Abstract
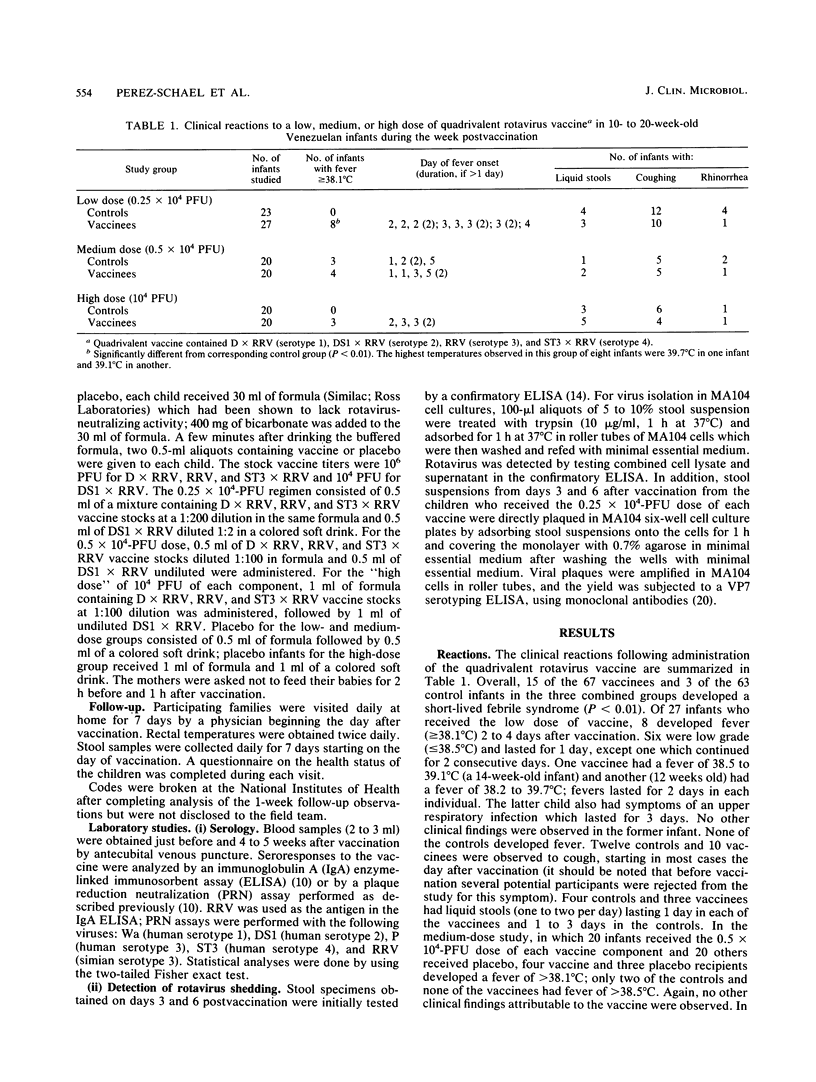
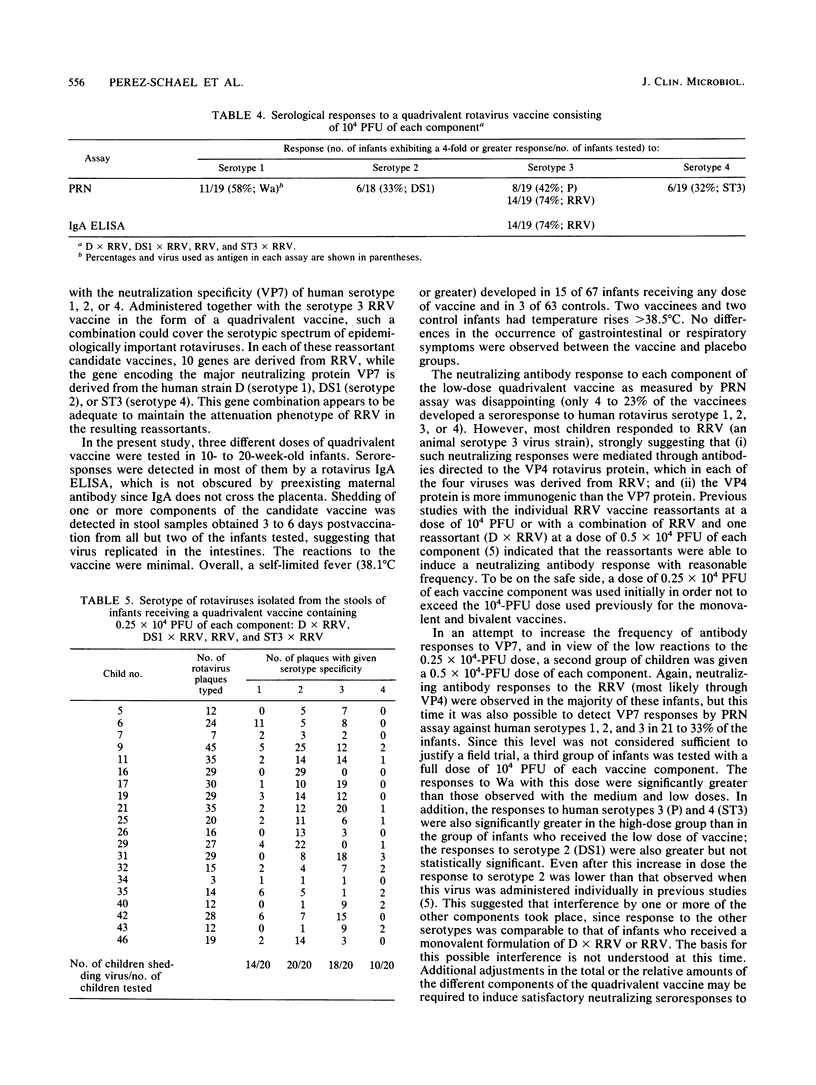
Phase I studies of an oral quadrivalent rotavirus vaccine were conducted in 130 Venezuelan infants 10 to 20 weeks of age. The vaccine consists of a mixture of equal amounts of rhesus rotavirus (RRV) vaccine (serotype 3 [VP7]) and each of three human rotavirus-RRV reassortant strains: D x RRV (serotype 1 [VP7]), DS1 x RRV (serotype 2 [VP7]), and ST3 x RRV (serotype 4 [VP7]). Three different doses of the quadrivalent vaccine (0.25 x 10(4), 0.5 x 10(4), and 10(4) PFU of each component) were evaluated sequentially for safety and antigenicity in placebo-controlled, double-blind trials. Starting the day after vaccination, the infants were monitored by daily home visits for 7 days. Only minor reactions were observed during this period; these were limited to mild transient febrile episodes which began day 2 or 3 after vaccination and lasted 1 to 2 days in 15 to 30% of the infants. Serological studies demonstrated that 68 to 96% of the infants developed a rotavirus serum immunoglobulin A response following vaccination. However, when tested by plaque reduction neutralization assay against individual human rotavirus serotype 1, 2, 3, or 4, the response rates ranged from 4 to 23% with the low dose, 21 to 33% with the medium dose, and 32 to 58% with the high dose. Most (73 to 79%) infants developed neutralizing antibodies to RRV following administration of each dose schedule. Vaccine virus shedding was analyzed by utilizing tissue culture isolation of virus from stool. All of the infants who received the lower of medium dose and 89% of those fed the high dose shed one or more components of the vaccine. Analyses of rotavirus serotypes isolated from the stool of infants who received the 0.25 x 10(4) -PFU dose revealed that DS1 x RRV was the most commonly shed vaccine component, followed by RRV, D x RRV, and ST3 x RRV in that order.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christy C., Madore H. P., Pichichero M. E., Gala C., Pincus P., Vosefski D., Hoshino Y., Kapikian A., Dolin R. Field trial of rhesus rotavirus vaccine in infants. Pediatr Infect Dis J. 1988 Sep;7(9):645–650. doi: 10.1097/00006454-198809000-00009. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Borian F. E., Bell L. M., Modesto K., Gouvea V., Plotkin S. A. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988 Sep;158(3):570–587. doi: 10.1093/infdis/158.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mol P., Zissis G., Butzler J. P., Mutwewingabo A., André F. E. Failure of live, attenuated oral rotavirus vaccine. Lancet. 1986 Jul 12;2(8498):108–108. doi: 10.1016/s0140-6736(86)91643-0. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Blanco M., Vilar M., Garcia D., Perez M., Daoud N., Midthun K., Kapikian A. Z. Reactions to and antigenicity of two human-rhesus rotavirus reassortant vaccine candidates of serotypes 1 and 2 in Venezuelan infants. J Clin Microbiol. 1989 Mar;27(3):512–518. doi: 10.1128/jcm.27.3.512-518.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Gonzalez M., Garcia D., Perez M., Daoud N., Cunto W., Chanock R. M., Kapikian A. Z. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet. 1987 Apr 18;1(8538):882–884. doi: 10.1016/s0140-6736(87)92858-3. [DOI] [PubMed] [Google Scholar]
- Halsey N., Galazka A. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bull World Health Organ. 1985;63(6):1151–1169. [PMC free article] [PubMed] [Google Scholar]
- Hanlon P., Hanlon L., Marsh V., Byass P., Shenton F., Hassan-King M., Jobe O., Sillah H., Hayes R., M'Boge B. H. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987 Jun 13;1(8546):1342–1345. doi: 10.1016/s0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Hoshino Y., Glass R. I., Midthun K., Gorziglia M., Chanock R. M. Rotavirus: the major etiologic agent of severe infantile diarrhea may be controllable by a "Jennerian" approach to vaccination. J Infect Dis. 1986 May;153(5):815–822. doi: 10.1093/infdis/153.5.815. [DOI] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Lim Y., Krall G., Kapikian A. Z., Levine M. M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988 Jun;7(6):388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Hoshino Y., Kapikian A. Z., Chanock R. M. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J Clin Microbiol. 1986 Nov;24(5):822–826. doi: 10.1128/jcm.24.5.822-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Schael I., Gonzalez M., Daoud N., Perez M., Soto I., Garcia D., Daoud G., Kapikian A. Z., Flores J. Reactogenicity and antigenicity of the rhesus rotavirus vaccine in Venezuelan children. J Infect Dis. 1987 Feb;155(2):334–338. doi: 10.1093/infdis/155.2.334. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., d'Hondt E., André F. E., Beards G. M., Flewett T. H. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985 Aug;107(2):189–194. doi: 10.1016/s0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- de Zoysa I., Feachem R. G. Interventions for the control of diarrhoeal diseases among young children: rotavirus and cholera immunization. Bull World Health Organ. 1985;63(3):569–583. [PMC free article] [PubMed] [Google Scholar]


