Abstract
Low-density lipoprotein receptor-related protein 1 (LRP1) functions in endocytosis and intracellular signaling for a variety of structurally diverse ligands. Although LRP1 has been implicated in several aspects of neuronal function, molecular mechanisms underlying the activity of neuronal LRP1 remain unclear. Here, we describe a signaling pathway whereby LRP1 transactivates Trk receptors. Binding of tissue-type plasminogen activator or α2-macroglobulin (α2M) to LRP1 resulted in Src family kinase (SFK) activation and SFK-dependent Trk receptor transactivation in PC12 cells and neurons. Trk receptor transactivation was necessary for activation of Akt and extracellular signal-regulated kinase, and for neurite outgrowth downstream of LRP1. Injection of the LRP1-binding domain of α2M into rat dorsal root ganglia induced Trk receptor phosphorylation, which was blocked by receptor-associated protein, an antagonist of ligand binding to LRP1. Trk receptor transactivation provides a mechanism by which diverse LRP1 ligands may demonstrate neurotrophic activity.
INTRODUCTION
Low-density lipoprotein receptor-related protein 1 (LRP1) is a 600 kDa type 1 transmembrane receptor that mediates the endocytosis of diverse ligands, including proteases, growth factors, extracellular matrix proteins, lipoproteins, and other membrane proteins (1). LRP1 also regulates signaling directly, in response to ligand binding (2–6), as well as indirectly, by controlling the cell surface abundance of other receptors, such as urokinase-type plasminogen activator receptor (uPAR) (7) and tumor necrosis factor receptor 1 (8). LRP1, which is present in neurons (9), is implicated in diverse aspects of neuronal function, including synaptic transmission (10), neurite outgrowth (6, 11), cell survival (4), and long-term potentiation (12). LRP1 also is present in non-neuronal cells in the nervous system, where it may influence the permeability of the blood-brain barrier (13).
The cytoplasmic tail of LRP1 contains binding sites for signaling adaptor proteins, such as Shc, JIP1 [c-Jun N-terminal kinase (JNK) interacting protein 1], and JIP2 (1); however, little is known regarding how ligand binding to LRP1 initiates signaling or controls complex processes such as neurite outgrowth. We previously demonstrated that upon binding to LRP1, α2-macroglobulin (α2M) activates Akt and extracellular signal-regulated kinase (ERK) in PC12 cells and Schwann cells (6). The LRP1-binding domain of α2M (α2M RBD) showed equivalent signaling activity and promoted robust neurite outgrowth in PC12 cells. Signaling in response to the α2M RBD was blocked by co-incubation with receptor-associated protein (RAP), which inhibits ligand binding to LRP1 (14), or by LRP1 gene silencing in Schwann cells.
The goal of this study was to elucidate the pathway by which ligand binding to LRP1 promotes neuritogenesis. Our results demonstrate that tissue-type plasminogen activator (tPA) and α2M, which are structurally distinct LRP1 ligands, activate Akt and ERK1/2 and promote neurite outgrowth in PC12 cells and rat cerebellar granular neurons (CGNs) by a pathway that requires Src family kinases (SFKs) and transactivation of Trk receptors. In Schwann cells, in which TrkC is present but not TrkA (15, 16), LRP1 ligands activate ERK1/2 and Akt, but by an alternative, Trk receptor-independent pathway. Because of the variety of ligands that bind to LRP1 (1), Trk receptor transactivation downstream of LRP1 provides a mechanism by which diverse extracellular mediators may demonstrate neurotrophic activity.
RESULTS
Trk receptors are phosphorylated and necessary for activation of Akt and ERK1/2 by the α2M RBD in PC12 cells
PC12 cells were treated with the α2M RBD or with nerve growth factor β (NGF-β), which binds directly to TrkA and induces Trk receptor phosphorylation. Both proteins increased Trk phosphorylation (pTrk) to a similar extent, as assessed by immunoblotting with an antibody against phospho-Tyr490 (Fig. 1A). The α2M RBD and NGF-β also activated Akt and ERK1/2, as demonstrated previously (6). Next, PC12 cells were treated for 2 hours with the Trk receptor tyrosine kinase inhibitor, K252a (10 nM), and then with NGF-β or the α2M RBD. K252a blocked phosphorylation of Trk, Akt, and ERK1/2 in response to NGF-β, as anticipated, and also in response to the α2M RBD. These results suggested that Trk receptor tyrosine kinase activity may be required for α2M RBD-mediated activation of Akt and ERK1/2.
Fig. 1.
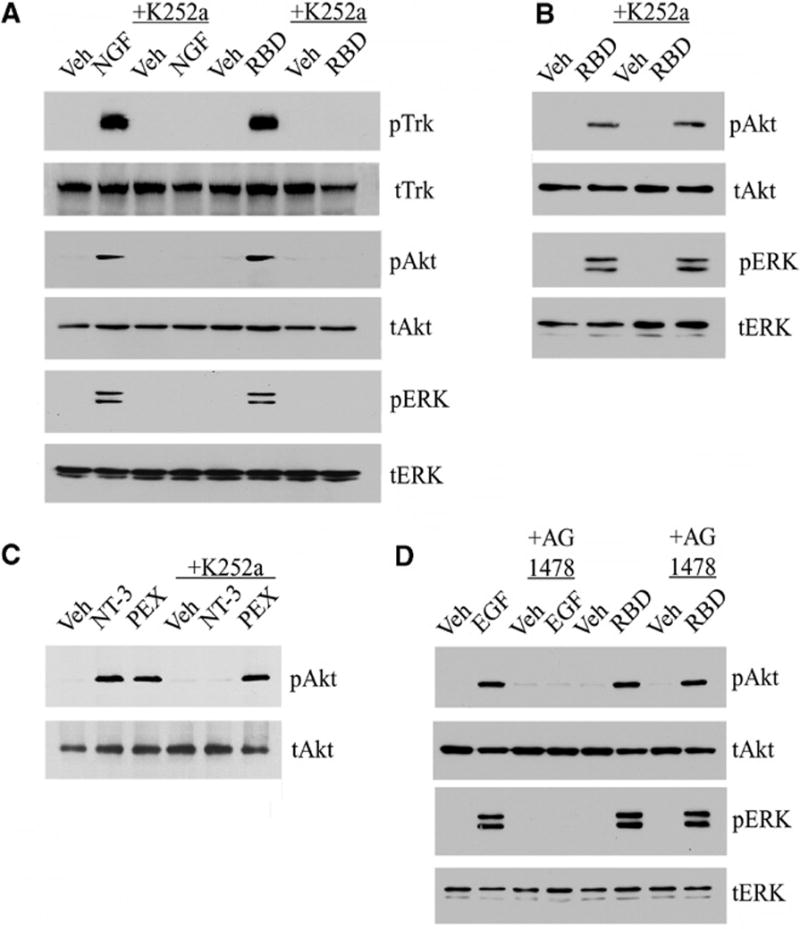
The α2M RBD induces Trk receptor phosphorylation. (A) PC12 cells were treated with serum-free medium (veh), NGF-β (100 ng/ml), or the α2M RBD (100 nM) for 10 min. Cultures were pre-treated with K252a (10 nM) for 2 hours as indicated. (B) Schwann cells were treated with the α2M RBD or vehicle after pre-treatment with K252a as indicated. (C) Schwann cells were treated with vehicle, NT-3 (10 nM), or the hemopexin domain of MMP-9 (PEX, 10 nM) for 10 min, with or without pretreatment of K252a (10 nM). (D) PC12 cells were pre-treated with AG1478 (50 nM) for 2 hours as indicated and then with EGF (10 ng/ml) or the α2M RBD (100 nM). For each experiment, cell extracts were immunoblotted for indicated proteins with antibodies specific for phosphorylated Trk (pTrk), phosphorylated Akt (pAkt), phosphorylated ERK1/2 (pERK), total Trk (tTrk), total Akt (tAkt), or total ERK1/2 (tERK).
K252a inhibits all three members of the Trk receptor family and may inhibit tyrosine kinases other than Trk receptors. Thus, as an initial specificity control, K252a was tested in primary cultures of rat Schwann cells, which have TrkC but not TrkA (15, 16). The α2M RBD activated Akt and ERK1/2 in Schwann cells (Fig. 1B), confirming our previous results (6); however, the response was not inhibited by K252a. Like the α2M RBD, the isolated hemopexin domain (PEX) of matrix metalloprotease-9 (MMP-9) binds directly to LRP1 and activates Akt and ERK1/2 (5). MMP-9 PEX activated Akt in Schwann cells and this response, like that observed with the α2M RBD, was not blocked by K252a. Neurotrophin-3 (NT-3), which binds directly to TrkC (15), was used to test whether K252a inhibits TrkC tyrosine kinase activity in Schwann cells. NT-3 activated Akt and the response was blocked by K252a (Fig. 1C), confirming that Trk receptors are inhibited by K252a in Schwann cells but not necessary for LRP1-dependent signaling to ERK1/2 and Akt.
As a second specificity control, we tested whether the epidermal growth factor (EGF) receptor, which is a receptor tyrosine kinase (RTK) like Trk, participates in LRP1-dependent signaling in PC12 cells. PC12 cells were treated with EGF or the α2M RBD in the presence and absence of the EGF receptor tyrosine kinase inhibitor, AG1478. AG1478 blocked activation of Akt and ERK1/2 in response to EGF, but not the α2M RBD (Fig. 1D). Thus, in PC12 cells, LRP1 appears to form a functional signaling system selectively with Trk receptors.
Structurally distinct LRP1 ligands activate Trk receptor-dependent signaling in PC12 cells
The α2M RBD contains only the 18 kDa LRP1-binding domain of intact α2M (17). Therefore, we examined the effects of K252a on signaling in PC12 cells in response to full-length α2M, which was purified from human plasma and converted by reaction with methylamine into the conformation that is recognized by LRP1 (18). Full-length α2M activated Akt and ERK1/2 in PC12 cells (Fig. 2A), as previously observed (6). Treatment of cells with K252a inhibited activation of Akt and ERK1/2 by α2M (Fig. 2A).
Fig. 2.
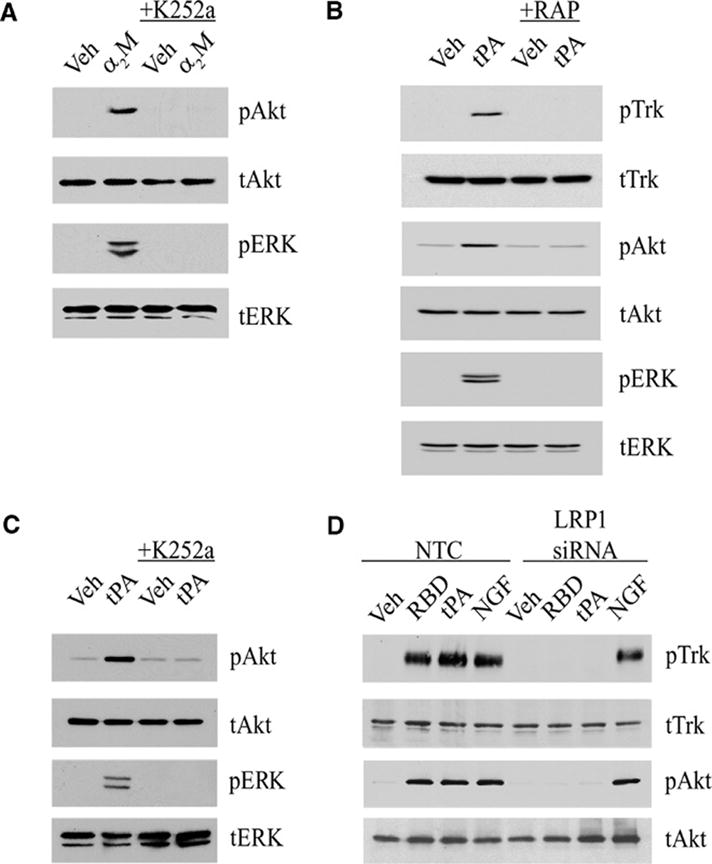
Trk receptors, Akt, and ERK1/2 are activated by two different LRP1 ligands. (A) PC12 cells were treated with 50 nM activated full-length α2M (α2M) or with vehicle (veh) for 10 min, with or without K252a pre-treatment. (B) PC12 cells were treated for 10 min with non-enzymatic tPA (10 nM) in the presence of GST-RAP (100 nM) or GST (100 nM) as a control. (C) PC12 cells were treated with non-enzymatic tPA (10 nM) after pretreatment with K252a as indicated. (D) PC12 cells transfected with LRP1-specific or NTC siRNA were treated for 10 min with vehicle, α2M RBD (100 nM), non-enzymatic tPA (10 nM) or NGF-β (100 ng/ml). For each experiment, cell extracts were immunoblotted with antibodies specific for pTrk, tTrk, pAkt, tAkt, pERK, and tERK.
Because Trk receptors are transactivated downstream of G-protein coupled receptors (GPCRs) (19–21), we hypothesized that Trk receptors may also be transactivated downstream of LRP1. Alternatively, α2M and the α2M RBD may bind directly to Trk receptors to trigger signaling. To distinguish between these two models, we tested another LRP1 ligand, tPA, which is distinct in structure from α2M but also activates signaling by binding to LRP1 (3). To eliminate the possibility that tPA activates signaling by a mechanism that requires its serine protease active site, a mutated variant of tPA that lacks enzymatic activity was used (3). tPA induced Trk receptor phosphorylation and activated Akt and ERK1/2 in PC12 cells (Fig. 2B). The ability of tPA to activate Akt and ERK1/2 was inhibited by RAP and by K252a (Fig. 2C). These results suggest that both α2M and tPA activate Akt and ERK1/2 in PC12 cells by a pathway that is dependent on LRP1 and Trk receptors.
RAP antagonizes ligand binding not only to LRP1 but also other closely related members of the low-density lipoprotein (LDL) receptor family, such as the very low-density lipoprotein (VLDL) receptor (1). The ability of RAP to block signaling in response to α2M and tPA argues against the independent involvement in PC12 cells of non-LDL receptor-family members that have been previously reported to mediate α2M-and tPA-initiated signaling in other cell types (22–24). To confirm the involvement of LRP1, PC12 cells were transfected with non-targeting control (NTC) siRNA or our previously characterized LRP1-specific siRNA, which has a silencing efficiency of >90% as determined by quantitative PCR (qPCR) (25). The NTC siRNA had no effect on the ability of tPA and the α2M RBD to induce Trk receptor phosphorylation or activate Akt (Fig. 2D). In contrast, LRP1-specific siRNA completely blocked the response to both LRP1 ligands (Fig. 2D), confirming an essential role for LRP1.
LRP1 undergoes constitutive endocytosis in clathrin-coated pits and is transported in endosomes that become acidified, promoting dissociation of ligand (if present) (26, 27). LRP1 then efficiently recycles back to the cell surface, where it is once again available to bind ligands. Because of this receptor trafficking pattern, we hypothesized that signaling in response to LRP1 ligands would be sustained. In PC12 cells that were treated with tPA or the α2M RBD, Trk phosphorylation was evident within 10 min and sustained for up to 6 hours, when the experiment was discontinued (Fig. 3). Thus, Trk receptor phosphorylation was indeed sustained.
Fig. 3.
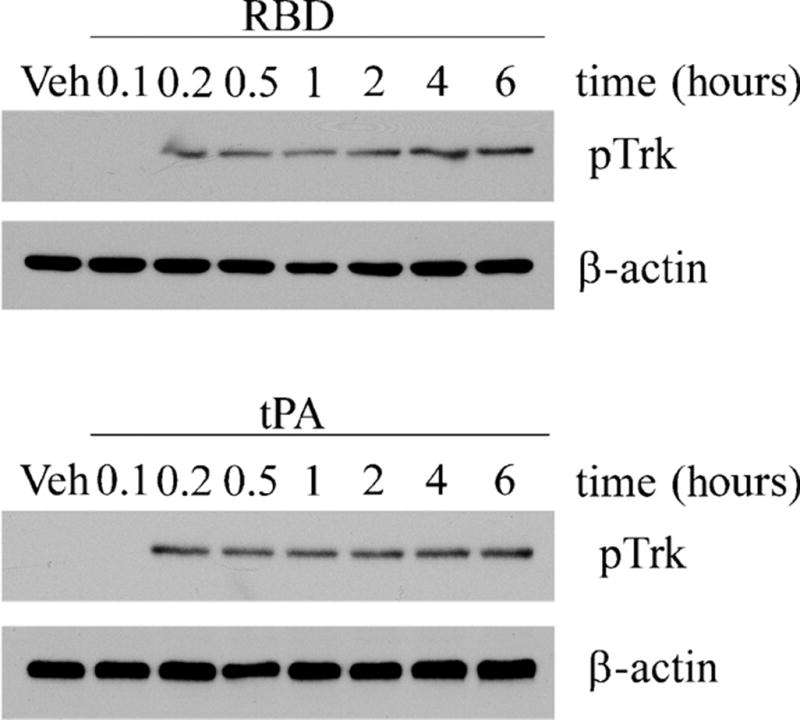
Trk receptor phosphorylation is sustained in PC12 cells treated with LRP1 ligands. Cells were treated for the indicated times with the α2M RBD (100 nM) or with tPA (10 nM). Cell extracts prepared at different time points were immunoblotted for pTrk. Blots were reprobed for β-actin as a loading control.
To confirm that K252a blocks signaling in response to LRP1 ligands by inhibiting Trk receptor tyrosine kinase activity, we silenced TrkA in PC12 cells. The extent of TrkA gene silencing was >90% as determined by immunoblot analysis (Fig. 4A). In cells in which TrkA was silenced, the α2M RBD failed to induce Trk receptor phosphorylation (Fig. 4A), which confirmed that the pTrk antibody detected Trk receptors. The α2M RBD and tPA also failed to activate Akt and ERK1/2 in TrkA-silenced cells (Fig. 4B). Activation of Akt and ERK1/2 was unaltered in control cells that were transfected with NTC siRNA.
Fig. 4.
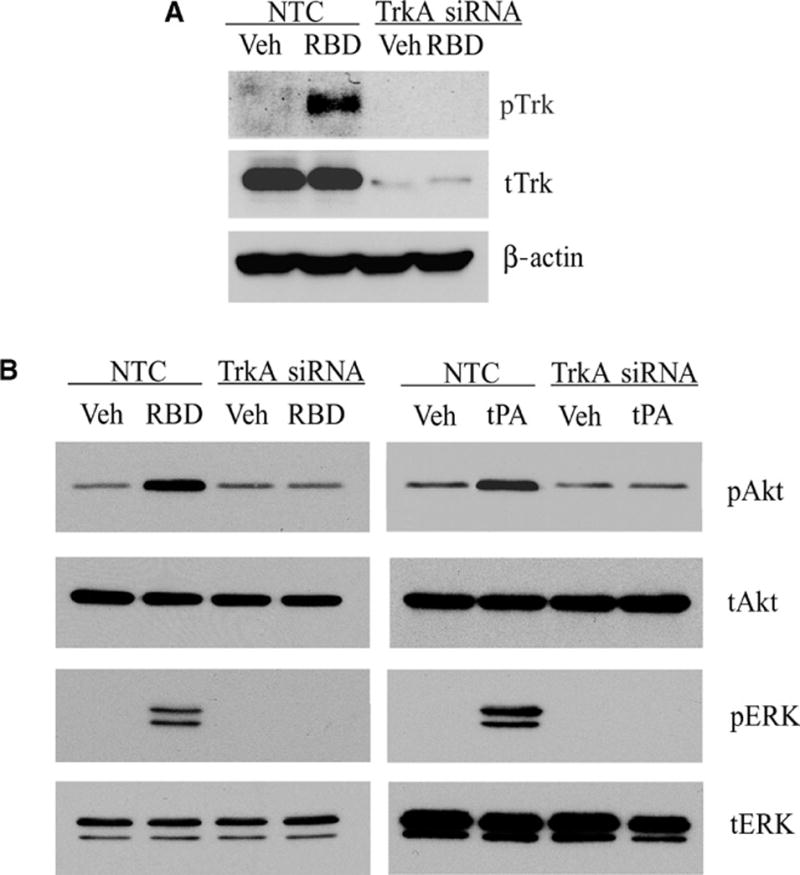
TrkA gene silencing blocks LRP1-initiated signaling. (A) PC12 cells transfected with TrkA or NTC siRNAs were stimulated with the α2M RBD or treated with vehicle. (B) PC12 cells transfected with TrkA or NTC siRNAs were treated with the α2M RBD, non-enzymatic tPA, or vehicle. For each experiment, cell extracts were immunoblotted with antibodies specific for pTrk, tTrk, pERK, tERK, pAkt, tAkt, and β-actin.
SFKs are activated and required for Trk receptor transactivation by LRP1 in PC12 cells
SFKs are required for Trk receptor transactivation following activation of GPCRs (21). To test whether SFKs are involved in Trk receptor activation in response to LRP1 ligands, PC12 cells were treated for 10 min with the α2M RBD or with tPA in the presence or absence of RAP. SFKs were immunoprecipitated and probed by immunoblotting with an antibody that recognizes phospho-Tyr418 in SFKs. Both LRP1 ligands increased SFK phosphorylation, and this response was inhibited by RAP (Fig. 5A) and by LRP1 gene silencing (Fig. 5B), confirming an essential role for LRP1.
Fig. 5.
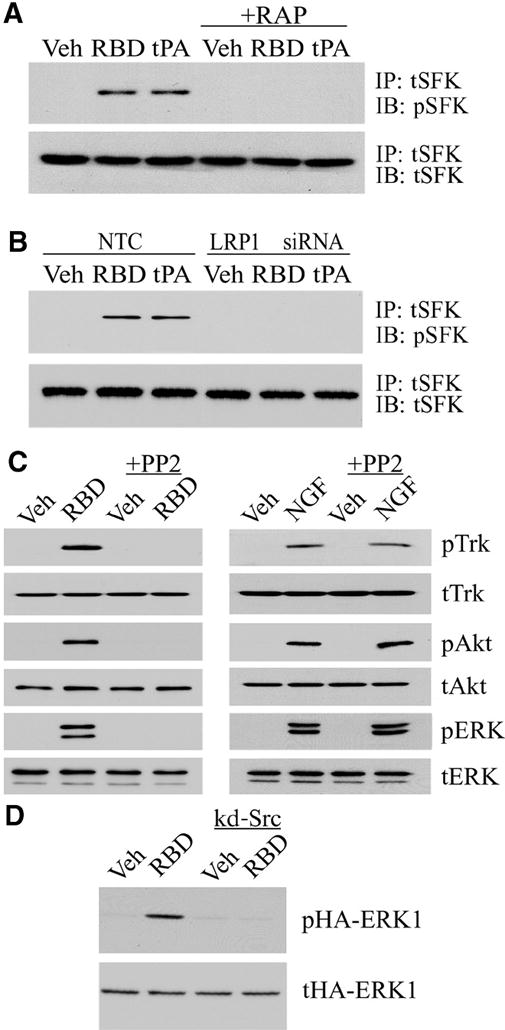
SFKs mediate Trk receptor transactivation by LRP1. (A) PC12 cells were treated with vehicle, the α2M RBD (100 nM), or non-enzymatic tPA (10 nM), with or without RAP pre-treatment. SFKs were immunoprecipitated (IP) from cell extracts, and precipitates were immunoblotted (IB) for phosphorylated SFK (pSFK) and total SFK (tSFK). (B) PC12 cells transfected with LRP1 or NTC siRNAs were treated with the α2M RBD, tPA, or vehicle. pSFK and tSFK were determined as described in A. (C) PC12 cells were pre-treated for 2 hours with PP2 (1 μM) in the indicated lanes. The cells were then stimulated with the α2M RBD (100 nM) or NGF-β (100 ng/ml). Cell extracts were immunoblotted for pTrk, tTrk, pAkt, tAkt, pERK, and tERK. (D) PC12 cells that were transfected with hemagglutinin (HA)-tagged ERK1 alone or with kinase defective (kd)-Src were stimulated with the α2M RBD or treated with vehicle. Cell extracts were immunoblotted for phosphorylated HA-ERK1 (pHA-ERK1) and total HA-ERK1 (tHA-ERK1).
Next, we tested whether SFK activity is necessary for Trk receptor phosphorylation and activation of Akt and ERK1/2 in response to LRP1 ligation. PC12 cells were pre-treated with the SFK-selective inhibitor, PP2, and then with the α2M RBD. PP2 blocked phosphorylation of Trk, Akt, and ERK1/2 in response to the α2M RBD (Fig. 5C), but had no effect on the response of PC12 cells to NGF-β, which binds directly to TrkA. These results support a model in which LRP1 ligands indirectly activate Trk receptors through an SFK-dependent transactivation pathway.
To confirm the role of SFKs in LRP1-dependent activation of ERK1/2 kinase, PC12 cells were transfected with a kinase-defective Src (kd-Src) which has an Ala430 →Val430 (A430V) mutation that confers dominant negative activity (28). ERK activation was monitored selectively in transfected cells by assessing the phosphorylation status of cotransfected hemagglutinin (HA)-tagged ERK1, as previously described (28). As anticipated, HA-ERK1 was phosphorylated in response to α2M RBD treatment, and kd-Src coexpression blocked HA-ERK1 phosphorylation (Fig. 5D).
Trk receptors are activated downstream of LRP1 in CGNs
To test whether Trk receptors are activated downstream of LRP1 in a second model system, we isolated neurons from the rat cerebellum. CGNs, which were identified by the characteristic single neurite extension, were positive for LRP1 immunoreactivity (Fig. 6A). Primary cultures of cerebellar neurons were treated with the α2M RBD for 10 min, and Trk receptor phosphorylation was assessed by immunofluorescence microscopy (Fig. 6B, C). The α2M RBD induced Trk receptor phosphorylation throughout the cell bodies of most CGNs. Pre-treatment of CGNs with RAP (100 nM) for 1 hour prior to introduction of the α2M RBD blocked Trk receptor phosphorylation, indicating a requirement for binding to LRP1.
Fig. 6.
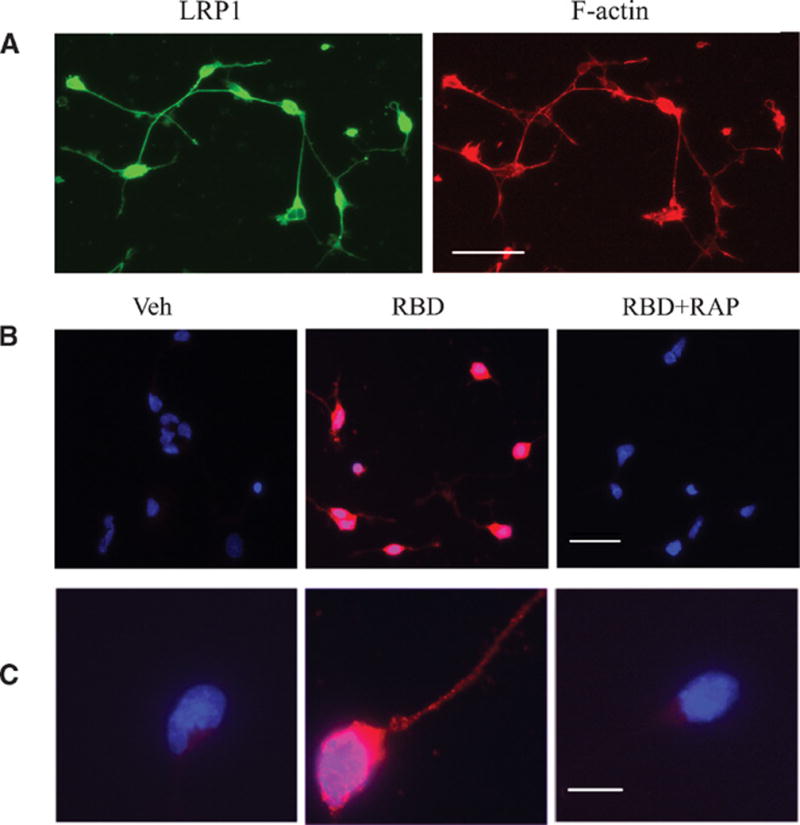
(A) LRP1 is present in cultured cerebellar neurons. Cerebellar neurons were labeled with an LRP1 antibody (green) and with phalloidin to detect actin (red). Scale bar: 40 μm. (B) Phosphorylation of Trk in α2M RBD-treated CGNs. Cultured cerebellar neurons were treated with the α2M RBD (100 nM) or with vehicle for 10 min. In the panels labeled RBD+RAP, the cells were pre-treated with 100 nM RAP for 1 h. Fluorescence microscopy was performed to detect phosphorylated Trk (pTrk; red). Nuclei were stained with DAPI (blue). Merged images are presented. Scale bar: 40 μm. (C) Merged images of a single representative neuron are shown. Scale bar: 10 μm.
Because Trk receptors were phosphorylated in CGNs and PC12 cells in response to the α2M RBD, we next determined whether the α2M RBD promotes neurite outgrowth in CGNs. Treatment of CGNs with the α2M RBD for 48 hours significantly increased neurite outgrowth and this response was blocked by RAP, K252a, or PP2 (Fig. 7, panels A and B). The α2M RBD also promoted neurite outgrowth in PC12 cells, as previously demonstrated (6), and this response was blocked by K252a and PP2 (Fig. 7C), suggesting that in both PC12 cells and CGNs, neurite outgrowth downstream of LRP1 requires SFKs and Trk receptor transactivation.
Fig. 7.
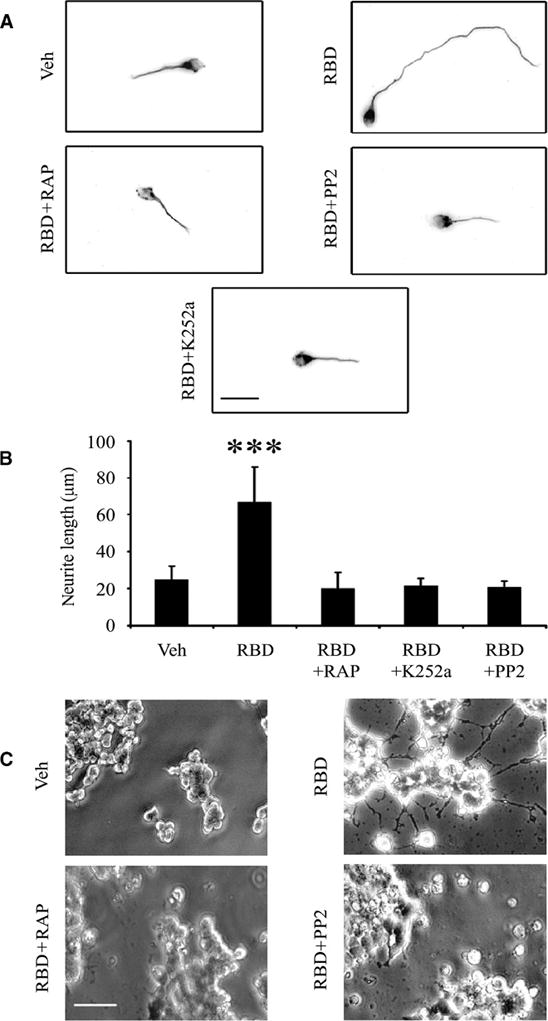
The α2M RBD promotes neurite outgrowth in CGNs and PC12 cells. (A) Representative CGNs are shown after culturing for 48 hours in the presence of the α2M RBD (100 nM) alone or with RAP, K252a, or PP2 as indicated. The neurons were stained with anti-tubulin antibody and imaged by fluorescence microscopy. Scale bar, 20 μm. (B) Neurite length for 50 CGNs in each condition was measured (***, p<0.001). (C) PC12 cells were treated with the α2M RBD (100 nM) for 48 hours, in the presence of K252a or PP2 as indicated. Phase contrast images are shown. Scale bar, 40 μm.
LRP1 ligation activates Trk receptors in vivo
In dorsal root ganglia (DRGs) isolated from adult rats, the cell bodies of primary sensory neurons were LRP1-positive, as determined by immunohistochemistry (Fig. 8A). The degree of immunopositivity was somewhat variable in neuronal cell bodies of different sizes; however, both large and small cell bodies were immunopositive. Satellite cells were robustly LRP1-positive (Fig. 8A, left, red arrow), although some of the immunofluorescent signal attributed to satellite cells may have overlapped with neuronal cell surface LRP1. LRP1 also was detected in axons (Fig. 8A, left, yellow arrow), as previously described (25). DRG extracts were immunoblotted with the 11H4 antibody, which recognizes the 85 kDa LRP1 β-chain (Fig. 8B). These studies confirmed the presence of LRP1 in DRGs.
Fig. 8.
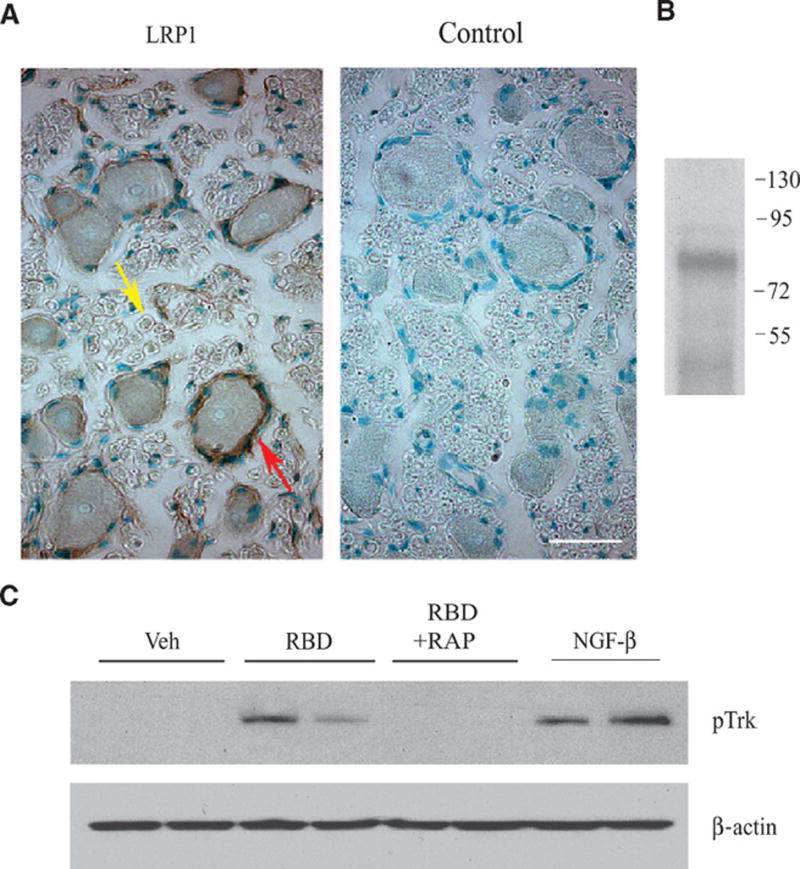
Trk receptors are phosphorylated by the α2M RBD in vivo. (A) Immunohistochemistry for LRP1 in rat DRGs detected immunopositive neuronal cell bodies, satellite cells (red arrow) surrounding neuronal cell bodies, and axons (yellow arrow). The panel labeled “control” was not stained with primary antibody. Scale bar: 10 μm. (B) DRG extracts were immunoblotted for LRP1 β-chain. Numbers to the right of the immunoblot indicate molecular mass markers in kilodaltons. (C) Vehicle, the α2M RBD, the α2M RBD+RAP, or NGF-β were injected directly into DRGs, which were collected 10 min later. Extracts were immunoblotted for pTrk, and then probed for β-actin as a loading control. Each lane shows extracts from a different animal.
To test whether Trk receptors are transactivated downstream of LRP1, we injected the α2M RBD directly into adult rat DRGs. 10 min after injection, Trk receptor phosphorylation was observed, and the degree of this response was nearly as great as that observed when NGF-β was injected (Fig. 8C). As anticipated, Trk receptor phosphorylation in response to the α2M RBD was inhibited by coinjection of RAP, suggesting that LRP1 binding was required.
DISCUSSION
Trk receptor transactivation by GPCRs is well characterized (19–21). In this study, we identified Trk receptors as transactivation targets downstream of LRP1. To our knowledge, this is the first example of a transactivation event initiated by LRP1. In PC12 cells, TrkA was necessary for LRP1-initiated signaling to Akt and ERK1/2 and for neurite outgrowth. In CGNs, Trk receptors were phosphorylated in response to the α2M RBD and were necessary for RBD-mediated neurite outgrowth. The α2M RBD also induced Trk receptor phosphoprylation in DRGs in vivo. These results support a model in which Trk receptors are part of the LRP1 signaling system in neurons. Furthermore, the link between LRP1 and Trk receptors suggests that a variety of LRP1 ligands may control Trk receptor activity. We identified activated α2M and tPA as LRP1 ligands that activate Trk receptors; other proteins that bind to LRP1 and thus may activate Trk receptors include growth factors, proteases, serpins, extracellular matrix proteins, and lipoproteins (1).
It was previously reported that α2M may bind directly to TrkA independently of LRP1 and antagonize TrkA-initiated signaling (29). Our results argue against this model, and against a direct interaction between α2M and Trk receptors as an explanation for the signaling activity of α2M. First, the structurally distinct LRP1 ligands, tPA and α2M, demonstrated equivalent activation of Trk, Akt, and ERK1/2, which was blocked by RAP, a general antagonist of LRP1 ligand binding. Furthermore, LRP1 gene silencing in PC12 cells blocked Trk receptor phosphorylation and Akt activation in response to the α2M RBD. Thus, LRP1 is an essential receptor for the signaling events described here.
Another possible explanation for our results is that LRP1 and Trk function as coreceptors, engaging ligands simultaneously at the cell surface. Coreceptor function is prevalent in the LDL receptor gene family. LRP5, LRP6 and Frizzled function as Wnt coreceptors (30–32). LRP4 and the muscle-specific RTK, MuSK, are coreceptors for agrin (33). However, our evidence argues against a coreceptor model. Blocking SFK activity prevented Trk receptor phosphorylation, suggesting that activated SFKs serve as intermediates between the initial event of tPA or α2M binding to LRP1 and Trk receptor phosphorylation. We were unable to co-immunoprecipitate LRP1 with Trk receptors in PC12 cells, even in the presence of the α2M RBD (Supplementary Fig. 1). When Trk receptors are phosphorylated in response to GPCR activation, phosphorylated Trk is present in subcellular compartments, separated from the GPCRs (20). Thus, we propose that tPA and activated α2M promote neurite outgrowth by transactivating Trk receptors through an SFK-dependent mechanism (Fig. 9), and hypothesize that multiple LRP1 ligands, in addition to tPA and α2M, may demonstrate neurotrophin-like activity through this pathway.
Fig. 9.
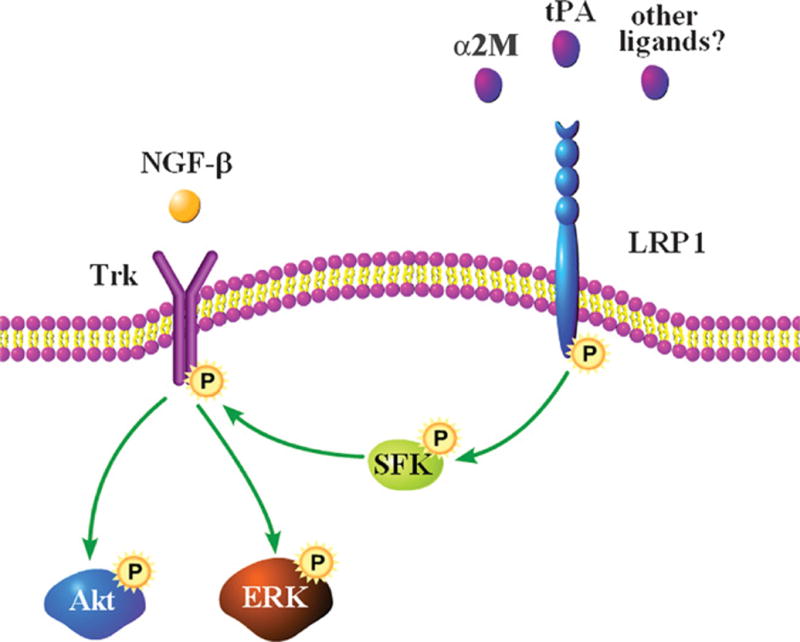
Proposed model for Trk receptor transactivation by LRP1. Although we show Trk receptor in the plasma membrane, transactivated Trk may be localized to different subcellular compartments, such as the Golgi. Future work will be needed to determine which LRP1 ligands transactivate Trk receptors.
In PC12 cells, TrkA gene silencing blocked activation of Akt and ERK1/2 in response to LRP1 ligation; however, more work will be necessary to determine which Trk receptors function in LRP1-dependent signaling. Trk receptor transactivation is required for neurite outgrowth in response to LRP1 ligation in CGNs even though the major Trk receptor in CGNs is TrkB rather than TrkA (34). TrkC, which is present in Schwann cells, mediated signaling in response to NT-3; however, TrkC did not appear to be necessary for activation of Akt and ERK1/2 by LRP1 ligands.
Another major question raised by our study is whether all LRP1 ligands or only a subset trigger signaling events that transactivate Trk receptors. Proteins that activate signaling by binding to LRP1 include tPA, α2M, apoE, MMP-9, and connective tissue growth factor (3, 5, 6, 26, 27). RAP is an exception that, in multiple cell culture models, blocks signaling triggered by other LRP1 ligands (2, 3, 5, 6). RAP is an intracellular chaperone, not normally present outside the cell; however, like other LRP1 ligands, RAP binds to clusters of complement-like repeats on the extracellular 515 kDa LRP1 α-chain (14, 35, 36). Further work will be necessary to determine the molecular characteristics of RAP that make this protein an LRP1 signaling antagonist as opposed to an agonist.
Injury to the peripheral nervous system increases LRP1 abundance in Schwann cells (25). LRP1 signaling supports Schwann cell survival, assuring the formation of extracellular scaffolds that are necessary for axonal regeneration at a later time (37). Thus, in this cell type, a function for LRP1 signaling is defined. Our results suggest that LRP1 signaling in neurons also may be involved in repair of injuries to the nervous system due to the effects of LRP1 signaling on neurite outgrowth. The function of this pathway would depend on Trk receptors, which may be present in decreased abundance in certain types of nervous system injury (38). Because of its ability to support neurite outgrowth, LRP1 also may function in nervous system development. Although a previous study did not report histoanatomical abnormalities in the brain of mice in which LRP1 was conditionally deleted in neurons, Cre recombinase was expressed under the control of a promoter that is active only in differentiated neurons and not neural progenitors (10). Thus, additional investigation will be needed to understand the integrated activity of LRP1 signaling in the normal nervous system and in injury.
MATERIALS AND METHODS
Proteins and reagents
α2M was purified from human plasma by the method of Imber and Pizzo (39) and activated for binding to LRP1 by dialysis against 200 mM methylamine HCl, as previously described (18). Modification of α2M by methylamine was confirmed by demonstrating the characteristic increase in α2M electrophoretic mobility by nondenaturing PAGE (40). The α2M RBD, which includes amino acids 1242–1451 of the mature α2M structure, was expressed as a glutathione-S-transferase (GST) fusion protein in bacteria, purified to homogeneity, and characterized for activity as previously described (6). Non-enzymatic tPA was purchased from Molecular Innovations. The hemopexin domain of MMP-9 (PEX) was prepared and characterized as previously described (5). RAP was expressed as a GST fusion protein (GST-RAP) in bacteria and purified as previously described (41), and as a control, we expressed GST in bacteria transformed with the empty vector, pGEX-2T. Purified α2M RBD, PEX, GST-RAP, and GST were subjected to chromatography on Detoxi-Gel endotoxin-removing columns (Pierce). Murine NGF-β was from Invitrogen, NT-3 from Abcam, K252a and AG1478 from Calbiochem, and EGF from R&D System. Expression constructs encoding HA-tagged ERK1 and kd-Src have been previously described (28, 42).
Antibodies
The following primary antibodies were used for immunoblotting, immunoprecipitation, and immunofluorescence: rabbit polyclonal antibody 2629, which recognizes the LRP1 α-chain (a kind gift from Dr. Dudley Strickland, University of Maryland School of Medicine, Baltimore, Maryland); monoclonal LRP1 antibody 11H4, which recognizes the β-chain (purified from the conditioned medium of hybridoma cells; ATCC); rabbit polyclonal anti-phospho-Akt (Cell Signaling Technologies); rabbit polyclonal anti-phospho-ERK (Cell Signaling Technologies); rabbit polyclonal anti-ERK (Cell Signaling Technologies); anti-phospho-Trk (Cell Signaling Technologies and Sigma); mouse monoclonal-anti-SFK (Millipore); polyclonal anti-SFK (Invitrogen); polyclonal anti-Trk (Santa Cruz Biotechnology); and mouse monoclonal anti-β-actin (Sigma). Horseradish peroxidase-conjugated secondary antibodies were purchased from Cell Signaling Technologies.
Cell culture
PC12 rat pheochromocytoma cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; high glucose; Gibco) containing 10% fetal bovine serum (FBS; Hyclone), 5% heat-inactivated horse serum (Omega Scientific Inc.), 100 U/ml penicillin, and 100 mg/ml streptomycin at 37° C.
Schwann cells were isolated from the sciatic nerves of 1 day-old Sprague Dawley rats and further selected from fibroblasts using fibronectin-specific antibody and rabbit complement, as previously described (43). Final preparations contained at least 95% Schwann cells, as assessed by immunofluorescence for S100. Primary cultures of Schwann cells were maintained in DMEM containing 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 21 μg/ml bovine pituitary extract, and 4 μM forskolin (complete medium) at 37° C under humidified 5.0% CO2.
Primary cultures of cerebellar neurons were prepared from postnatal day 1 rats as previously described (44, 45). In brief, cells were isolated from tissue by mechanical dissociation in a solution that contained papain (0.5 mg/ml) and DNase (0.6 μg/ml) for 20 min at 37°C. The cells were plated on glass coverslips coated with poly-D-lysine (0.5 mg/ml) and fibronectin (5 μg/ml) at low density (100,000 cells/400 mm2) and cultured in Neurobasal medium with 25 μM glutamine, 1% penicillin-streptomycin, and B27 supplement (Invitrogen) under a 5% CO2 atmosphere at 37° C.
Gene silencing
Rat Trk A-specific siRNA ON-TARGETplus SMARTpool (CGAGUUACCUGGACGUUCU, GUGGAGAAGAAGGACGAAA, CCAAUGAGACCAUGCGGCA, GCUCAUGGUCUUCGAGUAC), LRP1-specific siRNA L2 (CGAGCGACCUCCUAUCUUUUU) (6, 25), and pooled NTC siRNA were purchased from Dharmacon. PC12 cells (2 × 106) were transfected with TrkA-specific siRNA (50 nM), LRP1-specific siRNA L2 (25 nM), or with NTC siRNA (25–50 nM) by electroporation using the Cell Line nucleofector Kit V (Amaxa), as previously described (25). At the protein level, the degree of TrkA gene silencing was greater than 90% at 48–72 hours post-electroporation, as determined by immunoblot analysis and densitometry. At the mRNA level, the degree of LRP1 gene silencing was 90–95%, as determined by qPCR (24). Experiments were performed 48 hours after siRNA transfection.
Activation of Trk, Akt, and ERK
PC12 cells were plated in 100 mm dishes at a density of 2 × 106 cells/well in serum-containing medium and cultured until approximately 70% confluent. The cultures were then transferred into serum-free medium for 5 hours prior to adding vehicle or candidate stimulants, such as the α2M RBD, activated α2M, non-enzymatic tPA, or NGF-β. Pharmacologic inhibitors, including K252a (10 nM) or PP2 (1 μM), were added 2 hours prior to the addition of the stimulants. RAP (100 nM) was added 1 hour before other LRP1 ligands; GST was added as a control. Stimulants were allowed to incubate with cells for 10 min unless otherwise described. The cells then were rinsed twice with ice-cold phosphate-buffered saline. Cell extracts were prepared in radioimmune precipitation assay buffer (phosphate-buffered saline with 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitor mixture, and sodium orthovanadate). The protein concentration in cell extracts was determined by bicinchoninic acid assay. An equivalent amount of cellular protein (40 μg) was subjected to 8% or 12% SDS-PAGE and electrotransferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in 20 mM Tris-HCl, 150 mM NaCl, pH 7.4 with Tween 20 and incubated with primary antibodies. The membranes then were washed and treated with horseradish peroxidase-conjugated secondary antibodies for 1 hour. Immunoblots were developed by enhanced chemiluminescence (Perkin Elmer). All presented studies were performed at least in duplicate and typically with n=3–5.
PC12 cells were transfected with vectors encoding HA-tagged ERK1 (0.4 μg) and kd-Src (1.6 μg) or with the vector encoding HA-ERK1 alone, using the Cell Line Nucleofector Kit V (Amaxa, Gaithersburg, MD) as described previously (28). Cells were maintained in serum-containing medium for 48 hours and then transferred into serum-free medium for 5 hours. After 10 min treatment with the α2M RBD or vehicle, cells were extracted in ice-cold radioimmune precipitation assay buffer. The extracts were incubated with HA-specific antibody 12CA5 and protein G-Sepharose for 3 hours at 4°C. Pellets were washed three times and analyzed by SDS-PAGE and immunoblot analysis.
Analysis of SFK activation
PC12 cells were treated with the α2M RBD (100 nM), non-enzymatic tPA (10 nM), or vehicle. Some cultures were pre-treated with RAP (100 nM) or with GST (as a control). Cultures were extracted in ice-cold 25 mM Tris-HCl, 0.15 mM NaCl, pH 7.4, containing 1% Triton X-100, 5 mM EDTA, sodium orthovanadate, and protease inhibitor cocktail. Cell extracts were cleared by centrifugation at 12,000 × g. SFK-specific monoclonal antibody (4 μg) and Protein G-Sepharose beads (General Electric Healthcare), which were pre-incubated for 2 hours at 4°C and then washed, were mixed with the cell extracts. Incubations were allowed to proceed at 4°C for 12 hours. The beads then were washed three times with ice-cold cell extraction buffer and eluted in SDS-PAGE sample buffer for SDS-PAGE. Phospho-SFK and total SFK were detected by immunoblot analysis using polyclonal antibodies to avoid cross-reaction of the secondary antibody with the antibody used for immunoprecipitation.
Detection of LRP1 in cerebellar neurons
Cerebellar neurons were plated on glass coverslips coated with poly-D-lysine and fibronectin (5 μg/ml) and cultured for seven days. The cells then were fixed with 4% paraformaldehyde and incubated with LRP1-specific antibody (1:100) and with Alexa Fluor 568-phalloidin (1:100, Invitrogen). The secondary antibody was Alexa Fluor 488-conjugated chicken anti-mouse IgG (4 μg/ml). Immunostaining was analyzed using a Leica fluorescence microscope equipped with a Hamamatsu CCD camera and SimplePCI software.
Detection of pTrk in CGNs
Cerebellar neurons were treated with the α2M RBD, with or without RAP pretreatment for 1 hour, and then fixed in 4% paraformaldehyde and permeabilized in 0.1% Triton X-100. Primary antibodies were introduced at 4°C overnight. The primary antibody was rabbit anti-phospho-Trk (1:100). The secondary antibody was Alexa Fluor 594-conjugated chicken anti-rabbit IgG (4 μg/ml). Nuclei were stained with DAPI. Immunostaining was analyzed as described for LRP1.
Neurite outgrowth
PC12 cells were plated at 2 × 105 cells/cm2 and maintained in serum-containing medium for 24 hours. The medium was then replaced with serum-free medium supplemented with the α2M RBD and K252a, PP2, or vehicle, and cells were cultured for an additional 48 hours. Axodendritic process formation was assessed by phase contrast microscopy, using a Leica microscope equipped with a Hamamatsu CCD camera and SimplePCI software.
CGNs were treated with the α2M RBD, in the presence of RAP, K252a, PP2, or vehicle for 48 hours, fixed with 4% paraformaldehyde, and stained with mouse anti-tubulin primary antibody (1:500; Sigma) followed by Alexa Fluor 594-conjugated chicken anti-mouse IgG secondary antibody (4 μg/ml). Experimental and control samples were encoded for blind analysis. CGNs (50 per study, three separate studies) were randomly selected and imaged using a Leica microscope. The length of axons was determined by measuring the distance between its tip and the cell body using ImageJ software. Statistical analysis was performed using GraphPad Software. Statistical differences for multiple groups were assessed by one-way ANOVA followed by Newman-Keuls post hoc tests.
Detection of LRP1 and pTrk in DRGs
Experiments were performed using adult male Sprague-Dawley rats (200 g) from Harlan Laboratories (San Diego, CA), which were housed in pairs with a 12 hour:12 hour light:dark cycle and ad libitum access to food and water. For surgery, rats were anesthetized with 2.0% isoflurane (IsoSol; VedCo, St. Joseph, MO). Using a sterile field, laminectomies were performed to expose the L5 DRG bilaterally. Euthanasia was accomplished by intraperitoneal injection of an overdose of anesthetic cocktail containing ketamine (100 mg/kg; Phoenix Scientific, St Joseph, MO) and xylazine (10 mg/kg; Boerhinger Pharmaceutical, St. Joseph, MO) followed by cervical dislocation. All procedures were performed according to protocols approved by the University of California, San Diego Committee on Animal Research, and conform to the NIH Guidelines for Animal Use.
For immunohistochemistry, the DRGs were isolated and fixed in 4% paraformaldehyde. Paraffin sections (10 μm) were cut, deparaffinized, incubated in antigen retrieval buffer, and then in 3% hydrogen peroxide for 5 min. Nonspecific antibody binding was blocked by pre-incubation with 10% normal goat serum. The sections then were immunostained with polyclonal antibody 2629, which recognizes the LRP1 α-chain in 0.1% goat serum, followed by biotinylated HRP-conjugated goat anti-rabbit secondary antibody and avidin-biotin complex. Control slides were not treated with primary antibody. Sections were developed with 3,3′-diaminobenzidine. Methyl green was used as a counterstain.
To assess Trk receptor phosphorylation in response to LRP1 ligation in vivo, exposed DRGs in anesthetized animals were injected with 2.0 μL of GST (2 μM), 2.0 μL of α2M RBD (2 μM), 1.0 μL of α2M RBD (4 μM) + 1.0 μL RAP (4 μM) or 2.0 μL of NGF-β (2 μM). All injections were administered directly into the center of the left L5 DRG. Sham-operated animals were injected with vehicle (2.0 μL). All DRGs were harvested 10 min later. Extracts of DRGs were isolated in RIPA buffer for immunoblot analysis.
Supplementary Material
References and Notes
- 1.Strickland DK, Gonias SL, Argraves WS. Diverse roles for the LDL receptor family. Trends Endocrinol Metab. 2002;13:66–74. doi: 10.1016/s1043-2760(01)00526-4. [DOI] [PubMed] [Google Scholar]
- 2.Bacskai BJ, Xia MQ, Strickland DK, Rebeck GW, Hyman BT. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 2000;97:11551–11556. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27:1933–1941. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, Campana WM. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci. 2008;28:11571–11582. doi: 10.1523/JNEUROSCI.3053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantuano E, Mukandala G, Li X, Campana WM, Gonias SL. Molecular dissection of the human alpha2-macroglobulin subunit reveals domains with antagonistic activities in cell signaling. J Biol Chem. 2008;283:19904–19911. doi: 10.1074/jbc.M801762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb DJ, Nguyen DH, Gonias SL. Extracellular signal-regulated kinase functions in the urokinase receptor-dependent pathway by which neutralization of low density lipoprotein receptor-related protein promotes fibrosarcoma cell migration and matrigel invasion. J Cell Sci. 2000;113( Pt 1):123–134. doi: 10.1242/jcs.113.1.123. [DOI] [PubMed] [Google Scholar]
- 8.Gaultier A, Arandjelovic S, Niessen S, Overton CD, Linton MF, Fazio S, Campana WM, Cravatt BF, 3rd, Gonias SL. Regulation of tumor necrosis factor receptor-1 and the IKK-NF-kappaB pathway by LDL receptor-related protein explains the antiinflammatory activity of this receptor. Blood. 2008;111:5316–5325. doi: 10.1182/blood-2007-12-127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf BB, Lopes MB, VandenBerg SR, Gonias SL. Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am J Pathol. 1992;141:37–42. [PMC free article] [PubMed] [Google Scholar]
- 10.May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, Noebels JL, Beffert U, Sweatt JD, Weeber EJ, Herz J. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu Z, Hyman BT, Rebeck GW. Apolipoprotein E receptors mediate neurite outgrowth through activation of p44/42 mitogen-activated protein kinase in primary neurons. J Biol Chem. 2004;279:34948–34956. doi: 10.1074/jbc.M401055200. [DOI] [PubMed] [Google Scholar]
- 12.Zhuo M, Holtzman DM, Li Y, Osaka H, DeMaro J, Jacquin M, Bu G. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams SE, Ashcom JD, Argraves WS, Strickland DK. A novel mechanism for controlling the activity of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Multiple regulatory sites for 39-kDa receptor-associated protein. J Biol Chem. 1992;267:9035–9040. [PubMed] [Google Scholar]
- 15.Carter BD, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm-Matthaei R, Baeuerle PA, Barde YA. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi J, Chan JR, Shooter EM. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc Natl Acad Sci U S A. 2003;100:14421–14426. doi: 10.1073/pnas.2336152100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang W, Dolmer K, Liao X, Gettins PG. NMR solution structure of the receptor binding domain of human alpha(2)-macroglobulin. J Biol Chem. 2000;275:1089–1094. doi: 10.1074/jbc.275.2.1089. [DOI] [PubMed] [Google Scholar]
- 18.Gonias SL, Reynolds JA, Pizzo SV. Physical properties of human alpha 2-macroglobulin following reaction with methylamine and trypsin. Biochim Biophys Acta. 1982;705:306–314. doi: 10.1016/0167-4838(82)90252-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem. 2002;277:9096–9102. doi: 10.1074/jbc.M107421200. [DOI] [PubMed] [Google Scholar]
- 20.Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajagopal R, Chao MV. A role for Fyn in Trk receptor transactivation by G-protein-coupled receptor signaling. Mol Cell Neurosci. 2006;33:36–46. doi: 10.1016/j.mcn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz-Zapater E, Peiro S, Roda O, Corominas JM, Aguilar S, Ampurdanes C, Real FX, Navarro P. Tissue plasminogen activator induces pancreatic cancer cell proliferation by a non-catalytic mechanism that requires extracellular signal-regulated kinase 1/2 activation through epidermal growth factor receptor and annexin A2. Am J Pathol. 2007;170:1573–1584. doi: 10.2353/ajpath.2007.060850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci. 2002;22:3352–3358. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 2006;281:13694–13707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- 25.Campana WM, Li X, Dragojlovic N, Janes J, Gaultier A, Gonias SL. The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J Neurosci. 2006;26:11197–11207. doi: 10.1523/JNEUROSCI.2709-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bu G, Cam J, Zerbinatti C. LRP in amyloid-beta production and metabolism. Ann N Y Acad Sci. 2006;1086:35–53. doi: 10.1196/annals.1377.005. [DOI] [PubMed] [Google Scholar]
- 27.Cam JA, Zerbinatti CV, Li Y, Bu G. Rapid endocytosis of the low density lipoprotein receptor-related protein modulates cell surface distribution and processing of the beta-amyloid precursor protein. J Biol Chem. 2005;280:15464–15470. doi: 10.1074/jbc.M500613200. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen DH, Webb DJ, Catling AD, Song Q, Dhakephalkar A, Weber MJ, Ravichandran KS, Gonias SL. Urokinase-type plasminogen activator stimulates the Ras/Extracellular signal-regulated kinase (ERK) signaling pathway and MCF-7 cell migration by a mechanism that requires focal adhesion kinase, Src, and Shc. Rapid dissociation of GRB2/Sps-Shc complex is associated with the transient phosphorylation of ERK in urokinase-treated cells. J Biol Chem. 2000;275:19382–19388. doi: 10.1074/jbc.M909575199. [DOI] [PubMed] [Google Scholar]
- 29.Koo PH, Qiu WS. Monoamine-activated alpha 2-macroglobulin binds trk receptor and inhibits nerve growth factor-stimulated trk phosphorylation and signal transduction. J Biol Chem. 1994;269:5369–5376. [PubMed] [Google Scholar]
- 30.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 31.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 32.Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 33.Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Courtney MJ, Akerman KE, Coffey ET. Neurotrophins protect cultured cerebellar granule neurons against the early phase of cell death by a two-component mechanism. J Neurosci. 1997;17:4201–4211. doi: 10.1523/JNEUROSCI.17-11-04201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bu G, Geuze HJ, Strous GJ, Schwartz AL. 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J. 1995;14:2269–2280. doi: 10.1002/j.1460-2075.1995.tb07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willnow TE. Receptor-associated protein (RAP): a specialized chaperone for endocytic receptors. Biol Chem. 1998;379:1025–1031. [PubMed] [Google Scholar]
- 37.Ide C. Peripheral nerve regeneration. Neurosci Res. 1996;25:101–121. doi: 10.1016/0168-0102(96)01042-5. [DOI] [PubMed] [Google Scholar]
- 38.Hajebrahimi Z, Mowla SJ, Movahedin M, Tavallaei M. Gene expression alterations of neurotrophins, their receptors and prohormone convertases in a rat model of spinal cord contusion. Neurosci Lett. 2008;441:261–266. doi: 10.1016/j.neulet.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 39.Imber MJ, Pizzo SV. Clearance and binding of two electrophoretic “fast” forms of human alpha 2-macroglobulin. J Biol Chem. 1981;256:8134–8139. [PubMed] [Google Scholar]
- 40.Barrett AJ, Brown MA, Sayers CA. The electrophoretically ‘slow’ and ‘fast’ forms of the alpha 2-macroglobulin molecule. Biochem J. 1979;181:401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb DJ, Hussaini IM, Weaver AM, Atkins TL, Chu CT, Pizzo SV, Owens GK, Gonias SL. Activated alpha 2-macroglobulin promotes mitogenesis in rat vascular smooth muscle cells by a mechanism that is independent of growth-factor-carrier activity. Eur J Biochem. 1995;234:714–722. doi: 10.1111/j.1432-1033.1995.714_a.x. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen DH, Catling AD, Webb DJ, Sankovic M, Walker LA, Somlyo AV, Weber MJ, Gonias SL. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol. 1999;146:149–164. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campana WM, Hiraiwa M, O’Brien JS. Prosaptide activates the MAPK pathway by a G-protein-dependent mechanism essential for enhanced sulfatide synthesis by Schwann cells. FASEB J. 1998;12:307–314. doi: 10.1096/fasebj.12.3.307. [DOI] [PubMed] [Google Scholar]
- 44.Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 2006;26:1813–1822. doi: 10.1523/JNEUROSCI.4091-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moeller ML, Shi Y, Reichardt LF, Ethell IM. EphB receptors regulate dendritic spine morphogenesis through the recruitment/phosphorylation of focal adhesion kinase and RhoA activation. J Biol Chem. 2006;281:1587–1598. doi: 10.1074/jbc.M511756200. [DOI] [PubMed] [Google Scholar]
- 46.This work was supported by grants from the National Institutes of Health to S.L.G. (R01 NS054571) and to W.M.C. (R01 NS057456). G.I. was supported by the Uehara Memorial Foundation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


