Abstract
Adolescence is the transition period that prepares individuals for fulfilling their role as adults. Most conspicuous in this transition period is the peak level of risk-taking behaviors that characterize adolescent motivated behavior. Significant neural remodeling contributes to this change. This review focuses on the functional neuroanatomy underlying motivated behavior, and how ontogenic changes can explain the typical behavioral patterns in adolescence. To help model these changes and provide testable hypotheses, a neural systems-based theory is presented. In short, the Triadic Model proposes that motivated behavior is governed by a carefully orchestrated articulation among three systems, approach, avoidance and regulatory. These three systems map to distinct, but overlapping, neural circuits, whose representatives are the striatum, the amygdala and the medial prefrontal cortex. Each of these system-representatives will be described from a functional anatomy perspective that includes a review of their connectivity and what is known of their ontogenic changes.
INTRODUCTION TO ADOLESCENCE
Adolescence is the transition period during which individuals acquire and refine cognitive, emotional, and social skills in preparation for their ultimate role as responsible, independent and sexually mature adults. This period is marked by typical physical and behavioral changes. These changes result from an interplay of biological and environmental factors. The biological factors arise from the genetic, hormonal and neural domains. These factors promote changes that occur along a set timeline determined by a “developmental clock”. A number of processes contribute interactively to this developmental clock, including the potential role of clock genes (James et al., 2007; Perrin et al., 2006; Plant and Barker-Gibb, 2004) and the cumulative effects of hormonal and neural maturational cascades that generate sharp irreversible new states.
Puberty is one of these new states. Some confusion between the boundaries of adolescence and puberty often occurs. Puberty is not synonymous with adolescence. However, it is one of the most important and conspicuous set of processes taking place during this period. Puberty is most often defined from an endocrine perspective as the activation of the hypothalamic-pituitary-gonadal axis and its consequences on physical growth and sexual maturation (Dorn et al., 2006; Sisk and Foster, 2004).
The fairly stereotypical pattern of behavioral changes that is observed during adolescence includes increased risk-taking and alteration of the social landscape. It can also be understood from an evolutionary perspective (Steinberg and Belsky, 1996). Accordingly, these behaviors are essential to facilitate the separation of the adolescents from their families. This move away from the familiar and towards novelty provides genetic diversity and avoids the deleterious effects of genetic inbreeding. As such, adolescent behavioral changes permit to fulfill the ultimate goal of species survival and reproduction. Unfortunately, these behaviors come with a cost, manifest as unmatched rates of morbidity (i.e., psychiatric disorders) and mortality, mainly from accidents and suicides (Arnett, 1992). Thus, understanding the mechanisms underlying these critical behavioral and emotional changes may provide approaches for interventions that could reduce cost without altering benefit.
This review focuses on the functional anatomy and ontogeny of key neural systems involved in the control of behavior. These key neural systems are integrated within a theoretical three-system framework termed the Triadic Model (Ernst et al., 2006). The Triadic Model has been proposed as a neurobiological framework for examining the neural mechanisms underlying motivated behavior and, most relevant here, the behavioral changes occurring during adolescence. To place this model in the context of explanatory models of the neural underpinning of behavior, a brief survey of highly prominent work in this field is presented first.
NEURAL SYSTEMS MODELS
A number of greatly influential theories of prefrontal cortical function have been proposed in an effort to understand the mechanisms underlying complex behavior (to cite a few, Dehaene and Changeux, 1997; Duncan, 2001; Fuster, 1997; Goldman-Rakic, 1998; Grafman, 2002; Miller and Cohen, 2001; Rolls, 1996; Shallice and Burgess, 1996; Sigman and Dehaene, 2008). For example, Baddeley (2000) proposed a model of working memory that recognized two main functional components ‘holding stimuli online’ and ‘manipulating memory content’. These components have been linked to the ventrolateral PFC and the dorsolateral PFC, respectively (Bor et al., 2003; Owen et al., 1996). Shallice and Norman (Shallice, 1982) formulated the role of PFC as a ‘supervisory attentional system’, based on the observation that deficits on routine or automatic tasks can be dissociated from deficits on non-routine, internally generated, tasks in patients with distinct PFC lesions. This hiearchical organization has been challenged by proposals such as recurrent connections (Botvinick and Plaut, 2004) or simple cognitive branching determined by the level of reward associated with actions (Koechlin and Hyafil, 2007). Whereas these PFC models relate to cognitive strategy and planning, others have stressed the interplay of emotional and cognitive processes on behavior.
The latter formulations typically have included cortical (particularly the ventral/orbital PFC) and subcortical regions (particularly the amygdala) to account for responses to environmental stimuli (e.g., Damasio, 1994). These theories also arose largely from studies of patients with brain lesions affecting the ventral prefrontal region (see review, Bechara and Van Der, 2005) and the amygdala (Adolphs et al., 1995; Bechara et al., 1999; Damasio et al., 1985; Lee et al., 1988; Lee et al., 1995; Tranel and Hyman, 1990). Among them, the somatic marker hypothesis, developed by Damasio and colleagues (Damasio, 1994), has been prominent. This theory, inspired by the James-Lange hypothesis of the origin and nature of emotions (James, 1884; Lange, 1887), was built upon observations of impaired decision-making in patients with ventromedial PFC lesions. The tenet of this work holds that emotion-related bodily signals (somatic markers) modulate cognitive processes engaged in decision-making.
Other constructs emerged from neuroanatomical studies. These theories stressed that the processing of incoming information follows circuitries that link distinct PFC sectors to functionally related regions of the striatum in a cascading series of serial as well as parallel loops (Alexander et al., 1986; Haber et al., 2000). The involvement of cortico-striatal loops highlights dopaminergic mechanisms in the control of complex behavior, which have fueled an enormous body of research (Grace et al., 2007; Jentsch et al., 2000; Robbins and Everitt, 1992; Schultz, 1997).
Finally, models encompassing all three PFC, amygdala and striatum functional nodes have raised interest for a long time (Alheid and Heimer, 1988; Cardinal et al., 2002; Chambers et al., 2003; Grace et al. 2007), although the complexity of systematically evaluating the functional integration of three distinct neural systems represents a huge undertaken, which is reflected by the related limited literature. In summary, each of these single-, dual- or tri-system models provides a unique framework within which fundamental questions can be empirically addressed. The Triadic Model was originally developed to address specifically the neural mechanisms underlying the typical behavioral developmental changes of adolescence. Its development was greatly influenced by Chambers et al. (2003) who proposed neural mechanisms that promote the enhanced vulnerability to addiction in adolescence.
THE TRIADIC MODEL
Prior to describing the model, the term motivated behavior needs clarification. Indeed, a variety of terms are used in the literature to refer to motivated behavior. Such terms include ‘goal-directed behavior’, ‘conscious behavior’, or ‘decision-making’, to cite only a few. To avoid confusion, we will use selectively the term ‘motivated behavior’, which we define operationally as actions taken in response to stimuli to achieve a goal. Schematically, two sets of behaviors can be generated in response to stimuli, approach or avoidance (including no action, or withdrawal).
The Triadic Model provides a simple dynamic scheme of brain function that underlies motivated behavior (Figure 1). It is based on the premises that responses to stimuli represent the output of the functional integration of three distinct, although overlapping, systems. One system exerts a preferential role in approach behavior and relies on striatal circuits. The second system has a dominant role in avoidance behavior and relies on amygdala circuits. The third system exerts a regulatory control over both approach and avoidance responses and relies on the medial prefrontal cortex. Although these systems are heterogeneous with respect to both anatomy and function (Table 1), ample evidence indicates a dominant role for each of them along the lines proposed by the Triadic Model (see review, Ernst et al. 2006).
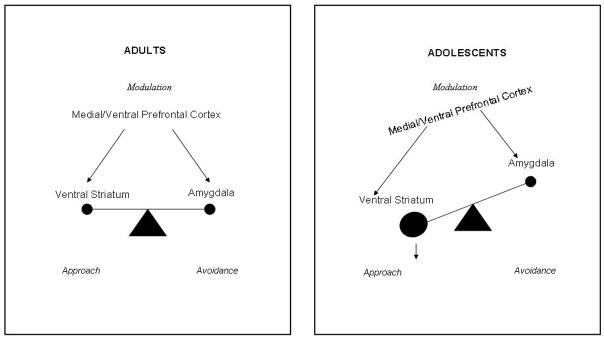
Figure 1.
Triadic Model. This model is a heuristic representation of a neural systems approach to the neurobiology of motivated behavior. Generically, behavior results from the integration of signals underlying the coding of approach, the coding of avoidance and the reciprocal modulation of both latter signals. Taken as the gold standard, the adult pattern is shown as a balanced system. Compared to this balanced system, the pattern expected in adolescents is tilted towards approach behavior. In this model, the representatives of the three systems include amygdala for avoidance, striatum for approach and medial prefrontal cortex for modulation. Arrows represent relative medial prefrontal cortical control over striatum and amygdala rather than the array of anatomic connections.
Table 1.
Anatomical and functional heterogeneity of the triadic nodes
| ANATOMY | ||
|---|---|---|
| AMYGDALA | STRIATUM | MEDIAL PFC |
| BLNG | Caudate Nucleus | Frontal Pole (area 10) |
| Central nucleus | Putamen | Medial orbital (areas 13a, b) |
| Medial nucleus | Nucleus Accumbens | Anterior Cingulate (areas 25, 24) |
| FUNCTION | ||
| AMYGDALA | STRIATUM | MEDIAL PFC |
| Attention Orienting | Motor Responses | Self-Assessment |
| Conditioned fear response | Habits | Conflict Monitoring |
| Affective intensity | Motivation | Action Planning |
| Salience detector | Incentive learning | Conditioning |
| Reward Processing | Reward Processing | Affective Value |
| DOMINANT ROLE | ||
| AMYGDALA | STRIATUM | MEDIAL PFC |
| Avoidance | Approach | Modulation |
This scheme integrates four sources of data, (1) behavioral deficits observed in human lesion studies (e.g., Bechara and Van Der, 2005; Fellows and Farah, 2005; Seitz et al., 2006); (2) findings from functional neuroimaging of the developing brain (Ernst and Hardin, 2008), (3) cognitive neuroscience theories of motivated behavior (functional specialization of brain systems) (see review, Ernst and Paulus, 2005), and (4) a dual-system theory of behavior applied to temperament and personality research (Gray, 1972; Pickering and Gray, 2001).
First, lesion studies in humans provide invaluable information on the role of brain regions in behavior. They are particularly useful for identifying “essential” or “dominant” functions of these structures. However, a number of caveats limit the generalizability of the findings. Among them is the difficulty of assessing accurately the anatomical boundaries of the lesions. As a consequence, the over- or under-inclusion of anatomical regions involved in these lesions can provide inaccurate links between brain structures and clinical pictures. Another caveat is the absence of pre-lesion neuropsychological assessment limits the ability to definitively attribute deficits to the lesions. This issue is often compounded by limited sample sizes. Finally, the nature of the pathology leading to the brain lesions may itself be responsible for some aspects of the behavioral deficits.
With regard to the triadic nodes, certain pathologies affecting the amygdala are very specific (e.g., Urbache-Weithe disease) and provide important information on the function of this structure (see below). In contrast, lesions of the striatum are usually large, often secondary to anoxic events (Caine and Watson, 2000), and may not be quite specific to the function of these structures. Similarly, prefrontal cortical lesions are variable and specific regions are more difficult to assess with precision, although meta-analyses of lesion studies are beginning to provide reliable findings (e.g., Seitz et al., 2006). The most consistent reports suggest that amygdala lesions are associated with deficits in response to negative stimuli (Adolphs et al., 1994; Berntson et al., 2007) and striatal regions are associated with amotivation and reduction of motor initiation (e.g., Adam et al., 2008). Prefrontal cortical regions highly depend highly on the regions being affected. The ventromedial PFC has been consistently associated with deficits in inhibition and decision-making (see review, Bechara and Van Der, 2005). In contrast, the medial prefrontal cortex has been found to contribute to self-reflection, regarding action more dorsally, and emotion more ventrally (Seitz et al. 2006). These findings of human lesion studies seem to support the general lines of the Triadic Model.
Second, neuroimaging findings suggest functional differences between adolescents and adults in response to reward-related stimuli. Some of these studies reflect enhanced responsivity of the striatal system to appetitive stimuli, reduced involvement of the amygdala in response to negative stimuli, and reduced contribution of prefrontal cortex in choice selection in adolescents compared to adults, although these findings are not all consistent (Ernst and Hardin, 2008).
Third, cognitive neuroscience has provided a systematic approach to the study of complex processes such as decision-making or motivated behaviors, based on the decomposition of these processes into smaller elements more amenable to scientific scrutiny. This approach is typically used to develop paradigms and to provide a strategy for the neuroimaging analyses of these paradigms.
Fourth, the dual-system theory is based on a reduction of behavior patterns into two separate response modes, approach, and avoidance, that are subserved by two separate neurobehavioral systems (Gray, 1972). This framework is used extensively in research on temperament and personality (Pickering and Gray, 2001). In general, the neuroimaging literature in adults tend to support the notion of separate, yet overlapping, systems coding for approach and avoidance. In addition, an overall supervisory system (the prefrontal cortex) has been described as a regulator of behavior.
Neuroimaging studies in adults consistently report recruitment of striatal regions during reward-related processes (Breiter et al., 2001; Delgado et al., 2000; Ernst et al., 2005; Knutson et al., 2001; Knutson et al., 2003; O’Doherty et al., 2004; Rogers et al., 2004). Conversely, amygdala, hippocampus, and insula are most commonly recruited in response to unfavorable or aversive outcomes (Becerra et al., 2001; LeDoux, 2000; Tom et al., 2007; Zald and Pardo, 1997). Recruitment of the striatum in conjunction with negative outcomes (Becerra et al. 2001; Jensen et al., 2003; Seymour et al., 2007), and amygdala in conjunction with positive outcome is also reported, however less frequently (Becerra et al. 2001; Jensen et al. 2003; Seymour et al. 2007; Zalla et al., 2000). Medial prefrontal structures are recognized for their role in modulating affective and cognitive processes.
Regional functional specialization is also emerging from the extant literature. For example, orbital and medial prefrontal regions have been implicated in the representations of affective values (O’Doherty et al., 2003; O’Doherty, 2007; Rolls, 2004), inhibition (Elliott and Deakin, 2005; Yucel et al., 2007), response reversal (Schoenbaum et al., 2007), and conflict resolution (Yeung et al., 2004). The anterior medial PFC has been associated with metacognition (Fletcher et al., 1995; Gallagher et al., 2000), self-evaluation (Amodio and Frith, 2006; Kelley et al., 2002; O’Doherty et al. 2003) and rule formation (Bunge et al., 2005). These high-level cognitive functions integrate information about endogenous (physical and emotional state) and exogenous environment to modulate behavioral output. Some of this information is provided by loops through the amygdala and striatum.
The main point of this discussion is three-fold: (1) the processing of outcome is occurring throughout the triadic system, engaging amygdala, striatum, and prefrontal cortex. (2) However, each node is engaged distinctly, and the relative contribution of these regions depends on a number of factors, one of them being outcome valence, i.e., how attractive or aversive the outcome is. Other factors include experimental conditions, such as the stage of decision-making under study (selection, vs. anticipation, vs. outcome), the modulation of probability or timing of outcomes, or the use of contingencies to the delivery of rewards, such as performing well on a cognitive task. Because of the influence of experimental conditions on regional activation, discrepant results among studies can clearly be an effect of the different paradigms being used. (3) Finally, the most consistent finding across studies is a bias of the amygdala (and associated circuitry) to be recruited in the presence of negative stimuli, and of the striatum (and associated circuitry) to be recruited in the presence of positive stimuli. This valence-related bias emerges against a role of these structures in the coding of both positive and negative affective polarities.
These three nodes of behavioral control, centered on the striatum, amygdala, and prefrontal cortex, constitute the backbone of the Triadic Model. The functional connections among these three centers and their development are beginning to be mapped out, as discussed in the following section.
ANATOMY, CONNECTIVITY, ONTOGENY OF THE TRIADIC NODES
Amygdala
Anatomy and connectivity
The amygdala is a complex structure composed of highly interconnected nuclei that resides in the anterior temporal lobe (Price and Russchen, 1987) (Figure 2). These nuclei can be functionally distinguished based on gene expression patterns, which correlate with unique embryologic origins (Zirlinger et al., 2001). They also have unique sets of interconnections with one another (Amaral and Insausti, 1992; Pitkanen et al., 1997). We describe the general organization of the amygdala subregions and highlight how specific subregions may influence specific behaviors. However, this is not an exhaustive review of amygdaloid circuitry.
Figure 2.
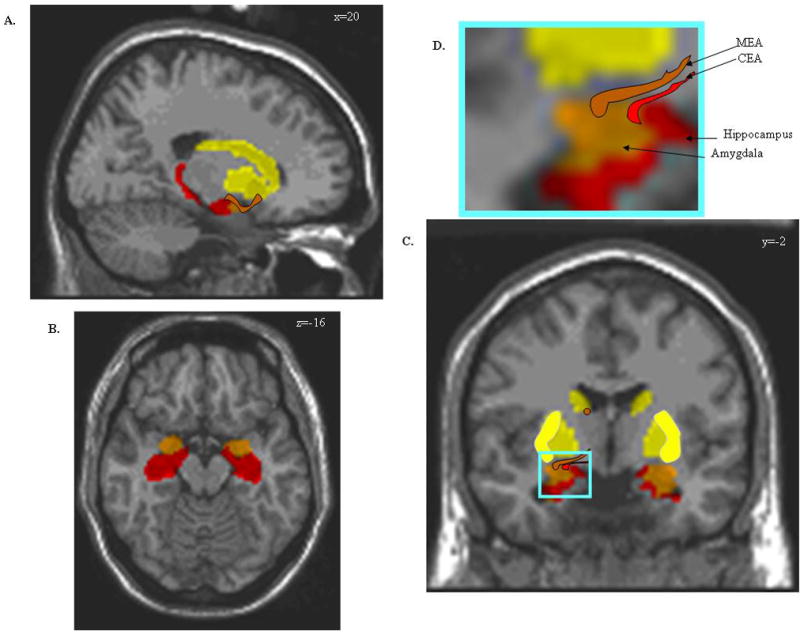
A–D. Rendering of the approximate boundaries for the amygdala (orange), hippocampus (red) and striatopallidal structures (yellow) on a 3-D structural MRI. The boundaries of amygdala and hippocampal subregions cannot be reliably resolved; therefore the images are drawn based on the location of these structures in human postmortem sections. The planes intersect at the following Talairach coordinates x=20mm, y=−16mm and z=−2mm.
On the sagittal view (A.), the location of the extended amygdala is represented as an orange strip bridging the dorsal amygdala and bed nucleus of the stria terminalis. Both the medial or central extended amygdala follow this rostrocaudal trajectory. The axial view (B.) is from a plane that passes right below the striatum and the bed nucleus of the amygdala, and reveals the amygdala (orange) positioned anteriorly to the hippocampus (red). Both structures form the medial wall of the temporal horn of the lateral ventricle.
The coronal view (C.) delineates the putamen (bright yellow). The medial (red with black outline) and central extended amygdala (orange with black outline) are illustrated on the left hemisphere. The superior part of the bed nucleus of the stria terminalis is also being represented at the inferior part of the caudate nucleus (orange circle).
In D., this area (boxed D.) is enlarged to provide a better vision of the relative location of these structures.
MEA = Medial Extended Amygdala; CEA = Central Extended Amygdala.
The ‘cortical-like’, or ‘deep’, nuclei of the amygdala form the greater part of this structure. They include three subunits, the lateral, basal, and accessory basal nuclei, and are often collectively referred to as the ‘basolateral nuclear group (BLNG)’.
These large nuclei resemble the cortex, without the apparent layering: they contain glutamatergic projection neurons in the form of pyramidal cells, are interconnected with the cortex, and project to the same striatal sectors as their reciprocal cortical targets (Alheid and Heimer, 1988; Van Hoesen et al., 1981). These nuclei are the main ‘receiving’ subunits, onto which converge inputs from sensory association cortex, prefrontal cortex, and hippocampus (Carmichael and Price, 1996; Ghashghaei and Barbas, 2002; Rosene and Van Hoesen, 1977; Saunders et al., 1988; Stefanacci and Amaral, 2000; Turner et al., 1980).
The BLNG receives information about characteristics of the environment, including sensory and incentive values of stimuli as well as affective context. However, within the BLNG, different types of information flow along distinct paths. Sensory association cortices project to the lateral nucleus along a general topography determined by sensory modality (Pitkanen and Amaral, 1998; Stefanacci and Amaral, 2000; Turner et al. 1980). The lateral nucleus, in turn, projects to the basal and accessory basal nuclei. In primates, the basal and accessory basal nuclei also receive the majority of inputs from the hippocampus and orbital and medial prefrontal cortex (OMPFC) (Carmichael and Price, 1996; Ghashghaei and Barbas, 2002; Saunders et al. 1988), areas that mediate information about the emotional context (via hippocampus) and incentive value (via OMPFC) of a stimulus.
The BLNG has a complex relationship with one of the main output regions of the amygdala, the central nucleus. The neuronal output of the BLNG is modulated by small GABAergic cells, known as the intercalated islands. These GABAergic neuron clusters are important in setting the inhibitory tone of amygdala pathways. They are positioned strategically among the main BLNG nuclei to modulate intrinsic passage of information from the BLNG to the central nucleus (Pare et al., 2003). This modulation follows a ‘feedforward’ inhibitory mechanism, by which excitatory fibers from the BLNG, which stimulate the central nucleus, also send collaterals to the intercalated islands. This has the effect of driving inhibitory output of the intercalated islands, which in turn modulate the central nucleus (Figure 3). This organization serves as an important internal ‘braking’ system of the central nucleus output to brainstem ‘fear centers’ controlling startle, freezing, and visceral responses to aversive stimuli.
Figure 3.
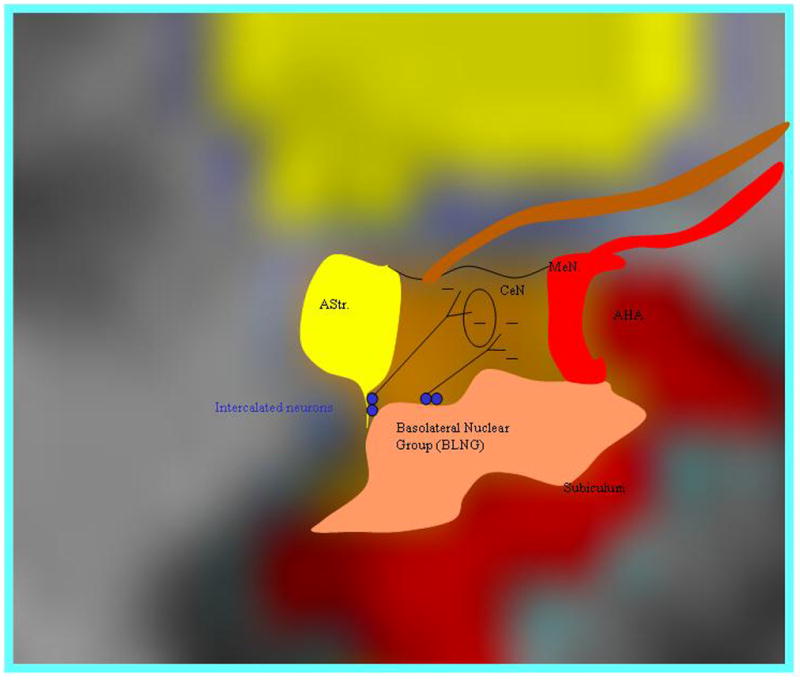
Simplified rendering of the general locations of amygdala subregions superimposed on a coronal section at the level of the amygdalohippocampal transition (AHA). The main basolateral nuclear group (BLNG, in tan) sends excitatory inputs to the CeN. These excitatory inputs also collateralize to innervate the GABAergic intercalated neurons (blue dots), which inhibit CeN activity. This arrangement provides a means for feedforward inhibitory modulation of CeN outputs. The intercalated islands are also importantly innervated by PFC inputs (see text). AStr= amygdalostriatal area, BLNG= basolateral nuclear group, CeN= central nucleus, MeN= medial nucleus
Anatomical studies in nonhuman primates indicate that the intercalated islands are themselves strongly influenced by the caudal orbitofrontal cortex (Ghashghaei and Barbas, 2002) and the serotonin system (Bauman and Amaral, 2005; O’Rourke and Fudge, 2006). Studies in rats suggest that the infralimbic prefrontal cortex (considered most analogous to Brodmann area 25 of the anterior cingulate cortex in primate) may also be involved. Indeed, its stimulation damps amygdala output, while increasing expression of neural activation markers (e.g., detected by c-fos expression) in the intercalated islands (Berretta et al., 2005; Quirk et al., 2003). These studies suggest that cortical inhibition of amygdala outputs via the central nucleus is at least partly mediated through projections to the inhibitory intercalated islands.
The medial and central nuclei are relatively smaller than the BLNG. They do not have the cortical characteristics of the BLNG. Instead, they are considered more ‘striatal’ in character by some authors, a concept which remains controversial (Cassell et al., 1999; Heimer et al., 1997; Swanson and Petrovich, 1998; Zahm, 1998). Regardless, the predominant cell type is GABAergic rather than glutamatergic, and the cells are medium sized stellate (medial nucleus) or medium spiny type (central nucleus). They are important modulators of subcortical and brainstem centers, but do not project to the cortex like the BLNG (see review, Alheid and Heimer, 1988; Fudge and Emiliano, 2003; Zahm et al., 1999).
The medial and central nuclei have recently been considered part of a larger macrostructure known as the ‘extended amygdala,’ (Alheid and Heimer, 1988; De Olmos and Ingram, 1972). The extended amygdala is so-named because it includes the medial and central nucleus and their forebrain ‘extensions’ into the bed nucleus of the stria terminalis (Figures 2. C–D and 3.). Under the microscope, cells of the medial and central nuclei flow into the forebrain in chains of cells that ‘extend’ into the bed nucleus of the stria terminalis. The extended amygdala has been divided into the medial extended amygdala, which includes the medial nucleus of the amygdala and corresponding medial bed nucleus of the stria terminalis, and the central extended amygdala, which includes the central nucleus of the amygdala and lateral bed nucleus of the stria terminalis (Figure 3). The ‘central extended amygdala’ receives massive BLNG projections, and channels this information to the hypothalamus and brainstem, modulating autonomic, visceral, and reflexive responses to rapidly changing environmental stimuli. In contrast, the medial extended amygdala structures receive inputs from the periamygdaloid cortex and amygdalalohippocampal regions, with some overlapping input from the BLNG. It projects to somewhat different regions of the hypothalamus and periacqueductal gray (Price and Russchen, 1987) and has been associated with social and reproductive behaviors (see below).
Animal studies show that the extended amygdala is involved in responses to both positive and negative environmental cues. In rodent studies, the medial extended amygdala is involved in unconditioned “instinctual” responses to social threat (social avoidance) (Blanchard et al., 2005; Kollack-Walker et al., 1997) and to reproductive and maternal social behaviors (social approach) (Numan et al., 1998; Parfitt and Newman, 1998). For example, infant monkeys with amygdala lesions can do all these behaviors, but apply them indiscriminately. Furthermore, rats with lesions of the amygdala medial nucleus or its specific output regions in hypothalamus and periaqueductal gray show changes in the appropriate expression of agonistic behaviors, suggesting a loss of inhibitory control (see review, Albert and Walsh, 1984).
The central extended amygdala is considered an effector of motor and autonomic responses such as freezing and startle to fear stimuli (Hitchcock and Davis, 1991; Parfitt and Newman, 1998; Walker and Davis, 1997; Wilensky et al., 2006). However the rostral component (bed nucleus of the stria terminalis) and caudal component (central nucleus) likely play somewhat different roles (Lee and Davis, 1997; Sullivan et al., 2004; Walker and Davis, 1997). The lateral bed nucleus of the stria terminalis mediates unconditioned fear responses, while the central nucleus is involved in conditioned fear responses, as well as acquistion and consolidation of fear-stimulus cues. The central nucleus is also involved in balancing approach and avoidance of stimuli during changes in the predictive value of environmental cues (Holland and Gallagher, 2006; Killcross et al., 1997). These latter effects may be partly mediated through inputs to the dopamine neurons of the substantia nigra, which modulate striatal and cortical function (Fudge and Haber, 2000; Lee et al., 2005; Lee et al., 2006). In summary, considerable evidence now suggests that integrating competing stimuli in the service of performing behavioral switches (between approach and avoidance) begins at the level of the central nucleus of the amygdala. We hypothesize that this ‘integrative’ function matures as the amygdala itself matures.
As can be seen, maturational changes in amygdala function are bound to have important ‘downstream’ effects on the expression of social defensive behaviors mediated through the hypothalamus (medial extended amygdala), and of automatic behavioral responses mediated through the dopamine system and caudal brainstem (central extended amygdala).
Ontogeny
The amygdala is the first formed structure of the telencephalon, and its nuclear subdivisions can be appreciated as early as 8–9 weeks gestational age in the human (Muller and O’Rahilly, 1990). Development continues over the ensuing gestation, with differential developmental trajectories for specific nuclei (Kordower et al., 1992).
A key question is whether and how the primate amygdala matures over human postnatal life. Volumetric studies using structural MRI in human children, adolescents and young adults, reveal that the amygdala enlarges with age, in a linear fashion relative to temporal lobe volume, and more in boys than in girls (Giedd et al., 1996; Giedd et al., 1999). However, these findings are preliminary because of the large interindividual variability in growth, and the related need for very large sample sizes to draw firm conclusions.
Histological studies indicate that immature neurons persist throughout postnatal life and beyond in both humans and monkeys (Bernier et al., 2002; Crosby and Humphrey, 1941; Fudge, 2004; Meyer et al., 1989; Millhouse, 1986; Yachnis et al., 2000). These immature cells are specifically distributed in the paralaminar nucleus (located in the remnant of the germinal zone) and among the intercalated islands. Their presence suggests that the postnatal primate amygdala has the capacity for neuroplastic changes well into adulthood. The question remains as to whether these cells can or do integrate into existing amygdala circuits, and if so, when and in what circumstances.
Since the time in 1939 of Kluver-Bucy’s lesions experiments (removal of bilateral temporal lobes in rhesus monkeys) (1997), newer, more limited lesioning techniques have helped to better define the role of the amygdala, and its function over development. These studies support the idea that the primate amygdala matures postnatally, since ibotenic acid lesions placed in infancy result in different behavioral profiles than lesions introduced in adulthood (Bauman et al., 2004; Prather et al., 2001). Importantly, the developmental consequences of amygdala lesions depend on the behavioral context, in particular its social nature. This seems to be consistent across species, and suggests that social learning is a protracted phase that coincides with juvenile and adolescent periods, before adult patterns of social stimulus-response are stabilized.
Non-human primates
Both adult and infants monkeys with amygdala lesions have blunted fear responses to non-social cues, such as snakes (Izquierdo et al., 2005; Kalin et al., 2001; Meunier et al., 1999; Prather et al. 2001; Stefanacci et al., 2003). This suggests a role in signaling non-social, perhaps ‘hard-wired’, fear responses in both adulthood and infancy.
In contrast to the age-independent effects of amygdala lesions on responses to non-social cues, non-human primate studies show that the age of the animal at the time of lesioning and testing does influence responses to social cues (presence of conspecifics). For example, amygdala-lesioned adult monkeys show increased excitability and exploratory behaviors with other animals, suggesting a loss of learned social fear associations (Emery et al., 2001; Machado and Bachevalier, 2006). However, lesioned infant monkeys show more fear behaviors and less aggression towards novel animals, while approach responses to familiar caregivers remain normal (Bauman and Amaral, 2005; Goursaud and Bachevalier, 2007; Jensen et al. 2003; Prather et al. 2001).
These data suggest that the infant amygdala mediates social emotional learning, laying down engrams of what is familiar and safe, what is familiar and dangerous, and what is unknown and therefore potentially dangerous in the social environment. Of interest are the human studies of children with Williams syndrome, a genetic neurodevelopmental disorder. This disorder is marked by an abnormally large amygdala volume and a distinct behavior of ‘hypersociability’. A possible interpretation is that these children exhibit abnormally low amygdala responses to social threat compared to normal children (Meyer-Lindenberg et al., 2005; Reiss et al., 2004).
Rodents
A fascinating series of studies in rats suggests that amygdala connectivity is developmentally timed and sensitive to social context (particularly maternal presence), and has important consequences for the emergence of appropriate avoidance behaviors to aversively conditioned stimuli (Moriceau and Sullivan, 2006; Shionoya et al., 2006).
Very early on, rat pups that are confined to the nest exhibit a prepotent appetitive learning (approach), and attenuated aversion learning (avoidance) tendencies (Camp and Rudy, 1988; Haroutunian and Campbell, 1979; Levine, 2000; Sullivan et al., 2000). This approach bias in behavioral response is mediated by an inability to engage the amygdala (Moriceau and Sullivan, 2006; Roth and Sullivan, 2005). In the transitional period when pups gain independence and leave the nest (days 12–15, the “juvenile period” prior to puberty) they readily learn to avoid an odor paired with an aversive stimulus, an adaptive mechanism for the world beyond mother. In mother’s absence, odor-shock conditioning produces amygdala activation and learned avoidance (Moriceau and Sullivan, 2006). However, with mother present in the testing apparatus, the same conditioning produces odor preference and attenuates avoidance learning. The maternal effect has been shown to be associated with lower corticosterone levels in the amygdala. Indeed, when corticosterone is injected systemically or in the amygdala, aversion learning and amygdala activation are rescued.
As a whole, this series of experiments indicates that maternal cues provide a powerful environmental ‘switch’, which can set the timing of amygdala participation in limbic circuits. Because many goal-directed approach behaviors, such as foraging for food, largely depend on ventral striatal circuits, we hypothesize that the coming ‘on line’ of the amygdala is expected to be a key modulator of approach and avoidance behavior during this transitional period. Similarly, hypothalamic and midbrain structures are likely to come under the influence of the amygdala (via ‘extended amygdala’ pathways) during this period, triggering the expression of more ‘automatic’ responses to environmental cues (freezing, startle, sexual and affiliative behaviors).
Humans
Historically, lesion studies in patient populations have provided an important way to understand the function of specific brain regions. However, amygdalectomy studies have been hampered by an absence of controls. While many patients with temporal lobe epilepsy have undergone resection of the amygdala, there is significant variability in the size of surgical lesions as well as the issue of pre-existing functional compromise. A general decrease in emotional output, and a decrease in the ability to recognize emotional expressions in such patients has been reported (Aggleton, 1992).
While recognized early on in primate studies that the timing of amygdala lesion influences behavioral phenotype (Bachevalier, 1994), this important variable has only recently been addressed in humans. Adolphs first reported on a case of amygdala disruption due to Urbache-Weithe disease, a congenital disease causing aberrant calcium deposition in the amygdala bilaterally. This patient had little in the way of psychiatric symptoms, but was unable to recognize fear in facial expressions on standard tests. Furthermore, she exhibited deficits in recognizing complex (more than one emotion) facial expressions (Adolphs et al. 1994). The authors recently proposed that deficits in spontaneous fixation on the eye region during free viewing of emotional faces may explain this abnormal emotion recognition, since the deficit disappeared when patients were explicitly instructed to look at the eyes (Adolphs et al., 2005).
Another example of a congenital amygdala ‘lesion’ is Williams syndrome, a genetic disease characterized by abnormal emotional processing. Children with Williams syndrome, are not anxiety-free but have an unusual phenotype characterized by high sociability and abnormally low social fear (Bellugi et al., 1999; Dykens, 2003; Klein-Tasman and Mervis, 2003). Such children often have mental retardation, limiting controlled studies. However, comparison of a select group of Williams syndrome subjects with normal intelligence and normal controls reveal interesting findings in the amygdala (Meyer-Lindenberg et al. 2005). While normal children had higher amygdala activation for emotional faces compared to scenes, the opposite was true for the patients. Amygdala activation to threatening faces was especially diminished in the Williams syndrome group, suggesting a specific deficit in recognizing social threat.
One way to compare early versus late amygdala dysfunction involves comparing adult patients with dyembryoplastic neuroepithelial tumors (DNET) of the amygdala with patients having amygdala resection. DNETs are thought to arise in early childhood, if not before (Daumas-Duport et al., 1988), and have a characteristic appearance on MRI (Stanescu et al., 2001). DNETs are space-occupying lesions often associated with epilepsy. Comparison of ‘early amygdala lesion’ groups (adults with DNET lesions) with ‘late amygdala lesion’ subjects (adults with surgical resections for intractable epilepsy not due to DNET) reveals that subjects with ‘early lesions’ have reduced ability to interpret complex social cues, such as detecting ironic, tactless, or flirtatious statements and behaviors. The ability to understand the motives and emotional states of others, known as ‘theory of mind’, is thus thought to require early amygdala involvement. Once acquired, possibly at a critical period, theory of mind capabilities may be subsumed by other brain regions, or rely instead on distributed networks less dependent on amygdala function (Shaw et al., 2004; Shaw et al., 2005). These findings are especially intriguing in light of the fact that late childhood and adolescence are key periods for learning complex social cues, an ability important for forming peer groups and finding a mate. The development of theory of mind tasks for adolescent populations will be of great utility in tracking the functional development of the triadic nodes in normal and at-risk children.
Functionally, neuroimaging work in humans is beginning to identify differences in amygdala responses to affective stimuli in adolescents compared to adults. Overall, the amygdala seems to be more responsive in adolescents than in adults during identification of negatively valenced social face stimuli (Guyer et al., 2008; Killgore and Yurgelun-Todd, 2004; Monk et al., 2003), but less influenced by processes involved in negative monetary outcomes (Ernst et al. 2005). This functional pattern suggests either lower modulation of this structure by regulatory systems mainly originating from prefrontal regions (immaturity of top-down modulatory systems), or intrinsic cellular/molecular/neurochemical immaturity within the structure. Changes in neurochemical modulation may include not only ontogenic alterations of neurotransmitter systems, but also steroid hormones.
Although highly speculative, it is conceivable that the medial extended amygdala subsystems, which are associated more specifically with social threat, matures differently across adolescence than the central extended amygdala, which mediates conditioned response to threat. These specific subsystems could contribute distinctly to the adolescent changes in social and stress responses, particularly given the recognized dissociation of the type of steroid receptors that populate these two sub-systems: sex-steroid receptors (e.g., androgen receptor, estrogen receptor α) predominantly present in the medial extended amygdala (Michael et al., 2005; Michael and Rees, 1982; Osterlund and Hurd, 2001; Perlman et al., 2004; Roselli et al., 2001) and stress-steroid receptors (for glucocorticoids and corticotropin releasing factor [CRF]) in the central extended amygdala (Perlman et al. 2004; Sanchez et al., 1999; Sanchez et al., 2000; Sarrieau et al., 1986).
Responses to social stimuli undergo drastic changes in adolescence, establishing the primacy of peer relationships with the emergence of exquisite sensitivity to social appraisal and romantic involvement. Such magnification of social processes may be partly mediated by pubertal changes in sex hormones, whose role in social behavior is well established (Newman, 1999; Wirth and Schultheiss, 2006). On the other hand, changes in stress response have also been noted in adolescence, indicating an extended hormonal stress response in prepubertal animals compared to adult animals (Romeo et al., 2006). These changes in stress steroid function may have implications for motivated behaviors within a stressful context, although their significance at the behavioural level is still unknown.
The intercalated islands, which have important inhibitory functions on the central nucleus—as well as the central nucleus itself-- are especially enriched in CRF and glucocorticoid receptors (Perlman et al. 2004; Sanchez et al. 1999). This may be consistent with the central extended amygdala’s role in detecting unexpected stimuli, or ‘surprise’, in the environment (Holland and Gallagher, 2006). The impact of CRF and corticosteroids on such a system would be expected to enhance both appetitive and aversive learning in the setting of arousing or ‘unexpected’ stimuli. Separation of the medial and central extended amygdala into distinct emotional subsystems mediating ‘social’ and ‘nonsocial’ emotional learning is tempting but overly simplistic. However, clarifying both the separation and overlap of circuits influenced by gonadal steroids vs. stress hormones may help us understand changes in these areas during adolescence.
In summary, the extended amygdala has two main subdivisions—medial and central—which have been broadly conceptualized based on their connectivities (Alheid, 2003; McDonald et al., 1999; Pitkanen et al., 1997; Zahm, 1998; Zahm et al. 1999). The medial extended amygdala receives inputs from the medial amygdaloid nuclei (periamygdaloid cortex, parvicellular accessory basal nucleus, cortical nucleus, and amygdalohippocampal area), while the BLNG provides the main inputs to the central extended amygdala. These broad connectional differences are mirrored in the distribution of gonadal receptors, which tend to aggregate in the medial nucleus and its afferents, and glucocorticoids/CRF receptors, which are densely distributed in the central nucleus and its afferents. Thus, the medial extended amygdala has been considered a mediator of reproductive and social defensive behaviors, while the central extended amygdala has been considered a mediator of fear responses. These general functional assignments will surely be refined once the details of this circuitry are better understood, particularly in higher species. The study of adolescent development may benefit from elucidation of the differential maturation of these specific subregions, as well as a better understanding of their points of convergence. Hence, another focus of future research should be the maturation of amygdaloid subregions, where ‘stress’ hormones and gonadal hormones both have an effect (e.g., the periamygdaloid cortex and amygdalohippocampal area), and the common output targets of the medial and central extended amygdala systems.
Striatum
Anatomy and connectivity
The striatum is composed of the caudate nucleus and putamen, and is populated largely by medium-size spiny neurons (Graveland and DiFiglia, 1985), which send information beyond the striatum through basal ganglia circuits. Chemically, spiny striatal neurons are heterogeneous; that is, most contain more than one neurotransmitter, although gamma-aminobutyric acid (GABA) is the primary neurotransmitter. Other neurotransmitters include substance P and enkephalin. The striatum and the globus pallidus are referred to as the basal ganglia, although some define the basal ganglia more broadly as also encompassing midbrain nuclei (substantia nigra, ventral tegmental area and subthalamic nuclei).
Information flows from the cortex, to the striatum, where it is channeled to the globus pallidus, then the thalamus and finally back to the cortex (Figure 4). In fact, the cortico-striatal projection is one of the major projections from the prefrontal cortex in primates (Goldman and Nauta, 1977; Kemp and Powell, 1970). The highly specialized medium-spiny neurons are recipients of a large number of excitatory afferent fibers from the prefrontal cortex and ‘cortical-like’ amygdala (BLNG), which terminate mainly on the many spine heads covering each dendrite (Johnson et al., 1994; Lapper et al., 1992). This anatomic arrangement allows for multiple cortical inputs to modulate individual striatal cells, and in turn, determine basal ganglia output. The midbrain dopamine neurons also send afferents throughout the striatum, and regulate which cortical inputs ultimately result in striatal cell firing (Grace et al. 2007) (see below).
Figure 4.
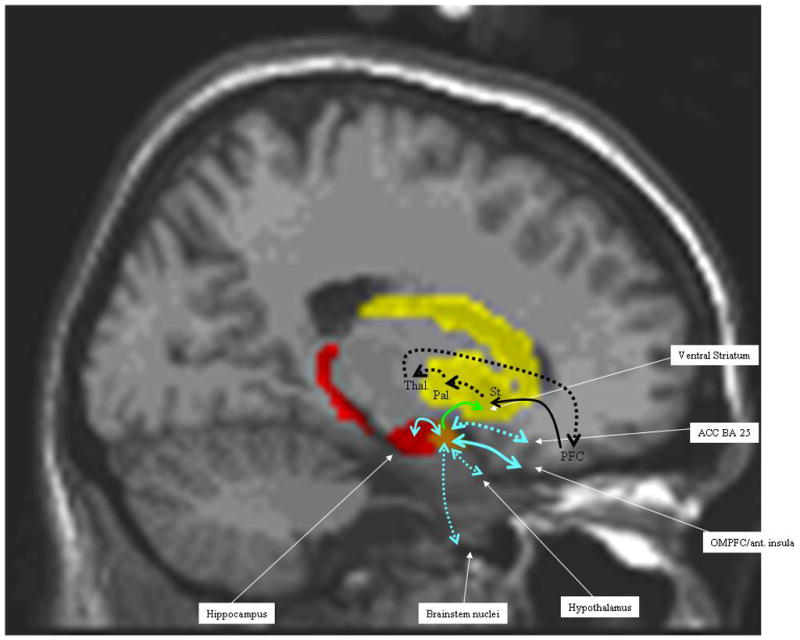
Representation of the projections of the amygdala (blue) and cortico-striato-pallido-thalamo-cortical loops (black). The hatched lines signify that these connections are not in this sagittal plane, but go more medially. The solid lines are in the plane of the section. The amygdala projection to the ventral striatum is the only one that is unidirectional (green arrow). All other amygdala projections are bidirectional (blue) OMPFC= orbito-medial prefrontal cortex; ant. Insula = anterior insula; BA 25 = Brodmann area 25; Pal. = pallidum; PFC = prefrontal cortex; Thal. = thalamus; Str. = striatum
The topography of striatal inputs from the limbic, association, and motor-related regions of the prefrontal cortex has led to the idea that the striatum can be divided into discrete functional domains. Yet, significant debate exists as to whether cortical inputs to the striatum are organized in a strictly topographic manner (Alexander and Crutcher, 1990), or whether there are opportunities for convergence of information from different functional regions of the cortex (Haber et al., 2006).
In general, the amygdala and those cortical regions most directly influenced by the amygdala (‘limbic-related’) project to the ventral striatum (Chikama et al., 1997; Fudge et al., 2002; Haber et al., 1995; Hemphill et al., 1981; Kunishio and Haber, 1994; Russchen et al., 1985; Selemon and Goldman-Rakic, 1985; Yeterian and Pandya, 1991; Yeterian and Van Hoesen, 1978). This region includes the ‘shell’ and ‘core’ of the nucleus accumbens, and the ventromedial areas of caudate nucleus and putamen. Cortical regions involved in sensorimotor processes project to the dorsolateral striatum (Flaherty and Graybiel, 1991; Jones et al., 1977; Kemp and Powell, 1970; Kunzle, 1978). Associative, or ‘cognitive-related’, regions such as the dorsolateral prefrontal cortex (Brodmann areas 46, 9) project to central regions of the striatum that lie interposed between the ventral striatum and dorsolateral striatum (Cavada and Goldman-Rakic, 1991; Kunzle and Akert, 1977; Selemon and Goldman-Rakic, 1985; Selemon and Goldman-Rakic, 1988; Stanton et al., 1988).
However, recent evidence shows that inputs from diverse functional domains of the cortex converge in patches into the striatum, creating ‘hot spots’ where information can be integrated (Haber et al. 2006). Thus, for example, afferent fibers from limbic-related and associative areas of the prefrontal cortex converge in patches within the central striatum (Haber et al. 2006). It is not yet known whether such foci of cortical afferent convergence correspond to the cellular islands that arise during development (see below), or how and when such convergence occurs. In summary, while the concept of a ‘functional topography’ of the striatum based on segregated cortical inputs has heuristic value, new data indicate that this scheme may be overly simplistic.
The overall functional topography of the striatum exists along its entire rostrocaudal extent, a fact which has been under-appreciated. Thus, the functional gradient traditionally attached to the rostral striatum (anterior to the decussation of the anterior commissure) extends caudally. In a series of elegant experiments, Selemon demonstrated that cortical projections terminate along the ventromedial (limbic) to dorsolateral (sensorimotor) gradient following the entire rostrocaudal extent of the striatum (Selemon and Goldman-Rakic, 1985). Anterograde tracer injections into the amygdala have also indicated that afferent fibers terminate in the caudal ventromedial striatum, as well as in the nucleus accumbens (Fudge et al. 2002; Fudge et al., 2004; Russchen et al. 1985). Based on inputs from the amygdala and ’limbic’ prefrontal cortex (i.e., PFC regions receiving amygdala inputs), as well as cellular and neurochemical similarities to the nucleus accumbens, we have proposed that the caudal ‘limbic’ striatal region includes the ventromedial putamen and medial tail of the caudate nucleus, and their transition with the amygdala, the lateral amygdalostriatal area (Fudge et al. 2004; Fudge, 2004; Fudge and Haber, 2002).
The identification of such ‘limbic-related’ anatomical ensembles in the striatum, which are innervated by specific regions of PFC and amygdala, may provide a unique opportunity to examine the foundations of the Triadic Model. In our view, the ‘limbic striatum’ can be defined as that part of the striatum that receives inputs from the amygdala (Fudge et al. 2002; Fudge et al. 2004). As mentioned, the amygdala projects to a broad rostrocaudal extent of the striatum, expanding the definition of the ‘limbic striatum’ far beyond the nucleus accumbens. These regions receive variable inputs from prefrontal cortical areas including the anterior cingulate cortex, medial and lateral orbital cortex, and the agranular insula (Carmichael and Price, 1996; Ferry et al., 2000; Haber et al. 2006; Selemon and Goldman-Rakic, 1985). Determining the detailed overlap between amygdala and prefrontal cortical afferents in the striatum will help us understand how ‘emotional’ inputs from the amygdala may facilitate ‘throughput’ from discrete prefrontal cortical regions.
Taken a step further, the resultant signal of the “limbic striatum” may vary along a gradient across this structure. This variation could represent different types of combination of amygdala/PFC afferents. Approach behaviors might be mediated by a unique set of afferents, while avoidance behaviors by another set of afferents. Indeed, a recent fMRI study of financial reward and loss shows that the anterior ventral striatum has relative selectivity for reward prediction, while more posterior striatum signals prediction of loss (Seymour et al. 2007). This is consistent with appetitive/aversive ventral striatal gradients detected in rats using pharmacologic probes (Reynolds and Berridge, 2001; Reynolds and Berridge, 2002; Reynolds and Berridge, 2003). Such a scheme could be integrated into the Triadic Model by modifying the schematic unimodal function of the nodes. As a way to incorporate the functional heterogeneity into the Triadic Model, each node (amygdala, striatum and prefrontal cortex) could be defined along a triadic organization underlying approach, avoidance and modulation. Such representation of the Triadic Model is akin to a fractal organization (Figure 5). This framework can help formulate questions and develop experiments related to functional connectivity and maturational changes that affect the balance of this triadic system.
Figure 5.
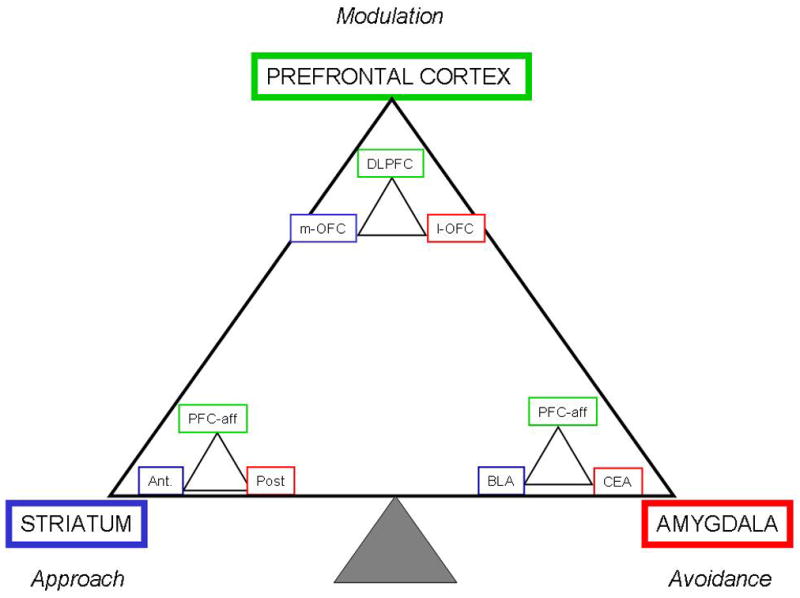
Fractal Triadic Model.
The fractal Triadic Model is a more comprehensive representation of the Triadic Model, because it takes into account the heteromodal role of each of the nodes of the triad. Each of the nodes of approach, avoidance and regulatory control is itself the seat of a triadic formation that mirrors the overall organization. Examples of regions previously identified to be associated in some studies with either approach or avoidance responses are indicated. However, similarly to the original Triadic Model, this representation is highly schematic and not all-inclusive.
DLPFC=dorsolateral prefrontal cortex, m-OFC=medial orbital frontal cortex, l-OFC lateral orbital frontal cortex, PFC-aff, prefrontal cortical afferents, Ant=anterior striatum, Post=posterior striatum, BLA=basolateral amygdala, CEA=central amygdala
Ontogeny
Most of our knowledge on the development of primate striatum stems from early work in nonhuman primates. In general, cortico-striatal pathways develop before cortico-cortical connections (Goldman-Rakic, 1987). Cortical afferents reach the striatum early in the second trimester of life and steadily increase their innervation over the rest of gestation. The striatum undergoes a shift several weeks after cortico-striatal innervation begins. The cytoarchitectural pattern changes from a homogeneous mass of densely packed cells to cellular islands of densely packed cells encapsulated by fibers and surrounded by a relatively less densely packed matrix of neurons (Goldman-Rakic, 1981). The reason for this transformation is unclear, but it is speculated that either migration of clusters of new neurons and/or the entry and retraction of afferent contacts plays a role in this development. There is some indication that the dopamine innervation of the striatum contributes to maintaining the integrity of the cellular islands, which persist throughout life (van der Kooy, 1996).
In monkeys at birth, recognizable neuronal subtypes still exist in various stages of growth, as do undifferentiated cells near the ventricular wall. Rapid increases in synaptic density levels off, but ultrastructural changes in synaptic modeling continue. Spine density on dendrites increases progressively over at least the first four months postnatally in monkeys (Brand and Rakic, 1984), and with this, the proportion of axon contacts shifts toward axospinous rather than axodendritic types. This shift is functionally significant because spines increase the overall length of the dendrite, and hence its electrical resistance. Such enhanced electrical resistance of dendritic membranes has the effect of decreasing the contribution of any individual input to the total excitatory post-synaptic potential of the cell (Sharpe and Tepper, 1998; Wilson, 1984). Consequently, the multiplication of afferents arriving in the striatum during postnatal life may be modulated by the concurrent proliferation of spines, which may serve to attenuate the effects of any particular excitatory input to a cell. These changes collectively lead to a refinement of striatal modulation (Sharpe and Tepper, 1998). The evolution of axospinous over axodendritic contacts, along with continuing degeneration of some afferent axons, suggests important alterations in information processing at least during the first months of life, and perhaps well beyond.
Volumetric studies in humans using MRI indicate that the striatum, specifically the caudate nucleus, undergoes volume loss starting around 10 years of age in girls and 14 years of age in boys, and exhibiting a steeper volume decrease in girls than in boys (Lenroot et al. 2007). Furthermore, Sowell and colleagues found that a significant volume reduction in the ventromedial putamen (an area we consider part of the ‘limbic’ striatum due to its afferents from the amygdala) differed between adolescents and young adults (Sowell et al., 1999a).
A few developmental functional neuroimaging studies in humans have targeted striatal function, particularly through the use of reward-related paradigms. Findings depend largely on the nature of the paradigms and cognitive processes being investigated. Anticipation of rewards/motivation-to-act activates the striatum less strongly in adolescents than in adults (Bjork et al., 2004), but the process of decision-making itself as well as the response to the receipt to rewards activate this structure more in adolescents than in adults (Ernst et al. 2005; Galvan et al., 2006). Here again, the paucity of studies calls for caution in interpreting the results. With this caveat in mind, one hypothesis is that this pattern of striatal activity in adolescence may suggest a more reactive system for coding positively valenced stimuli, and a more efficient system for integrating rewards into action. Alternatively, researchers have proposed an overall less efficient striatal system in adolescents for processing reward stimuli. In both cases, the behavioral output would be consistent with enhanced reward seeking because of enhanced sensitivity to reward in the first interpretation, or because of a need to maintain the reward system at an homeostatic level in the second interpretation. These interpretations are currently being tested in several laboratories.
Prefrontal cortex (PFC)
Anatomy and connectivity with amygdala
The primate cortex in general ranges from a simple one-two layered primitive cortex (known as the allocortex) to six-layered isocotex (see review, Mesulam, 2000). Between these extremes are the ‘periallocortical’ regions. The allocortical zones include the hippocampus and piriform cortex. These allocortical regions are most tightly interconnected with the amygdala. The periallocortex can show a range of transitional features, but is more differentiated than the allocortex. In general, periallocortial regions have poorly demarcated layers II and IV and relatively rudimentary layer V. The rostral anterior cingulate cortex and medial wall (Brodmann areas 25, 32, 14) and the caudal orbital cortex (now recognized as the ‘rostral agranular insula’ (Carmichael and Price, 1994) comprise important periallocortical regions of the prefrontal cortex. These areas are the most strongly interconnected with the amygdala, compared to other prefrontal cortical regions (Amaral, 1986; Carmichael and Price, 1996; Freedman et al., 2000; Ghashghaei and Barbas, 2002).
Many amygdalar subregions that send efferents to the orbital and medial prefrontal cortices, are the recipients of return projections (Amaral, 1986; Carmichael and Price, 1996; Freedman et al. 2000; Ghashghaei and Barbas, 2002; Porrino et al., 1981). The major amygdalar inputs to the orbital and medial prefrontal cortices originate from the basal nucleus, and terminate in the medial wall (Brodmann areas 25, 32 and 14) and the caudal orbital surface (i.e., the agranular insula) (Carmichael and Price, 1996; Porrino et al. 1981). In turn, the caudal orbital surface of the prefrontal cortex returns afferents to the intercalated neurons, modulating the output of the BLNG (Ghashghaei and Barbas, 2002). In contrast, the medial wall of the orbital and medial prefrontal cortices provides the main projections to the BLNG, i.e., both basal and lateral nuclei.
Other periallocortical regions of the PFC, while still less differentiated than the isocortex, are relatively more differentiated than Brodmann areas 25, 32, 14, and the agranular insula. These regions, including rostral Brodmann areas 13, 11, and 12, receive relatively modest afferents from the amygdala, and project back sparingly. In contrast, the isocortex forms the largest part of the primate cortex, is involved in associative and motor control functions, and is not directly interconnected with the amygdala. Nonetheless, isocortical areas, specifically those involved in working memory and planning (Brodmann areas 9/46), receive inputs from the ‘limbic cortex’ described above (Carmichael and Price, 1996; Petrides and Pandya, 1999). This organization provides an anatomical substrate for affective processing to influence cognitive operations including planning, and sequencing via cortical-cortical interactions.
Anatomy and connectivity with striatum
As mentioned above, the ‘limbic’ striatum can be defined as the striatal regions that receive inputs from the amygdala. Typically, these striatal regions also receive inputs from areas of the prefrontal cortex, which themselves are innervated by the amygdala. This organization creates a ‘triangular’ set of inputs. It is important to remember that, by this definition—as well as based on functional data—, the ‘ventral striatum’ extends well beyond the nucleus accumbens.
For example, the ventromedial prefrontal cortex (Brodmann area 25), which is strongly interconnected with the amygdala, projects to the shell of the nucleus accumbens but also to the adjacent ventromedial caudate nucleus. More dorsal sectors of the anterior cingulate cortex (Brodmann areas 24a and b), which also receive amygdala inputs, albeit somewhat fewer, project to the head of the caudate nucleus, the central rostral putamen, and the core of the nucleus accumbens (Ferry et al., 2000; Haber et al., 2006; Selemon and Goldman-Rakic, 1985; Yeterian and Van Hoesen, 1978). The caudolateral orbitofrontal cortex (known as the rostral agranular insula), innervated by the amygdala, projects to the lateral nucleus accumbens and ventromedial putamen, including caudal aspects posterior to the decussation of the anterior commissure (Ferry et al., 2000; Fudge et al., 2005). Prefrontal cortical regions that receive few amygdala inputs (such as association areas, like Brodmann areas 46/9,) project to dorsolateral regions of the striatum. However, their terminals do overlap with those from more ‘limbic’ regions such as the dorsal anterior cingulate cortex, suggesting potential convergence of limbic and cognitive information at the level of the striatum (Haber et al. 2006).
Ontogeny
Similar to the temporal lobe, the prefrontal cortex is significantly expanded in higher primates compared to lower species (Gloor, 1997; Jerison, 1973). This expansion accompanies a protracted developmental period, which continues through late childhood and adolescence. A cellular explanation for this expansion, at least with respect to the prefrontal cortex, is offered by Kornack and Rakic (1998). They have demonstrated that the duration of the cell cycle (during cell division) is up to five times longer in primates compared to rodents. At the same time, many more total rounds of division occur during the neurogenetic period in monkeys, accelerating toward the end of development (Kornack and Rakic, 1998). Together these findings provide a basis for understanding the greater size and lamination of the primate cortex compared to rodents, and why its development occurs over a lengthy period postnatally.
Recently, structural neuroimaging has provided information on human brain development during childhood and adolescence using relatively crude measures of volumetric estimations of discrete brain regions. An overall increase in cortical size was reported during childhood (Durston et al., 2001; Giedd et al., 1996), which seems to co-occur with changes in dendritic density and increased myelination (Paus et al., 1999; Yakovlev et al., 2001). Between childhood and adolescence, cortical gray matter changes were found to be diffusely distributed in dorsal frontal and parietal regions, whereas between adolescence and adulthood, cortical changes seemed to be segregated within large frontal regions comprising dorsal, medial, and orbital areas (Giedd et al., 1999; Sowell et al., 1999b).
Synaptogenesis in the PFC wanes after age two years based on human postmortem studies, (Huttenlocher, 1979) with synaptic pruning resulting in dendritic densities resembling adult levels by about age 16 years (Huttenlocher, 1979). The rate of synaptic eliminations varies by regions, ending first in primary sensory cortices (around age 12 years), and last in the prefrontal cortex by age 16 years (Huttenlocher and Dabholkar, 1997). In addition, there is active maturation of synapse morphology, specifically of presynaptic terminals during periadolescence (Huttenlocher, 1979).
Progressive myelination through childhood and adolescence has also been documented in post-mortem samples (Yakovlev, 1967), and more recently suggested with MRI (Benes et al., 1994; Giedd et al., 1996; Paus et al., 1999). Significant age-related increases in white matter ‘density’ (generated with 3-D masks) occurs in the arcuate fasciculus on the dominant side, and in the posterior internal capsule (Paus et al., 1999). These tracts carry axons joining the frontal and temporal speech areas (Broca’s and Wernicke’s areas), and cortex and brainstem (cortico-spinal tract), respectively. An increase in tract size is likely due to a combination of increasing axonal diameter, coupled with myelination of the tracts. The relationship between age-related changes in regional white matter density and competence in specific cognitive and motor functions remains to be explored.
In general, recent developmental functional neuroimaging studies in humans report more diffuse activation of the prefrontal cortex during cognitive challenges in pre-adolescence, suggesting that less efficient or well-formed networks carry out specific processing (Luna and Sweeney, 2004). More specifically, a shift from diffuse to focal activation has been proposed as a general developmental pattern of cortical engagement in cognitive function (Durston and Casey, 2006). During the processing of cognitive tasks, accessory regions become less involved, whereas critical regions to the cognitive processes under scrutiny are recruited more readily and strongly. Hence, the narrowing of recruited regions may reflect enhanced specificity and a more efficient use of neural networks for task completion.
CONCLUSION
In summary, this review underscores the complexity of the anatomical and functional organization of key structures underlying motivated behavior. Most notably, it provides a template along which to coordinate future research on the role of these structures and associated systems in the control of behavior.
The fractal Triadic Model is ideally suited for the study of ontogenic neural changes and their consequences on behavior, as evidenced by the emerging functional neuroimaging studies of reward systems and decision-making in adolescents. Basic neuroscience research provides invaluable information about the functional cohesion of this model and its integration with knowledge on connectivity and development of neural systems. The field is ripe for a rich reciprocal translational work between basic and clinical neuroscience research.
We close this review by highlighting the most salient points regarding the amygdala, striatum and prefrontal cortex contributions to motivated behavior and its ontogeny.
Three critical aspects dominate the review on the amygdala. First, lesion studies have different long-term behavioral impacts as a function of the developmental stage at which the lesion occurs. This underscores the notion of windows of vulnerability. Second, this differential impact does not apply indiscriminately to all behavioral responses. Innate responses, such as avoidance of snakes by monkeys, seem to be impervious to the notion of sensitive periods (at least in the age groups examined so far), whereas social behavior appears to be highly dependent on the timing of amygdala lesion. And third, developmental changes of the intact amygdala manifests opposite functional patterns as a function of the stimuli: challenged by threat related stimuli, the amygdala shows greater activation in adolescents than in adults. However, challenged by receipt of losses, the amygdala shows diminished responses in adolescents relative to adults. This functional heterogeneity of the amygdala is consistent with the complexity of its structure (multi-nuclei) and connectivity, and the anatomic evidence of immature neurons that persist throughout adolescence and into adulthood. As a result, it is critical to avoid over-generalization of a given pattern of response, be it developmental or to distinct stimuli.
The striatum receives direct inputs, in an organized fashion, from the totality of the prefrontal cortex. The segregation of functions that comprise sensory-motor, cognitive and affective processing, has been classically aligned along a dorsoventral gradient. This somatotopic organization is shown to be more complex, with the existence of a convergence of these functional paths onto distinct “patches”, as well as the realization that the functional ventrodorsal gradient is present along the whole length of the rostro-caudal (antero-posterior) extent of the striatum. It is unknown whether such organization is present since birth or how it evolves across childhood and adolescence. Clearly, the few developmental histological studies suggest a refinement of striatal modulation. Similarly to the amygdala, developmental patterns of activation in humans depend on the type of stimuli/events under study. Relative to adults, adolescents show enhanced striatal activity to receipt of rewards or punishments and selection of risky choices, but reduced activity in anticipation of rewards. Adolescent responses to distinct types of salient stimuli, compared to the adult, may reflect a relative lack of development of some striatal ensembles over others.
Finally, the prefrontal cortex is the most complex structure with the longest protracted period of maturation compared to the amygdala and striatum. It receives information from the amygdala directly and striatum indirectly (via cortico-basal ganglia loops), and in turn, modulates both structures. This protracted maturation can be expected to influence, and be influenced by the other nodes of the triad. For example, delayed maturation of amygdala circuits that process social cues (e.g., social disapproval), would affect the PFC regions regulating social judgement and the striatal regions involved in approach towards a novel or interesting, but socially disapproved, stimulus.
Future research will examine how the balance among these three systems changes with development, but also in disease states that impact behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam J, Baulac M, Hauw JJ, Laplane D, Duyckaerts C. Behavioral symptoms after pallido-nigral lesions: a clinico-pathological case. Neurocase. 2008;14:125–130. doi: 10.1080/13554790802032200. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP. The functional effects of amygdala lesions in humans: A comparison with findings from monkeys. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss Inc.; New York: 1992. pp. 485–503. [Google Scholar]
- Albert DJ, Walsh ML. Neural systems and the inhibitory modulation of agonistic behavior: a comparison of mammalian species. Neurosci Biobehav Rev. 1984;8:5–24. doi: 10.1016/0149-7634(84)90017-4. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Amaral DG. Amygdalohippocampal and amygdalocortical projections in the primate brain. Adv Exp Med Biol. 1986;203:3–17. doi: 10.1007/978-1-4684-7971-3_1. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp Brain Res. 1992;88:375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Reckless Behavior in adolescence: a developmental perspective. Developmental Review. 1992;12:339–373. [Google Scholar]
- Bachevalier J. Medial temporal lobe structures and autism: a review of clinical and experimental findings. Neuropsychologia. 1994;32:627–648. doi: 10.1016/0028-3932(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Amaral DG. The distribution of serotonergic fibers in the macaque monkey amygdala: an immunohistochemical study using antisera to 5-hydroxytryptamine. Neuroscience. 2005;136:193–203. doi: 10.1016/j.neuroscience.2005.07.040. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Van Der LM. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Lichtenberger L, Mills D, Galaburda A, Korenberg JR. Bridging cognition, the brain and molecular genetics: evidence from Williams syndrome. Trends Neurosci. 1999;22:197–207. doi: 10.1016/s0166-2236(99)01397-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bechara A, Damasio H, Tranel D, Cacioppo JT. Amygdala contribution to selective dimensions of emotion. Soc Cogn Affect Neurosci. 2007;2:123–129. doi: 10.1093/scan/nsm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Bor D, Duncan J, Wiseman RJ, Owen AM. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37:361–367. doi: 10.1016/s0896-6273(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Plaut DC. Doing without schema hierarchies: a recurrent connectionist approach to normal and impaired routine sequential action. Psychol Rev. 2004;111:395–429. doi: 10.1037/0033-295X.111.2.395. [DOI] [PubMed] [Google Scholar]
- Brand S, Rakic P. Cytodifferentiation and synaptogenesis in the neostriatum of fetal and neonatal rhesus monkeys. Anat Embryol (Berl) 1984;169:21–34. doi: 10.1007/BF00300583. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wallis JD, Parker A, Brass M, Crone EA, Hoshi E, Sakai K. Neural circuitry underlying rule use in humans and nonhuman primates. J Neurosci. 2005;25:10347–10350. doi: 10.1523/JNEUROSCI.2937-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine D, Watson JD. Neuropsychological and neuropathological sequelae of cerebral anoxia: a critical review. J Int Neuropsychol Soc. 2000;6:86–99. doi: 10.1017/s1355617700611116. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Topographic segregation of corticostriatal projections from posterior parietal subdivisions in the macaque monkey. Neuroscience. 1991;42:683–696. doi: 10.1016/0306-4522(91)90037-o. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability 19. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby E, Humphrey T. Studies of the vertebrate telencephalon: the nuclear pattern of the anterior olfactory nucleus, turberculum olfactorium and the amygdaloid complex in adult man. Journal of Comparitive Neurology. 1941;74:309–347. [Google Scholar]
- Damasio AR. Descartes’ error: emotion, reason, and the human brain. Grosset/Putnam; New York: 1994. [Google Scholar]
- Damasio AR, Eslinger PJ, Damasio H, Van Hoesen GW, Cornell S. Multimodal amnesic syndrome following bilateral temporal and basal forebrain damage. Arch Neurol. 1985;42:252–259. doi: 10.1001/archneur.1985.04060030070012. [DOI] [PubMed] [Google Scholar]
- Daumas-Duport C, Scheithauer BW, Chodkiewicz JP, Laws ER, Jr, Vedrenne C. Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizures. Report of thirty-nine cases. Neurosurgery. 1988;23:545–556. doi: 10.1227/00006123-198811000-00002. [DOI] [PubMed] [Google Scholar]
- De Olmos JS, Ingram WR. The projection field of the stria terminalis in the rat brain. An experimental study. J Comp Neurol. 1972;146:303–334. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. A hierarchical neuronal network for planning behavior. Proc Natl Acad Sci U S A. 1997;94:13293–13298. doi: 10.1073/pnas.94.24.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dorn L, Dahl R, Woodward H, Biro F. Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescence: A user’s guide to assessing pubertal status adn pubertal timing in research with adolecents. Applied Developmental Science. 2006;10:30–56. [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44:2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Dykens EM. Anxiety, fears, and phobias in persons with Williams syndrome. Dev Neuropsychol. 2003;23:291–316. doi: 10.1080/87565641.2003.9651896. [DOI] [PubMed] [Google Scholar]
- Elliott R, Deakin B. Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: evidence from functional magnetic resonance imaging studies in healthy human subjects. Int Rev Neurobiol. 2005;65:89–116. doi: 10.1016/S0074-7742(04)65004-5. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:515–544. [PubMed] [Google Scholar]
- Ernst M, Hardin M. Goal-directed behavior: Evolution and ontogenhy Goal-directed behavior: Evolution and Ontogeny. In: Rumsey J, Ernst M, editors. Neuroimaging in Developmental Clinical Neuroscience. Cambridge University; Cambridge, UK: 2008. [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of Decision Making: A Selective Review from a Neurocognitive and Clinical Perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic Model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations. J Neurophysiol. 1991;66:1249–1263. doi: 10.1152/jn.1991.66.4.1249. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- Fudge JL. Bcl-2 immunoreactive neurons are differentially distributed in subregions of the amygdala and hippocampus of the adult macaque. Neuroscience. 2004;127:539–556. doi: 10.1016/j.neuroscience.2004.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005;490:101–118. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, McClain C. Amygdaloid inputs define a caudal component of the ventral striatum in primates. J Comp Neurol. 2004;476:330–347. doi: 10.1002/cne.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Emiliano AB. The extended amygdala and the dopamine system: another piece of the dopamine puzzle. J Neuropsychiatry Clin Neurosci. 2003;15:306–316. doi: 10.1176/jnp.15.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience. 2000;97:479–494. doi: 10.1016/s0306-4522(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. Defining the caudal ventral striatum in primates: cellular and histochemical features. J Neurosci. 2002;22:10078–10082. doi: 10.1523/JNEUROSCI.22-23-10078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology and neuropsychology of the frontal lobe. Lippincott-Raven; Philadelphia, PA: 1997. [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gloor P. Comparative anatomy of the temporal lobe and of the limbic system. In: Gloor P, editor. The Temporal Lobe and Limbic System. Oxford University Press; USA: 1997. pp. 21–111. [Google Scholar]
- Goldman-Rakic PS. Prenatal formation of cortical input and development of cytoarchitectonic compartments in the neostriatum of the rhesus monkey. J Neurosci. 1981;1:721–735. doi: 10.1523/JNEUROSCI.01-07-00721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58:601–622. [PubMed] [Google Scholar]
- Goldman-Rakic PS. The Prefrontal Cortex: Executive and Cognitive Functions. Oxford University Press; Oxford, UK: 1998. [Google Scholar]
- Goldman PS, Nauta WJ. An intricately patterned prefronto-caudate projection in the rhesus monkey. J Comp Neurol. 1977;72:369–386. doi: 10.1002/cne.901710305. [DOI] [PubMed] [Google Scholar]
- Goursaud AP, Bachevalier J. Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala and orbital frontal cortex. Behav Brain Res. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grafman J. In: The Frontal Lobes. Stuss DTH, Knight RT, editors. Oxford University Press; Oxford, UK: 2002. pp. 292–310. [Google Scholar]
- Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327:307–311. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Gray JA. The psychophysiological nature of introversion-extraversion: A modification of Eysenck’s theory. Academic Press; New York: 1972. [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm S, Leibenluft E, Pine DS, Ernst M. A Developmental Examination of Amygdala Response to Facial Expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, De Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Hemphill M, Holm G, Crutcher M, Delong M, Hedreen J. Afferent connections of the nucleus accumbens in the monkey. In: Chronister R, DeFrance J, editors. Neurobiology of the Nucleus Accumbens. Oxford University Press; Oxford: 1981. pp. 75–81. [Google Scholar]
- Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci. 1991;105:826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. J Neurosci. 2006;26:3791–3797. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James FO, Cermakian N, Boivin DB. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep. 2007;30:1427–1436. doi: 10.1093/sleep/30.11.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res. 2000;126:433–453. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]
- Jerison H. Evolution of the Brain and Intelligence. Academic Press; New York: 1973. [Google Scholar]
- Johnson LR, Aylward RL, Hussain Z, Totterdell S. Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience. 1994;61:851–865. doi: 10.1016/0306-4522(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Burton H, Porter R. Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol. 1977;173:53–80. doi: 10.1002/cne.901730105. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The cortico-striate projection in the monkey. Brain. 1970;93:525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Perceptual and Motor Skills. 2004;99:371–391. doi: 10.2466/pms.99.2.371-391. [DOI] [PubMed] [Google Scholar]
- Klein-Tasman BP, Mervis CB. Distinctive personality characteristics of 8-, 9-, and 10-year-olds with Williams syndrome. Dev Neuropsychol. 2003;23:269–290. doi: 10.1080/87565641.2003.9651895. [DOI] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. 1939. J Neuropsychiatry Clin Neurosci. 1997;9:606–620. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. J Comp Pysiol Psychol. 2003;47:419–427. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Piecinski P, Rakic P. Neurogenesis of the amygdaloid nuclear complex in the rhesus monkey. Brain Res Dev Brain Res. 1992;68:9–15. doi: 10.1016/0165-3806(92)90242-o. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc Natl Acad Sci U S A. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishio K, Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J Comp Neurol. 1994;350:337–356. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- Kunzle H. An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (areas 6 and 9) in macaca fascicularis. Brain Behav Evol. 1978;15:185–234. doi: 10.1159/000123779. [DOI] [PubMed] [Google Scholar]
- Kunzle H, Akert K. Efferent connections of cortical, area 8 (frontal eye field) in Macaca fascicularis. A reinvestigation using the autoradiographic technique. J Comp Neurol. 1977;173:147–164. doi: 10.1002/cne.901730108. [DOI] [PubMed] [Google Scholar]
- Lange C. A philosophical essay on probabilities. Republished by Dover (New York); 1887. Ueber Gemuthsbewgungen. 3, 8. Laplace, P. S. De (1796) 1951. [Google Scholar]
- Lapper SR, Smith Y, Sadikot AF, Parent A, Bolam JP. Cortical input to parvalbumin-immunoreactive neurones in the putamen of the squirrel monkey. Brain Res. 1992;580:215–224. doi: 10.1016/0006-8993(92)90947-8. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee GP, Arena JG, Meador KJ, Smith JR, Loring DW, Flanigin HF. Changes in autonomic responsiveness following bilateral amygdalotomy in humans. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:119–129. [Google Scholar]
- Lee GP, Reed MF, Meador KJ, Smith JR, Loring DW. Is the amygdala crucial for cross-modal association in humans? Neuropsychologia. 1995;9:236–245. [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. J Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, MJ O, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. J Neurosci. 2006;26:6077–6081. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Mesulam M. The human frontal lobes: transcending the default mode through contingent encoding in Principles of Frontal Lobe Function. Oxford University Press; Oxford: 2000. [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Malkova L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, Berman KF. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Meyer G, Gonzalez-Hernandez T, Carrillo-Padilla F, Ferres-Torres R. Aggregations of granule cells in the basal forebrain (islands of Calleja): Golgi and cytoarchitectonic study in different mammals, including man. J Comp Neurol. 1989;284:405–428. doi: 10.1002/cne.902840308. [DOI] [PubMed] [Google Scholar]
- Michael RP, Clancy AN, Zumpe D. Mating activates estrogen receptor-containing neurons in the female monkey brain. Physiol Behav. 2005;85:404–413. doi: 10.1016/j.physbeh.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Michael RP, Rees HD. Autoradiographic localization of 3H-dihydrotestosterone in the preoptic area, hypothalamus, and amygdala of a male rhesus monkey. Life Sci. 1982;30:2087–2093. doi: 10.1016/0024-3205(82)90450-7. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, O’Rahilly R. The human brain at stages 21–23, with particular reference to the cerebral cortical plate and to the development of the cerebellum. Anat Embryol (Berl) 1990;182:375–400. doi: 10.1007/BF02433497. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Marzella SR, Palumbo A. Expression of c-fos, fos B, and egr-1 in the medial preoptic area and bed nucleus of the stria terminalis during maternal behavior in rats. Brain Res. 1998;792:348–352. doi: 10.1016/s0006-8993(98)00257-1. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann N Y Acad Sci. 2007;1121:254–272. doi: 10.1196/annals.1401.036. [DOI] [PubMed] [Google Scholar]
- O’Rourke H, Fudge JL. Distribution of serotonin transporter labeled fibers in amygdaloid subregions: implications for mood disorders. Biol Psychiatry. 2006;60:479–490. doi: 10.1016/j.biopsych.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol. 2001;64:251–267. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Owen AM, Morris RG, Sahakian BJ, Polkey CE, Robbins TW. Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain. 1996;119:1597–1615. doi: 10.1093/brain/119.5.1597. [DOI] [PubMed] [Google Scholar]
- Pare D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Newman SW. Fos-immunoreactivity within the extended amygdala is correlated with the onset of sexual satiety. Horm Behav. 1998;34:17–29. doi: 10.1006/hbeh.1998.1459. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol Psychiatry. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Segall LA, Harbour VL, Woodside B, Amir S. The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. Proc Natl Acad Sci U S A. 2006;103:5591–5596. doi: 10.1073/pnas.0601310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Pickering AD, Gray JA. The Neuroscience of Personality. In: Pervin LA, John OP, editors. Handbook of Personality: Theory and Research. Guilford Press; 2001. pp. 277–299. [Google Scholar]
- Pitkanen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Hum Reprod Update. 2004;10:67–77. doi: 10.1093/humupd/dmh001. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol. 1981;198:121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Russchen FT. The limbic region. II The amygdaloid complex. In: Hokfelt BT, Swanson LW, Amsterdam E, editors. Handbook of Neuroanatomy. 1987. pp. 279–381. [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, Reynolds MF, Kwon H, Galaburda A. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci. 2004;24:5009–5015. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Seminars in the neurosciences. Saunders; London, UK: 1992. [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1433–1443. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. Anat Rec. 2004;281A:1212–1225. doi: 10.1002/ar.a.20126. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J Comp Neurol. 2001;439:208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985;329:241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrieau A, Dussaillant M, Agid F, Philibert D, Agid Y, Rostene W. Autoradiographic localization of glucocorticosteroid and progesterone binding sites in the human post-mortem brain. J Steroid Biochem. 1986;25:717–721. doi: 10.1016/0022-4731(86)90300-6. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Rosene DL, Van Hoesen GW. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. J Comp Neurol. 1988;271:185–207. doi: 10.1002/cne.902710203. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Stalnaker TA. Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Ann N Y Acad Sci. 2007;1121:320–335. doi: 10.1196/annals.1401.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Nickel J, Azari NP. Functional modularity of the medial prefrontal cortex: involvement in human empathy. Neuropsychology. 2006;20:743–751. doi: 10.1037/0894-4105.20.6.743. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J. Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. J Neurosci. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philos Trans R Soc Lond B Biol Sci. 1996;351:1405–1411. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Sharpe NA, Tepper JM. Postnatal development of excitatory synaptic input to the rat neostriatum: an electron microscopic study. Neuroscience. 1998;84:1163–1175. doi: 10.1016/s0306-4522(97)00583-6. [DOI] [PubMed] [Google Scholar]
- Shaw P, Bramham J, Lawrence EJ, Morris R, Baron-Cohen S, David AS. Differential effects of lesions of the amygdala and prefrontal cortex on recognizing facial expressions of complex emotions. J Cogn Neurosci. 2005;17:1410–1419. doi: 10.1162/0898929054985491. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lawrence EJ, Radbourne C, Bramham J, Polkey CE, David AS. The impact of early and late damage to the human amygdala on ‘theory of mind’ reasoning. Brain. 2004;127:1535–1548. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Lunday L, Miner C, Roth TL, Sullivan RM. Development switch in neural circuitry underlying odor-malaise learning. Learn Mem. 2006;13:801–808. doi: 10.1101/lm.316006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, Dehaene S. Brain mechanisms of serial and parallel processing during dual-task performance. J Neurosci. 2008;28:7585–7598. doi: 10.1523/JNEUROSCI.0948-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999a;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999b;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Stanescu CR, Varlet P, Beuvon F, Daumas DC, Devaux B, Chassoux F, Fredy D, Meder JF. Dysembryoplastic neuroepithelial tumors: CT, MR findings and imaging follow-up: a study of 53 cases. J Neuroradiol. 2001;28:230–240. [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey: I. Subcortical pathways and topography of striatal and thalamic terminal fields. J Comp Neurol. 1988;271:473–492. doi: 10.1002/cne.902710402. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: a retrograde tracing study. J Comp Neurol. 2000;421:52–79. doi: 10.1002/(sici)1096-9861(20000522)421:1<52::aid-cne4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Clark RE, Zola SM. Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behav Neurosci. 2003;117:1029–1043. doi: 10.1037/0735-7044.117.5.1029. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Belsky J. A sociobiological perspective on psychopathology in adolescence. In: Cicchetti D, Toth S, editors. Rochester Symposium on Developmental Psychopathology. University of Rochester Press; Rochester, NY: 1996. pp. 93–124. [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, LeDoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Tranel D, Hyman BT. Neuropsychological correlates of bilateral amygdala damage. Arch Neurol. 1990;47:349–355. doi: 10.1001/archneur.1990.00530030131029. [DOI] [PubMed] [Google Scholar]
- Turner BH, Mishkin M, Knapp M. Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. J Comp Neurol. 1980;191:515–543. doi: 10.1002/cne.901910402. [DOI] [PubMed] [Google Scholar]
- van der Kooy D. Early postnatal lesions of the substantia nigra produce massive shrinkage of the rat striatum, disruption of patch neuron distribution, but no loss of patch neurons. Brain Res Dev Brain Res. 1996;94:242–245. doi: 10.1016/0165-3806(96)00062-4. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Yeterian EH, Lavizzo-Mourey R. Widespread corticostriate projections from temporal cortex of the rhesus monkey. J Comp Neurol. 1981;199:205–219. doi: 10.1002/cne.901990205. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. Passive cable properties of dendritic spines and spiny neurons. Journal of Neuroscience. 1984:281–297. doi: 10.1523/JNEUROSCI.04-01-00281.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MM, Schultheiss OC. Effects of affiliation arousal (hope of closeness) and affiliation stress (fear of rejection) on progesterone and cortisol. Horm Behav. 2006;50:786–795. doi: 10.1016/j.yhbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Yachnis AT, Roper SN, Love A, Fancey JT, Muir D. Bcl-2 immunoreactive cells with immature neuronal phenotype exist in the nonepileptic adult human brain. J Neuropathol Exp Neurol. 2000;59:113–119. doi: 10.1093/jnen/59.2.113. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Di X, Movsesyan V, Mullins PG, Wang G, Boulares H, Zhang J, Xu M, Faden AI. Presence of DNA fragmentation and lack of neuroprotective effect in DFF45 knockout mice subjected to traumatic brain injury. Mol Med. 2001;7:205–216. [PMC free article] [PubMed] [Google Scholar]
- Yakovlev P. The mylogenetic cycles of regional maturation of the brain. Blackwell Scientific Publishing; Boston: 1967. [Google Scholar]
- Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol. 1991;312:43–67. doi: 10.1002/cne.903120105. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Van Hoesen GW. Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res. 1978;139:43–63. doi: 10.1016/0006-8993(78)90059-8. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Yucel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, Clarke K, Phillips ML, Kyrios M, Velakoulis D, Pantelis C. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64:946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Is the caudomedial shell of the nucleus accumbens part of the extended amygdala? A consideration of connections. Crit Rev Neurobiol. 1998;12:245–265. doi: 10.1615/critrevneurobiol.v12.i3.50. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Jensen SL, Williams ES, Martin JR., III Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur J Neurosci. 1999;11:1119–1126. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalla T, Koechlin E, Pietrini P, Basso G, Aquino P, Sirigu A, Grafman J. Differential amygdala responses to winning and losing: a functional magnetic resonance imaging study in humans. Eur J Neurosci. 2000;12:1764–1770. doi: 10.1046/j.1460-9568.2000.00064.x. [DOI] [PubMed] [Google Scholar]
- Zirlinger M, Kreiman G, Anderson DJ. Amygdala-enriched genes identified by microarray technology are restricted to specific amygdaloid subnuclei. Proc Natl Acad Sci U S A. 2001;98:5270–5275. doi: 10.1073/pnas.091094698. [DOI] [PMC free article] [PubMed] [Google Scholar]