Abstract
Background
Studies on the association between smoking and dementia are necessarily on those who have “survived” smoking. We examined the association between smoking and cognition in middle-age and estimate the risk of death and non-participation among smokers.
Methods
Data come from the Whitehall II study of 10308 participants, aged 35–55 years at baseline (phase 1; 1985–1988). Smoking history was assessed at Phases 1 and 5 (1997–1999). Cognitive data (memory, reasoning (AH4-I), vocabulary, semantic and phonemic fluency) were available on 5,346 participants at Phase 5, 4,630 of these were retested 5-years later.
Results
Smokers at Phase 1 were at higher risk of death (Hazard Ratio (HR), 2.00 (95% Confidence Interval (CI), 1.58–2.52) in men, and HR, 2.46 (1.80–3.37) in women) and non-participation in cognitive tests (Odds Ratio (OR), 1.32 (1.16–1.51) in men, OR, 1.69 (1.41– 2.02) in women). At Phase 5, in age- and sex-adjusted analyses, smokers compared to “never smokers” were more likely to be in the lowest quintile of cognitive performance. After adjustment for multiple covariates, this risk remained for memory (OR, 1.37 (1.10–1.73)). Ex-smokers at Phase 1 had 30% lower risk of poor vocabulary and verbal fluency. In longitudinal analysis, the evidence for an association between smoking history and cognitive decline was not consistent. Finally, stopping smoking during the follow-up was associated with improvement in other health behaviours.
Conclusions
Smoking was associated with greater risk of poor memory. The loss to follow-up was significant among smokers. Ex-smokers had lower risk of poor cognition, possibly due to improvement in other health behaviours.
Keywords: Adult, Chi-Square Distribution, Cognition Disorders, diagnosis, epidemiology, etiology, Female, Health Behavior, Humans, Logistic Models, London, epidemiology, Male, Middle Aged, Proportional Hazards Models, Questionnaires, Risk Factors, Smoking, adverse effects, epidemiology
The association between smoking and dementia has been much discussed in recent years1–3 with a recent meta-analysis concluding that smoking is a risk factor for dementia4. This association is thought to be primarily through the effect of smoking on vascular disease2, 4. Examining this effect in the elderly is problematic due to loss during follow-up, misdiagnosis of dementia, and smoking related premature mortality before the onset of dementia2, 3. In order to avoid some of these problems, one approach entails exploring the association between smoking and cognition before the onset of dementia. There is increasing evidence to suggest the importance of midlife risk factors for later dementia5. Furthermore, the link between cognitive impairment and later life dementia 6–8 is clearly established. Thus, it is important to examine if the risk of cognitive impairment in smokers is also present in midlife 9–15; evidence of this association at younger ages would support the hypothesis that smoking is involved in the pathogenesis of preclinical cognitive deficit and decline.
We aim to investigate the association between history of tobacco consumption (smoking status, pack-years of smoking) and multiple domains of cognition in middle-aged individuals. We examine associations with cognitive performance and change in cognitive function over a five-year period in analysis adjusted for the effects of socioeconomic status, health behaviours and a range of health indicators. A further objective was to assess the extent to which even middle-aged smokers are lost to follow-up, either through death or through non-participation.
METHODS
Data are drawn from the Whitehall II study, established in 1985 to examine the socioeconomic gradient in health and disease among 10,308 civil servants (6,895 men and 3,413 women)16. All civil servants aged 35–55 years in 20 London based departments were invited to participate by letter, and 73 percent agreed. Baseline examination (Phase 1) took place during 1985–1988, and involved a clinical examination and a self-administered questionnaire containing sections on demographic characteristics, health, lifestyle factors as smoking habits, work characteristics, social support and life events. Clinical examination included measures of blood pressure, anthropometry, biochemical measurements, neuroendocrine function, and subclinical markers of cardiovascular disease. Subsequent phases of data collection have alternated between postal questionnaire alone (Phases 2 (1988–1990), 4 (1995–1996), 6 (2001) and 8 (2006)) and postal questionnaire accompanied by a clinical examination (Phases 3 (1991–1994), 5 (1997–1999) and 7 (2002–2004)). Participants gave written consent to participate in the study and the University College London ethics committee approved the study.
Smoking History
Data on smoking were collected at every phase using questions on smoking status (current, past, never), age at which the participant started smoking, average number of cigarettes per day, number of cigars/cigarillos smoked, ounces of tobacco smoked in a pipe or in hand-rolled cigarettes per week (see figure 1). Ex-smokers were asked the age at which they had stopped smoking. The smoking history variable was created with the following categories: “current smoker at Phase 5”, “recent ex-smoker” (stopped smoking between Phases 1 and 5), “long-term ex-smoker” (those who stopped before Phase 1) and “never smoker”. Among “smokers” at Phase 5, we further used the amount of tobacco smoked, in total grams of tobacco per day (one cigarette=1 g, one cigar/cigarillos=3 g)17, to calculate pack-years of smoking (average daily number of grams of tobacco divided by 20 and multiplied by the number of years of smoking).
Figure 1.
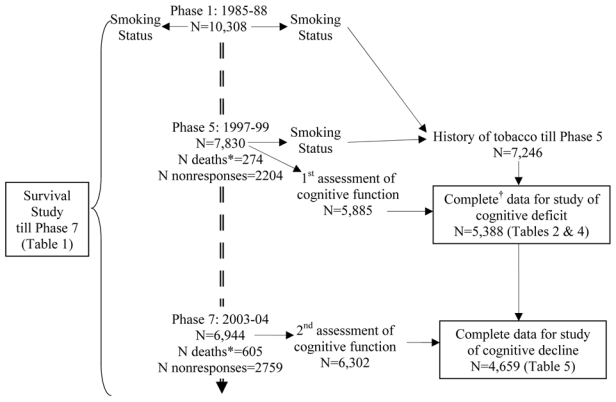
Flow chart, (1985–2004).
* Number of deaths since Phase 1
† N with missing sociodemographic variables =154, N with missing behavioral variables =236, N with missing health variables =156
Cognition
Cognitive function was assessed at Phases 5 and 7 using a battery of five standard tasks, described below.
Short-term verbal memory was assessed with a 20-word free recall test. Participants were presented a list of 20 one or two syllable words at two second intervals and were then asked to recall in writing as many of the words in any order and had two minutes to do so.
The AH4-I (Alice Heim 4-I) is composed of a series of 65 verbal and mathematical reasoning items of increasing difficulty18. It tests inductive reasoning, measuring the ability to identify patterns and infer principles and rules. Participants had 10 minutes to do this section.
Vocabulary was assessed using the Mill Hill Vocabulary test 19, used in its multiple format, consisting of a list of 33 stimulus words ordered by increasing difficulty and six response choices.
We used two measures of verbal fluency: phonemic and semantic20. Phonemic fluency was assessed via “S” words and semantic fluency via “animal” words. Subjects were asked to recall in writing as many words beginning with “s” and as many animal names as they could. One minute was allowed for each test.
Covariates
Socio-demographic variables used were age, sex, socioeconomic position (using the British civil service grade of employment - high (administrative), intermediate (professional or executive) and low (clerical or support) grades), education (no or lower primary school, lower secondary school, higher secondary school, university, and higher university degree) and marital status (married/cohabiting, single, widowed, and divorced/separated).
Health behaviours were as follows. Alcohol consumption, assessed via questions on the number of alcoholic drinks (“measures” of spirits, “glasses” of wine, and “pints” of beer) consumed in the last seven days, converted to number of units of alcohol. Frequency of fruit and vegetable consumption, assessed using the question “How often do you eat fresh fruit or vegetables?”; responses were on an eight-point scale, ranging from ‘seldom or never’ to ‘two or more times a day’. Physical activity, calculated as the sum of the hours of mild, moderate, and vigorous physical activities in response to a 20-item questionnaire on the frequency and duration of participation in walking, cycling, sports, gardening, housework, and home maintenance21.
Health measures were drawn from Phase 5. Coronary heart disease prevalence was based on clinically verified events and included myocardial infarction and definite angina22. Stroke and diabetes were assessed using self-reports of doctor diagnosis. Blood pressure, systolic and diastolic, was measured at the Phase 5 clinical examination, twice in the sitting position after five minutes rest with an automated Omron 907 devise. The average of two measures was taken to be the measured blood pressure. Serum cholesterol was measured within 72 h in serum stored at 4°C using enzymatic colorimetric methods.
Statistical methods
The association between smoking status at Phase 1 (never smoker, ex-smoker and current smoker) and mortality till Phase 7 was assessed using Cox regression and that with non-participation in cognitive tests at Phase 7 using logistic regression.
Descriptive analyses as a function of smoking history at Phase 5 were carried out and tested using chi-square analysis for trend for categorical variables and by fitting a linear trend for continuous variables. We first assessed the association between smoking history and continuous measures of cognition using linear mixed-effects models to account for unequal time interval between the two clinical examinations, between 3.9 and 7.1 years. The independent variables were smoking history, time, interaction term between smoking history and time since first cognitive assessment, and other covariates. The dependent variables were the cognitive measures. Next, we examined the association between smoking history and the dichotomised measures of cognition in logistic regression, where the reference group was the “never smoker” category. Cognitive scores in the lowest sex-specific quintile were seen to represent cognitive deficit at Phase 5 and those in the worst sex-specific quintile of change to represent decline. The time interval between the two measures of cognition has been adjusted for in the analyses of change using logistic regression. The analyses were adjusted first for age and sex, then for socio-demographic measures (education and age as continuous, all others as categorical), and finally for health behaviours (all continuous) and health measures (all vascular risk factors as continuous).
Other ways of looking at smoking history (age of starting smoking, time since stopping smoking, etc.) were also examined in exploratory analysis but are not presented here, except for analysis using “pack-years” of smoking for current smokers, as the results are not strikingly different. In addition, we undertook post-hoc analysis to examine changes in health behaviours (consumption of alcohol and of fruits and vegetable) between Phases 1 and 7 in the four smoking history categories. All analyses were performed using SAS statistical software, version 8.
RESULTS
Sample description and missing data
Of the 10,308 participants at Phase 1 (1985–1988), 7,830 participated in at least one part of Phase 5 (1997–1999), 2,204 were non responders and 274 dead (figure 1). At Phase 5, data on cognitive function, smoking history and all covariates were available for 5,388 respondents. Compared to baseline, this group was younger (55.5 years versus 56.1 years) and composed of fewer women (27.6% versus 33.1%) and fewer low socioeconomic position participants (14.6% versus 22.7%) (p < 0.001). From this population, calculation of cognitive decline, implying participation in cognitive tests at Phase 7, was possible for 4,659 participants (figure 1). Here again, missing data were similarly influenced by age, gender and socioeconomic position compared to data available for analysis on cognitive deficit (N=5,388).
In order to assess whether the smoking-cognition association is underestimated due to premature mortality among smokers, we examined the association between smoking status at Phase 1 and mortality during the 17.1 (standard deviation=2.3) years of follow-up till Phase 7 (table 1). “Current smokers” at Phase 1 had a higher risk of dying during follow-up compared to “never smokers” after adjustment for age, socioeconomic position and marital status among men (Hazard Ratio (HR), 2.00 (95% confidence interval (CI), 1.58–2.52)) and women (HR, 2.46 (1.80–3.37)). Ex-smokers at Phase 1 did not have a higher risk of death during the period of follow-up examined (HR, 1.09 (0.84–1.41) among men, HR, 1.23 (0.84–1.79) among women). Among survivors at Phase 7 (N=9,625), we examined the association between smoking status at Phase 1 and non-participation in the cognitive tests at Phase 7. In analyses adjusted for age, socioeconomic position and marital status, “current smokers” at Phase 1 were more likely to be non-participants among men (Odds Ratio (OR), 1.32 (1.16–1.51)) and women (OR, 1.69 (1.41–2.02)). In order to examine the persistence of this association, we repeated the analysis with smoking history at Phase 5 and participation in cognitive tests at Phase 7 (N=7,221). Greater numbers of both male (OR, 1.47 (1.20–1.81)) and female smokers (OR, 1.81 (1.35–2.43)) did not undertake the cognitive tests. “Long-term ex-smokers” and “recent ex-smokers” at Phase 5 were not different from “never smokers”.
Table 1.
Association between smoking and mortality and non-participation (2002–2004).
| Smoking status at Phase 1 (1985–1988) | Sex | Never smoker | Ex-smoker | Current smoker | |
|---|---|---|---|---|---|
| Association with mortality till Phase 7 † | M | 1 | 1.09 (0.84–1.41) | 2.00 (1.58–2.52)* | |
| F | 1 | 1.23 (0.84–1.79) | 2.46 (1.80–3.37)* | ||
| Association with non participation at Phase 7 cognitive tests‡ | M | 1 | 1.06 (0.93–1.21) | 1.32 (1.16–1.51)* | |
| F | 1 | 1.08 (0.90–1.29) | 1.69 (1.41–2.02)* | ||
| Smoking status at Phase 5 (1997–1999) | Never smoker | Long-term ex-smoker | Recent ex-smoker | Current smoker | |
| Association with non participation at Phase 7 cognitive tests§ | M | 1 | 0.95 (0.79–1.15) | 1.04 (0.80–1.35) | 1.47 (1.20–1.81)* |
| F | 1 | 0.96 (0.74–1.25) | 1.23 (0.83–1.82) | 1.81 (1.35–2.43)* | |
p<0.05
N= 6,841 men, N=3,371 women.
N= 6,449 men, N=3,176 women, excluding participants lost to follow-up at Phase 7 due to death.
N= 5,064 men, N=2,157 women, excluding participants lost to follow-up at Phase 7 due to death.
Characteristics of individuals included in the analyses on smoking and cognitive deficit at Phase 5 are shown in table 2. The test for trend shows that smoking status was associated with socioeconomic position, education, alcohol and fruit and vegetable consumption (p < 0.0001). Prevalence of CHD, stroke and diabetes was not associated with smoking history. Among the vascular risk factors, smoking history was associated only with cholesterol (p < 0.0001). Cognitive scores at Phase 5 as a function of health measures are presented in Table 3.
Table 2.
Characteristics of the study population at Phase 5 (1997–1999).*
| Never smoker | Long-term ex-smoker | Recent ex-smoker | Current smoker | P trend | |
|---|---|---|---|---|---|
| N (N, %) | 2,543 (47.2) | 1,519 (28.2) | 511 (9.5) | 815 (15.1) | |
| Age (M, SD) | 55.2 (6.0) | 56.1 (6.0) | 56.2 (6.0) | 55.0 (5.7) | 0.24 |
| Women (N, %) | 826 (32.5) | 387 (25.5) | 97 (18.2) | 177 (21.7) | <.0001 |
| High SEP (N, %) | 932 (36.7) | 525 (34.6) | 180 (35.2) | 231 (28.3) | <.0001 |
| University degree or higher (N, %) | 901 (35.4) | 434 (28.6) | 132 (25.8) | 171 (21.0) | <.0001 |
| Married/cohabiting (N, %) | 1,908 (75.0) | 1,217 (80.1) | 395 (77.3) | 600 (73.6) | 0.87 |
| Alcohol units/week (M, SD) | 10.5 (11.9) | 15.3 (14.5) | 17.1 (15.8) | 20.6 (22.1) | <.0001 |
| Hours of physical activity/week (M, SD) | 21.9 (15.1) | 22.7 (15.0) | 23.1 (16.0) | 21.5 (15.8) | 0.98 |
| Consumption of fruits & vegetable† (N, %) | 1,966 (77.3) | 1,152 (75.8) | 373 (73.0) | 509 (62.5) | <.0001 |
| CHD (N, %) | 143 (5.6) | 87 (5.7) | 48 (9.4) | 48 (5.9) | 0.18 |
| Stroke (N, %) | 18 (0.7) | 13 (0.9) | 3 (0.6) | 8 (1.0) | 0.55 |
| Diabetes (N, %) | 60 (2.4) | 41 (2.7) | 14 (2.7) | 12 (1.5) | 0.30 |
| SBP (mmHg) (M, SD) | 121.9 (16.4) | 123.6 (16.6) | 124.1 (16.9) | 122.0 (15.6) | 0.24 |
| DBP (mmHg) (M, SD) | 77.2 (10.6) | 77.8 (10.3) | 78.4 (11.2) | 76.8 (10.0) | 0.91 |
| Cholesterol (mmol/l) (M, SD) | 5.8 (1.0) | 6.0 (1.0) | 5.9 (1.0) | 6.0 (1.1) | <.0001 |
Analysis restricted to those with complete data (N=3,901 men, 1,487 women).
Denotes at least daily consumption of fruits and vegetables.
M: Mean, SD: Standard deviation, CHD Coronary heart disease, SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure.
Table 3.
Cognitive function (mean (standard deviation)) as a function of health measures at Phase 5.*
| Memory | AH4-I | Mill Hill | Phonemic fluency | Semantic fluency | |
|---|---|---|---|---|---|
| Range | 0–20 | 0–65 | 0–33 | 0–35 | 0–36 |
| CHD | |||||
| No | 7.0 (2.4) | 47.3 (10.7) | 25.2 (4.3) | 17.0 (4.4) | 16.6 (4.1) |
| Yes | 6.4 (2.3) | 44.4 (12.0) | 24.4 (4.9) | 16.1 (4.4) | 15.4 (4.3) |
| Stroke | |||||
| No | 6.9 (2.4) | 47.1 (10.7) | 25.2 (4.3) | 17.0 (4.4) | 16.5 (4.2) |
| Yes | 6.6 (2.6) | 43.8 (10.7) | 25.1 (3.8) | 14.5 (3.3) | 14.9 (3.5) |
| Diabetes | |||||
| No | 6.9 (2.4) | 47.3 (10.7) | 25.2 (4.3) | 17.0 (4.4) | 16.5 (4.1) |
| Yes | 6.4 (2.3) | 41.3 (13.1) | 23.1 (5.6) | 15.6 (4.6) | 15.0 (4.3) |
| SBP (mmHg) | |||||
| <140 | 7.0 (2.4) | 47.5 (10.5) | 25.2 (4.2) | 17.1 (4.4) | 16.6 (4.1) |
| ≥140 | 6.6 (2.3) | 44.8 (11.9) | 24.7 (4.8) | 16.1 (4.3) | 15.9 (4.2) |
| DBP (mmHg) | |||||
| <90 | 6.9 (2.4) | 47.1 (10.7) | 25.2 (4.3) | 17.0 (4.4) | 16.5 (4.2) |
| ≥90 | 6.7 (2.3) | 46.9 (11.4) | 25.1 (4.5) | 16.7 (4.2) | 16.4 (4.1) |
| Cholesterol (mmol/l) | |||||
| <4.92 (<240 mg/dL) | 7.0 (2.3) | 47.4 (10.8) | 25.3 (4.5) | 17.1 (4.5) | 16.8 (4.2) |
| ≥4.92 (≥240 mg/dL) | 6.9 (2.4) | 47.1 (10.8) | 25.1 (4.3) | 16.9 (4.4) | 16.5 (4.2) |
Analysis restricted to those with complete data (N=3,901 men, 1,487 women).
CHD Coronary heart disease, SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure.
Smoking history and cognitive function at Phase 5
The fully-adjusted mixed-effects model showed that smoking history was associated with memory (p=0.01), reasoning (p=0.0004), vocabulary (p<0.0001), phonemic (p<0.0001), and semantic fluency (p=0.0009). Table 4 presents results of the logistic regression using binary cognitive outcomes; the sex-specific cut-offs used are also shown. In age- and sex-adjusted models, “current smokers” were more likely to have cognitive deficits on all tests: memory (OR, 1.54 (1.25–1.90)), AH4-I (OR, 1.53 (1.27–1.85)), Mill Hill (OR, 1.42 (1.18–1.70)), phonemic (OR, 1.32 (1.09–1.60)) and semantic fluency (OR, 1.30 (1.08–1.57)). In fully adjusted models, the association remained for memory (OR, 1.37 (1.10–1.73)). Compared to “never smokers”, the “long-term ex-smokers” were less likely to have deficits in memory (OR, 0.79 (0.65–0.96)), the Mill Hill (OR, 0.73 (0.60–0.87)), phonemic (OR, 0.73 (0.61–0.87)) and semantic fluency (OR, 0.75 (0.63–0.89)) in fully adjusted models. “Recent ex-smokers” also had a reduced risk of poor vocabulary score (OR, 0.65 (0.49–0.85)) and semantic fluency (OR, 0.72 (0.55–0.94)).
Table 4.
Odds ratio of being in the worst quintile of cognitive function at Phase 5 as a function of smoking status (1997–1999), N=5,388.†
| Never smoker N=2,543 | Long-term ex-smoker N=1,519 | Recent ex-smoker N=511 | Current smoker N=815 | |
|---|---|---|---|---|
| Memory (<5) | ||||
| Adjusted for age & sex | 1 | 0.80 (0.66–0.97)* | 1.17 (0.90–1.51) | 1.54 (1.25–1.90)* |
| + sociodemograhics‡ | 1 | 0.77 (0.63–0.93)* | 1.10 (0.85–1.44) | 1.33 (1.07–1.65)* |
| + behaviours§ & health# | 1 | 0.79 (0.65–0.96)* | 1.12 (0.86–1.47) | 1.37 (1.10–1.73)* |
| AH4-I (<42 in men; <31 in women) | ||||
| Adjusted for age & sex | 1 | 0.96 (0.82–1.14) | 0.94 (0.74–1.20) | 1.53 (1.27–1.85)* |
| + sociodemograhics‡ | 1 | 0.87 (0.73–1.05) | 0.81 (0.61–1.06) | 1.11 (0.90–1.37) |
| + behaviours§ & health# | 1 | 0.91 (0.76–1.10) | 0.83 (0.63–1.10) | 1.20 (0.96–1.49) |
| Mill Hill (<24 in men; <20 in women) | ||||
| Adjusted for age & sex | 1 | 0.87 (0.74–1.02) | 0.84 (0.66–1.08) | 1.42 (1.18–1.70)* |
| + sociodemograhics‡ | 1 | 0.72 (0.60–0.86)* | 0.67 (0.51–0.88)* | 0.97 (0.79–1.19) |
| + behaviours§ & health# | 1 | 0.73 (0.60–0.87)* | 0.65 (0.49–0.85)* | 0.92 (0.74–1.15) |
| Phonemic fluency (<14 in men; <13 in women) | ||||
| Adjusted for age & sex | 1 | 0.76 (0.64–0.90)* | 1.00 (0.79–1.27) | 1.32 (1.09–1.60)* |
| + sociodemograhics‡ | 1 | 0.70 (0.59–0.84)* | 0.91 (0.71–1.17) | 1.04 (0.85–1.28) |
| + behaviours§ & health# | 1 | 0.73 (0.61–0.87)* | 0.95 (0.74–1.22) | 1.10 (0.89–1.35) |
| Semantic fluency (<14 in men; <13 in women) | ||||
| Adjusted for age & sex | 1 | 0.80 (0.65–1.05) | 0.82 (0.65–1.05) | 1.30 (1.08–1.57)* |
| + sociodemograhics‡ | 1 | 0.73 (0.61–0.87)* | 0.72 (0.56–0.93)* | 0.97 (0.80–1.19) |
| + behaviours§ & health# | 1 | 0.75 (0.63–0.89)* | 0.72 (0.55–0.94)* | 0.98 (0.79–1.21) |
Analysis restricted to those with complete data.
p<0.05
Socio-demographic variables: socioeconomic position, education and marital status.
Health behaviours: alcohol, fruit and vegetable consumption and hours of physical activity at Phase 5.
Health variables: prevalence of CHD, stroke and diabetes at Phase 5 and systolic blood pressure, diastolic blood pressure and cholesterol measurements at Phase 5.
Among current smokers at phase 5, in fully adjusted models, there was no evidence of a dose-response association between pack-years of smoking and cognitive deficit (memory, p=0.97; AH4-I, p=0.13; Mill Hill, p=0.33; phonemic, p=0.25; semantic fluency, p=0.97).
Smoking history and cognitive decline between Phases 5 & 7
The interaction term between smoking history and time in the fully-adjusted mixed-effects model showed that smoking history was associated with cognitive decline in reasoning (p=0.0004), but not with memory (p=0.64), vocabulary (p=0.68), phonemic (p=0.63), and semantic fluency (p=0.61); detailed results shown in the Appendix. Further analysis on decline (table 5) uses the worst quintile of change, implying decrease greater than 1 point for memory and the Mill Hill, 7 points for the AH4-I and 3 points for the fluency measures. In fully adjusted models, both “current smokers” (OR, 1.40 (1.11–1.75)) and “recent ex-smokers” (OR, 1.38 (1.07–1.77)) were more likely to decline on the AH4-I. No other association was evident. Further adjustment for health behaviours at Phase 7 did not much change these results.
Table 5.
Odds ratio of being in the worst quintile of change in cognitive function between Phase 5 (1997–1999) and Phase 7 (2002–2004), N=4,659.
| Never smoker N=2,218 | Long-term ex-smoker N=1,338 | Recent ex-smoker N=443 | Current smoker N=660 | |
|---|---|---|---|---|
| Memory (<−1) | ||||
| Adjusted for age & sex† | 1 | 0.91 (0.78–1.06) | 0.95 (0.75–1.21) | 1.01 (0.83–1.23) |
| + sociodemograhics‡ | 1 | 0.91 (0.78–1.07) | 0.95 (0.75–1.21) | 1.01 (0.83–1.23) |
| + behaviours§ & health# | 1 | 0.91 (0.77–1.06) | 0.96 (0.75–1.22) | 0.99 (0.80–1.22) |
| AH4-I (<−7) | ||||
| Adjusted for age & sex† | 1 | 0.98 (0.82–1.18) | 1.41 (1.10–1.82)* | 1.46 (1.18–1.81)* |
| + sociodemograhics‡ | 1 | 0.97 (0.81–1.16) | 1.40 (1.09–1.80)* | 1.45 (1.09–1.80)* |
| + behaviours§ & health# | 1 | 0.96 (0.80–1.16) | 1.38 (1.07–1.77)* | 1.40 (1.11–1.75)* |
| Mill Hill (<−1) | ||||
| Adjusted for age & sex† | 1 | 1.04 (0.88–1.23) | 1.01 (0.78–1.30) | 1.01 (0.81–1.25) |
| + sociodemograhics‡ | 1 | 1.00 (0.85–1.19) | 0.97 (0.75–1.25) | 0.93 (0.75–1.25) |
| + behaviours§ & health# | 1 | 1.01 (0.85–1.20) | 0.97 (0.75–1.26) | 0.95 (0.75–1.19) |
| Phonemic fluency (<−3) | ||||
| Adjusted for age & sex† | 1 | 1.00 (0.85–1.19) | 1.01 (0.79–1.30) | 0.97 (0.79–1.21) |
| + sociodemograhics‡ | 1 | 1.02 (0.86–1.20) | 1.03 (0.80–1.32) | 1.00 (0.81–1.24) |
| + behaviours§ & health# | 1 | 1.00 (0.84–1.18) | 1.01 (0.78–1.30) | 0.97 (0.78–1.21) |
| Semantic fluency (<−3) | ||||
| Adjusted for age & sex† | 1 | 1.05 (0.88–1.25) | 0.94 (0.72–1.24) | 1.08 (0.86–1.35) |
| + sociodemograhics‡ | 1 | 1.03 (0.86–1.23) | 0.94 (0.72–1.24) | 1.09 (0.87–1.37) |
| + behaviours§ & health# | 1 | 1.02 (0.85–1.23) | 0.94 (0.71–1.24) | 1.09 (0.86–1.38) |
p<0.05
Analysis restricted to those with complete data and adjusted for time interval between Phases 5 & 7.
Socio-demographic variables: socioeconomic position, education and marital status.
Health behaviours: alcohol, fruit and vegetable consumption and hours of physical activity at Phase 5.
Health variables: prevalence of CHD, stroke and diabetes at Phase 5 and systolic blood pressure, diastolic blood pressure and cholesterol measurements at Phase 5.
Among current smokers at Phase 5, in fully adjusted models, there was no dose-response association between pack-years of smoking and cognitive decline (memory, p=0.22; AH4-I, p=0.88; Mill Hill, p=0.54; phonemic, p=0.30; semantic fluency, p=0.94).
Post-hoc analysis
This analysis was aimed at the exploration of changes in other health behaviours along with change in smoking status (giving up smoking) over the follow-up period. Those who stopped smoking between Phases 1 and 5 (“recent ex-smokers”) had the smallest increase in consumption of alcohol between Phases 1 and 7 (0.82 g of alcohol per week) compared to the other groups (1.46 g among “never smokers”). In terms of healthy eating, the percentage of participants consuming at least one fruit or vegetable per day increased more among “recent ex-smokers” than among “never smokers”. Figure 2 shows that “recent ex-smokers” were at the same level of fruit and vegetable consumption as “current smokers” at Phase 1 but by Phase 7 they had reached the same level as “long-term ex-smokers” and “never smokers”.
Figure 2.
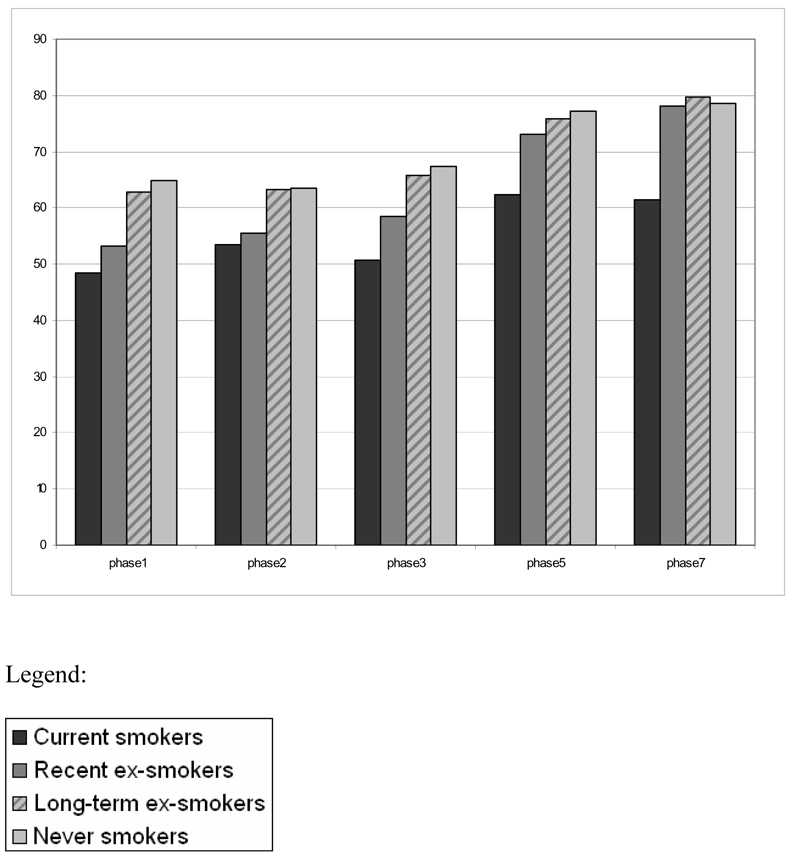
Participants (%) consuming at least one fruit or vegetable per day as a function of smoking history at Phase 5.
COMMENT
This study presents four key findings. First, smoking in middle is associated with memory deficit and decline in reasoning abilities. Second, “long-term ex-smokers” are less likely to have cognitive deficits in memory, vocabulary and in verbal fluency. Third, giving up smoking in midlife is accompanied by improvement in other health behaviours. Finally, our results based on a large prospective cohort study of middle-aged British civil servants suggest that the association between smoking and cognition, even in late midlife, could be underestimated due to higher risk of death and non-participation among smokers.
Public health messages on smoking over the past twenty years have led to changes in smoking behaviour23–25. Thus, estimation of the association between smoking and any health outcome needs to assess smoking behaviour over time, explore whether change in smoking status is also accompanied by other changes, and to examine possible underestimation of the association due to premature mortality or greater loss to follow-up among smokers. Our analyses show all three aspects outlined above to be important. Exploration of the association between smoking and dementia in the elderly is complicated by the fact that it can only be among those who have “survived” long enough to become demented2, 3. The alternative is to examine cognitive deficit and decline at earlier ages. Cognition in midlife is clinically relevant as research suggests that individuals with mild cognitive impairment progress to clinically diagnosed dementia at an accelerated rate6–8.
Comparison with others studies
Studies using global cognitive tests (the Mini Mental State Examination, etc.) have found smoking to be associated with cognitive impairment26–29 and decline30. Smokers have also been reported to have poorer vocabulary31, psychomotor speed11, visuospatial performance12, 32, memory12, 31, 32 and reasoning27. Our results suggest poorer performance on memory and reasoning. Thus, the current evidence does not allow conclusions to be drawn about the association between smoking and specific cognitive domains.
Few studies9–15 have examined the association between smoking and cognition in a middle-aged population and only two of them12, 15 reported analysis on cognitive decline in this age group. Smoking was found to be associated with decline in memory in one study12 but no association was found in the other15. Our results suggest a greater risk of deficit but not of decline in memory among smokers. A recent study suggests that the effect of smoking on decline in memory is confined to those over 75 years of age32. Future studies need to replicate these analyses in order to estimate the age at which smoking related decline in memory become apparent. Our results also show a decline in reasoning abilities among both recent ex-smokers and current smokers.
One could expect survival bias due to premature death of smokers to be limited among middle-aged individuals. Few studies33, 34 have measured this bias or the bias introduced by greater loss to follow-up amongst smokers. In our study, smoking was associated with loss to follow-up, both through death and non-participation. “Current smokers” at Phase 1 were twice as likely to die during the follow-up and those who were current smokers at Phase 1 or at Phase 5 were less likely to participate in the cognitive tests. These effects, either due to death or non-participation, were not evident among ex-smokers and their results on the association between smoking and cognition are likely not to be biased. Thus, the risk of cognitive deficit and decline among “current smokers” in our analyses may have been under-estimated. It is possible that those who are missing, either due to death or non-participation, had higher risk of cognitive deficit35.
Previous results on the association between smoking and cognition in ex-smokers are mixed. In the EURODEM study, ex and never smokers did not differ on cognitive impairment30. Others have found the risk of cognitive impairment to be lower among ex-smokers compared to never smokers, even though the differences were not significant26, 28. Apart from a few studies12, 28, 29, most have looked at ex-smoking status without distinguishing between “long-term” and “recent” ex-smokers11, 26, 27, 30, 31. In the 1946 British Birth Cohort study12, ‘long-term ex-smokers” had better memory and a slower decline in memory compared to “never smokers”. In the Honolulu-Asia Aging Study28, “long-term ex-smokers” did not have a lower risk of cognitive impairment than “never smokers” and “recent ex-smokers” had the same increased risk of impairment as “current smokers”. Our results show that “long-term ex-smokers” were consistently less likely to have cognitive deficits for Mill Hill and verbal fluency. Future studies need to separate out the long-term ex-smokers from the recent ex-smokers.
The association between smoking and cognition could be explained by the fact that smoking is a risk factor of atherosclerotic disease36, which is itself related to higher risk of cognitive deficit37, 38. However, we did not find a dose-response association between pack-years of smoking and cognitive deficit/decline. Some studies have also reported the lack of a dose-response association26–28 while others have found this effect to be inconsistent12, 30. It is possible that the loss of the heavy smokers, through death and non-participation, biases the results using pack-years of smoking. In relation to results among ex-smokers, it has been suggested that some of the differences in cognitive performance between groups defined by their smoking habit may be the consequence of self-selection out of the smoking groups. Thus, smokers with higher cognitive function scores would be more likely to quit and become ex-smokers27. This hypothesis is plausible. However, a competing hypothesis is that those who stop smoking also change other health behaviours and possibly other aspects of their life as well. In our population, those who stopped smoking in the ten years preceding cognitive testing improved their other health behaviours (consumption of alcohol, fruits and vegetables) considerably when compared to others.
Strengths
This study has several strengths. The detailed prospective assessment allowed a precise lifelong smoking history to be established and several confounders and explanatory variables were included in the analysis. We were able to examine changes in health behaviours longitudinally. Furthermore, the design of the Whitehall II study allowed us to get a handle on the underestimation of the association between smoking and cognition by evaluating the extent of missing data, related to death during follow-up and to non-participation.
Limits
First, although the sample covered a wide socioeconomic range, the data are from white-collar civil servants and cannot be assumed to represent general populations. Second, smoking habits were self-reported and may have been under-reported. Third, the requirement to write down answers for tests of verbal fluency may have led to a restriction in response range. Finally, it is important to note that change between two time points is not enough to examine intra-individual change and analyses on further waves of data are necessary.
In conclusion, our results show an association between smoking and risk of memory deficit and reasoning decline. These results may have been under-estimated because of premature death and lower participation among smokers. Stopping smoking in middle-age was associated with improvement in other health behaviours and little residual adverse effect of smoking on cognition. Public health messages on smoking should continue to target smokers at all ages.
Acknowledgments
AS-M is supported by a “Chaire d’excellence” award from the French Ministry of Research and a “European Young Investigator Award” from the European Science Foundation. MM is supported by an MRC research professorship. The Whitehall II study has been supported by grants from the British Medical Research Council (MRC); the British Heart Foundation; the British Health and Safety Executive; the British Department of Health; the National Heart, Lung, and Blood Institute (grant HL36310); the National Institute on Aging (grant AG13196); the Agency for Health Care Policy and Research (grant S06516); and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socioeconomic Status and Health.
Footnotes
Conflict of interest: none
Reference List
- 1.Almeida OP, Hulse GK, Lawrence D, Flicker L. Smoking as a risk factor for Alzheimer’s disease: contrasting evidence from a systematic review of case-control and cohort studies. Addiction. 2002 Jan;971:15–28. doi: 10.1046/j.1360-0443.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- 2.Brayne C. Smoking and the brain. BMJ. 2000 Apr 22;3207242:1087–8. doi: 10.1136/bmj.320.7242.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kukull WA. The association between smoking and Alzheimer’s disease: effects of study design and bias. Biol Psychiatry. 2001 Feb 1;493:194–9. doi: 10.1016/s0006-3223(00)01077-5. [DOI] [PubMed] [Google Scholar]
- 4.Anstey KJ, von SC, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007 Aug 15;1664:367–78. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 5.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006 Sep;59:735–41. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 6.Chertkow H. Mild cognitive impairment. Curr Opin Neurol. 2002 Aug;154:401–7. doi: 10.1097/00019052-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001 Dec;5812:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001 Mar;583:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 9.Cerhan JR, Folsom AR, Mortimer JA, et al. Correlates of cognitive function in middle-aged adults. Atherosclerosis Risk in Communities ARIC. Study Investigators. Gerontology. 1998;442:95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005 Jan 1;571:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am J Epidemiol. 2002 Nov 15;15610:936–44. doi: 10.1093/aje/kwf135. [DOI] [PubMed] [Google Scholar]
- 12.Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am J Public Health. 2003 Jun;936:994–8. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starr JM, Deary IJ, Fox HC, Whalley LJ. Smoking and cognitive change from age 11 to 66 years: a confirmatory investigation. Addict Behav. 2007 Jan;321:63–8. doi: 10.1016/j.addbeh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Whalley LJ, Fox HC, Deary IJ, Starr JM. Childhood IQ, smoking, and cognitive change from age 11 to 64 years. Addict Behav. 2005 Jan;301:77–88. doi: 10.1016/j.addbeh.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001 Jan 9;561:42–8. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 16.Marmot MG, Smith GD, Stansfeld S, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991 Jun 8;3378754:1387–93. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 17.Bernaards CM, Twisk JW, Snel J, Van MW, Kemper HC. Is calculating pack-years retrospectively a valid method to estimate life-time tobacco smoking? A comparison between prospectively calculated pack-years and retrospectively calculated pack-years. Addiction. 2001 Nov;9611:1653–61. doi: 10.1046/j.1360-0443.2001.9611165311.x. [DOI] [PubMed] [Google Scholar]
- 18.Heim AW. AH 4 group test of general Intelligence. Windsor, UK: NFER-Nelson Publishing Company Ltd; 1970. [Google Scholar]
- 19.Raven JC. Guide to using the Mill Hill vocabulary test with progressive matrices. London, UK: HK Lewis; 1965. [Google Scholar]
- 20.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologica. 1967;5:135–40. [Google Scholar]
- 21.Singh-Manoux A, Hillsdon M, Brunner E, Marmot M. Effects of physical activity on cognitive functioning in middle age: evidence from the Whitehall II prospective cohort study. Am J Public Health. 2005 Dec;9512:2252–8. doi: 10.2105/AJPH.2004.055574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrie JE, Langenberg C, Shipley MJ, Marmot MG. Birth weight, components of height and coronary heart disease: evidence from the Whitehall II study. Int J Epidemiol. 2006 Dec;356:1532–42. doi: 10.1093/ije/dyl184. [DOI] [PubMed] [Google Scholar]
- 23.Lee DJ, Fleming LE, Arheart KL, et al. Smoking Rate Trends in U.S. Occupational Groups: The 1987 to 2004 National Health Interview Survey. J Occup Environ Med. 2007 Jan;491:75–81. doi: 10.1097/JOM.0b013e31802ec68c. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor RJ, Giovino GA, Kozlowski LT, et al. Changes in nicotine intake and cigarette use over time in two nationally representative cross-sectional samples of smokers. Am J Epidemiol. 2006 Oct 15;1648:750–9. doi: 10.1093/aje/kwj263. [DOI] [PubMed] [Google Scholar]
- 25.Cummings KM. Programs and policies to discourage the use of tobacco products. Oncogene. 2002 Oct 21;2148:7349–64. doi: 10.1038/sj.onc.1205810. [DOI] [PubMed] [Google Scholar]
- 26.Cervilla JA, Prince M, Mann A. Smoking, drinking, and incident cognitive impairment: a cohort community based study included in the Gospel Oak project. J Neurol Neurosurg Psychiatry. 2000 May;685:622–6. doi: 10.1136/jnnp.68.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elwood PC, Gallacher JE, Hopkinson CA, et al. Smoking, drinking, and other life style factors and cognitive function in men in the Caerphilly cohort. J Epidemiol Community Health. 1999 Jan;531:9–14. doi: 10.1136/jech.53.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galanis DJ, Petrovitch H, Launer LJ, Harris TB, Foley DJ, White LR. Smoking history in middle age and subsequent cognitive performance in elderly Japanese-American men. The Honolulu-Asia Aging Study. Am J Epidemiol. 1997 Mar 15;1456:507–15. doi: 10.1093/oxfordjournals.aje.a009138. [DOI] [PubMed] [Google Scholar]
- 29.Launer LJ, Feskens EJ, Kalmijn S, Kromhout D. Smoking, drinking, and thinking. The Zutphen Elderly Study. Am J Epidemiol. 1996 Feb 1;1433:219–27. doi: 10.1093/oxfordjournals.aje.a008732. [DOI] [PubMed] [Google Scholar]
- 30.Ott A, Andersen K, Dewey ME, et al. Effect of smoking on global cognitive function in nondemented elderly. Neurology. 2004 Mar 23;626:920–4. doi: 10.1212/01.wnl.0000115110.35610.80. [DOI] [PubMed] [Google Scholar]
- 31.Stewart MC, Deary IJ, Fowkes FG, Price JF. Relationship between lifetime smoking, smoking status at older age and human cognitive function. Neuroepidemiology. 2006;262:83–92. doi: 10.1159/000090253. [DOI] [PubMed] [Google Scholar]
- 32.Reitz C, Luchsinger J, Tang MX, Mayeux R. Effect of smoking and time on cognitive function in the elderly without dementia. Neurology. 2005 Sep 27;656:870–5. doi: 10.1212/01.wnl.0000176057.22827.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edelstein SL, Kritz-Silverstein D, Barrett-Connor E. Prospective association of smoking and alcohol use with cognitive function in an elderly cohort. J Womens Health. 1998 Dec;710:1271–81. doi: 10.1089/jwh.1998.7.1271. [DOI] [PubMed] [Google Scholar]
- 34.Wang HX, Fratiglioni L, Frisoni GB, Viitanen M, Winblad B. Smoking and the occurrence of Alzheimer’s disease: cross-sectional and longitudinal data in a population-based study. Am J Epidemiol. 1999 Apr 1;1497:640–4. doi: 10.1093/oxfordjournals.aje.a009864. [DOI] [PubMed] [Google Scholar]
- 35.Tyas SL, Salazar JC, Snowdon DA, et al. Transitions to mild cognitive impairments, dementia, and death: findings from the nun study. Am J Epidemiol. 2007 Jun 1;16511:1231–8. doi: 10.1093/aje/kwm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolego C, Poli A, Paoletti R. Smoking and gender. Cardiovasc Res. 2002 Feb 15;533:568–76. doi: 10.1016/s0008-6363(01)00520-x. [DOI] [PubMed] [Google Scholar]
- 37.Singh-Manoux A, Britton AR, Marmot M. Vascular disease and cognitive function: evidence from the Whitehall II Study. J Am Geriatr Soc. 2003 Oct;5110:1445–50. doi: 10.1046/j.1532-5415.2003.51464.x. [DOI] [PubMed] [Google Scholar]
- 38.Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994 Feb;572:202–7. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]


