Abstract
The 66 KDa isoform of Shc and its signalling properties have attracted in the past years major interest in aging research. Here, we summarize p66Shc functions and outline a specific signalling route leading to mitochondrial import, that accounts for its pro-apoptotic activity upon oxidative stress. This model, that could explain the alterations of mitochondrial Ca2+ homeostasis observed after oxidative stress, highlights novel pharmacological targets in age-related disorders.
Keywords: calcium homeostasis, apoptosis, ROS, cell death, Pin1, PKC
A Multifactorial View of Aging
Aging, the process of organism deterioration with time depends on a combination of genetic and environmental factors. Maximum life span is not entirely clock-driven as it is influenced by exposure to damage or, conversely, the enhanced efficiency of somatic maintenance functions.
Thus, there is no single route to cell aging, but rather the synergy or antagonism of multiple mechanisms. A central problem in aging studies is understanding how cells accumulate damage through time and how changes at the cellular level produce age-related dysfunction and disease within tissues and organs. A leading theory of aging is based on the concept that damage, either due to normal toxic products of metabolism or inefficient repair/defensive systems, accumulates throughout the entire lifespan and gradually leads to organ dysfunction.1,2
There is substantial support for a role of reactive oxygen species (ROS) in the aging process. The free radical theory of aging proposed by Harman in 1956 suggested that ROS are responsible for macromolecular damage leading to age-related degeneration.3 Oxidative stress and altered mitochondrial function have consistently been proposed to parallel organism deterioration, and overexpression of antioxidants have shown to extend the lifespan of transgenic animals.4–6
Different types of antioxidants exhibit protective effects, ranging from vitamins C and E to enzymes such as superoxide dismutase, catalase and glutathione peroxidase. Indeed, mammalian cells posses a broad repertoire of ROS scavengers, directly proving the potential hazard imposed by ROS exposure.
The identification of single genes that extend lifespan in diverse model organisms has revealed an additional surprising result: many of these deficiencies that extend lifespan cluster to what appears to be a common pathway, the IGF-1/Insulin like signalling pathway.7 Members of this pathway have been discovered to extend maximum lifespan in organisms as diverse as Caenorhabditis elegans,8 Drosophila melanogaster9 and Mus musculus,10 suggesting that a similar mechanism could operate in humans.
The only non-genetic mechanism known to extend lifespan in mice is caloric restriction. Caloric restriction also appears to function across species boundaries, and affects many biochemical parameters. Thus it appears to underlie a complex mechanism, that cannot be explained by a simple reduction in metabolism or slower growth of the organism.11–13 Interestingly, in Caenorhabditis elegans, the transcription factor PHA-4 (orthologous to genes encoding the mammalian family of Foxa transcription factors) has been recently shown to play a role in lifespan control by dietary restriction, because it is not required for the increased longevity caused by other genetic pathways that regulate aging. Moreover, the PHA-4 protein seems to act independently from the insulin pathway.14
In the past years, another gene and its signalling properties have attracted major interest in aging research: i.e., that encoding the 66 KDa isoform of Shc (p66Shc).15–20
The proto-oncogenes Shc were initially recognized as ‘adaptor’ proteins, which specifically bind to phosphorylated tyrosines on the cytoplasmic motif of growth factor receptors. In mice, three isoforms of Shc are expressed, of 46, 52 and 66 kD.21 All three isoforms of Shc are tyrosine-phosphorylated after growth factor receptor activation, and form stable complexes with Grb2, an adaptor protein for the Ras exchange factor SOS. However, p66Shc has little, if any, effect on Ras or, more in general, Ras-downstream effectors (Raf-1, MAPK), strongly indicating that the target of p66Shc is not Ras (Bonfini et al., 1996). p66Shc is activated by oxidative stress,22 through the phosphorylation of a critical serine (Ser36). After dissociation from an inhibitory complex,23 activated p66Shc sensitises to apoptotic stimuli, and ablation of the p66Shc gene causes lifespan prolongation, with no pathological consequence (such as endocrinological abnormalities or increase in tumor frequency).18 Mouse Embryo Fibroblasts (MEFs) from p66Shc−/− transgenics are resistant to apoptotic death induced by oxidative stress.18 This death is p53-dependent, and knockout of either p53 or p66Shc causes resistance, suggesting that p66Shc is downstream of p53 in the pathway from ROS to apoptosis.19
Mitochondrial Ca2+ Signals in Cell Death
Apoptosis, the process that allows multicellular organisms to eliminate unnecessary, dangerous or damaged cells without evoking inflammation or tissue damage, takes place in a wide number of physiological and pathological situations, minimizing risk to the overall organism.24 However, the occurrence of apoptosis in nonproliferating cells could underlie degenerative disorders and even the progressive loss of organ function during aging.25,26 Thus, an understanding of the regulation of apoptosis is potentially very important for the mechanistic understanding of several pathophysiological conditions, including those occurring in aging, and may support the development of new therapeutic interventions.
In the past years, mitochondria have been shown to be a critical checkpoint, capable of releasing structural components into the cytoplasm of cells doomed to die via apoptosis.27 These proteins normally retained in the organelle (that include an important component of the respiratory chain, cytochrome c, as well as newly discovered proteins, such as AIF and Smac/Diablo) are released into the cytoplasm, where they activate effector caspases and drive cells to apoptotic cell death.28 Interestingly, Ca2+ has been shown to play an important function in this process, thus representing a complex signal within the organelle, that can be decoded into radically different biological consequences (reviewed in refs. 29 and 30–34). Indeed, work from various labs has revealed that the alteration of the Ca2+ signal reaching the mitochondria and/or the combined action of apoptotic agents or pathophysiological conditions (e.g., oxidative stress, Cadmium injury) can induce a profound alteration of organelle structure and function.35,36 Different effects have been described. Szalai et al. showed in hepatocytes that, upon treatment with sub-optimal doses of the lipid mediator of apoptosis ceramide, the repetitive spiking of cytosolic Ca2+ concentration ([Ca2+] c) rather than triggering the activation of matrix dehydrogenases (and thus the stimulation of aerobic metabolism), causes PTP opening, mitochondrial swelling and the release of cytochrome c.37 Thus, a mechanism of coincidence detection of physiological agonists and apoptotic stimuli allows the specific induction of the apoptotic program (with mitochondria acting as the site where this differential decoding is operated). Subsequently, we have shown that in HeLa cells ceramide directly causes the release of Ca2+ from the Endoplasmic reticulum (ER), and Ca2+ loading in mitochondria possibly in combination with a direct activity of the lipid mediator causes the morphological alteration of the organelle (fragmentation, swelling) and the ensuing release of cytochrome c and other caspase cofactors.35
In all cases, the amount of Ca2+ released from the ER and the ability of mitochondria to accumulate it appear to play a critical role. In agreement with this view, the antiapoptotic Bcl-2 protein protects cells from C2 ceramide induced cell death by decreasing the releasable Ca2+ pool of the ER38 while the ER-resident fraction of the proapoptotic Bax protein increases the steady state of ER Ca2+ concentration ([Ca2+]er), thus amplifying Ca2+-mediated cell death.39,40 Conversely, amplification of Ca2+ signals has a pro-apoptotic effect. In this respect, both in neurons and in hepatic cells it was observed that apoptotic signals (staurosporin in the former case, the proapoptotic X protein of Hepatitis B virus in the latter) cause the activation of caspase 3, with ensuing cleavage of the plasma membrane Ca2+ ATPase (that contain a consensus for caspase cleavage in its sequence).41,42 Thus, the mechanism that allows the termination of the agonist induced Ca2+ signals is impaired, and [Ca2+]c rises are enhanced and prolonged. This causes mitochondrial Ca2+ overload and the apoptotic morphological and functional alterations of the organelles.30
Oxidative Stress, p66Shc and Mitochondrial Signalling
Compared to the knowledge on the role of Ca2+ and mitochondria (for which we summarized above a broad body of data), the information on oxidative stress in mitochondrial signalling is very scant, and its mechanism of action and real significance in apoptosis and are still largely unclear.
Among the most important players of oxidative stress are ROS originating from exogenous sources such as ultraviolet and ionizing radiations, or from several intracellular sources. Mitochondria are quantitatively the most important source of intracellular ROS and leak from the electron transfer chain is supposed to be the main route.43 Recently, a totally new, unexpected pathway has emerged, that involves p66Shc in mitochondria ROS production. In higher organisms about 20% of fibroblast p66Shc is localized to mitochondria and, in response to oxidative stress, part of the cytosolic pool of p66Shc translocates to mitochondria.23 Within mitochondria p66Shc then binds cytochrome c and acts as an oxidoreductase, shuttling electrons from cytochrome c to molecular oxygen.44 The redox activity of p66Shc is likely to be the explanation of the increase in ROS production caused by the recombinant expression of p66Shc, and the decrease in ROS levels typical of p66Shc knockout cells.45
ROS production in mitochondria is regulated by metabolic activity, substrate supply, and mitochondrial membrane potential. Interestingly, p66Shc−/− fibroblasts have altered mitochondrial bio-energetic properties. In particular, p66Shc−/− cells have a reduction in both their resting and maximal oxidative capacity, suggesting that the ablation of p66Shc may extend life span by repartitioning metabolic energy conversion away from oxidative and toward glycolytic pathways.46
Given the information summarized above, the mitochondrial effects of p66Shc in response to oxidative stress are of great interest and significance. Indeed, p66Shc is one of a handful of identified mammalian aging genes, and it is one of three with a small effect on body size.47 One interpretation of the data is that p66Shc mediates deleterious triggering of apoptosis,48 and its ablation induces a significant expansion of the lifespan. The mitochondrial effects of p66Shc may represent the apoptotic “switch,” by controlling the increased mitochondrial production of ROS, and a variety of consequences on mitochondrial functions such as Ca2+ homeostasis and organelle morphology.
The Effects of p66Shc on Mitochondrial Ca2+ Signalling
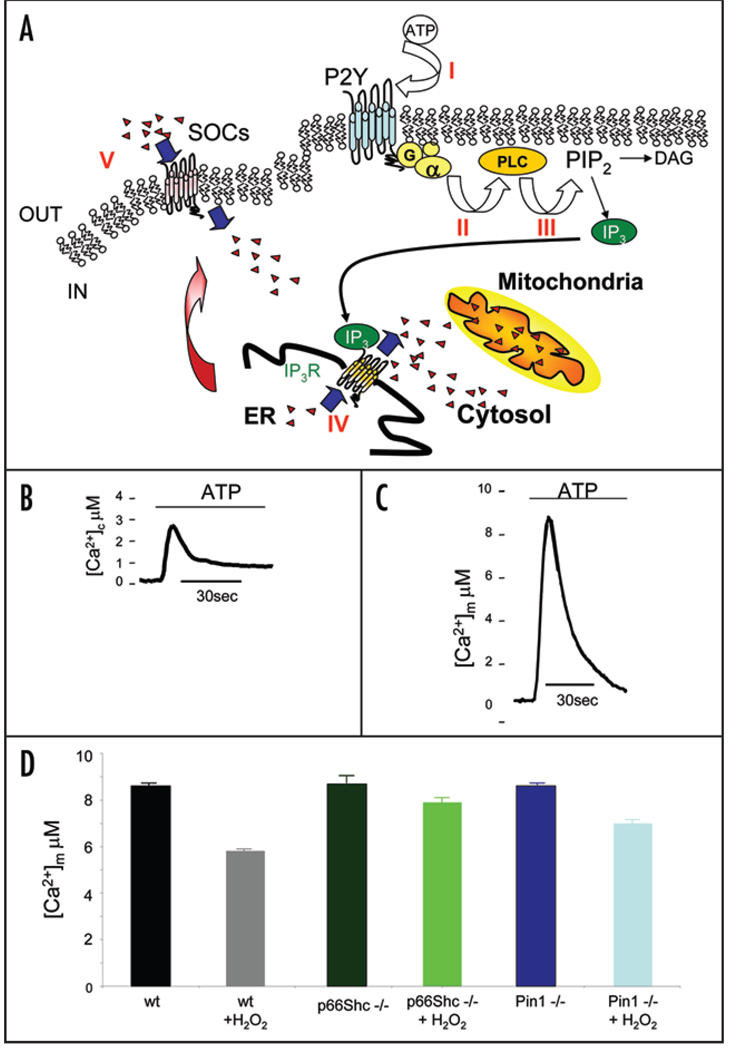
Mitochondrial Ca2+ homeostasis is dramatically compromised during apoptotic cell death. In MEFs, application of ATP, an extra-cellular agonist acting on a Gq-coupled P2Y receptor, causes the production of inositol 1,4,5 trisphosphate and thus the release of Ca2+ from the ER and the transient increase of cytosolic (Fig. 1B) and mitochondrial [Ca2+] (Fig. 1C). In wild-type MEFs, applying oxidative stress causes a drastic reduction in the Ca2+ spike evoked by agonist stimulation (Fig. 1D), as an early consequence of mitochondrial damage. The ablation of the p66Shc gene confers mitochondria insensitiveness to oxidative stress. Indeed the mitochondrial Ca2+ response of p66Shc−/− MEFs (Fig. 1D) is almost unaffected by the ROS-induced cell death.49
Figure 1.
(A) Intracellular Ca2+ homeostasis during agonist stimulation. When an extracellular agonist, e.g., ATP, binds its receptor (I), a Gq-coupled receptor is activated (II) with consequent stimulation of phospholipase C (III). Thus, phosphatidylinositol-4,5-bisphosphate hydrolysed to diacylglycerol and IP3, which diffuses through the cytosol and binds to its IP3 receptors on internal stores of Ca2+. Ca2+ is released (IV) and an increase of [Ca2+]c and [Ca2+]m occurs. Emptying of the store is detected and is transmitted to the store-operated channel (SOCs) to induce Ca2+-influx across the plasma membrane (V). (B and C) Subcellular analysis of Ca2+ homeostasis in MEFs. The traces show representative [Ca2+] measurements performed in the cell cytoplasm (B) and mitochondria (C). To monitor [Ca2+] in the different compartments MEFs were transfected with specifically targeted aequorin chimeras.56 Cells were perfused with Krebs-Ringer saline solution and challenged, where indicated, with 100 µM ATP. (D) Agonist dependent mitochondrial Ca2+ response in MEFs wt, p66Shc−/− and Pin1−/− in control and after H2O2 treatment. Mitochondrially targeted aequorin (mtAEQ)—transfected cells were treated with 100 µM ATP.
The effect of the oxidizing agent was dose-dependent and developed through time, as revealed by the experiment in which the reduction of the [Ca2+]m peak in p66Shc−/− and control cells was correlated with the H2O2 concentration and with the duration of its application. In control cells, the effect ranged from a ~25% to ~50% reduction for an application of 1 mM for 5 and 180 min, respectively, to ~10% to ~30% reductions for 30 minutes applications of doses from 0.4 to 1 mM. In all cases, the effect was drastically smaller in p66Shc−/− cells, in which at the highest dose and application time the reduction was <20%. This alteration was uniquely mitochondrial, as no difference was detected between p66Shc−/− and control H2O2-treated cells in the [Ca2+]c responses evoked by ATP application. As to the effect on organelle morphology, a major mitochondrial alteration (fragmentation and swelling) was detected in p66Shc+/+, but not in p66Shc−/− cells, after application of the oxidative stress.49
These data do not reveal the mechanism that links oxidative stress to the activation of the “aging” (pro-apoptotic) effect of p66Shc. To address this issue, we focused our attention on experimental evidence, on the site in the p66Shc molecule that appears to be targeted by the oxidative trigger.44 Upon oxidative challenge, Ser36 is phosphorylated, and mutagenesis of Ser36 results in a p66Shc variant incapable of inducing apoptosis. Thus, Ser36 appears to be a critical regulatory site for the apoptotic activity of p66Shc. We thus looked for the kinase pathways leading to Ser36 phosphorylation.
Several pieces of indirect evidence suggested that a PKC-mediated signalling route could play an important role in linking mitochondrial ROS production to the aging effects of p66Shc. PKCs form a heterogeneous group of protein kinases differing in their stimulatory mechanisms (the so called “classical” are activated by Ca2+ and diacylglycerol, the “novel” by diacylglycerol alone, and the “atypical” are insensitive to both), substrate specificity, and cellular distribution. This allows them to play very different functions, and indeed, as far as apoptosis is concerned, different PKC isoforms have been demonstrated to play opposite roles, i.e., to stimulate (e.g., PKCα) or inhibit (e.g., PKCζ) the apoptotic process. The molecular routes by which PKCs intervene in the apoptotic process are however largely unknown. We investigated whether PKC recruitment could affect mitochondrial structure and function. Again, in a first series of experiments, attention was focused on Ca2+ signalling, which controls key organelle activities yet is promptly affected by relatively minor mitochondrial dysfunctions. In these experiments, we co-transfected the PKC isoform of interest and targeted probes for the mitochondria, the cytosol and the intracellular Ca2+ store (the ER). This allowed us not only to observe alterations in mitochondrial Ca2+ homeostasis, but also to clarify whether the alteration, if detected, reflects a primary mitochondrial effect or is rather a consequence of a more general modification of cellular Ca2+ signalling. The experiments provided a very diversified and interesting picture. Indeed, they revealed that some PKC isoforms, such as PKCε, do not affect Ca2+ homeostasis in any investigated compartment, while conversely PKCα induces a global alteration (a reduction in Ca2+ release from the ER, with Ca2+ signals thus detected both in the cytosol and in the mitochondria). However, a few isoforms displayed a “pure” mitochondrial effect: they did not affect Ca2+ release from the ER (and thus the Ca2+ rise in the cytosol), but increased (PKCζ) or decreased (PKCβ) the mitochondrial Ca2+.50 Thus, they probably act on a mitochondrial effector that either stimulates the Ca2+ uptake machinery or affects the thermodynamic driving force for cation accumulation into the matrix.
Interestingly, PKCβ is activated by oxidative stress and it is required for phosphorylation of the Ser36 of p66Shc, suggesting that p66Shc is the pro-apoptotic (i.e., “aging”) effector system. A series of experiments suggest that this is the key mechanism. First, the effect of PKCβ overexpression on mitochondrial Ca2+ signalling was not observed in p66Shc−/− cells (implying that p66Shc is a downstream effector of this kinase pathway), and interestingly, the mitochondrial consequences of hydrogen peroxide are also blocked by hispidin, a specific PKCβ inhibitor. Together, these results highlight a novel, unexpected signalling pathway bridging the oxidative challenge of a cell to the activation of the p66Shc-controlled apoptotic pathway: PKCβ is activated by oxidation, causes phosphorylation of p66Shc and triggers its mitochondrial pro-apoptotic effects. But, is mitochondrially localized p66Shc phosphorylated on Ser36 in response to oxidative challenges? The answer is no, and this apparent contradiction is solved by an other intriguing player in this process: the peptidyl-prolyl-isomerase Pin1, which induces conformational changes in phosphorylated proteins in response to different cellular challenges, including oxidative stress. Pin1 catalyzes conformational changes in a large number of phosphorylated proteins, and thus it has been implicated in the regulation of major cellular events, such as cell cycle progression, transcription, proliferation and differentiation (reviewed in refs. 51 and 52). Pin1 recognizes a phosphorylated sequence present in p66Shc and phosphorylated by PKC-beta. Moreover, it has been shown that, the phosphorylated sites, once isomerized by Pin1 become targets for dephosphorylation by PP2A.51 In Pin1−/− cells the H2O2-dependent reduction of the [Ca2+]m rise was markedly smaller than in control cells as well as alterations of mitochondrial morphology in Pin1−/− were minimal (Fig. 1D). Even more interestingly, in Pin1−/− cells, the mitochondrial fraction of p66Shc was markedly smaller, both at rest (reflecting probably the basal inputs occurring in culture conditions) and after the oxidative challenge or treatment with PKCβ activators. However, in Pin1−/− MEFs, a modest increase after H2O2 treatment (not reaching the resting levels of wt MEFs) was observed and it was not reduced by hispidin, revealing that other PKCβ and Pin1-independent stress pathways may cooperate, with a minor role, in p66Shc translocation during oxidative stress conditions. On contrary, the p66Shc translocation induced by TPA treatment (and therefore by PKC) is strictly Pin1 dependent as no p66Shc translocation was observed in Pin1−/− under those conditions.
The Model
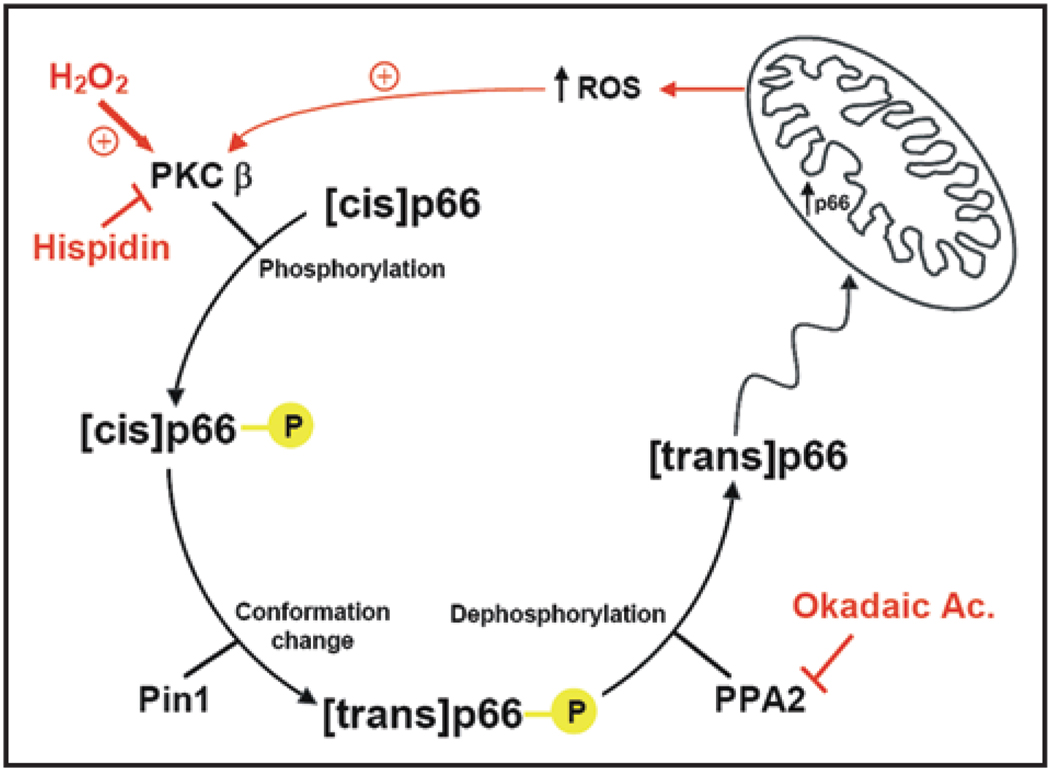
The pathway emerging from these data is the following (Fig. 2): during oxidative stress PKCβ is activated and induces p66Shc phosphorylation, thus allowing p66Shc to be recognized by Pin1, isomerised and imported into mitochondria after dephosphorylation by type 2 protein serine/threonine phosphatase (PP2A). At this point, the protein translocated into the appropriate cell domain, can exert the oxidoreductase activity, generating H2O2 and inducing the opening of PTP. This event in turn perturbs mitochondria structure and function (as revealed by the reduced Ca2+ responsiveness and the alteration of mitochondrial three-dimensional structure). Caspase cofactors, such as cytochrome c, are released and the cell progresses into apoptosis. By this way p66Shc may regulate the mitochondrial clock controlling the lifespan. Overall, these results thus identify and clarify a novel signalling mechanism, that is operative in the pathophysiological condition of oxidative stress, and may open new possibilities for pharmacologically addressing the process of organ deterioration during aging. In addition to this route, p66Shc appears to act through different pathways. Indeed, it has been shown that p66Shc enhances oxidative stress-induced apoptosis also by participating in the phosphorylation-induced repression of Forkhead transcription factors, which regulate expression of several antioxidant enzymes.53 Consistent with this, p66Shc knockout mice exhibit higher catalase activity, and mice with extra catalase in their mitochondria lived about 20% more than controls and were less likely to develop cataracts, but they did not appear to age more slowly and their extended lifespan appeared to derive from a decrease in cardiac diseases throughout the entire lifespan54 further emphasizing the mitochondrial role of p66Shc.
Figure 2.
Signal transduction pathway of p66Shc in oxidative condition. PKCβ is activated by H2O2 (ROS) and induces p66Shc phosphorylation thus allowing p66Shc to be recognized by Pin1, isomerised and imported into mitochondria where induces the opening of PTP. This event in turn perturbs mitochondria structure and function (as revealed by the reduced Ca2+ responsiveness and the alteration of mitochondrial three-dimensional structure). Caspase cofactors, such as cytochrome c, are released and the cell progresses into apoptosis.
Finally, it should be remembered that the “aging” role of p66Shc appears in contrast with a recent publication showing that p66Shc is highly expressed in fibroblasts from centenarians.55 An intriguing reconciling hypothesis could be the existence of some defects on the mitochondrial pathway of p66Shc (presented in Fig. 2) on centenarians that prevents the mitochondrial import of p66Shc and in turn its pro-aging properties, while leaving other biological functions (e.g., the production of low levels of ROS necessary in differentiation) unaffected.
Acknowledgements
The authors are deeply indebted to past and present collaborators. This work was supported by Telethon grant GGP05284, the Italian Association for Cancer Research (AIRC), local funds from the University of Ferrara, the Italian University Ministry, the EU (fondi strutturali Obiettivo 2), the PRRIITT program of the Emilia Romagna Region, the Italian Space Agency (ASI), NIH (Grant: A mitochondrial longevity pathway: p66shc mechanisms) and the United Mitochondrial Disease Foundation (UMDF).
References
- 1.Rattan SI. Theories of biological aging: genes, proteins and free radicals. Free Radic Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- 2.de Magalhaes JP, Church GM. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol. 2006;41:1–10. doi: 10.1016/j.exger.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 4.Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–86. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 5.Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 6.Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, Ishizaki-Koizumi S, et al. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4:693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 7.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 8.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 9.Helfand SL, Rogina B. Genetics of aging in the fruit fly, Drosophila melanogaster. Annu Rev Genet. 2003;37:329–348. doi: 10.1146/annurev.genet.37.040103.095211. [DOI] [PubMed] [Google Scholar]
- 10.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 11.Weindruch R. Caloric restriction, gene expression and aging. Alzheimer Dis Assoc Disord. 2003;17:58–59. doi: 10.1097/00002093-200304002-00008. [DOI] [PubMed] [Google Scholar]
- 12.Ingram DK, Anson RM, de Cabo R, Mamczarz J, Zhu M, Mattison J, et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–423. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 13.Lane MA, Black A, Handy A, Tilmont EM, Ingram DK, Roth GS. Caloric restriction in primates. Ann N Y Acad Sci. 2001;928:287–295. doi: 10.1111/j.1749-6632.2001.tb05658.x. [DOI] [PubMed] [Google Scholar]
- 14.Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 15.Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- 16.Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G, et al. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, et al. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 18.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 19.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, et al. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 20.Purdom S, Chen QM. p66(Shc): at the crossroad of oxidative stress and the genetics of aging. Trends Mol Med. 2003;9:206–210. doi: 10.1016/s1471-4914(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 21.Luzi L, Confalonieri S, Di Fiore PP, Pelicci PG. Evolution of Shc functions from nematode to human. Curr Opin Genet Dev. 2000;10:668–674. doi: 10.1016/s0959-437x(00)00146-5. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson MA, Pollock SS, Coleman CN, Calderwood SK. X-irradiation, phorbol esters and H2O2 stimulate mitogen-activated protein kinase activity in NIH-3T3 cells through the formation of reactive oxygen intermediates. Cancer Res. 1994;54:12–15. [PubMed] [Google Scholar]
- 23.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, et al. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004;279:25689–25695. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 24.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hetts SW. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279:300–307. doi: 10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- 26.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 27.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 28.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 29.Distelhorst CW, Shore GC. Bcl-2 and calcium: controversy beneath the surface. Oncogene. 2004;23:2875–2880. doi: 10.1038/sj.onc.1207519. [DOI] [PubMed] [Google Scholar]
- 30.Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- 31.Hajnoczky G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochem Biophys Res Commun. 2003;304:445–454. doi: 10.1016/s0006-291x(03)00616-8. [DOI] [PubMed] [Google Scholar]
- 32.Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007;14:1267–1274. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- 33.Nicotera P, Orrenius S. Ca2+ and cell death. Ann N Y Acad Sci. 1992;648:17–27. doi: 10.1111/j.1749-6632.1992.tb24520.x. [DOI] [PubMed] [Google Scholar]
- 34.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 35.Pinton P, Ferrari D, Rapizzi E, Di Virgilio FD, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biagioli M, Pifferi S, Ragghianti M, Bucci S, Rizzuto R, Pinton P. Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium. 2007;43:184–195. doi: 10.1016/j.ceca.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, et al. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 40.Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwab BL, Guerini D, Didszun C, Bano D, Ferrando-May E, Fava E, et al. Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ. 2002;9:818–831. doi: 10.1038/sj.cdd.4401042. [DOI] [PubMed] [Google Scholar]
- 42.Chami M, Ferrari D, Nicotera P, Paterlini-Brechot P, Rizzuto R. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J Biol Chem. 2003;278:31745–31755. doi: 10.1074/jbc.M304202200. [DOI] [PubMed] [Google Scholar]
- 43.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Migliaccio E, Giorgio M, Pelicci PG. Apoptosis and aging: role of p66Shc redox protein. Antioxid Redox Signal. 2006;8:600–608. doi: 10.1089/ars.2006.8.600. [DOI] [PubMed] [Google Scholar]
- 46.Nemoto S, Combs CA, French S, Ahn BH, Fergusson MM, Balaban RS, et al. The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. J Biol Chem. 2006;281:10555–10560. doi: 10.1074/jbc.M511626200. [DOI] [PubMed] [Google Scholar]
- 47.Quarrie JK, Riabowol KT. Murine models of life span extension. Sci Aging Knowledge Environ. 2004;5 doi: 10.1126/sageke.2004.31.re5. [DOI] [PubMed] [Google Scholar]
- 48.Pellegrini M, Pacini S, Baldari CT. p66SHC: the apoptotic side of Shc proteins. Apoptosis. 2005;10:13–18. doi: 10.1007/s10495-005-6057-8. [DOI] [PubMed] [Google Scholar]
- 49.Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, et al. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 50.Pinton P, Leo S, Wieckowski MR, Di Benedetto G, Rizzuto R. Long-term modulation of mitochondrial Ca2+ signals by protein kinase C isozymes. J Cell Biol. 2004;165:223–232. doi: 10.1083/jcb.200311061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wulf G, Finn G, Suizu F, Lu KP. Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat Cell Biol. 2005;7:435–441. doi: 10.1038/ncb0505-435. [DOI] [PubMed] [Google Scholar]
- 52.Joseph JD, Yeh ES, Swenson KI, Means AR, Winkler The peptidyl-prolyl isomerase Pin1. Prog Cell Cycle Res. 2003;5:477–487. [PubMed] [Google Scholar]
- 53.Smith WW, Norton DD, Gorospe M, Jiang H, Nemoto S, Holbrook NJ, et al. Phosphorylation of p66Shc and forkhead proteins mediates Abeta toxicity. J Cell Biol. 2005;169:331–339. doi: 10.1083/jcb.200410041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 55.Pandolfi S, Bonafe M, Di Tella L, Tiberi L, Salvioli S, Monti D, et al. p66(shc) is highly expressed in fibroblasts from centenarians. Mech Ageing Dev. 2005;126:839–844. doi: 10.1016/j.mad.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Pinton P, Rimessi A, Romagnoli A, Prandini A, Rizzuto R. Biosensors for the detection of calcium and pH. Methods Cell Biol. 2007;80:297–325. doi: 10.1016/S0091-679X(06)80015-4. [DOI] [PubMed] [Google Scholar]