing the cytokine profile of both the porcine endothelium and human blood by using species-selective assays. The role of cytokines in xenotransplantation is interesting given their well-established role in the pathogenesis of allograft rejection (17, 18), and numerous reports describing elevated levels in xenotransplantation (19, 20). However, their dependence on Gal-mediated immune activation upon exposure of xenograft endothelium has not been explored. Human polymorphonuclear cells (PMN) represent a major source of proinflammatory factors, including TNF-α, IL-1, IL-8, MIP-1α, and MIP-1β, and recruit additional PMN to the sites of inflammation (21, 22). PAECs also induce IL-1β, PGE2, and TNF-α production in human monocytes (23).
There are different roles for Gal in direct human leukocyte interactions with porcine endothelial cells (EC). Gal on porcine EC constitutes potential binding sites for human PMN, NK cells, and monocytes. Human NK cells may induce porcine EC activation by direct interaction in the absence of Gal (24, 25) and no significant reduction of direct NK cytotoxicity or NK cell and PMN adhesion on porcine ECs was found in the absence of, or upon blocking, Gal. In contrast, monocyte adhesion to porcine ECs depends on Gal-mediated activation (26).
In a recent study (16), we observed a marked reduction in human serum-induced E-selectin (CD62E) expression in PAECs lacking the Gal (Gal−/−) when compared with normal (Gal+/+) cells. In the present study, the mutual inflammatory response of the PAECs and the human blood cells was studied by establishing a species-specific detection system to measure porcine and human mediators in the same sample. Furthermore, specific complement inhibitors were used to evaluate the role of complement in this response.
Materials and Methods
Cell culture
Primary PAECs from GalT-knockout (KO; Gal−/− PAECs) or control littermate (Gal+/+ PAECs) pigs were provided by R. J. Hawley (formerly Immerge Biotherapeutics), and used up to passage number 20. The immortalized PAEC line PEDSV.15 (Gal+/+) was generated as previously described in detail (27). The Gal−/− cell line PED2*3.51 was generated from PEDSV.15 by homologous recombination, panning, and limiting dilution cloning (28). All cells were cultured in DMEM (Invitrogen Life Technologies) supplemented with 10% FCS (BioWhittaker), 1 mM sodium pyruvate, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 20 mM HEPES, 50 µg/ml gentamicin (all obtained from Invitrogen Life Technologies), and 0.25 µg/ml amphotericin B (BioWhittaker).
Human whole blood
Blood from five healthy volunteers was collected into sterile polypropylene tubes (4.5-ml Nunc cryotubes; Nagle Nunc International) containing 50 µg/ml lepirudin (refludan; Hoechst) as anticoagulant. Samples were obtained immediately before each experiment and kept on ice. Informed written consent was obtained from each donor. The study was approved by the local ethical committee.
In vitro pig-to-human transplantation model
Gal+/+ and Gal−/− PAECs (both primary and immortalized) where grown to confluence in 96-well cell culture plates (Corning). The cells where then exposed to 100 µl of human whole blood, diluted to 50% in culture medium, for 24 h on a shaker in a CO2 incubator. After incubation, cell supernatants were collected, centrifuged, and immediately frozen at −70°C. PAECs were then washed, trypsinized, washed with medium containing 10% FCS, and centrifuged at 300 × g for 5 min. PE-conjugated anti-CD62E (E-selectin) clone 1.2B6 or PE-conjugated isotype control IgG1 (both obtained from Southern Biotechnology Associates) were added directly to the pellet and cells were incubated for 30 min at 4°C, and subsequently analyzed by flow cytometry (FACScan; BD Biosciences). Cells incubated with TNF-α (Sigma-Aldrich) were used as positive control. Results are given as median fluorescence intensity (MFI).
Porcine cytokine measurement
Pilot samples from the PAEC-human whole blood model were examined in a panel of seven porcine cytokine ELISAs (TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-12(p70), IFN-γ), performed according to the instructions from the manufacturer (R&D Systems). The porcine ELISAs are quantitative sandwich immunoassays, precoated with the actual anti-porcine cytokine Ab (usually monoclonal). Briefly, standards and samples were added to the wells. After washing, an enzyme-linked Ab (usually polyclonal) reacting with the porcine cytokine was added. Following a final wash, the plates were subsequently developed with substrate solution for 30 min at room temperature, stop solution was added, and OD was measured at 405 nm using a microplate reader (Asys Hightech). Data were analyzed with MicroWin software (Mikrotek Laborsysteme). A control sample with known concentrations of the porcine cytokine was included on every plate.
Human cytokine measurement
Multiplex technology was used to measure the concentration of a broad panel of human cytokines, chemokines, and growth factors (Human Cytokine 27-plex-kit; Bio-Rad). This is a bead-based sandwich immunoassay, which simultaneously measure the following 27 human biomarkers: IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17, TNF-α, IFN-γ, IFN-γ-inducible protein 10 (IP-10), fibroblast growth factor (FGF)-basic, MIP-1α, MIP-1β, MCP-1, G-CSF, GM-CSF, platelet-derived growth factor (PDGF)-BB, eotaxin, RANTES, and vascular endothelial growth factor (VEGF). The analyses were performed according to instructions from the manufacturer. Briefly, beads of defined spectral properties are conjugated to bind specific capture Abs. The biomarker in each sample will bind to the capture Abs on the beads. Then biomarker-specific biotinylated detector Abs added will bind to the appropriate immobilized analytes. Finally, streptavidin conjugated to the fluorescent protein R-PE binds to the biotinylated detector Abs associated with the immune complexes of the beads, forming a solid-phase sandwich. The beads were analyzed with a Luminex 100, Bio-Plex array reader (Bio-Rad). Standard curves were generated for each biomarker using the mixed analyze standard provided with the kit.
Species specificity of the porcine and human cytokine detection assays
The experimental model of PAECs incubated with human whole blood requires that the cytokines in the plasma supernatant are detected in species-specific assays for correct interpretation of the results. Human whole blood was incubated with Neisseria meningitis bacteria (strain H44/76, isolated from a patient with invasive meningococcal disease (29)), and porcine whole blood was incubated with Escherichia coli (American Type Culture Collection) (both 108 bacteria/ml blood) to produce plasma samples with large amounts of human and porcine cytokines, respectively (T. E. Mollnes et al., unpublished data). These two samples where tested in both species assays described above. Based on the high specificity of these assays (Table I), the current study was undertaken.
Table I.
Species specificity in porcine inflammatory mediator assaysa
| Mediator | Human Plasma | Porcine Plasma | Detected in the Present Model |
|---|---|---|---|
| IL-1β | 22 | 9,000 | |
| IL-6 | 0 | 150 | X |
| IL-8 | 0 | 60,000 | X |
| IL-10 | 0 | 700 | |
| IL-12(p70) | 0 | 500 | |
| IFN-γ | 0 | 40 | |
| TNF-α | 0 | 10,000 |
Plasma obtained from human and porcine blood incubated with Gram-negative bacteria examined in ELISAs for detection of porcine inflammatory mediators (values are given in picograms per milliliter). Cytokines marked X (right column) were detected in the present pig-to-human in vitro whole blood model using both primary and immortalized PAECs.
Complement inhibitors
Compstatin is a 13-aa cyclic peptide that binds and inhibits cleavage of complement component C3 (30, 31). The sequence is Ac-I[CV(1MeW)QDWGAHRC]T and the molecular mass is 1628 Da. A linear-inactive analog to compstatin, differing in only one amino acid, was used as a control. The sequence is Ac-IAVVQDWGHHRAT and the molecular mass is 1532 Da. To block complement C5aR on leukocytes, a small cyclic hexapeptide that acts as a selective C5aR antagonist (C5aRa) was used (32, 33). The sequence is AcF-[OPdChaWR] and the molecular mass is 896 Da. The inhibitors and the control peptide were produced in the laboratory of J. D. Lambris.
Statistical analysis
The Wilcoxon test for paired samples was used. Values of p < 0.05 were considered statistically significant.
Results
E-selectin expression is strongly up-regulated on Gal+/+, but not Gal−/−, PAECs incubated with human whole blood
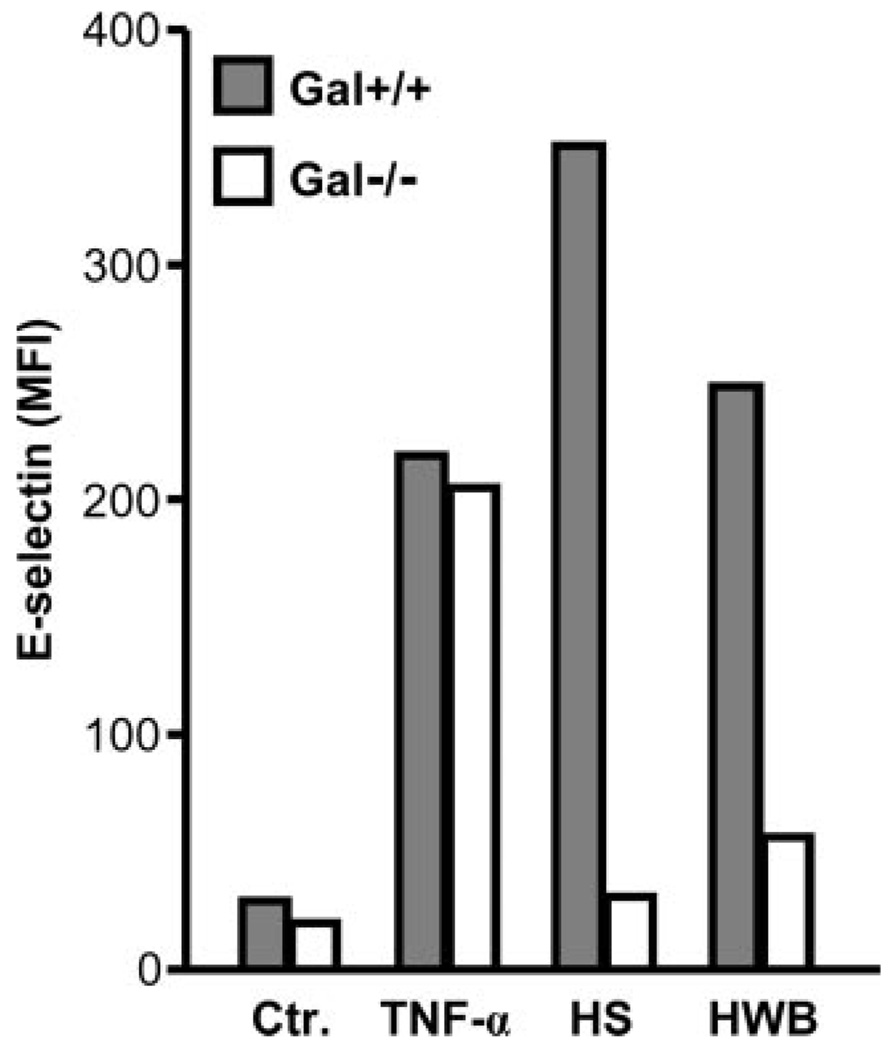
TNF-α, used as positive control, caused a marked up-regulation of E-selectin on both Gal+/+ (220 MFI) and Gal−/− (205 MFI) immortalized PAECs compared with resting cells (29 and 19 MFI, respectively) (Fig. 1). Human serum induced a substantial up-regulation of E-selectin on Gal+/+ PAECs, whereas virtually no effect was seen on Gal−/− cells (351 and 31 MFI, respectively). The effect of human whole blood on porcine E-selectin expression was comparable to the response seen with serum alone (248 MFI on Gal+/+ and 56 MFI on Gal−/− cells) (Fig. 1), indicating that plasma factors rather than blood cells were the main inducers of this effect. No staining of the cells was observed using an isotypematched control Ab.
FIGURE 1.
E-selectin expression on immortalized Gal+/+ and Gal−/− PAECs. PAECs were incubated 4 h with TNF-α, 50% human serum (HS), and 50% human whole blood (HWB). E-selectin cell surface expression was measured by flow cytometry. Data represent MFI in one representative experiment.
Human blood-induced E-selectin up-regulation is reversed by complement inhibition
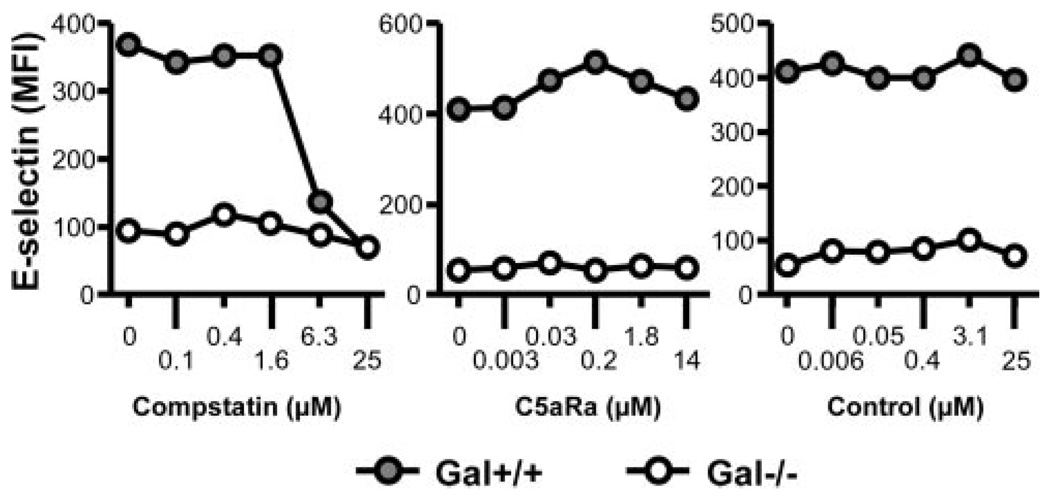
The increase in E-selectin on immortalized Gal+/+ cells was dose-dependently and completely reduced by the C3 inhibitor compstatin, whereas the C5aR antagonist and the control peptide had no effect (Fig. 2, closed circles), consistent with a C5b-9-mediated effect in the whole blood model as previously shown for serum (16). By contrast, the minor up-regulation of E-selectin on Gal−/− cells incubated with human whole blood was not affected by any of the complement inhibitors (Fig. 2, open circles).
FIGURE 2.
Effect of complement blocking on E-selectin expression on immortalized Gal+/+ and Gal−/− PAECs. Gal+/+ (●) and Gal−/− (○) PAECs were incubated with human whole blood and increasing concentrations of the complement-inhibitors compstatin (left panel) and a C5aR antagonist (middle panel) as well as a control peptide (right panel). E-selectin expression on incubated PAEC was determined by flow cytometry. Representative data from one blood donor are presented.
Species-specific detection of porcine and human inflammatory mediators
Porcine and human plasma was obtained from whole blood incubated with Gram-negative bacteria. These plasma samples were already known to contain high amounts of a number of cytokines (see Materials and Methods). To document species specificity of the assays used in this study, the samples were first tested in seven porcine ELISAs (Table I, left and middle columns). Reactivity was found for all of these mediators in porcine plasma. Six of the seven mediators were completely negative when human plasma, known to contain high amounts of the corresponding human variants, were tested, whereas IL-1β showed a weak reactivity (22 pg/ml in human plasma compared with 9000 pg/ml in porcine plasma). When the same samples were tested in a multiplex assay for detection of human inflammatory markers, reactivity was seen for 18 of 27 markers in human plasma (Table II, left and middle columns). Fifteen of the 18 were completely negative when porcine plasma was tested in the assay, whereas MCP-1, PDGF-bb, and RANTES showed various degree of cross-reactivity. Because species specificity requires that the mediator in question is documented to be present in both the porcine and the human plasma sample, whereas reactivity is found only in the corresponding assay, species specificity was concluded for IL-6, IL-8, IL-10, IFN-γ, and TNF-α. In addition, IL-1β was highly specific in the human assay and virtually specific in the porcine assay.
Table II.
Species specificity of human inflammatory mediator assaysa
| Mediator | Human Plasma | Porcine Plasma | Detected in the Present Model |
|---|---|---|---|
| IL-1β | 3,480 | 0 | |
| IL-1ra | 3,800 | 0 | X |
| IL-6 | 17,000 | 0 | X |
| IL-8 | 14,000 | 0 | X |
| IL-9 | 80 | 0 | X |
| IL-10 | 77 | 0 | |
| Eotaxin | 113 | 0 | X |
| FGF-basic | 131 | 0 | Xb |
| G-CSF | 180 | 0 | |
| IFN-γ | 460 | 0 | Xc |
| IP-10 | 1,400 | 0 | X |
| MCP-1 | 191 | 196 | X |
| MIP-1α | 6,800 | 0 | X |
| MIP-1β | 15,000 | 0 | X |
| PDGF-bb | 12,200 | 2,700 | X |
| RANTES | 29,000 | 223 | X |
| TNF-α | 40,000 | 0 | Xc |
| VEGF | 112 | 0 | Xc |
Plasma obtained from human and porcine blood incubated with Gram-negative bacteria (same samples as presented in Table I) examined in a multiplex assay for detection of human inflammatory mediators (values are given in picograms per milliliter). Cytokines marked X (right column) were detected in the present pig-to-human in vitro model using both primary and immortalized PAECs.
Detected only using primary cells.
Detected only using immortalized cells.
Cytokines and chemokines released upon coculture of human whole blood with PAEC
The incubation with human whole blood induced the secretion of porcine IL-6 and IL-8 in both immortalized and primary Gal+/+ PAECs (Table I, right column). In the human cells, IL-1ra, IL-6, IL-8, IL-9, IP-10, MIP-1α MIP-1β, MCP-1, PDGF-BB, eotaxin, and RANTES secretion was induced by both immortalized and primary Gal+/+ PAEC. In contrast, human TNF-α, IFN-γ, and VEGF were only detected using immortalized cells, whereas FGF-basic was only induced using primary cells (Table II, right column).
Dose-dependent effect of complement inhibition on porcine and human cytokine release
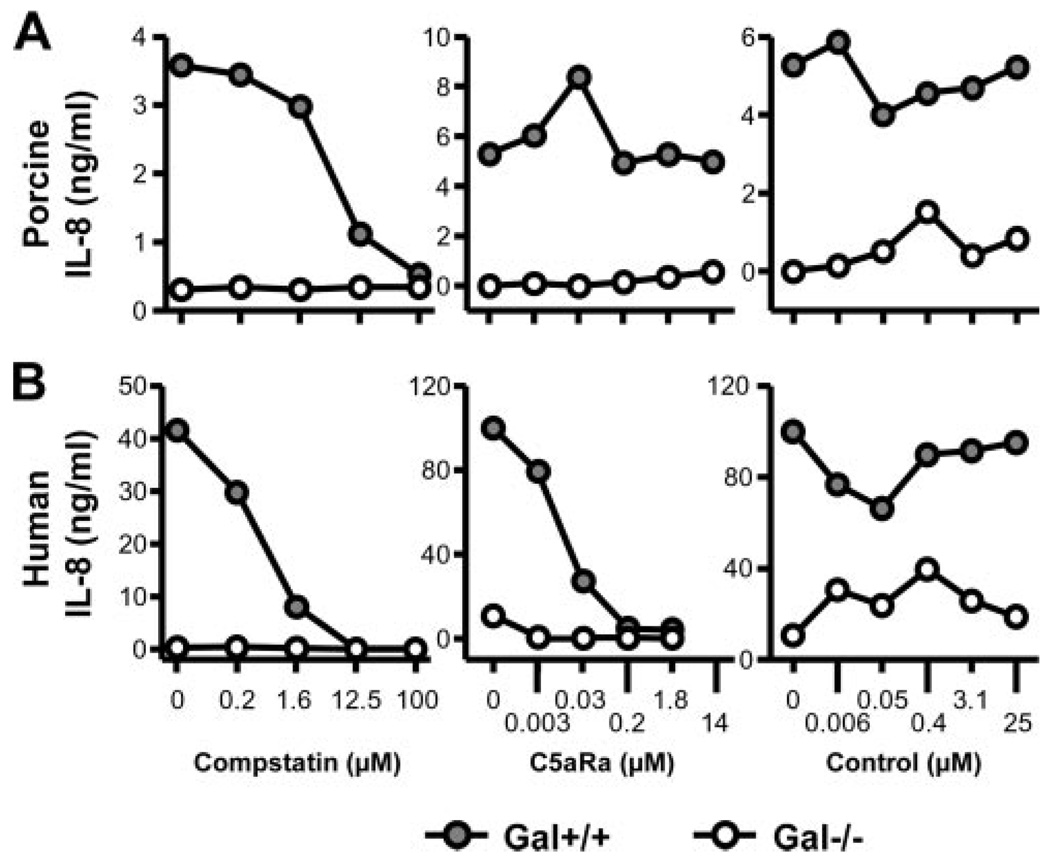
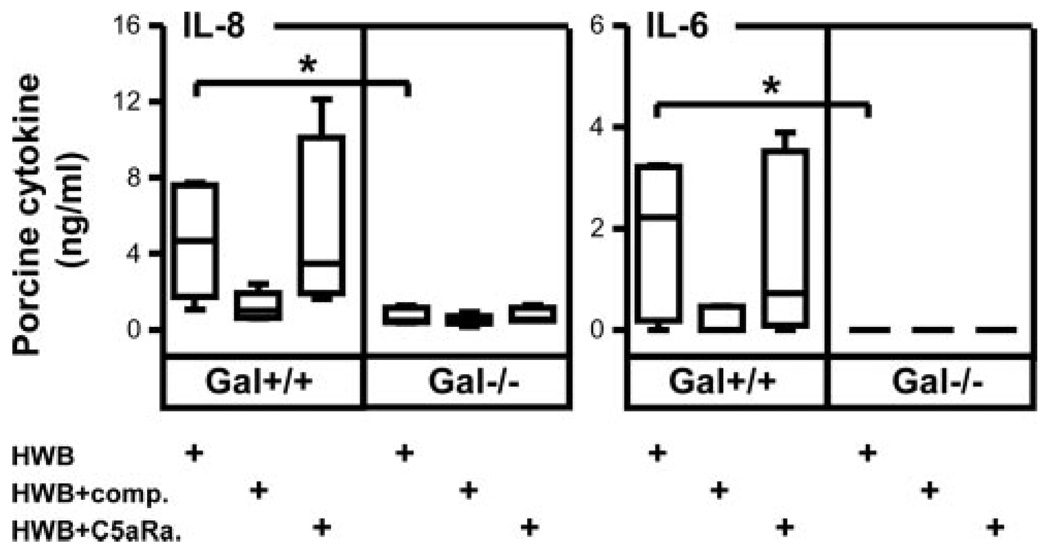
The differential effect of complement on porcine and human cytokine release as described above was further investigated in dose-response experiments using human whole blood and immortalized PAEC with IL-8 as a readout. In contrast to Gal+/+ cells, Gal−/− cells induced neither porcine nor human IL-8 (Fig. 3). Human IL-8 secretion decreased dose-dependently to nadir by both the C3 inhibitor compstatin and a C5aR antagonist (Fig. 3, lower panel). In contrast, porcine IL-8 production by Gal+/+ PAEC was reduced to nadir with increasing concentrations of compstatin, whereas no effect was seen using the C5aR antagonist or the control peptide (Fig. 3, upper panel). Accordingly, human whole blood incubation induced a significant IL-6 production in Gal+/+, but not Gal−/−, PAECs, which was virtually abolished by compstatin but not by the C5aR antagonist (Fig. 4). Thus, complement is essential for both the porcine and the human response, but the effector mechanisms are strikingly different.
FIGURE 3.
Effect of complement blocking on porcine and human cytokine production. Immortalized Gal+/+ (●) and Gal−/− (○) PAECs were incubated with human whole blood to measure porcine (A) or human (B) IL-8 production. Complement inhibitors compstatin (left panel) and a C5aR antagonist (middle panel) as well as a control peptide (right panel) were added in increasing concentrations (x-axis). Representative data from one blood donor are presented. Cytokine data are presented as concentration (nanograms per milliliter).
FIGURE 4.
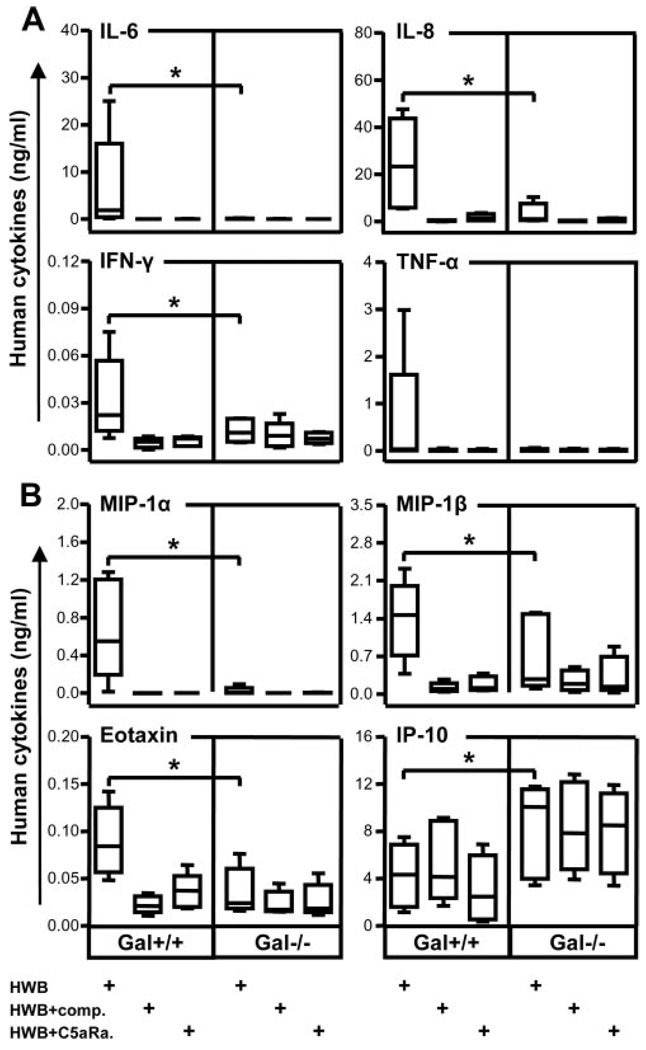
Detection of porcine cytokines in cell supernatants. Porcine IL-8 (left panel) and IL-6 (right panel) concentrations were analyzed in supernatants from immortalized Gal+/+ and Gal−/− PAECs incubated with human whole blood (HWB) alone, HWB with complement inhibitor C5aR (C5aRa, 14 µM), or HWB with complement inhibitor compstatin (comp., 100 µM). Both IL-8 and IL-6 concentration was significantly higher from Gal+/+ compared with Gal−/− porcine PAECs (*, p = 0.03). Data are presented as box plots showing median, quartiles, and minimum and maximum values from five experiments, each using a different donor.
Differential induction of human inflammatory mediators by immortalized Gal+/+ and Gal−/− PAECs
Significantly higher concentrations of human IL-6, IL-8, and IFN-γ were detected after whole blood incubation with Gal+/+ PAECs as compared with Gal−/− cells, whereas the difference for TNF-α was not statistically significant due to large interindividual variation (Fig. 5A). For the chemokines where cross-reactivity could not be determined, significantly higher concentrations of the chemokines MIP-1α, MIP-1β, and eotaxin were measured in cell supernatants from cultures with Gal+/+ compared with Gal−/− PAECs (Fig. 5B). In contrast significantly higher concentrations of IP-10 were recorded in cell supernatants from Gal−/− PAECs as compared with Gal+/+ cells (Fig. 5B, lower right panel). For IL-9, VEGF, and IL-1ra, no difference was seen between Gal+/+ and Gal−/− PAEC cultures (data not shown). Of the mediators with a certain degree of documented species cross-reactivity, RANTES was significantly higher in the supernatant from Gal+/+ than from Gal−/− cells, whereas no difference were seen for PDGF-bb and MCP-1 (data not shown). In line with its effect on human IL-8, compstatin virtually abolished the production of IL-6, IL-8, IFN-γ, TNF-α, MIP-1α, MIP-1β, and eotaxin (Fig. 5). Moreover, in contrast to the production of porcine cytokines, the C5aR antagonist efficiently inhibited all human cytokines except IP-10 (Fig. 5), again indicating that the cytokine production by human whole blood induced by Gal+/+ cells was C5a mediated. In contrast, the IP-10 production was complement independent.
FIGURE 5.
Detection of human cytokines in cell supernatants. Cytokine production was detected in cell supernatants when immortalized Gal+/+ and Gal−/− PAECs were incubated with human whole blood (HWB) alone or with complement inhibitors C5aR antagonist (C5aRa, 14 µM) or compstatin (comp., 100 µM). A, Human cytokines (IL-6, IL-8, IFN-γ, and TNF-α) for which cross-reactivity to porcine cytokines was excluded in the human multiplex system (Table II). B, Human cytokines (MIP-1α, MIP-β, eotaxin, and IP-10) for which cross-reactivity with porcine cytokines could not be determined. Significant differences were found between human cytokines detected in the Gal+/+ compared with Gal−/− PAECs for all cytokines (*, p = 0.03). Data are presented as box plots showing median, quartiles, and minimum and maximum values from five experiments, each using a different donor.
Comparable results using immortalized and primary PAECs in cocultures with whole human blood
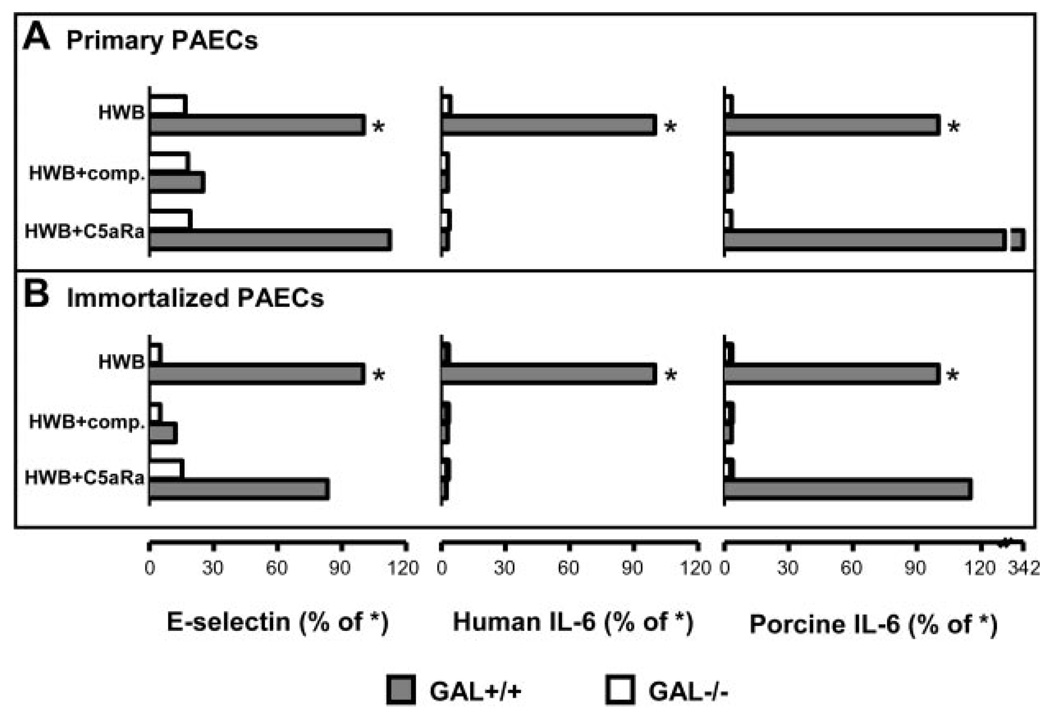
The validity of using the immortalized Gal+/+ PAECs line PESDV.15 and the corresponding Gal−/− variant has been questioned, e.g., with respect to expression of VCAM-1 (24). We therefore performed a series of coculture experiments using primary PAECs obtained from GalT-KO pigs or their native control animals, and the immortalized lines described above. Primary Gal+/+ and Gal−/− PAECs incubated with human whole blood showed E-selectin up-regulation and induced IL-6-responses virtually identical with their corresponding immortalized cell lines (Fig. 6). In addition, the effect of compstatin and the C5aR antagonist was equal in the primary and immortalized cell cultures.
FIGURE 6.
Comparison of immortalized and primary Gal+/+ and Gal−/− PAECs. Primary (A) and immortalized PAECs (B) were compared with respect to E-selectin expression and human and porcine cytokines in cell supernatants (here shown as IL-6) after incubation with human whole blood (HWB), HWB with compstatin (Comp.), or HWB with a C5aR antagonist (C5Ra). E-selectin expression data represents percent median fluorescent intensity of Gal+/+ PAECs incubated with HWB (marked with an asterisk (*)). Cytokine data are presented as concentration (nanograms per milliliter).
Discussion
In the present study, we explored the importance of Gal and non-Gal epitopes in the inflammatory reaction induced when PAECs are exposed to human whole blood, an issue which has become particularly important after the advent of cloned GalT-KO pigs (34, 35). Organs from GalT-KO animals are still rejected by AHXR and show features of thrombotic microangiopathy. Possible explanations for these later rejection processes include molecular incompatibility between pig and primates (36, 37), T cell-independent mechanisms due to natural non-Gal Abs, or T cell-dependent mechanisms due to elicited non-Gal Abs (38). Growing evidence supports a close relation between inflammation and hemostasis, e.g., as illustrated by the cross-talk between complement and coagulation (39). Thus, control of the inflammatory reaction, as reflected by cytokine release, is required to increase the compatibility between pig and primate organs.
Differences in cytokine production during rejection of cells and organs from GalT-KO compared with wild-type pigs are largely unknown. To investigate this, we used an established human whole blood model (40) to activate Gal+/+ and Gal−/− PAECs and documented large differences between Gal+/+ and Gal−/− PAECs with respect to production of both endogenous porcine cytokines and induction of cytokine production from human blood cells. This model is based on anticoagulation with lepirudin, a recombinant hirudin analog which is a highly specific thrombin inhibitor that does not influence complement activation, in contrast to heparin and other available anticoagulants (40). Establishing a whole blood model without using an anticoagulant is complicated, and attempts show that blood coagulates within 2 h (41). Because a time period of several hours is required to study cytokine production, anticoagulation is mandatory and despite inhibition of the last step of coagulation (thrombin), to our knowledge, the protocol used herein represents the most physiological relevant model to study whole blood in vitro. There are no data published on differences between the different endothelial cells used in this study with respect to expression of thrombin receptors or their response to lepirudin. This is a limitation of the current study. Comparing the consistent data obtained from the different cell lines we used, however, there is no indication that lepirudin influenced the results.
The model was extended to include primary PAECs from Gal−/− pigs in addition to the immortalized cell lines. We are aware that endothelial cells may undergo senescence by numerous passages in vitro. However, these cells have been cultured up to passage 25 without difficulties and with retained typical endothelial morphology and phenotype, including expression of receptors such as CD31, CD54, CD62E, CD62P, and CD106 (24). Furthermore, the cell lines were examined and compared at the same number of passages. The physiologic relevance of the Gal−/− immortalized cells used by our group and others have been questioned because they lack VCAM-1 (CD106) in their resting state (24, 42), a molecule important for adhesion of PBMC (43, 44). However, there have been technical difficulties in detecting up-regulation of VCAM-1 even on activated naive Gal+/+ PAECs, probably because good anti-porcine VCAM-1 Abs, to our knowledge, are not commercially available. One concern using primary cells is that they cannot be cultured indefinitely, and may alter properties over short periods of time. The primary cells used in this study are used from passage 15 to 20. This may be somewhat longer than generally accepted, but even at these passage numbers, the primary PAECs exhibited virtually the same results as the immortalized cells lines (Fig. 6) and displayed an EC-typical phenotype including the expression of adhesion receptors important for human leukocyte adhesion (24). The observations that the immortalized cells in this study showed the same results as the primary PAECs, lends support to the validity of previous results obtained using these cell lines as a reliable model for in vitro studies on the role of Gal in pig-to-human xenotransplantation (16, 24, 45), where the immortalized Gal−/− were used. The fact that immortalized and primary Gal+/+ PAEC induced comparable cytokine production patterns in human cells, except for only three cytokines (IFN-γ, TNF-α, and VEGF) exclusively detected when using immortalized cells, support the assumption that the primary and immortalized cells differ only slightly phenotypically, and that these cytokines might not be physiologically relevant in a clinically setting. Compared with the serum-based model we previously used (16), the present whole blood model is physiologically more relevant and allows us to explore cellular mechanisms in the mutual interaction between PAECs and blood cells.
Inflammatory cytokines have been associated with AHXR of xenografts (20), and Al-Mohanna et al. (46) demonstrated that neutrophils are one of the major sources of cytokines, even in the absence of xenoantibodies and complement, and that this cytokine release was independent of Gal structures. Our results definitely indicate that Gal has a dominant role in the induction of cytokine production in the presence of xenoantibodies, and that this cytokine response is significantly higher than that mediated by direct cellular interactions, as studied by Al-Mohanna et al. (46). The whole blood model, however, does not discriminate between the contributions of the different cells present, but rather reflects the total amount of cytokines produced by the cells present. A major advantage with the present model is the opportunity to selectively detect and quantify both human and porcine cytokines and chemokines in the same sample. A broad panel of human cytokines was found not to cross-react with pig, whereas the number of porcine-specific cytokines was more limited, mainly due to fewer available reagents.
IL-8 serves as a potent chemoattractant for granulocytes to the site of inflammation and is associated with EC activation (47, 48), and the level of porcine IL-8 correlates with E-selectin expression in the current model (own unpublished data). In the present study, substantial differences were seen between Gal+/+ and Gal−/− PAECs both with regard to human and porcine IL-8 production, consistent with complement activation induced by anti-Gal Abs leading to C5b-9 deposition on the PAECs and C5a release to the fluid phase, both effects known to mediate IL-8 release. IL-6 is a prototypic proinflammatory cytokine which has been associated with rejection of cardiac allo- (49) and xenograft (20, 50). We found that the Gal epitope was also critically important for the release of both human and porcine IL-6, with low levels induced by Gal−/− cells.
Substantially higher levels of human IFN-γ were found in supernatants of Gal+/+ PAECs incubated with human whole blood compared with Gal−/− PAECs. Whether IFN-γ promotes or protects against xenograft rejection is unclear. A study by Wang et al. (51) found that IFN-γ together with IL-12 was protective against AHXR, whereas other studies have demonstrated that high levels of IFN-γ are associated with rejection (52–55). The precise role of IFN-γ in xenotransplantation remains to be elucidated.
The role of IL-1 in xenograft rejection was demonstrated by the finding that treatment with an IL-1ra reduced the expression of a number of inflammatory genes and increased graft survival in rats receiving guinea pig hearts (56). In our study, we recorded no up-regulation of human IL-1, but interestingly IL-1ra was detected and the concentration was equally or slightly stronger induced by Gal−/− than Gal+/+ PAECs, suggesting a possible endogenous protection against Gal−/− graft failure.
In accordance with our observations, Solomon et al. (57) found up-regulation of the β chemokines such RANTES, MIP-1α, MIP-1β, and eotaxin in a pig-to-murine islet transplantation model. In our study, their human counterparts showed significantly higher levels in supernatants from Gal+/+ PAECs compared with those from Gal−/− PAECs, indicating that Gal is important for chemokine-induced inflammation. In contrast, up-regulation of human MCP-1 was not mediated by the Gal epitope in our study, whereas increased MCP-1 levels have been associated with rejection in other xenotransplantation models (20, 57). Up-regulation of the T cell-recruiting chemokine IP-10 has been associated with xenograft rejection in several models (19, 57, 58), although a recent study showed no change in IP-10 (59). Notably, we found that human IP-10, in contrast to all other chemokines, was produced to a greater extent when blood was incubated with Gal−/− PAECs than when Gal+/+ PAECs were used, indicating that other factors than Gal was responsible for the induction of IP-10. Importantly, IP-10 was the only chemokine which was not reduced by complement inhibition using Gal+/+ cells (see Fig. 5B). This implies that IP-10 is induced by a different, complement-independent, mechanism, which is enhanced on cells lacking the Gal epitope. Further investigations are needed to reveal the role of IP-10 in xenotransplantation.
The role of growth factors in xenotransplantation is unknown, while in allotransplantation both VEGF and PDGF are associated with graft rejection (60, 61). We could not document any difference in VEGF or PDGF concentrations when eliminating the Gal epitope on PAECs stimulated with human whole blood.
The mechanism of complement-mediated induction of cytokines and chemokines was revealed by inhibition of C3 activation, using the C3 inhibitor compstatin, and by blocking the C5aR using a C5aR antagonist. Consistent with previous data from the serum model, Gal+/+ PAEC activation was independent of C5a and mediated by the C5b-9 complex (16). In contrast, activation of human blood cells and production of human cytokines and chemokines was completely dependent on C5a, consistent with the role of C5a in induction of inflammation induced by leukocytes in whole human blood. The minor up-regulation of E-selectin on Gal−/− PAECs stimulated with human whole blood seemed to be independent of complement; however, we do not exclude a possible inhibition of complement if the sensitivity of the assay was higher. Similarly, we cannot exclude a certain effect of anti-non-Gal Abs in the up-regulation of cytokines in a Gal−/− system, which might have passed undetected in our experiments due to the sensitivity of the assay. In any case, our data indicate that naturally occurring anti-non-Gal Abs play a minor role compared with anti-Gal Abs for this response.
In conclusion, we demonstrate a crucial role for Gal in xenoantibody-dependent cellular production of both porcine and human IL-6 and IL-8 in an in vitro model of xenograft rejection. Furthermore, human cytokines and chemokines, including IFN-γ, MIP-1α, MIP-1β, and eotaxin, were highly dependent on Gal. The present model and current findings contribute to further understanding of the inflammatory mechanisms involved in rejection of cells and organs from Gal+/+ vs Gal−/− pigs and can be used for subsequent studies to evaluate the compatibility of the next generation of gene-targeted pigs intended for human transplantation.
Footnotes
This work was supported by The Norwegian Research Council, The Research Council of Rikshospitalet, The Norwegian Council on Cardiovascular Disease, The Family Blix Foundation, The Odd Fellow Foundation, Swiss National Science Foundation Research Grant 3200B0-109921, and National Institutes of Health Grants GM-62134 and AI-068730.
Abbreviations used in this paper: HAR, hyperacute rejection; Gal, Galα1–3Gal; AHXR, acute humoral xenograft rejection; PAEC, porcine aortic endothelial cell; KO, knockout; PMN, polymorphonuclear cell; EC, endothelial cell; MFI, median fluorescence intensity; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; IP-10, IFN-γ-inducible protein 10; VEGF, vascular endothelial growth factor.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J. Biol. Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 2.Oriol R, Ye Y, Koren E, Cooper DK. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56:1433–1442. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, Neethling FA, Ye Y, Romano E, Zuhdi N. Identification of α-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl. Immunol. 1993;1:198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 4.Dalmasso AP, Vercellotti GM, Fischel RJ, Bolman RM, Bach FH, Platt JL. Mechanism of complement activation in the hyperacute rejection of porcine organs transplanted into primate recipients. Am. J. Pathol. 1992;140:1157–1166. [PMC free article] [PubMed] [Google Scholar]
- 5.McCurry KR, Kooyman DL, Alvarado CG, Cotterell AH, Martin MJ, Logan JS, Platt JL. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nat. Med. 1995;1:423–427. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- 6.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat. Med. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 7.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 8.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, et al. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platt JL, Lin SS, McGregor CG. Acute vascular rejection. Xenotransplantation. 1998;5:169–175. doi: 10.1111/j.1399-3089.1998.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 10.Rieben R, Seebach JD. Xenograft rejection: IgG1, complement and NK cells team up to activate and destroy the endothelium. Trends Immunol. 2005;26:2–5. doi: 10.1016/j.it.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat. Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, Wise Y, Liu Y, Xiang Y, Copeman L, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat. Med. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper DK. Xenoantigens and xenoantibodies. Xenotransplantation. 1998;5:6–17. doi: 10.1111/j.1399-3089.1998.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Sun H, Yang H, Kubelik D, Garcia B, Luo Y, Xiang Y, Qian A, Copeman L, Liu W, et al. The role of anti-non-Gal antibodies in the development of acute humoral xenograft rejection of hDAF transgenic porcine kidneys in baboons receiving anti-Gal antibody neutralization therapy. Transplantation. 2006;81:273–283. doi: 10.1097/01.tp.0000188138.53502.de. [DOI] [PubMed] [Google Scholar]
- 15.Zhong R. Gal knockout and beyond. Am. J. Transplant. 2007;7:5–11. doi: 10.1111/j.1600-6143.2006.01615.x. [DOI] [PubMed] [Google Scholar]
- 16.Saethre M, Baumann BC, Fung M, Seebach JD, Mollnes TE. Characterization of natural human anti-non-Gal antibodies and their effect on activation of porcine Gal-deficient endothelial cells. Transplantation. 2007;84:244–250. doi: 10.1097/01.tp.0000268815.90675.d5. [DOI] [PubMed] [Google Scholar]
- 17.Marshall SE, Welsh KI. The role of cytokine polymorphisms in rejection after solid organ transplantation. Genes Immun. 2001;2:297–303. doi: 10.1038/sj.gene.6363795. [DOI] [PubMed] [Google Scholar]
- 18.Dallman MJ, Clark GJ. Cytokines and their receptors in transplantation. Curr. Opin. Immunol. 1991;3:729–734. doi: 10.1016/0952-7915(91)90104-9. [DOI] [PubMed] [Google Scholar]
- 19.Hardstedt M, Finnegan CP, Kirchhof N, Hyland KA, Wijkstrom M, Murtaugh MP, Hering BJ. Post-transplant upregulation of chemokine messenger RNA in non-human primate recipients of intraportal pig islet xenografts. Xenotransplantation. 2005;12:293–302. doi: 10.1111/j.1399-3089.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 20.Candinas D, Belliveau S, Koyamada N, Miyatake T, Hechenleitner P, Mark W, Bach FH, Hancock WW. T cell independence of macrophage and natural killer cell infiltration, cytokine production, and endothelial activation during delayed xenograft rejection. Transplantation. 1996;62:1920–1927. doi: 10.1097/00007890-199612270-00042. [DOI] [PubMed] [Google Scholar]
- 21.Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 22.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 23.Millan MT, Geczy C, Stuhlmeier KM, Goodman DJ, Ferran C, Bach FH. Human monocytes activate porcine endothelial cells, resulting in increased E-selectin, interleukin-8, monocyte chemotactic protein-1, and plas-minogen activator inhibitor-type-1 expression. Transplantation. 1997;63:421–429. doi: 10.1097/00007890-199702150-00016. [DOI] [PubMed] [Google Scholar]
- 24.Baumann BC, Schneider MK, Lilienfeld BG, Antsiferova MA, Rhyner DM, Hawley RJ, Seebach JD. Endothelial cells derived from pigs lacking Gal α(1,3)Gal: no reduction of human leukocyte adhesion and natural killer cell cytotoxicity. Transplantation. 2005;79:1067–1072. doi: 10.1097/01.tp.0000157231.11083.7c. [DOI] [PubMed] [Google Scholar]
- 25.He Z, Ehrnfelt C, Kumagai-Braesch M, Islam KB, Holgersson J. Aberrant expression of α-Gal on primary human endothelium does not confer susceptibility to NK cell cytotoxicity or increased NK cell adhesion. Eur. J. Immunol. 2004;34:1185–1195. doi: 10.1002/eji.200324683. [DOI] [PubMed] [Google Scholar]
- 26.Peterson MD, Jin R, Hyduk S, Duchesneau P, Cybulsky MI, Waddell TK. Monocyte adhesion to xenogeneic endothelium during laminar flow is dependent on α-Gal-mediated monocyte activation. J. Immunol. 2005;174:8072–8081. doi: 10.4049/jimmunol.174.12.8072. [DOI] [PubMed] [Google Scholar]
- 27.Seebach JD, Schneider MK, Comrack CA, LeGuern A, Kolb SA, Knolle PA, Germana S, DerSimonian H, LeGuern C, Sachs DH. Immortalized bone-marrow derived pig endothelial cells. Xenotransplantation. 2001;8:48–61. doi: 10.1034/j.1399-3089.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- 28.Baumann BC, Forte P, Hawley RJ, Rieben R, Schneider MK, Seebach JD. Lack of galactose-α-1,3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J. Immunol. 2004;172:6460–6467. doi: 10.4049/jimmunol.172.10.6460. [DOI] [PubMed] [Google Scholar]
- 29.Holten E. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 1979;9:186–188. doi: 10.1128/jcm.9.2.186-188.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- 31.Katragadda M, Magotti P, Sfyroera G, Lambris JD. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J. Med. Chem. 2006;49:4616–4622. doi: 10.1021/jm0603419. [DOI] [PubMed] [Google Scholar]
- 32.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J. Immunol. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 33.Lappegard KT, Riesenfeld J, Brekke OL, Bergseth G, Lambris JD, Mollnes TE. Differential effect of heparin coating and complement inhibition on artificial surface-induced eicosanoid production. Ann. Thorac. Surg. 2005;79:917–923. doi: 10.1016/j.athoracsur.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 35.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, et al. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tai HC, Ezzelarab M, Hara H, Ayares D, Cooper DK. Progress in xenotransplantation following the introduction of gene-knockout technology. Transpl. Int. 2007;20:107–117. doi: 10.1111/j.1432-2277.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 37.Cowan PJ. Coagulation and the xenograft endothelium. Xenotransplantation. 2007;14:7–12. doi: 10.1111/j.1399-3089.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 38.Tseng YL, Kuwaki K, Dor FJ, Shimizu A, Houser S, Hisashi Y, Yamada K, Robson SC, Awwad M, Schuurman HJ, et al. α1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 39.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegard KT, Kohl J, Lambris JD. Essential role of the C5a receptor in E. coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- 41.Banz Y, Cung T, Korchagina EY, Bovin NV, Haeberli A, Rieben R. Endothelial cell protection and complement inhibition in xenotransplantation: a novel in vitro model using whole blood. Xenotransplantation. 2005;12:434–443. doi: 10.1111/j.1399-3089.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 42.Baumann BC, Forte P, Hawley RJ, Rieben R, Schneider MK, Seebach JD. Lack of galactose-α-1,3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J. Immunol. 2004;172:6460–6467. doi: 10.4049/jimmunol.172.10.6460. [DOI] [PubMed] [Google Scholar]
- 43.Robinson LA, Tu L, Steeber DA, Preis O, Platt JL, Tedder TF. The role of adhesion molecules in human leukocyte attachment to porcine vascular endothelium: implications for xenotransplantation. J. Immunol. 1998;161:6931–6938. [PubMed] [Google Scholar]
- 44.Schneider MK, Strasser M, Gilli UO, Kocher M, Moser R, Seebach JD. Rolling adhesion of human NK cells to porcine endothelial cells mainly relies on CD49d-CD106 interactions. Transplantation. 2002;73:789–796. doi: 10.1097/00007890-200203150-00023. [DOI] [PubMed] [Google Scholar]
- 45.Baumann BC, Forte P, Hawley RJ, Rieben R, Schneider MK, Seebach JD. Lack of galactose-α-1,3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J. Immunol. 2004;172:6460–6467. doi: 10.4049/jimmunol.172.10.6460. [DOI] [PubMed] [Google Scholar]
- 46.Al-Mohanna F, Saleh S, Parhar RS, Khabar K, Collison K. Human neutrophil gene expression profiling following xenogeneic encounter with porcine aortic endothelial cells: the occult role of neutrophils in xenograft rejection revealed. J. Leukocyte Biol. 2005;78:51–61. doi: 10.1189/jlb.0904494. [DOI] [PubMed] [Google Scholar]
- 47.Goodman DJ, Von AM, Willson A, Millan MT, Bach FH. Direct activation of porcine endothelial cells by human natural killer cells. Transplantation. 1996;61:763–771. doi: 10.1097/00007890-199603150-00016. [DOI] [PubMed] [Google Scholar]
- 48.Gilli UO, Schneider MK, Loetscher P, Seebach JD. Human polymorphonuclear neutrophils are recruited by porcine chemokines acting on CXC chemokine receptor 2, and platelet-activating factor. Transplantation. 2005;79:1324–1331. doi: 10.1097/01.tp.0000155429.44902.44. [DOI] [PubMed] [Google Scholar]
- 49.Deng MC, Plenz G, Labarrere C, Marboe C, Baba HA, Erre M, Itescu S. The role of IL6 cytokines in acute cardiac allograft rejection. Transpl. Immunol. 2002;9:115–120. doi: 10.1016/s0966-3274(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 50.Matsumiya G, Gundry SR, Nehlsen-Cannarella S, Fagoaga OR, Morimoto T, Arai S, Fukushima N, Zuppan CW, Bailey LL. Serum interleukin-6 level after cardiac xenotransplantation in primates. Transplant. Proc. 1997;29:916–919. doi: 10.1016/s0041-1345(96)00718-x. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, DeVries ME, Deng S, Khandaker MH, Pickering JG, Chow LH, Garcia B, Kelvin DJ, Zhong R. The axis of interleukin 12 and γ interferon regulates acute vascular xenogeneic rejection. Nat. Med. 2000;6:549–555. doi: 10.1038/75029. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka K, Yamagami S, Streilein JW. Evidence that T-helper type 2 cell-derived cytokines and eosinophils contribute to acute rejection of ortho-topic corneal xenografts in mice. Transplantation. 2005;79:1317–1323. doi: 10.1097/01.tp.0000158714.09346.a6. [DOI] [PubMed] [Google Scholar]
- 53.Holan V, Pindjakova J, Zajicova A, Krulova M, Zelezna B, Matousek P, Svoboda P. The activity of inducible nitric oxide synthase in rejected skin xenografts is selectively inhibited by a factor produced by grafted cells. Xenotransplantation. 2005;12:227–234. doi: 10.1111/j.1399-3089.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang XG, Lu Y, Wang B, Li H, Yu L, Liu C, Wu Z, Liu XM. Cytokine production during the inhibition of acute vascular rejection in a concordant hamster-to-rat cardiac xenotransplantation model. Chin. Med. J. 2007;120:145–149. [PubMed] [Google Scholar]
- 55.Lorant T, Krook H, Wilton J, Olausson M, Tufveson G, Korsgren O, Johnsson C. Intragraft cytokine mRNA expression in rejecting and non-rejecting vascularized xenografts. Xenotransplantation. 2003;10:311–324. doi: 10.1034/j.1399-3089.2003.02032.x. [DOI] [PubMed] [Google Scholar]
- 56.Saadi S, Takahashi T, Holzknecht RA, Platt JL. Pathways to acute humoral rejection. Am. J. Pathol. 2004;164:1073–1080. doi: 10.1016/S0002-9440(10)63194-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solomon MF, Kuziel WA, Mann DA, Simeonovic CJ. The role of chemokines and their receptors in the rejection of pig islet tissue xenografts. Xenotransplantation. 2003;10:164–177. doi: 10.1034/j.1399-3089.2003.01146.x. [DOI] [PubMed] [Google Scholar]
- 58.Lee EM, Park JO, Kim D, Kim JY, Oh KH, Park CG, Oh BH, Kim S, Ahn C. Early up-regulation of CXC-chemokine expression is associated with strong cellular immune responses to murine skin xenografts. Xenotransplantation. 2006;13:328–336. doi: 10.1111/j.1399-3089.2006.00311.x. [DOI] [PubMed] [Google Scholar]
- 59.Kim JY, Kim D, Lee EM, Choi I, Park CG, Kim KS, Ha J, Kim SJ, Yang J, Kim YS, et al. Inducible nitric oxide synthase inhibitors prolonged the survival of skin xenografts through selective down-regulation of proinflammatory cytokine and CC-chemokine expressions. Transpl. Immunol. 2003;12:63–72. doi: 10.1016/S0966-3274(03)00013-3. [DOI] [PubMed] [Google Scholar]
- 60.Nykanen AI, Krebs R, Tikkanen JM, Raisky O, Sihvola R, Wood J, Koskinen PK, Lemstrom KB. Combined vascular endothelial growth factor and platelet-derived growth factor inhibition in rat cardiac allo-grafts: beneficial effects on inflammation and smooth muscle cell proliferation. Transplantation. 2005;79:182–189. doi: 10.1097/01.tp.0000147199.60464.f9. [DOI] [PubMed] [Google Scholar]
- 61.Tambur AR, Pamboukian S, Costanzo MR, Heroux A. Genetic polymorphism in platelet-derived growth factor and vascular endothelial growth factor are significantly associated with cardiac allograft vasculopathy. J. Heart Lung Transplant. 2006;25:690–698. doi: 10.1016/j.healun.2006.02.006. [DOI] [PubMed] [Google Scholar]