Abstract
The ability of antigen-presenting cells to sample distinct intracellular compartments is crucial for microbe detection. Major histocompatibility complex class I and class II molecules sample the cytosol or the late endocytic compartment, allowing detection of microbial peptide antigens that arise in distinct intracellular compartments. In contrast, CD1a and CD1b molecules mediate the presentation of lipid and glycolipid antigens and differentially sample early recycling endosomes or late endocytic compartments, respectively, that contain distinct sets of lipid antigens. Here, we show that, unlike the other CD1 isoforms or major histocompatibility complex molecules that each sample restricted only intracellular compartments, CD1c is remarkable in that it distributes broadly throughout the endocytic system and is expressed in both recycling endosomes and late endocytic compartments. Further, in contrast to CD1b, which requires an acidic environment to function, antigen presentation by CD1c was able to overcome dependence on vesicular acidification. Because CD1c is expressed on essential antigen-presenting cells, such as epidermal Langerhans cells (in the absence of CD1b), or on B cells (without CD1a or -b), we suggest that CD1c molecules allow a comprehensive survey for lipid antigens throughout the endocytic system even in the absence of other CD1 isoforms.
In contrast to the ability of T cells to recognize peptide antigens presented by major histocompatibility complex (MHC)-encoded antigen-presenting molecules, CD1 molecules present microbial lipid and glycolipid antigens to a variety of effector T cells. Mycobacteria-infected dendritic cells are detected and lysed by group 1 CD1 (CD1a, CD1b, and CD1c)-restricted T cells that recognize mycobacterial lipids, including mycolic acids, lipoarabinomannan, and isoprenoid glycolipids (1–4). The CD8+ CD1-restricted cytotoxic T cells also contain granulysin that can directly kill released mycobacteria, underscoring a role of CD1 in clearing mycobacterial infection (5). These T cells also produce inflammatory (Th1) cytokines, such as interferon-γ (6). Group 2 CD1 (CD1d)-reactive T cells have been shown to be potent interferon-γ and interleukin (IL)-4 producers that may have immunoregulatory effects and control humoral immune responses to glycosylphosphatidylinositol-anchored protein antigens in parasitic infection with plasmodia and trypanosomas (7). Thus, it seems likely that independent recognition of the distinct chemical classes of antigens, namely proteins and lipids, allows MHC and CD1 molecules to survey for distinct antigens and mediate independent pathways for antigen presentation and T cell activation against microbial infection.
MHC class I, class II, and CD1 molecules appear designed to sample particular intracellular compartments that may contain microbial antigens. Many intracellular viral infections, as well as some bacteria that are taken up in phagosomes then escape from the endocytic compartments, enter the MHC class I pathway via the cytosol. In contrast, peptide antigens derived from phagosome-resident bacteria penetrate deeply into the endocytic system and are detected by MHC class II molecules. Thus, MHC class I and class II molecules sample distinct intracellular compartments and coordinately elicit efficient cell-mediated immune responses against pathogens. Despite this potential for comprehensive antigen sampling, the MHC system samples only peptide antigens, and microbes have evolved evasive mechanisms that inhibit peptide antigen generation or its transport into the class I pathway or inhibit phagosome–lysosome fusion and vacuolar acidification that may disturb the class II pathway (8, 9).
Recently, we showed that CD1a and CD1b follow unique intracellular trafficking pathways that are distinct from one another and from MHC class I and class II molecules (10, 11). CD1b abundantly traffics to late endosomes and lysosomes, including the MHC class II compartment or MIIC, in which peptide antigen loading onto MHC class II is proposed to occur. However, CD1b uses its own cytoplasmic tail tyrosine-based sequence to mediate internalization from the cell surface via clathrin-coated pits and subsequent transport to late endocytic compartments. Disruption of this targeting sequence results in redistribution of CD1b from late endosomes to the cell surface and failure to efficiently present CD1b-restricted lipid antigens to T cells (10, 12). Collectively, these observations have given strong support to the assumption that CD1b, like MHC class II, samples acidified late endocytic compartments, but bound microbial lipid antigens rather than peptide antigens. In contrast, CD1a molecules, which lack the cytoplasmic tail tyrosine-based endosomal targeting motif, are excluded from these late endocytic compartments and do not require endosomal acidification for efficient presentation of endocytosed lipid antigens. After internalization from the cell surface, CD1a avoids entering the late endocytic system by sorting to a recycling pathway of the early endocytic system (11). Because lipid antigens also are transported intracellularly, based partly on the structure of their hydrophobic alkyl chains, either to recycling endosomes or to late endosomes and lysosomes (13), the nonoverlapping endosomal distribution of CD1a and CD1b predicts sampling of lipid antigens derived from intracellular microbes separately in these endocytic compartments (14).
However, the CD1a, -b, and -c antigen-presenting molecules are often not coordinately expressed. For example, B cells express CD1c alone, whereas epidermal Langerhans cells express CD1a and -c with little expression of CD1b (15, 16). This predicts holes in the lipid antigen sampling capacity of antigen-presenting cells that do not express both CD1a and -b. Moreover, CD1c molecules seem to present distinct classes of lipid antigens and to be important targets for recognition by the major subset of tissue γδ T cells (4, 17). Here, we show that CD1c molecules localize intracellularly in a pattern that is distinct from the other CD1 and MHC class I and class II molecules. We propose that CD1c is a broad surveyor of the endocytic system of professional antigen-presenting cells, allowing its expression in the absence of CD1a or CD1b to sample lipid antigens throughout the endocytic system.
Materials and Methods
Cell Lines and Antibodies.
We developed J.RT3 cells reconstituted with the mycobacterial lipid antigen-specific, CD1-restricted T cell receptors (TCRs) (CD8–2/J.RT3, LDN5/J.RT3, and CD8–1/J.RT3) (18). These TCR-reconstituted cells were cultured in RPMI 1640 complete medium (GIBCO/BRL) [10% heat-inactivated fetal calf serum/20 mM Hepes/2 mM l-glutamine/1 mM sodium pyruvate/55 μM 2-mercaptoethanol (all from GIBCO/BRL)], containing G418 (1 mg/ml) (GIBCO/BRL), and hygromycin B (0.5 mg/ml) (GIBCO/BRL). We developed human B lymphoblastoid JY cells transfected with either CD1a or CD1b (11). To generate JY cells expressing CD1c, JY cells were transfected with CD1c cDNA in pCEP4 by electroporation and cultured in RPMI 1640 complete medium containing hygromycin B (0.2 mg/ml). The CD1c-expressing cells were enriched by labeling with the F10/21A3 anti-CD1c antibody (18), followed by positive selection with magnetic beads coated with goat anti-mouse IgG (Dynal). To obtain HeLa cells expressing CD1c, HeLa cells were transfected with CD1c in pSRα-neo by a calcium phosphate precipitation method and cultured in DMEM supplemented with 10% fetal calf serum and G418 (0.5 mg/ml). The CD1c-expressing HeLa cells were enriched by labeling with the F10/21A3 anti-CD1c antibody, followed by positive selection with magnetic beads coated with goat anti-mouse IgG. IL-2-dependent HT-2 cells (18) were cultured in RPMI complete medium supplemented with 1 nM recombinant IL-2 (Ajinomoto, Kawasaki, Japan). The mouse mAbs 10H3 (anti-CD1a) (19) and 4A7.6 (anti-CD1b) (19) as well as rabbit polyclonal antisera against lysosome-associated membrane protein-1 (LAMP-1; ref. 20) and ADP-ribosylation factor-6 (ARF6; ref. 21) have been described.
T Cell Transfectants Stimulation Assay.
Untransfected JY cells and JY cells stably transfected with CD1a (JY/CD1a), CD1b (JY/CD1b), or CD1c (JY/CD1c) were incubated overnight with the chloroform/methanol extract of Mycobacterium tuberculosis (M.tb) H37Ra sonicate (6) (1.56 μg/ml), washed, and fixed with 0.08% glutaraldehyde. The CD8–1/J.RT3 cells (5 × 104 cells per well) were cultured with these fixed antigen-presenting cells (1 × 105cells per well) in the presence of 10 ng/ml phorbol 12-myristate 13-acetate using 96-well, flat-bottomed microtiter plates (200 μl of medium per well). Aliquots of the culture supernatants were collected after 24 h, and the amounts of IL-2 released into the supernatants were measured by using the HT-2 indicator cells (18). To examine the effect of concanamycin B, monocyte-derived dendritic cells were generated from human peripheral blood monocytes that were induced to differentiate by incubation with GM-CSF and IL-4 as described (22) and used as antigen-presenting cells. The cells were preincubated with 10 nM concanamycin B (gift of Hidde Ploegh, Harvard Medical School, Boston) for 30 min and then washed and incubated for 4 h with indicated concentrations of specific M.tb antigen preparations [the semipurified M.tb antigen for CD8–2/J.RT3 (11), purified glucose monomycolate for LDN5/J.RT3 (23), and the chloroform/methanol extract of H37Ra sonicate for CD8–1/J.RT3) (6)] either in the presence or absence of 10 nM concanamycin B. The cells (1 × 105 cells per well) were then washed, fixed, and incubated with the corresponding TCR transfectant cells (5 × 104 cells per well), and IL-2 production by the T cells was measured as described above.
Confocal Microscopy.
Monocyte-derived dendritic cells were adhered on glass slides by a cytospin procedure, fixed, and permeabilized, and double-labeling with mouse antibodies against CD1 and rabbit antibodies against LAMP-1 was carried out as described (11). HeLa cell transfectants expressing CD1c were grown on coverslips and supertransfected with ARF6-T27N cDNA by a calcium phosphate precipitation method. Thirty hours after transfection, the cells were fixed and permeabilized, and double-labeling with mouse anti-CD1c antibody and rabbit anti-ARF6 antiserum was performed as described (11). The labeled cells were examined by using the Leica TCS-NT confocal laser scanning microscope fitted with krypton and argon lasers as described (11).
Immunogold-Labeled Transmission Electron Microscopy.
Monocyte-derived dendritic cells were fixed with 2% paraformaldehyde and 0.2% glutaraldehyde and processed for ultrathin cryosectioning as described (24). Cryo sections were incubated with the F10/21A3 anti-CD1c antibody for 60 min, washed, and incubated with rabbit anti-mouse IgG antibodies (Dako) for 20 min, followed by incubation with protein-A gold (EM Laboratory, Utrecht University) for 20 min. Labeled sections were viewed with a Philips (Eindhoven, The Netherlands) CM10 electron microscope.
Results
Endocytosis of Lipid Antigen Is Required for Presentation by CD1c.
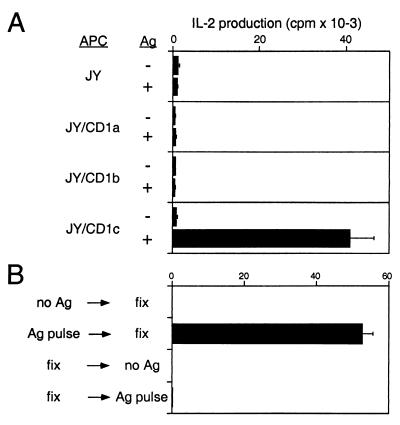
Specific pairs of variable TCR α and β chains mediate dual recognition of both CD1 and bound lipid antigen (18). To study the pathway for CD1c-mediated antigen presentation, the TCRs derived from a mycobacterial lipid antigen-specific, CD1c-restricted T cell line (CD8–1) were reconstituted in TCR-deficient Jurkat cells (J.RT3) by transfection with cDNAs encoding the specific TCR α and β chains. The TCR transfected cells (CD8–1/J.RT3) produced IL-2 in response to mycobacteria-derived lipid antigens only when JY cell transfectants expressing CD1c (JY/CD1c), but not untransfected JY nor JY cells expressing either CD1a (JY/CD1a) or CD1b (JY/CD1b), were used as antigen-presenting cells (Fig. 1A). Thus, reconstitution with the pair of TCR α and β chains was sufficient to transfer both antigen specificity and CD1c restriction.
Figure 1.
Presentation of lipid antigen to the CD1c-restricted CD8–1/J.RT3 cells. (A) Untransfected JY cells and JY cells stably transfected with either CD1a (JY/CD1a), CD1b (JY/CD1b), or CD1c (JY/CD1c) were incubated overnight either with or without 1.56 μg/ml of chloroform/methanol extract of M.tb H37Ra sonicate, fixed with 0.08% glutaraldehyde, and used as antigen-presenting cells. The J.RT3 transfectants expressing the TCR α and β chains derived from CD8–1 (CD8–1/J.RT3) were cultured with these fixed antigen-presenting cells for 24 h in the presence of phorbol 12-myristate 13-acetate (10 ng/ml), and IL-2 production was measured, using the HT-2 indicator cells. (B) Monocyte-derived dendritic cells were incubated for 4 h with the chloroform/methanol extract of H37Ra sonicate (5 μg/ml) either before or after fixation with 0.04% glutaraldehyde. The cells were then washed and incubated with CD8–1/J.RT3, and IL-2 release into the media was measured.
To gain insight into the antigen presentation pathway mediated by CD1c, we first examined whether endocytosis of lipid antigens into antigen-presenting cells was required for CD1c antigen presentation. Antigen-presenting cells that were fixed with glutaraldehyde to block endocytosis were tested for their ability to present lipid antigens to T cells. Recognition of M.tb-derived lipid antigen by CD8–1/J.RT3 occurred when antigen-presenting cells were pulsed with antigen and then fixed, but not when pulsed with antigen after fixation (Fig. 1B). Thus, uptake of lipid antigen and its intracellular delivery were required for CD1c-mediated antigen presentation.
Vacuolar Acidification Dependent and Independent Antigen Presentation by CD1c.
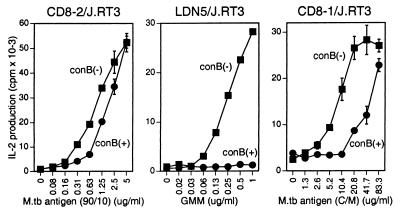
Endocytosed lipid antigens are transported to late endosomes and lysosomes, including the MIIC compartment, to which CD1b molecules also are directed (10, 25). In addition, CD1b-mediated antigen presentation is fully dependent on vacuolar acidification (11, 22), making it most likely that CD1b binds lipid antigens in these acidic compartments of the late endocytic system. Because the cytoplasmic domain of CD1c contains a tyrosine-based endosomal targeting motif (Lys-Lys-His-Cys-Ser-Tyr-Gln-Asp-Ile-Leu, where the sequence corresponding to the motif is underlined) similar to that found in the CD1b cytoplasmic tail, we initially hypothesized that CD1c antigen presentation, like CD1b, might be highly sensitive to vacuolar acidification. To address this possibility, the effect of a vaculolar proton ATPase inhibitor, concanamycin B (26, 27), upon lipid antigen presentation to CD8–1/J.RT3 was analyzed. As we previously showed, antigen presentation to the CD1b-restricted LDN5/J.RT3 cells was completely blocked by concanamycin B (Fig. 2, Center) (11). In contrast, little if any effect of this drug was detected on antigen presentation to the CD1a-restricted CD8–2/J.RT3 T cells (within twofold difference in antigen concentration) (Fig. 2, Left). Under identical conditions, the antigen-specific, CD1c-restricted response by CD8–1/J.RT3 was impaired in the presence of concanamycin B at limiting antigen concentrations, but readily overcame drug sensitivity at antigen concentrations that exceeded the half-maximal dose. Thus, the acidic environment was not stringently required and could be overcome for CD1c antigen presentation. This finding raised the possibility that antigen presentation by CD1c might not occur exclusively in highly acidified late endosomes and lysosomes but might also occur in nonacidified endosomal compartments.
Figure 2.
Effect of concanamycin B on CD1a-, CD1b-, and CD1c-mediated antigen presentation. Monocyte-derived dendritic cells were preincubated with 10 nM concanamycin B (con B) for 30 min and then washed and incubated for 4 h with indicated concentrations of specific M.tb antigen preparations [the semipurified M.tb lipid antigen preparation for CD8–2/J.RT3, purified glucose monomycolate (GMM) for LDN5/J.RT3, the chloroform/methanol extract of H37Ra for CD8–1/J.RT3] either in the presence or absence of 10 nM concanamycin B. The cells were then washed, fixed, and incubated with CD8–2/J.RT3 (Left), LDN5/J.RT3 (Center), and CD8–1/J.RT3 (Right), respectively, and IL-2 release into the media was measured.
Heterogenous Colocalization of CD1c and LAMP-1.
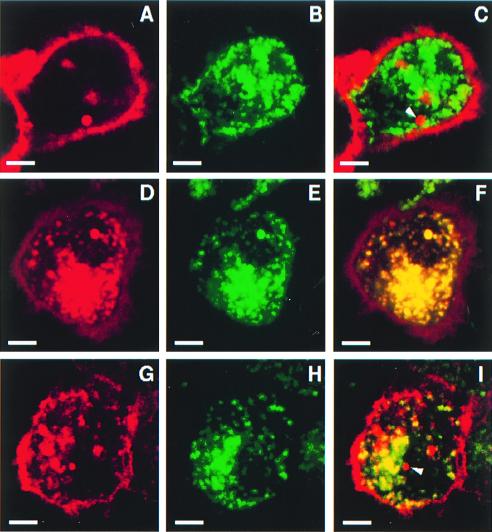
To detect possible differences in the endocytic distribution between CD1b and CD1c, colocalization with LAMP-1 (a marker of late endosomes and lysosomes) was examined for each of the CD1 isoforms. Thus, monocyte-derived dendritic cells that expressed CD1a, -b, and -c were analyzed by confocal microscopy after double labeling with antibodies to CD1 and LAMP-1. CD1b was found in numerous intracellular vesicles (red vesicles in Fig. 3D), with a nearly identical vesicular distribution to that observed for LAMP-1 expression (green vesicles in Fig. 3E) as revealed by their being almost entirely superimposable (yellow vesicles in Fig. 3F). In contrast, only very few CD1a+ intracellular vesicles were detected in the dendritic cells. The vesicles detected were mainly located at a juxtanuclear position of these dendtritic cells and lacked coexpression of LAMP-1 (Fig. 3C, indicated with an arrowhead). These data were consistent with CD1a being located in recycling endosomes of the early endocytic system, whereas CD1b was predominantly localized to the LAMP-1+ late endosomes and lysosomes.
Figure 3.
Colocalization of CD1a, -b, and -c with LAMP-1. Monocyte-derived dendritic cells were fixed and permeablized after a cytospin procedure. The cells were then double-labeled with mouse mAbs to CD1a (A), CD1b (D), or CD1c (G) (detected with Texas Red-conjugated donkey anti-mouse IgG) and a rabbit antiserum against human LAMP-1 (B, E, and H) (detected with FITC-conjugated donkey anti-rabbit IgG) and analyzed by confocal microscopy. The corresponding red and green fluorescent confocal images then were superimposed to detect any cellular compartments expressing both CD1 and LAMP-1 (C, F, and I, yellow vesicles). (Scale bars = 5 μm.)
The cellular distribution of CD1c appeared distinct from that for either CD1a or CD1b. CD1c was prominently expressed in intracellular vesicles although cell surface staining was relatively more intense when compared to that for CD1b (Fig. 3G). Moreover, only a moderate fraction of CD1c-containing vesicles expressed LAMP-1 (Fig. 3I, yellow vesicles), and vesicles that expressed CD1c, but not LAMP-1, were readily detected in many cells, some of which were located at a juxtanuclear position (Fig. 3I, red vesicles indicated with an arrowhead). Thus, the intracellular distribution of CD1c was not identical to that of CD1a or CD1b. In fact, the localization pattern of CD1c in intracellular vesicles appeared intermediate between that of CD1a and CD1b (compare Fig. 3 C, F, and I for CD1a, -b, and -c, respectively).
CD1c Is Broadly Distributed Throughout the Endocytic System.
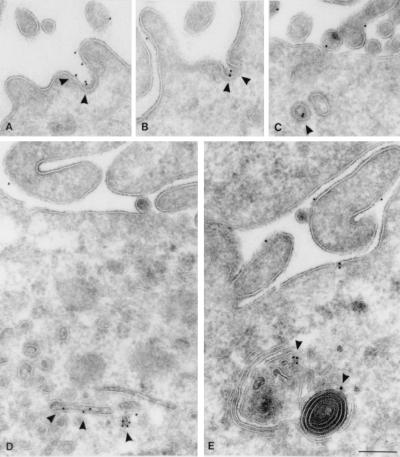
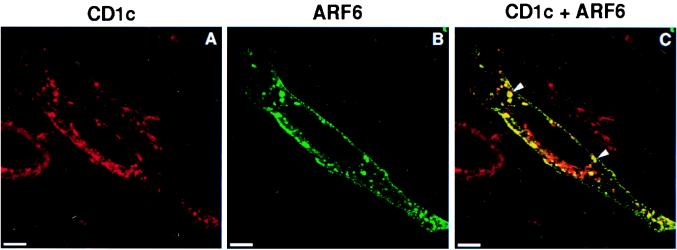
To gain further insight into the intracellular distribution of CD1c, monocyte-derived dendritic cells were analyzed by using immunogold-labeled transmission electron microscopy. Pronounced expression of CD1c was detected in plasma membrane-associated clathrin-coated pits (Fig. 4 A and B, indicated with arrowheads) and vesicles (Fig. 4C, arrowhead), suggesting that internalization of CD1c from the cell surface was a prominent pathway for its entry into the endocytic system. CD1c also was found in dense multilamellar or multivesicular organelles (Fig. 4E, arrowheads), characteristic of the MIIC, in which CD1b and MHC class II were prominently detected in our previous studies (10, 28). This result was consistent with the partial colocalization of CD1c with LAMP-1 detected by the confocal analysis because these MIICs are known to be LAMP-1+ lysosomal compartments (28). However, we also detected prominent CD1c expression in small intracellular vesicles of these monocyte-derived dendritic cells (Fig. 4D, arrowheads). These CD1c+ vesicles lacked an electron-dense coat structure and thus were distinct from clathrin-coated vesicles. ARF6, a small GTPase, regulates transport of recycling vesicles to the cell surface and overexpression of the T27N mutant of ARF6 (ARF6-T27N) perturbs the GTPase cycle, which results in an accumulation of recycling vesicles bearing ARF6 (21, 24). To examine whether a fraction of CD1c molecules might traffic in recycling vesicles, HeLa cells stably transfected with CD1c were transiently supertransfected with the ARF6-T27N mutant, double-labeled with antibodies to CD1c and ARF6, and viewed by confocal microscopy. In ARF6-T27N-transfected cells, a significant fraction of CD1c+ vesicles contained ARF6-T27N (Fig. 5C, yellow vesicles indicated with arrowheads). Thus, unlike CD1b, which primarily distributes to late endocytic compartments and does not traffic through the recycling pathway, CD1c was able to follow the recycling pathway of the early endocytic system as well as penetrate deeply into the late endocytic system, resulting in an intracellular distribution throughout the endocytic system.
Figure 4.
Immunoelectron micrographs of thin cryosections of monocyte-derived dendritic cells labeled with anti-CD1c antibody. CD1c was found in plasma membrane-associated clathrin-coated pits (A and B) and vesicles (C), in small tubular compartments (D), and in multilamellar or multivesicular compartments (E). (Scale bars = 200 nm.)
Figure 5.
Colocalization of CD1c with ARF6-T27N. HeLa cell transfectants expressing CD1c were grown on coverslips and transiently supertransfected with ARF6-T27N cDNA. The cells were then fixed and permeabilized, and double-labeling with mouse anti-CD1c antibody (detected with Texas Red-conjugated donkey anti-mouse IgG) and rabbit anti-ARF6 antibody (detected with FITC-conjugated donkey anti-rabbit IgG) was performed. Fluorescent confocal images were obtained for CD1c (A) and ARF6 (B). The two images then were superimposed to detect vesicles expressing both CD1c and ARF6 (C, yellow vesicles shown with arrowheads). (Scale bars = 5 μm.)
Discussion
For efficient antigen presentation, antigen-presenting molecules must be elaborately directed to appropriate intracellular compartments for antigen sampling. For peptide antigens, MHC class I and class II molecules differentially sample the cytosol and the late endocytic compartment. MHC class I molecules bind cytosol-derived peptide antigens in the endoplasmic reticulum and are then expressed on the cell surface without intersecting the endocytic route. In contrast, association with the invariant chain prevents MHC class II molecules from binding peptides in the endoplasmic reticulum and directs these molecules to the late endocytic compartment for binding endocytosed exogenous protein-derived antigens. Thus, separate pathways for peptide presentation mediated by these two classes of antigen-presenting molecules result in efficient sampling in distinct intracellular compartments (29). For presentation of lipid antigens, CD1a samples early recycling endosomes whereas the cytoplasmic signal directs CD1b to penetrate deeply into the endocytic system to sample the late endocytic compartment. These two major arms of the endocytic pathway may contain distinct sets of lipid antigens and thus the differential trafficking of CD1a and CD1b predicts lipid antigen sampling separately in recycling endosomes or late endosomes (11). The present study has shown that CD1c trafficking is unique in that it distributes prominently to two anatomically and functionally distinct intracellular endocytic compartments that separately coexpress either CD1a or CD1b. This broad distribution of CD1c molecules in endocytic subcompartments may be crucial for efficient sampling of lipid antigens because CD1a, -b, and -c are differentially expressed in vivo on most professional antigen-presenting cells, depending on the cell types, degree of maturation, and the sites in which they reside. For example, epidermal Langerhans cells express CD1a and CD1c but little or no CD1b (16). Thus, CD1c may play a dominant role in sampling the late endocytic compartment in these CD1b-negative cells. In addition, CD1c is expressed on a significant fraction of B cells in the spleen, tonsils, and peripheral blood, and virtually all cord blood B cells that do not express either CD1a or CD1b (15). The individual expression of CD1c on B cells and its ability to broadly sample endocytic compartments appear crucial for lipid antigen presentation in these cells. Because mAbs are frequently directed against glycolipids, such antigen-specific B cells may be particularly efficient in antigen presentation as is the case for B cells expressing antibodies to protein antigens (30).
Molecular mechanisms that control differential endosomal localization of CD1b and CD1c remain to be established. Both molecules contain similar tyrosine-based endosomal targeting motifs in their cytoplasmic domain. However, we recently found, by using a yeast two-hybrid system, that the cytoplasmic tails of CD1b and LAMP-1, but not CD1c, were capable of interacting with the AP-3 adaptor complex that is proposed to mediate protein trafficking to lysosomes (ref. 31 and unpublished observations). AP-3-dependent transport of CD1b may account for its enrichment in LAMP-1+ late endosomes and lysosomes, whereas CD1c can possibly diffuse broadly to the endocytic system by avoiding efficient signal-dependent delivery to these compartments.
Trafficking of endocytosed lipid antigen to either early recycling endosomes or late endosomes is controlled at least in part by the length and the degree of saturation of the hydrophobic alkyl chain(s) of the lipid. Whereas lipids with saturated long alkyl chains are sorted toward late endosomes and lysosomes, those with unsaturated short alkyl chains are preferentially transported to recycling endosomes (13). Recently, the CD8–1 T cell line and two other independently isolated CD1c-restricted T cell lines were found to recognize semisynthetic mannosyl phoshodolichol, which contains several unsaturated double bonds in the alkyl chain (4). Further, the T cell response was inversely proportional to prenyl length, favoring the hypothesis that the antigen presented to CD8–1 might be captured by CD1c in early endocytic compartments. Because CD1c also is expressed in late endosomes and lysosomes (Figs. 3 and 4), it may bind certain types of lipid antigens in these highly acidified compartments like CD1b. However, its ability to overcome dependence on vesicular acidification as well as its broad endocytic distribution may allow sampling of early endocytic compartments where CD1b does not function.
The ability of CD1c to bind antigen even in vacuoles whose acidification is blocked by concanamycin B may be of physiological relevance. Phagocytosed live mycobacteria often reside in phagosomes that appear to actively exclude the vesicular proton ATPase, resulting in their failure to acidify (9). This may be a major mechanism by which mycobacteria can survive and replicate in phagosomes and abrogate antigen presentation by CD1b and MHC class II molecules (8). CD1c expression is detected in phagosomes (data not shown) and its ability to bind mycobacterial lipid antigens in vesicles with impaired acidification predict that CD1c may be in an ideal position to sample mycobacterial lipid antigens upon phagocytosis and present them to T cells. Indeed, the reactivity of peripheral blood T cells to mannosyl phosphodolicol that the CD1c-restricted CD8–1 T cells recognize was detected in patients with active tuberculosis (4). Further, the fact that the CD8–1 cells recognize and lyse mycobacteria-infected dendritic cells and secrete granulysin that can directly kill released mycobacteria (3, 5) makes it likely that CD1c may mediate effective anti-mycobacteria immune responses at an early phase of infection. In addition, we recently found that the major tissue subset of granulysin-containing cytolytic γδ T cells recognize CD1c (17). Together, these features of its broad sampling within the endocytic system, presentation of distinct subclasses of lipid antigens, and expression alone or along with other CD1 isoforms offer the immune system a valuable opportunity to sample the universe of lipid antigens and activate effector αβ and γδ T cells. It seems likely that CD1c, besides CD1a and CD1b, has been evolutionarily maintained to function against infection with microbes that constantly attempt to evolve evasive mechanisms.
Acknowledgments
We thank Drs. H. Ploegh, M. Fukuda, D. B. Moody, S. A. Porcelli, D. Olive, and C. Mawas for their gifts of reagents; Immunex for granulocyte/marcrophage colony-stimulating factor; Schering-Plough for IL-4; and Jean Lai for expert help with confocal microscopy. This work was supported by grants from the National Institutes of Health (to M.B.B.). M.S. was supported by the Leukemia and Lymphoma Society and N.v.d.W. by the Netherlands Leprosy Relief.
Abbreviations
- M.tb
Mycobacterium tuberculosis
- TCR
T cell receptor
- MHC
major histocompatibility complex
- LAMP-1
lysosome-associated membrane protein-1
- ARF6
ADP-ribosylation factor-6
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.150236797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.150236797
References
- 1.Beckman E M, Porcelli S A, Morita C T, Behar S M, Furlong S T, Brenner M B. Nature (London) 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 2.Sieling P A, Chatterjee D, Porcelli S A, Prigozy T I, Mazzaccaro R J, Soriano T, Bloom B R, Brenner M B, Kronenberg M, Brennan P J, et al. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 3.Stenger S, Mazzaccaro R J, Uyemura K, Cho S, Barnes P F, Rosat J P, Sette A, Brenner M B, Porcelli S A, Bloom B R, Modlin R L. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 4.Moody D B, Ulrichs T, Muhlecker W, Young D C, Gurcha S S, Grant E, Rosat J P, Brenner M B, Costello C E, Besra G S, Porcelli S A. Nature (London) 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 5.Stenger S, Hanson D A, Teitelbaum R, Dewan P, Niazi K R, Froelich C J, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, et al. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 6.Rosat J P, Grant E P, Beckman E M, Dascher C C, Sieling P A, Frederique D, Modlin R L, Porcelli S A, Furlong S T, Brenner M B. J Immunol. 1999;162:366–371. [PubMed] [Google Scholar]
- 7.Schofield L, McConville M J, Hansen D, Campbell A S, Fraser-Reid B, Grusby M J, Tachado S D. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann S H. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 9.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Science. 1994;263:678–181. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 10.Sugita M, Jackman R M, van Donselaar E, Behar S M, Rogers R A, Peters P J, Brenner M B, Porcelli S A. Science. 1996;273:349–352. doi: 10.1126/science.273.5273.349. [DOI] [PubMed] [Google Scholar]
- 11.Sugita M, Grant E P, van Donselaar E, Hsu V W, Rogers R A, Peters P J, Brenner M B. Immunity. 1999;11:743–752. doi: 10.1016/s1074-7613(00)80148-x. [DOI] [PubMed] [Google Scholar]
- 12.Jackman R M, Stenger S, Lee A, Moody D B, Rogers R A, Niazi K R, Sugita M, Modlin R L, Peters P J, Porcelli S A. Immunity. 1998;8:341–351. doi: 10.1016/s1074-7613(00)80539-7. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee S, Soe T T, Maxfield F R. J Cell Biol. 1999;144:1271–1284. doi: 10.1083/jcb.144.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugita M, Peters P J, Brenner M B. Traffic. 2000;1:295–300. doi: 10.1034/j.1600-0854.2000.010401.x. [DOI] [PubMed] [Google Scholar]
- 15.Durandy A, Thuillier L, Forveille M, Fischer A. J Immunol. 1990;144:60–65. [PubMed] [Google Scholar]
- 16.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. Nature (London) 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 17.Spada F M, Grant E P, Peters P J, Sugita M, Melian A, Leslie D S, Lee H K, van Donselaar E, Hanson D A, Krensky A M, et al. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant E P, Degano M, Rosat J P, Stenger S, Modlin R L, Wilson I A, Porcelli S A, Brenner M B. J Exp Med. 1999;189:195–205. doi: 10.1084/jem.189.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olive D, Dubreuil P, Mawas C. Immunogenetics. 1984;20:253–264. doi: 10.1007/BF00364207. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson S R, Roth J, Piller F, Fukuda M. J Biol Chem. 1988;263:18911–18919. [PubMed] [Google Scholar]
- 21.D'Souza-Schorey C, van Donselaar E, Hsu V W, Yang C, Stahl P D, Peters P J. J Cell Biol. 1998;140:603–616. doi: 10.1083/jcb.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porcelli S, Morita C T, Brenner M B. Nature (London) 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 23.Moody D B, Reinhold B B, Guy M R, Beckman E M, Frederique D E, Furlong S T, Ye S, Reinhold V N, Sieling P A, Modlin R L, et al. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 24.Peters P J, Hsu V W, Ooi C E, Finazzi D, Teal S B, Oorschot V, Donaldson J G, Klausner R D. J Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prigozy T I, Sieling P A, Clemens D, Stewart P L, Behar S M, Porcelli S A, Brenner M B, Modlin R L, Kronenberg M. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 26.Benaroch P, Yilla M, Raposo G, Ito K, Miwa K, Geuze H J, Ploegh H L. EMBO J. 1995;14:37–49. doi: 10.1002/j.1460-2075.1995.tb06973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugita M, Brenner M B. J Biol Chem. 1995;270:1443–1448. doi: 10.1074/jbc.270.3.1443. [DOI] [PubMed] [Google Scholar]
- 28.Peters P J, Neefjes J J, Oorschot V, Ploegh H L, Geuze H J. Nature (London) 1991;349:669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- 29.Germain R N, Margulies D H. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 30.Lanzavecchia A. Nature (London) 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 31.Dell'Angelica E C, Klumperman J, Stoorvogel W, Bonifacino J S. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]