Abstract
Introduction
The range of indications for vitreoretinal surgery has widened in recent years, and intraocular gas application is frequently performed as part of retinal surgery, with the aim of achieving long-acting tamponade.
Methods
Selective literature review.
Results
An intraocular gas bubble containing perfluoropropane (C3F8) or sulfur hexafluoride (SF6) can expand during anesthesia due to nitrous oxide diffusion and cause retinal ischemia and postoperative blindness. A decrease in atmospheric pressure associated with travel to high altitude can have the same effect. Case reports suggest that, considering physical properties of these gases and ocular physiology, patients remain at risk for at least three months after intraocular gas application.
Discussion
Both doctors and patients need to be well informed about the hazards of intraocular gas application as good communication may prevent complications. If in doubt, the anesthesiologist should avoid nitrous oxide, in particular in the unconscious patient.
Keywords: intraocular gas application, retinal ablation, complications, retinal surgery, nitrous oxide anesthesia
In recent years, surgical strategies in the treatment of retinal detachment have changed substantially, and intraocular surgical techniques are employed more and more (1, 2, 3). Further, the range of indications for vitreoretinal operations has widened considerably. Additional indications for intraocular gas application include macular foramina and circumscribed subretinal hemorrhages and foramina near the posterior pole of the eye (4, 5). Most of the operative methods require temporary intraocular gas injection, and sulfur hexafluoride (SF6) or perfluoropropane (C3F8) are the most commonly used gases. By using such gas injections, retinal tamponade can be achieved for two to six weeks owing to the high surface tension between gas bubble and retina – which is necessary to treat retinal disorders successfully. However, the procedure entails risks and can lead to blindness if patients receive repeated anesthesia within this time window or travel to high altitudes, as these factors lead to expansion of the intraocular gas bubble.
Intraocular gases
The gases most commonly used in ophthalmic surgery are air, sulfur hexafluoride (SF6), and perfluoropropane (C3F8); more rarely, perfluoroethane (C2F6) and perfluorobutane (C4F8) are used. The gases mentioned differ in terms of how long they will remain in the eye and in their expansion capacity (table 1).
Table 1. Physical attributes of commonly used intraocular gases.
| Gas | Expansion behavior at a concentration of 100% (factor) | Concentration that is usually applied (%) | Number of days that intraocular gas remains in the eye |
| Air | 0 | 5 to 7 | |
| SF6 | 2 | 20 | 10 to 14 |
| C2F6 | 3.5 | 16 | 30 to 35 |
| C3F8 | 4 | 12 | 55 to 65 |
modified from (15)
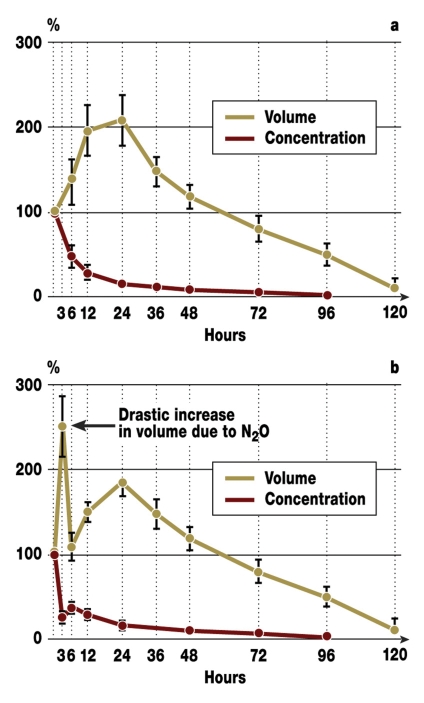
After application of pure (100%) SF6 or C3F8, when the patient is breathing ambient air, nitrogen, oxygen, and carbon dioxide will diffuse from the blood into the intraocular bubble. The volume of the gas bubble increases (expandable), because the gas applied earlier cannot diffuse at the same speed out of the vitreous space, owing to its extremely low solubility in a watery medium. The mixture that is therefore commonly used is 20% of gas in air, so that the gas bubble is less expandable. Its partial nitrogen pressure is almost identical to that of the surrounding air or blood, so that the bubble expands only to a limited extent. A bubble consisting of pure SF6 will expand to double its injected volume within 24 hours, whereas the volume of a pure C3F8 bubble quadruples over three to seven days (7, 8). After injecting SF6 or C3F8 into the eye, equilibrium between blood and gas bubble is reached within hours for O2 and CO2. For N2 equilibrium is reached only after several days (5, 24). Because of their poor solubility, SF6 or C3F8 leave the eye only slowly; the bubble volume increases or the pressure if the gas cannot expand. However, the bubble expands usually so slowly that the intraocular pressure remains constant thanks to an increase in drainage of anterior chamber fluid on the one hand and slow diffusion of the gas on the other hand (figure 1 a, b) (9, 10).
Figure 1.
Expansion behavior of an intraocular gas bubble filled with sulfur hexafluoride under conditions of ambient air ventilation (a) or (b) exposure to 67% nitrous oxide in sedated rabbits having local anesthesia. Liquefied vitreous was aspirated via the pars plana and pure sulfur hexafluoride injected afterwards. From: Kroll P, Reinhold R, Radig C, Eckard R, Ostmeier H: Zum Expansionsverhalten von intravitrealem Schwefelhexafluorid unter Lachgasexposition. Klin Monatsbl Augenheilkd 1986; 189: 36–8 (10), with kind permission from Georg Thieme Verlag KG Stuttgart.
Gases are well suited for intraocular tamponade because the marginal tension between gas and water is substantially higher than between oil and water, and the gas cannot penetrate into the subretinal area through a hole in the retina. A large gas bubble occupies a larger retinal area than a smaller bubble and therefore results in more effective tamponade.
As time passes, the gas applied into the eye diffuses slowly. A buffer zone consisting of partially equilibrated fluid forms between bubble and retina. During this process, the gas concentration from the inside to the outside falls steadily (diffusion path). In this way the concentration gradient, which drives the diffusion process according to Fick’s 1st law of diffusion (dm/dt = D A/d*[C1-C2]), is reduced locally (5, 6), and the diffusion of the gas into the blood is slowed down. Over time, the gas bubble is replaced by bodily fluids. In contrast to silicone oil tamponade, no further surgery is required to remove the endotamponade.
The length of time that the gas bubble will remain depends not only on the volume, concentration, and solubility of the administered gas but also on the choroidal circulation. The surgeon ideally selects the gas on the basis of individual findings. The less soluble a gas the longer it remains within the eye (11). The larger the injected volume, the greater the expansion effect. By doubling the concentration, the intraocular "lifespan" of a gas bubble is doubled. In a non-vitrectomized, phakic eye, the bubble remains twice as long as in a vitrectomized eye. Low intraocular pressure or low production of anterior chamber fluid also prolong the persistence of a gas bubble (12). Because of the influence of intraocular pressure, production of anterior chamber fluid, choroidal perfusion, and the eye’s preoperative condition, a precise prediction of the time to disappearance of the gas bubble is not possible in the individual patient.
What this means for the anesthesiologist
Laughing gas (nitrous oxide, N2O) has a blood gas distribution coefficient that is 35 times higher than that of nitrogen (0.46 versus 0.014). During anesthesia with nitrous oxide, N2O diffuses more quickly from the blood into an air filled cavity than nitrogen diffuses from the same cavity into the blood. The result: a notable increase in volume in expandable or an increase in pressure in non-expendable cavities. This means that during anesthesia, the intravitreous gas injection must be given only once the application of N2O has ceased for some 15 minutes and the N2O has been flushed out of the body. Otherwise, a dangerously fast increase in intraocular pressure is to be expected after intraocular gas injection. In reverse, after intraocular gas injection during N2O anesthesia, the gas bubble will rapidly shrink after N2O application has ceased, which will result in insufficient retinal tamponade (8).
Seemingly the opposite effect has been reported in studies when the intraocular pressure remained the same after injection of C3F8 during anesthesia with 65% N2O and anesthesia not using N2O. This effect seems to be due to the intraoperatively leaky seal of the sclerotomies, which would have enabled intraocular gas to escape during the operation. It thus seems that the risk lies less in the use of laughing gas during the initial procedure than rather in using N2O during a follow-up procedure, once the eye is "sealed" (13).
Reports about postoperative blindness after N2O anesthesia have increased with increasing numbers of intravitreous gas injections. Thirteen cases have been reported so far – the time lag between the initial administration of the gas and N2O anesthesia leading to blindness was up to six weeks (table 2). Assessors assume a high number of undetected cases. Whether the risk of developing blindness subsequent to intraocular gas injection persists after longer time intervals is not known.
Table 2. Case reports of visual loss after anesthesia with nitrous oxide.
| Patient’s age in years | Visual capacity before N2O anesthesia; patient sees: | Time lag to N2O anesthesia (days) | Visual capacity after N2O anesthesia | Type of subsequent procedure | Reference number |
| 71 | Hand movement | 1 | No sensation of light | Transurethral resection of the prostate (TURP) | (21) |
| 37 | Hand movement | 42 | No sensation of light | Femoroarterial bypass | (8) |
| 19 | Hand movement | 25 | No sensation of light | Transplantation of kidney or pancreas | (8) |
| 73 | Can count fingers | 22 | No sensation of light | Total pelvic endoprosthesis | (7) |
| 80 | Hand movement | 9 | No sensation of light | TURP | (7) |
| 75 | Not mentioned | 30 | Sensation of light | Femoral neck (hip)fracture | (7) |
| 61 | Can count fingers | 30 | Sensation of light | Prostate surgery (no more detail given) | (25) |
| Not known | Hand movement | 2 | No sensation of light | Vitrectomy | (25) |
| Not known | Hand movement | 25 | Sensation of light | Repair of arteriovenous fistula | (25) |
| Not known | Sensation of light | 6 | No sensation of light | Vitrectomy | (25) |
| 63 | Can count fingers | 14 | Sensation of light | Vitrectomy | (23) |
| 66 | 20/50 * | 5 | No sensation of light | Prostatectomy | (22) |
| 55 | 6/36 * | 37 | Hand movement | Femoropopliteal bypass | (20) |
*Snellen visual acuity scale
Using nitrous oxide in patients with intraocular gas bubbles can result in rapid bubble expansion with anterior shift of the lens-iris diaphragm and subsequent angle closure. The fluid in the anterior chamber subsequently cannot drain, which means that a crucial compensation mechanism to regulate intraocular pressure has been disabled (angle closure glaucoma) (8). If the intraocular pressure exceeds the perfusion pressure in the central artery the result will be retinal ischemia. Studies in monkeys have shown that occlusion of the central artery for more than 90 minutes causes irreversible ischemic damage to the retina with subsequent atrophy of the optic nerve (14). Disorders such as diabetes mellitus or atherosclerosis, but also old age, lessen the retinal perfusion pressure (15, 16) and can increase the risk of central artery occlusion after intraocular gas injection.
Which options remain if after N2O anesthesia it transpires that the patient has an intraocular gas bubble? The administration of nitrous oxide will have to be stopped immediately.
Ventilation with 100% oxygen results in accelerated flushing out of the nitrous oxide from the intraocular gas bubble than is achievable with ambient air. Arterial hypertension should be corrected in order to secure sufficient retinal perfusion pressure. An ophthalmologist should be consulted immediately, to control intraocular pressure and retinal vascularization – e.g., by using indirect ophthalmoscopy. During the waiting period, intraocular pressure can be assessed by palpating the eye. If the affected eye is "rock-hard" to the touch compared with the other eye, the risk of central arterial occlusion is high. Administration of acetazolamide is indicated to reduce the production of anterior chamber fluid, thereby lowering intraocular pressure.
If an occlusion of the central artery is visible through the ophthalmoscope or if the intraocular pressure is raised drastically, intraocular gas needs to be released immediately either via one of the pars plana sclerotomies that are already present or by transscleral puncture into the vitreous space at a distance of 3.5 mm from the corneal limbus.
Expansion of intraocular gases owing to reduced atmospheric pressure
Anecdotal reports have mentioned sudden vision loss when traveling to high altitudes or across the Alps, and sudden blindness on ascent with vision returning upon landing. These symptoms are presumably the consequences of fast volume changes of the gas bubble and may be regarded as transient retinal ischemia owing to raised intraocular pressure subsequent to reduced atmospheric pressure (17–19). Atmospheric pressure falls with increasing distance from the surface of the Earth. Starting at sea level, it falls by 1 hPa every 8 meters. According to the Boyle-Mariotte law (p *V = constant) the injected gas bubble therefore expands. If the body temperature remains constant the degree of expansion depends on the drop in atmospheric pressure over time – and therefore on the rate of ascent. The ascent rate of an aircraft on a scheduled flight is 2000 to 3000 ft/min (606 to 909 m/min). The cabin pressure compresses by a height equivalent of 90 to 150 m/min. This happens automatically, depends on the type of construction, and is characteristic of the type of aircraft. In a Boeing 747 at a traveling height of 11 200 m, the cabin pressure corresponds to a height of 1700 m, whereas in a McDonell Douglas DC9, the pressure is equivalent to a height of 2400 m when traveling at 11 200 m. The altitude of the starting airport is also important. The higher the airport, the lower the potential for expansion of the gas bubble.
To conclude, the extent of the intraocular pressure increase depends on the intraocular gas volume, the construction-related cabin compression of the aircraft, and the geographical altitude of the departure airport. Compensatory mechanisms for intraocular pressure rises are scleral expansion and increased drainage of anterior chamber fluid.
If the volume of the intraocular gas bubble is only 10% of the vitreous volume, the expansion of the gas can be compensated sufficiently, provided the compensatory mechanisms, such as drainage and reduction of anterior chamber fluid, are able to function. Both mechanisms may be disrupted in glaucoma patients. If the increase in intraocular pressure during the trip lasts less than 90 minutes then sequelae are unlikely in a healthy patient; however, in a glaucoma patient with prior damage to the optic nerve they cannot be excluded. Currently, drug treatment is not an option for influencing an increase in intraocular pressure. The only effective measure is to raise cabin pressure by reducing flight altitude. Administration of acetazolamide before the flight is not recommended because the pressure lowering effect is not sufficiently great to compensate for the later rise in intraocular pressure. Ocular hypotension is an additional undesirable potential effect that might be triggered (17–19).
Risk information
Every patient who receives a gas injection must be informed about associated risks. Such warnings should be given to the patient postoperatively or at discharge (at the latest) as a leaflet or pass. It must be ensured that the patient has understood this information. Providers of intraocular medical gases provide patient passes to their users. Further, a concluding report from the ophthalmologist to the patient’s general practitioner is desirable, in which explicit mention can be made of the risks in a future operation or travel to great heights or air travel. It is also recommended for legal reasons to document in the patient’s file the type and content of information provided.
Preventing complications
Often, patients with a gas bubble who are receiving elective or emergency care will require surgery or anesthesia outside an ophthalmic ward. In view of the potentially long lifespan of the gas bubble, it is of the utmost importance that the anesthesiologist is informed about this. The importance of a complete medical history is crucial: underlined by the fact that in all cases of blindness after anesthesia with nitrous oxide that have been reported in the literature the chain of information was interrupted. The question about earlier ophthalmic surgery should therefore be included in standard history taking before any anesthesia or surgical procedure. If any doubt remains the use of nitrous oxide will have to be avoided, especially in patients who cannot give their own history, e.g., unconscious patients.
An intraocular gas injection that dates back more than three months can be assumed to present no contraindication to the use of nitrous oxide or travel at high altitudes. In elective procedures patients should, however, undergo an ophthalmology examination, to rule out a possible risk of blindness.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Heimann H, Hellmich M, Bornfeld N, Bartz-Schmidt KU, Hilgers RD, Foerster MH. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment (SPR study): design issues and implications. SPR Study Report 1. Graefes Arch Clin Exp Ophthalmol. 2001;239:567–574. doi: 10.1007/s004170100319. [DOI] [PubMed] [Google Scholar]
- 2.Bornfeld N. Ablatiochirurgie. Von innen, von aussen, oder beides? Ophthalmologe. 2001;98:879–880. doi: 10.1007/s003470170066. [DOI] [PubMed] [Google Scholar]
- 3.Ezra E, Gregor ZJ. Surgery for idiopathic full-thickness macular hole: two-year results of a randomized clinical trial comparing natural history, vitrectomy, and vitrectomy plus autologous serum: Moorfields Macular Hole Study Group Report 1. Arch Ophthalmol. 2004;122:224–236. doi: 10.1001/archopht.122.2.224. [DOI] [PubMed] [Google Scholar]
- 4.Faude F, Wiedemann P. Intraokulare Gase in der Glaskörper- und Netzhautchirurgie. Teil II Klinik. Ophthalmologe. 1999;96:413–420. doi: 10.1007/s003470050429. [DOI] [PubMed] [Google Scholar]
- 5.Faude F, Wiedemann P. Intraokulare Gase in der Glaskörper- und Netzhautchirurgie. Teil I Grundlagen. Ophthalmologe. 1999;96:349–358. doi: 10.1007/s003470050417. [DOI] [PubMed] [Google Scholar]
- 6.Lockwood G. Expansion of air bubbles in aqueous solutions of nitrous oxide or xenon. Br J Anaesth. 2002;89:282–286. doi: 10.1093/bja/aef183. [DOI] [PubMed] [Google Scholar]
- 7.Vote BJ, Hart RH, Worsley DR, Borthwick JH, Laurent S, McGeorge AJ. Visual loss after use of nitrous oxide gas with general anesthetic in patients with intraocular gas still persistent up to 30 days after vitrectomy. Anesthesiology. 2002;97:1305–1308. doi: 10.1097/00000542-200211000-00038. [DOI] [PubMed] [Google Scholar]
- 8.Seaberg RR, Freeman WR, Goldbaum MH, Manecke GR., Jr Permanent postoperative vision loss associated with expansion of intraocular gas in the presence of a nitrous oxide-containing anesthetic. Anesthesiology. 2002;97:1309–1310. doi: 10.1097/00000542-200211000-00039. [DOI] [PubMed] [Google Scholar]
- 9.Jantzen JPAH. Anästhesie und intraokularer Druck. Anästhesist. 1998;37:458–469. [PubMed] [Google Scholar]
- 10.Kroll P, Reinhold R, Radig C, Eckard R, Ostmeier H. Zum Expansionsverhalten von intravitrealem Schwefelhexafluorid unter Lachgasexposition. Klin Monatsbl Augenheilkd. 1986;189:36–38. doi: 10.1055/s-2008-1050745. [DOI] [PubMed] [Google Scholar]
- 11.Schenk H. Experimentelle und klinische Untersuchungen zur Frage der Resorption gasförmiger Stoffe aus dem Glaskörper. Graef Arch Clin Exp Ophthalmo. 1959;161:252–281. [Google Scholar]
- 12.Chang S. Intraocular gases. In: Ryan SJ, editor. Retina. St. Louis: Mosby-Year Book; 1989. pp. 2115–2129. [Google Scholar]
- 13.Briggs M, Wong D, Groenewald C, McGalliard J, Kelly J, Harper J. The effect of anesthesia on the intraocular volume of the C3F6 gas bubble. Eye. 1997;11:47–52. doi: 10.1038/eye.1997.10. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87:75–78. doi: 10.1016/s0161-6420(80)35283-4. [DOI] [PubMed] [Google Scholar]
- 15.Recchia FM, Brown GC. Systemic disorders associated with retinal vascular occlusion. Curr Opin Ophthalmology. 2000;11:462–467. doi: 10.1097/00055735-200012000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Dallinger S, Findl O, Strenn K, Eichler HG, Wolzt M, Schmetterer L. Age dependence of choriodal blood flow. J Am Geriatr Soc. 1998;46:484–487. doi: 10.1111/j.1532-5415.1998.tb02471.x. [DOI] [PubMed] [Google Scholar]
- 17.Gandorfer A, Kampik A. Expansion intraokularer Gase infolge Reduktion des Atmosphärendruckes. Kasuistik und Literaturübersicht. Ophthalmologe. 2000;97:367–370. doi: 10.1007/s003470050539. [DOI] [PubMed] [Google Scholar]
- 18.Lincoff H, Weinberger D, Stergiu P. Air travel with intraocular gas. II. clinical considerations. Arch Ophthalmol. 1989;107:907–910. doi: 10.1001/archopht.1989.01070010929043. [DOI] [PubMed] [Google Scholar]
- 19.Lincoff H, Weinberger D, Reppucci V, Lincoff A. Air travel with intraocular gas. I. the mechanisms for compensation. Arch Ophthalmol. 1989;107:902–906. doi: 10.1001/archopht.1989.01070010924042. [DOI] [PubMed] [Google Scholar]
- 20.Lee EJ. Use of nitrous oxide causing severe visual loss 37 days after retinal surgery. Br J Anaesth. 2004;93:464–466. doi: 10.1093/bja/aeh213. [DOI] [PubMed] [Google Scholar]
- 21.Yang YF, Herbert L, Rüschen H, Colling RJ. Nitrous oxide anesthesia in the presence of intraocular gas can cause irreversible blindness. BMJ. 2002;325:532–533. doi: 10.1136/bmj.325.7363.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aström S, Kjellgren D, Mönestam E, Bäcklund U. Nitrous oxide anesthesia and intravitreal gastamponade. Acta Anaesthesiol Scand. 2003;47:361–362. doi: 10.1034/j.1399-6576.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 23.Kodjikian L, Fleury J, Garweg J, Rouberol F, Gambrelle J, Burillon C, Grange J-D. Cécité après une anesthésie comprenant du protoxyde d’azote en présence d’un tamponnement interne par gaz. J Fr Ophtalmol. 2003;26:967–971. [PubMed] [Google Scholar]
- 24.Thompson JT. The absorption of mixtures of air and perfluoropropane after pars plana vitrectomy. Arch Ophthalmol. 1992;110:1594–1597. doi: 10.1001/archopht.1992.01080230094028. [DOI] [PubMed] [Google Scholar]
- 25.Fu AD, et al. Complications of general anesthesia using nitrous oxide in eyes with preexisting gas bubbles. Retina. 2002;22:569–574. doi: 10.1097/00006982-200210000-00006. [DOI] [PubMed] [Google Scholar]