Abstract
Introduction
Magnetic resonance tomography (MRT) is the investigation of choice for diagnosing cerebral glioma, but its capacity to differentiate tumor tissue from non-specific tissue changes is limited. Positron emission tomography (PET) and single photon emission computerized tomography (SPECT) using radiolabeled amino acids add information which helps increase diagnostic accuracy.
Methods
Review based on the authors’ own research results and a selective literature review.
Results
The use of radiolabeled amino acids allows better delineation of tumor margins and improves targeting of biopsy and radiotherapy, and planning surgery. In addition, amino acid imaging appears useful in distinguishing tumor recurrence from non-specific post-therapeutic scar tissue, in predicting prognosis in low grade gliomas, and in monitoring metabolic response during treatment.
Discussion
The benefits of amino acid imaging in cerebral gliomas support arguments for its introduction into routine clinical practice in defined clinical situations; however, its influence on treatment quality remains to be demonstrated.
Keywords: amino acid, brain tumors, nuclear medicine, PET, SPECT
Malignant neoplasms of the nervous system arise with an incidence of 5 to 6 new cases per 100 000 persons per year (e1). Beside meningioma, glioma is the most common type of primary brain tumor. The conventional treatment of cerebral glioma consists of surgical resection, radiotherapy, and chemotherapy. The results of treatment, measured in terms of survival time and quality of life, remain unsatisfactory to this day.
Magnetic resonance imaging (MRI) is currently the method of first choice for the diagnosis and differential diagnosis of primary brain tumors. With MRI, the tumor can be reliably localized and, usually, well characterized in terms of its internal structure and the extent to which it disrupts the blood-brain barrier. Imaging with standard T1-weighted sequences serves to demonstrate the anatomical relationship of the tumor to the adjacent normal structures; when contrast medium is given, the tumor per se can be better delineated from the accompanying reaction in the surrounding brain tissue (edema) (e2). The differentiation of glioma tissue from surrounding edema is unreliable, however, particularly when the tumor is not sharply demarcated from normal brain tissue, and when the blood-brain barrier remains intact (e3).
Radiogenic changes in peritumoral brain tissue after treatment may result in pathological uptake of contrast medium that cannot be reliably distinguished from recurrent glioma (e4, e5, e6). T2-weighted sequences, supplemented with a proton-density or FLAIR (fluid-attenuated inversion recovery) sequence, can reveal the maximal extent of structural changes, but, like T1-weighted images, they do not permit reliable differentiation of tumor tissue from the surrounding edema (e2).
Metabolic studies, such as the measurement of glucose metabolism with 18F-fluorodeoxyglucose (FDG) and positron emission tomography (PET), have been used for many years as supplementary methods beyond morphological imaging. In cerebral gliomas, FDG uptake is correlated with the degree of malignancy of the tumor and with the patient’s outcome (e7, e8, e9, e56). Because of the high rate of glucose metabolism in normal brain tissue, however, it is often difficult to distinguish tumor tissue from normal brain tissue by FDG-PET (figure 1).
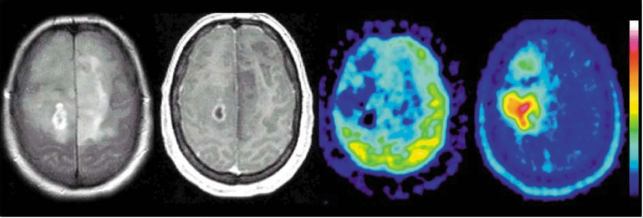
Figure 1.
Anaplastic glioma, WHO grade III: this extensive tumor is seen in the T2-weighted MR image (left) as a diffusely mottled, multifocal hyperintensity, and in the contrast-enhanced T1-weighted image (second from left) as a hypointensity with a small area of ring enhancement in the deep white matter of the right hemisphere. FDG-PET (second from right) shows reduced glucose uptake in the tumor, while FET-PET (right) shows increased FET uptake in the area of solid tumor, with a maximum lateral to the area of ring enhancement seen in the MRI. FET, O-(2-18F-fluoroethyl)-L-tyrosine; PET, positron emission tomography, FDG, 18F-fluorodeoxyglucose. (Institute for Neurosciences and Biophysics, Jülich Research Center; Clinic of Neurosurgery, University of Düsseldorf).
Aside from FDG, radiolabeled amino acids have also been used successfully for many years in positron emission tomography to measure tumor metabolism (e10). Because the uptake of amino acids by normal brain tissue is relatively low, cerebral gliomas can be distinguished from the surrounding normal tissue with high contrast.
Most PET studies of cerebral gliomas are performed with the amino acid 11C-methyl -L-methionine (MET) (e11), although the short half-life of 11C (20 minutes) limits the use of this technique to the few centers that are equipped with a cyclotron.
As an alternative to MET-PET, single photon emission computed tomography (SPECT) can be performed with the amino acid 123I iodo-alpha-methyl tyrosine (IMT); this method has been validated in many publications (e11, e12, e13, e14), but its poorer spatial resolution in comparison to PET is a disadvantage. A major advance in recent years has been the development of the 18F-labeled amino acid O-(2-18F-fluoroethyl)-L-tyrosine (FET), which, like FDG, can be transported from a cyclotron to multiple external PET centers. This enables a wider application of amino acid PET in clinical diagnosis (1, 2, e15). Unfortunately, these amino acids are not yet approved for use as pharmaceuticals. They can therefore be used only in a few centers and in experimental settings.
The purposes of this article are to provide an overview of the current state of development of amino acid diagnosis for cerebral glioma and to inform general practitioners as well as neurologists, neurosurgeons, radiologists, and radiation therapists about this diagnostic option. This review is based both on the authors’ own research findings and on a selective review of the literature.
The general properties of radiolabeled amino acids
The increased uptake of MET, IMT, and FET by cerebral glioma tissue is due almost entirely to increased transport via specific amino acid transporters (e16, e17, e18, e19). Comparative studies of these three substances have shown that all of them are taken up to roughly the same extent by cerebral glioma tissue (3, e20, 4, e19), so that the clinical experience gained with all three of them can be considered together. Whichever of these tracers is used, the radiation exposure to the patient remains within the same order of magnitude as that of conventional radiological studies (e21, e22). No side effects have been reported to date with the use of these tracers after several thousand studies have been performed worldwide. The duration of testing with any of these tracers is about 30 to 45 minutes. The cost of FET-PET is comparable to that of FDG-PET, i.e., about 1000 euros, including the cost of the tracer (this is the simple reimbursement in the German payment scheme for medical services, "GOÄ"). IMT-SPECT is not much cheaper, because of the relatively high cost of the iodine isotope, while MET-PET is substantially more expensive. The costs of these studies are partly reimbursable by private health insurance carriers, but the statutory health insurance carriers in Germany pay for ambulatory PET studies only in exceptional cases.
Showing the extent of tumor with amino acids for biopsy and treatment planning
An important aspect of the diagnostic assessment of cerebral glioma is the measurement of the extent of the tumor and the detection of the areas within the tumor in which the rate of cellular proliferation is highest. Representative tissue samples are vitally important for histological tumor diagnosis, prognostication, and treatment planning. The ability of MRI to show the most rapidly proliferating portions of inhomogeneous tumors is, unfortunately, limited, particularly when the tumor does not take up contrast medium (gadolinium). Multiple studies in which the radiological findings were compared with the histological findings in tissue obtained by biopsy or open surgery have provided clear evidence that MET-PET detects rapidly proliferating glioma tissue more reliably than either CT or MRI (5, 6, e23, e24, e25, e26). MET-PET can also be used to detect the regions with the most pronounced anaplastic changes inside a heterogeneous glioma (e27, e28). In a study involving 31 patients, tumor tissue was found in 94% of biopsies taken at sites where FET-PET had pointed to probable tumor tissue, but only in 53% of the suspicious areas identified by MRI. These figures impressively document the decidedly better diagnostic reliability of neuroimaging with radiolabeled amino acids (7) (figure 2).
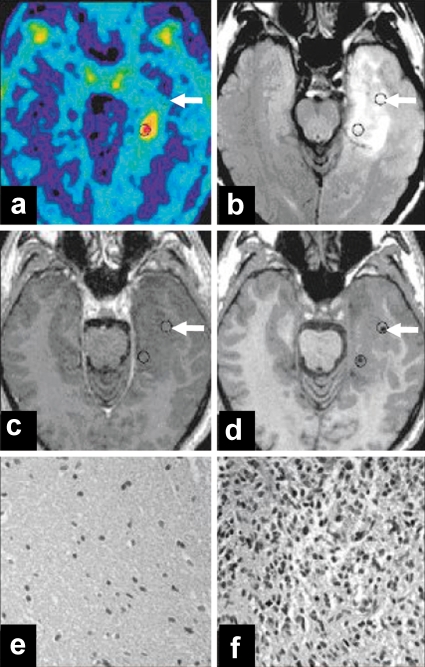
Figure 2.
Anaplastic astrocytoma, WHO grade III. MRI showed no enhancement with contrast medium, so that a differentiation of tumor tissue from non-specific tissue changes was not possible. PET shows a circumscribed area of FET uptake within an extensive zone of MRI signal changes. a) FET-PET; b) MRI-FLAIR; c) T1-weighted MR image after the injection of contrast medium; d) T1-weighted MR image after biopsy, with a titanium marker at the biopsy site. Histological study of the first tissue specimen (see arrows in a to d) revealed peritumoral tissue with reactive gliosis (e). The second tissue specimen in the area of FET uptake was found to contain dense glioma tissue (f) corresponding to an anaplastic astrocytoma, WHO grade III. FET, O-(2-18F-fluoroethyl)-L-tyrosine; PET, positron emission tomography. From: Pauleit D, Floeth F, Hamacher K, et al.: O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with Magnetic Resonance Imaging improves the diagnostic assessment of cerebral gliomas. Brain 2005; 128: 678-87 (7), with the kind permission of Oxford University Press.
No biopsy-controlled studies are available for IMT-SPECT. Nonetheless, the fact that, in comparative studies, IMT has been found to reveal tumor tissue in an identical manner to MET and FET implies that it is just as useful as MET and FET for revealing the extent of glioma tissue (3, 4, e20, e19, e29) (figure 3).
Figure 3.
Glioma, WHO grade II: T2-weighted MRI (left), contrast-enhanced T1-weighted MRI (second from left), IMT-SPECT (second from right), and FET-PET (right). The lesion appears homogenous in the MR images; IMT-SPECT and FET-PET reveal a circumscribed area of tracer uptake within it, corresponding to the portion of the tumor with the highest proliferative activity. FET, O-(2-18F-fluoroethyl)-L-tyrosine; PET, positron emission tomography; IMT, [123I] iodo-alpha-methyl tyrosine; SPECT, single photon emission computed tomography. (Institute for Neurosciences and Biophysics, Jülich Research Center; Clinic of Neurosurgery, University of Düsseldorf.)
Amino acid imaging thus optimizes the targeting biopsies and helps prevent the problem of non-diagnostic biopsies from non-specifically altered tissue. Amino acid imaging can, therefore, be expected to improve patient care, particularly in cases of glioma without any disruption of the blood-brain barrier (i.e. without contrast enhancement on MRI), and when the tumor lies adjacent to eloquent structures. FDG-PET is useful for biopsy guidance only for high-grade gliomas (e27, e28). Furthermore, amino acid imaging also improves the planning of treatment for glioma, both resective surgery and radiotherapy. A restriction of the target volume to the actual tumor tissue can substantially lessen the adverse effects of radiation therapy, while permitting treatment of the tumor itself with a higher dose than otherwise possible. Grosu et al. have reported that, in 29% of cerebral gliomas, pathological amino acid uptake is found outside the abnormal brain tissue revealed by MRI (8) (figure 4), while 90% of the hyperdense areas identified by T2-weighted MRI showed no pathological amino acid uptake. The authors recommend integrating amino acid imaging into CT- and MRI-based radiotherapeutic planning, particularly when high-precision radiotherapy is to be given or in the setting of dose-escalation studies or for the re-irradiation of recurrent tumors. An initial trial of amino acid imaging for radiotherapeutic planning in recurrent glioma has shown a significantly longer survival time than when the planning is based on CT and MRI alone (median, 9 months versus 5 months) (9).
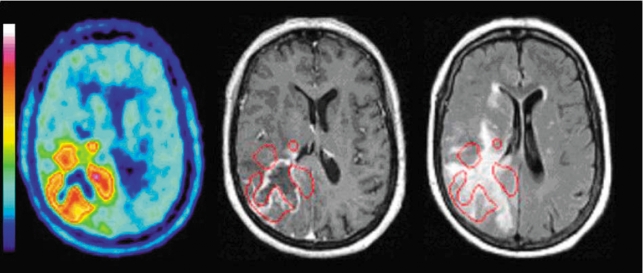
Figure 4.
FET-PET (left) and MRI with a T1-weighted, contrast-enhanced image (center) and a T2-FLAIR image (right); axial sections; glioblastoma, WHO grade IV, in the right occipital lobe. The computerized superimposition of the areas of elevated FET uptake (red border) onto the T1-weighted, contrast-enhanced MR image (center) reveals that FET uptake is not correlated with MR contrast enhancement. The areas of high FET uptake actually extend beyond the boundaries of the T2-FLAIR signal changes (right) and thus identify areas of tumor tissue that are morphologically normal in the MRI. The area of central necrosis appears in FET-PET as a site without any tracer uptake. FET, O-(2-18F-fluoroethyl)-L-tyrosine; PET, positron emission tomography. From: Langen KJ, Floeth F, Stoffels et al.: Verbesserte Diagnostik von zerebralen Gliomen mit der FET-PET. Zeitschr Med Physik 2007, 17 e57, with the kind permission of Elsevier GmbH, Munich.
The differential diagnosis of intracranial tumors of uncertain type
Although the uptake of radioactive amino acids is considered relatively specific for neoplastic masses, the possibility of non-specific enhancement must still be borne in mind. There have been reports of perifocal MET uptake around hematomas and areas of ischemia (e30, e31, e32), as well as of rare cases of MET and FET uptake in brain abscesses and foci of demyelination (e33, e34). In IMT-SPECT, no significant increase of IMT uptake has been found with non-neoplastic lesions; thus, these can easily be differentiated from high-grade gliomas (10). The occasional uptake of radiolabeled amino acids by non-neoplastic lesions reduces the specificity of this method to roughly 90%.
A recent study has shown the utility of combining FET-PET with 1H-magnetic resonance spectroscopy (1H-MRS) in the diagnostic evaluation of brain lesions. In a biopsy-controlled study of brain lesions of unknown type, a tumor was found by biopsy in 97% of cases where both FET-PET and MRS had indicated the presence of a tumor (11). On the other hand, a tumor was not found in any case in which both techniques had yielded a negative finding. In this study, the sensitivity and specificity of FET-PET alone for the differentiation of tumors from non-neoplastic changes were both 88%, while the sensitivity and specificity of 1H-MRS were 100% and 81%, respectively. It follows that, when a patient has a brain lesion of unknown type, the combination of amino acid imaging and 1H-MRS can be used to detect or rule out a tumor with a very high degree of diagnostic accuracy. If the findings of both FET-PET and 1H-MRS are normal, it would seem reasonable to dispense with a biopsy and follow the lesion further with MRI at regular intervals.
Glioma grading and prognosis
While FDG-PET is an accurate predictor of the grading and prognosis of cerebral gliomas (e7, e8, e9, e56), most studies employing amino acid imaging have shown that gliomas of different WHO grades overlap in their degree of amino acid uptake, so that the tumor grade cannot be reliably predicted with this technique (12, e26, e35, e36, e37). More recently, differences in the time course of FET uptake depending on tumor grade have been found (13, 14); this may enable a better differentiation of low- from high-grade gliomas.
The prognostic significance of the findings of amino acid imaging is currently debated. While some studies seem to show that the intensity of amino acid uptake is correlated with prognosis (37), a study with IMT-SPECT revealed no correlation either with the degree of malignancy of the tumor or with the patient’s survival time (e38).
Amino acid imaging does, however, play an important clinical role in prognostication for patients with low-grade gliomas (about 15% of the total population of glioma patients) (e1). Some of these patients will enjoy a stable course with an excellent quality of life for decades even without treatment, while others will have rapid tumor progression. A small number of prognostic factors have been identified, but the individual course remains unpredictable, and the optimal treatment strategy is controversial. A study with MET-PET showed that these patients benefit from a surgical procedure only when increased amino acid uptake can be demonstrated (15). FET-PET combined with MR morphology has also been found to be a statistically significant prognostic predictor for patients with low-grade gliomas (e50). Patients with grade II gliomas that are well demarcated in the MRI and do not take up FET have an excellent prognosis. Thus, combined assessment with FET-PET and MRI can identify a subgroup of patients who are best treated with observation alone.
The diagnostic assessment of recurrent tumors
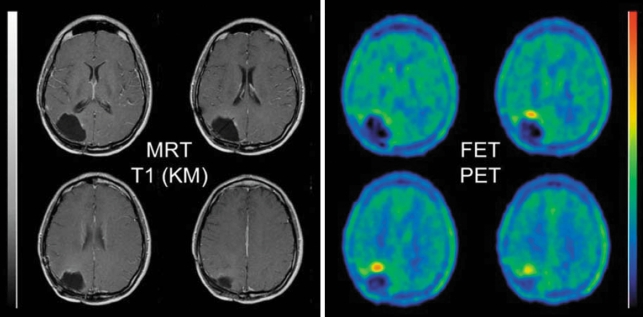
It is difficult to distinguish recurrent glioma from non-specific post-therapeutic changes with conventional MRI alone, because pathological enhancement with contrast medium may reflect either new growth of tumor or tissue necrosis after radio- or chemotherapy (e4, e5, e6). The role of FDG-PET in such cases has been called into question recently because of the frequency of non-specific uptake (e51). Multiple studies have shown, however, that MET-PET and IMT-SPECT are highly sensitive and specific for the differentiation of recurrent tumor from non-neoplastic changes (16, 17, e39, e40, e41, e42, e43, e44). In a recent FET-PET study involving 53 patients, a recurrent glioma was correctly recognized in 42 and correctly excluded in 11 patients (18) (figure 5). In another study directly comparing FET-PET with MRI and involving 46 patients, the sensitivity and specificity of FET-PET for the detection of recurrent tumor were 100% and 93%, respectively, compared with 93% and 50% for MRI (19).
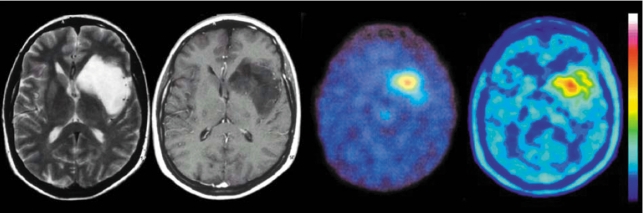
Figure 5.
Status post surgery, radiotherapy, and radioimmunotherapy of an anaplastic astrocytoma. The follow-up MRI (left) revealed no contrast enhancement and was judged to show no residual or recurrent tumor. FET-PET (right), however, shows focal tracer uptake in the ventral wall of the resection cavity. Biopsy confirmed the presence of tumor tissue, WHO grade IV. FET, O-(2-18F-fluoroethyl)-L-tyrosine; PET, positron emission tomography. From reference (18): Pöpperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K: Value of O-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 2004; 31: 1464-70, with the kind permission of Springer Verlag, Heidelberg.
Treatment monitoring
Changes in apparent tumor size or contrast enhancement that are seen in MRI and CT are taken as indicators of the response to therapy. It would also be worthwhile to measure metabolic changes within the tumor tissue for this purpose, but the experience with amino acid imaging for treatment monitoring has been limited to date. The currently available data suggest that a reduction of amino acid uptake by a glioma is a sign of a positive response to treatment (e45, e46, e47, e48). Initial longitudinal studies employing FET-PET after locoregional chemo- and radioimmunotherapy of gliomas have also shown a good correlation between FET uptake and treatment response (20, e49). Further comparative studies of the course of disease after surgery, radiation, and chemotherapy are needed, however, with histological verification of the findings, before any definite conclusions can be drawn about treatment-induced changes in amino acid uptake.
Alternative metabolic imaging with MRI
1H-magnetic resonance spectroscopy (1H-MRS) has been used for many years for the differential diagnosis of cerebral lesions of unknown type. This method enables the in vivo detection and quantification of metabolic products by measurement of shifts in the resonance signals of protons depending on their chemical environment. Living tumor tissue, for example, is characterized by an increased content of the cell membrane marker choline and a reduced content of the neuron marker N-acetylaspartate. Like amino acid imaging, 1H-MRS yields metabolic information that is markedly more specific than that obtainable by conventional MRI for the differentiation of tumor tissue from non-specific changes. It can be performed with any MRI scanner in the same sitting as conventional magnetic resonance imaging.
Unlike PET and SPECT, however, single-voxel MRS can only be used to analyze individually selected areas or partial areas in a single plane of section with "chemical shift imaging." With a combination of morphological imaging and spectroscopy, one can measure the chemical shifts in a grid of multiple, small volumes (voxels) that are chosen in a single plane of section during an MRI study. The data obtained in this way can be used to map the distribution of metabolites in the brain. Very long measuring times are needed to obtain data with an adequately high signal-to-noise ratio from small volumes of tissue (< 1.5 mL). Furthermore, the quality of the study can be impaired by susceptibility artefacts. Magnetic susceptibility, i.e., the magnetizability of a particular material by an externally applied magnetic field, varies among materials. In the near future, the use of 3 Tesla MRI scanners, parallel imaging, and faster MR spectroscopy sequences will shorten study times considerably. An initial comparative study of 1H-MRS with a 3 Tesla scanner versus IMT-SPECT has found the latter to be superior for the diagnosis of recurrent gliomas (22).
A biopsy-controlled study has shown that diffusion-weighted MRI (dwMRI) is not a suitable method for assessing the extent of cerebral gliomas (23). On the other hand, perfusion-weighted magnetic resonance imaging (pwMRI or PWI) yields information that is correlated with the degree of malignancy of gliomas and seems to be useful in biopsy planning. The potential of PWI as a means of assessing the extent of gliomas is currently still controversial (24, 25).
Perspectives for amino acid imaging
Diagnostic assessment in nuclear medicine by imaging with radiolabeled amino acids permits a more specific representation of the spatial extent of solid glioma tissue than is otherwise possible (box). This is very advantageous for the planning of biopsies, resections, and radiotherapy. Furthermore, recurrent tumors can be differentiated from post-therapeutic changes with a high degree of specificity, valuable prognostic information can be obtained for low-grade gliomas, and the treatment response can probably be judged early on in the course of treatment. Only a small number of studies of amino acid imaging for brain tumors in children have been performed to date, but here, too, the technique seems to be comparably useful (e52, e53, e54, e55). The scientifically documented utility of amino acid imaging of cerebral gliomas seems to justify its use as a routine diagnostic technique for certain indications, but it remains to be proved that this will improve the overall quality of care. The logistical prerequisites for amino acid imaging have become markedly less difficult to achieve in recent years with the introduction of IMT-SPECT and FET-PET. The costs of these diagnostic techniques would appear to be well justified by their clinical utility, not least because their timely application in a larger number of patients can be expected to save the costs incurred today by the use of other, less diagnostically reliable techniques.
Box. Improved diagnostic assessment of cerebral gliomas with amino acid PET/SPECT.
Primary diagnostic assessment
More specific demonstration of the extent of tumor than with MRI
Optimized biopsy planning, particularly for inhomogeneous gliomas that do not take up MR contrast medium
In combination with 1H-MRS, highly specific differentiation of benign from malignant brain lesions if the diagnosis is unclear
Prognosis
In combination with MR morphology, high prognostic value for low-grade gliomas
Treament planning
Optimized planning of the target volume for radiotherapy
Diagnostic assessment of possible recurrent tumor
More specific differentiation of recurrent tumor tissue from post-therapeutic changes than with conventional MRI
Treatment monitoring
Initial data show that these techniques are superior to conventional MRI for treatment monitoring
Acknowledgments
The authors thank the Neuro-Nuclear Medicine Working Group of the German Society of Nuclear Medicine (President: Prof. Dr. O. Sabri) for encouraging them to write this review article. They also thank Prof. Dr. Peter Bartenstein and Prof. Dr. Bernd J. Krause of Munich, PD Dr. Peter Brust of Leipzig, Prof. Dr. Heinz H. Coenen of Jülich, Prof. Dr. Frank Grünwald of Frankfurt, Prof. Dr. Torsten Kuwert of Erlangen, and Prof. Dr. Hans J. Steiger of Düsseldorf for their critical reading of the manuscript.
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors state that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Wester HJ, Herz M, Weber W, et al. Synthesis and radiopharmacology of O-(2[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med. 1999;40:205–212. [PubMed] [Google Scholar]
- 2.Langen KJ, Hamacher K, Weckesser M, et al. O-(2-[18F]fluoroethyl)-L-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol. 2006;33:287–294. doi: 10.1016/j.nucmedbio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Langen KJ, Ziemons K, Kiwit JCW, et al. [123I]-Iodo-α-methyltyrosine SPECT and [11C]-L-methionine uptake in cerebral gliomas: a comparative study using SPECT and PET. J Nucl Med. 1997;38:517–522. [PubMed] [Google Scholar]
- 4.Weber WA, Wester HJ, Grosu AL, et al. O-(2-[18F]Fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumors: initial results of a comparative study. Eur J Nucl Med. 2000;27:542–549. doi: 10.1007/s002590050541. [DOI] [PubMed] [Google Scholar]
- 5.Braun V, Dempf S, Weller R, Reske SN, Schachenmayr W, Richter HP. Cranial neuronavigation with direct integration of (11)C methionine positron emission tomography (PET) data - results of a pilot study in 32 surgical cases. Acta Neurochir. 2002;144:777–782. doi: 10.1007/s00701-002-0942-5. [DOI] [PubMed] [Google Scholar]
- 6.Pirotte B, Goldman S, Dewitte O, et al. Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: a report of 103 consecutive procedures. J Neurosurg. 2006;104:238–253. doi: 10.3171/jns.2006.104.2.238. [DOI] [PubMed] [Google Scholar]
- 7.Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with Magnetic Resonance Imaging improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678–687. doi: 10.1093/brain/awh399. [DOI] [PubMed] [Google Scholar]
- 8.Grosu AL, Feldmann H, Dick S, et al. Implications of IMT-SPECT for postoperative radiotherapy planning in patients with gliomas. Int J Radiat Oncol Biol Phys. 2002;54:842–854. doi: 10.1016/s0360-3016(02)02984-x. [DOI] [PubMed] [Google Scholar]
- 9.Grosu AL, Weber WA, Franz M, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET(SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:511–519. doi: 10.1016/j.ijrobp.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 10.Kuwert T, Morgenroth C, Woesler B, et al. Uptake of Iodine-(123I)α-methyltyrosine by gliomas and non-neoplastic brain lesions. Eur J Nucl Med. 1996;23:1345–1353. doi: 10.1007/BF01367590. [DOI] [PubMed] [Google Scholar]
- 11.Floeth FW, Pauleit D, Wittsack HJ, et al. Multimodal metabolic imaging of cerebral gliomas: positron emission tomography with [18F]fluoroethyl-L-tyrosine and magnetic resonance spectroscopy. J Neurosurg. 2005;102:318–327. doi: 10.3171/jns.2005.102.2.0318. [DOI] [PubMed] [Google Scholar]
- 12.Herholz K, Holzer T, Bauer B, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology. 1998;50:1316–1322. doi: 10.1212/wnl.50.5.1316. [DOI] [PubMed] [Google Scholar]
- 13.Weckesser M, Langen KJ, Rickert CH, et al. Initial experiences with O-(2-[18F]fluorethyl)-L-tyrosine PET in the evaluation of primary brain tumors. Eur J Nucl Med. 2005;32:422–429. doi: 10.1007/s00259-004-1705-8. [DOI] [PubMed] [Google Scholar]
- 14.Pöpperl G, Kreth FW, Herms J, et al. Analysis of 18F-FET-PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J Nucl Med. 2006;47:393–403. [PubMed] [Google Scholar]
- 15.Ribom D, Eriksson A, Hartman M, et al. Positron emission tomography (11)C-methionine and survival in patients with low-grade gliomas. Cancer. 2001;92:1541–1549. doi: 10.1002/1097-0142(20010915)92:6<1541::aid-cncr1480>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Kuwert T, Woesler B, Morgenroth C, et al. Diagnosis of recurrent glioma with SPECT and iodine-123-α-methyl tyrosine. J Nucl Med. 1998;39:23–27. [PubMed] [Google Scholar]
- 17.Henze M, Mohammed A, Schlemmer HP, et al. PET and SPECT for detection of tumor progression in irradiated low-grade astrocytoma: a receiver-operating characteristic analysis. J Nucl Med. 2004;45:579–586. [PubMed] [Google Scholar]
- 18.Pöpperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value of O-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging. 2004;31:1464–1470. doi: 10.1007/s00259-004-1590-1. [DOI] [PubMed] [Google Scholar]
- 19.Rachinger W, Goetz C, Pöpperl G, et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57:505–511. doi: 10.1227/01.neu.0000171642.49553.b0. [DOI] [PubMed] [Google Scholar]
- 20.Pöpperl G, Gotz C, Rachinger W, et al. Serial O-(2-[(18)F]fluoroethyl)-L-tyrosine-PET for monitoring the effects of intracavitary radioimmunotherapy in patients with malignant glioma. Eur J Nucl Med Mol Imaging. 2006;33:792–800. doi: 10.1007/s00259-005-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanfermann H, Herminghaus S, Pilatus U, Hattingen E, Zanella FE. Bedeutung der 1H-MR-Spektroskopie bei der Differenzialdiagnose und Graduierung intrakranieller Tumoren. Dtsch Arztebl. 2004;101(10):A 649–A 655. [Google Scholar]
- 22.Plotkin M, Eisenacher J, Bruhn H, et al. 123I-IMT-SPECT and 1H-MR-spectroscopy at 3T in the differential diagnosis of recurrent or residual gliomas: a comparative study. J Neurooncol. 2004;70:49–58. doi: 10.1023/b:neon.0000040810.77270.68. [DOI] [PubMed] [Google Scholar]
- 23.Pauleit D, Langen KJ, Floeth FW, et al. Can the apparent diffusion coefficient be used as a non-invasive parameter to distinguish tumor tissue from peritumoral tissue in cerebral gliomas? J Magn Reson Imaging. 2004;20:758–764. doi: 10.1002/jmri.20177. [DOI] [PubMed] [Google Scholar]
- 24.Chaskis C, Stadnik T, Michotte A, Van Rompaey K, D’Haens J. Prognostic value of perfusion-weighted imaging in brain glioma: a prospective study. Acta Neurochir. 2006;148:277–285. doi: 10.1007/s00701-005-0718-9. [DOI] [PubMed] [Google Scholar]
- 25.Di Costanzo A, Scarabino T, Trojsi F, et al. Multiparametric 3T-MR approach to the assessment of cerebral gliomas: tumor extent and malignancy. Neuroradiology. 2006;48:622–631. doi: 10.1007/s00234-006-0102-3. [DOI] [PubMed] [Google Scholar]
- e1.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- e2.Ernst S. Hirntumoren. In: Heindel W, Kugel H, Lackner K, editors. Rationelle MR-Untersuchungstechniken. Stuttgart: Thieme; 1997. pp. 3–7. [Google Scholar]
- e3.Jansen EP, Dewit LG, van Herk M, Bartelink H. Target volumes in radiotherapy for high-grade malignant gliomas of the brain. Radiother Oncol. 2000;56:151–156. doi: 10.1016/s0167-8140(00)00216-4. [DOI] [PubMed] [Google Scholar]
- e4.Byrne TN. Imaging of gliomas. Semin Oncol. 1994;21:162–167. [PubMed] [Google Scholar]
- e5.Leeds NE, Jackson EF. Current imaging techniques for the evaluation of brain neoplasms. Curr Opin Oncol. 1994;6:254–261. doi: 10.1097/00001622-199405000-00006. [DOI] [PubMed] [Google Scholar]
- e6.Nelson SJ. Imaging of brain tumors after therapy. Neuroimaging Clin N Am. 1999;9:801–819. [PubMed] [Google Scholar]
- e7.Barker FG, Chang SM, Valk PE, Pounds TR, P MD. 18-Fluorodeoxyglucose uptake and survival of patients with suspected recurrent malignant gliomas. Cancer. 1997;79:115–126. [PubMed] [Google Scholar]
- e8.Coleman RE, Hoffman JM, Hanson MW, Sostman HD, Schold SC. Clinical application of PET for the evaluation of brain tumors. J Nucl Med. 1991;32:616–622. [PubMed] [Google Scholar]
- e9.Goldman S, Levivier M, Pirotte B, et al. Regional methionine and glucose uptake in high-grade gliomas: a comparative study on PET-guided stereotactic biopsy. J Nucl Med. 1997;38:1459–1462. [PubMed] [Google Scholar]
- e10.Vaalburg W, Coenen HH, Crouzel C, et al. Amino acids for the measurement of protein synthesis in vivo by PET. Int J Rad Appl Instrum. 1992;19:227–237. doi: 10.1016/0883-2897(92)90011-m. [DOI] [PubMed] [Google Scholar]
- e11.Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med. 2001;42:432–445. [PubMed] [Google Scholar]
- e12.Biersack HJ, Coenen HH, Stöcklin G, et al. Imaging of brain tumors with L-3-[123I]iodo-α-methyl tyrosine and SPECT. J Nucl Med. 1989;30:110–112. [PubMed] [Google Scholar]
- e13.Langen KJ, Coenen HH, Roosen N, et al. SPECT-studies of brain tumors with L-3-[123I]iodo-α-methyl tyrosine: comparison with PET, 124IMT and first clinical results. J Nucl Med. 1990;31:281–286. [PubMed] [Google Scholar]
- e14.Langen KJ, Pauleit D, Coenen HH. [123I]iodo-α-Methyl-L-Tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol. 2002;29:625–631. doi: 10.1016/s0969-8051(02)00328-1. [DOI] [PubMed] [Google Scholar]
- e15.Hamacher K, Coenen HH. Efficient routine production of the 18F-labelled amino acid O-2-18F fluoroethyl-L-tyrosine. Appl Radiat Isot. 2002;57:853–856. doi: 10.1016/s0969-8043(02)00225-7. [DOI] [PubMed] [Google Scholar]
- e16.Riemann B, Stogbauer F, Kopka K, et al. Kinetics of 3-[(123)I]iodo-L-α-methyltyrosine transport in rat C6 glioma cells. Eur J Nucl Med. 1999;26:1274–1278. doi: 10.1007/pl00006652. [DOI] [PubMed] [Google Scholar]
- e17.Heiss P, Mayer S, Herz M, Wester HJ, Schwaiger M, Senekowitsch-Schmidtk R. Investigation of transport mechanism and uptake kinetics of O-(2-[18F]fluoroethyl)-L-tyrosine in vitro and in vivo. J Nucl Med. 1999;40:1367–1373. [PubMed] [Google Scholar]
- e18.Langen KJ, Mühlensiepen H, Holschbach M, Hautzel H, Jansen P, Coenen HH. Transport mechanisms of 3-[123I]iodo-α-methyl-L-tyrosine in a human glioma cell line: comparison with [Methyl-3H]-L-methionine. J Nucl Med. 2000;41:1250–1255. [PubMed] [Google Scholar]
- e19.Langen KJ, Jarosch M, Mühlensiepen H, et al. Comparison of fluorotyrosines and methionine uptake in F98 rat gliomas. Nucl Med Biol. 2003;30:501–508. doi: 10.1016/s0969-8051(03)00023-4. [DOI] [PubMed] [Google Scholar]
- e20.Langen KJ, Clauss RP, Holschbach M, et al. Comparison of iodotyrosines and methionine uptake in a rat glioma model. J Nucl Med. 1998;39:1596–1599. [PubMed] [Google Scholar]
- e21.Schmidt D, Gottwald U, Langen KJ, et al. 3-[123I]iodo-α-Methyl-L-Tyrosine uptake in cerebral gliomas: relationship to histopathological grading and prognosis. Eur J Nucl Med. 2001;28:855–861. doi: 10.1007/s002590100553. [DOI] [PubMed] [Google Scholar]
- e22.Pauleit D, Floeth F, Herzog H, et al. Whole-body distribution and dosimetry of O-(2-[18F]fluoroethyl)-L-tyrosine. Eur J Nucl Med Mol Imaging. 2003;30:519–524. doi: 10.1007/s00259-003-1118-0. [DOI] [PubMed] [Google Scholar]
- e23.Bergstrom M, Collins VP, Ehrin E, et al. Discrepancies in brain tumor extent as shown by computed tomography and positron emission tomography using [68Ga]EDTA, [11C]glucose, and [11C]methionine. J Comput Assist Tomogr. 1983;7:1062–1066. doi: 10.1097/00004728-198312000-00022. [DOI] [PubMed] [Google Scholar]
- e24.Mosskin M, von Holst H, Bergstrom M, et al. Positron emission tomography with 11C-methionine and computed tomography of intracranial tumors compared with histopathologic examination of multiple biopsies. Acta Radiol. 1987;28:673–681. [PubMed] [Google Scholar]
- e25.Mosskin M, Ericson K, Hindmarsh T, et al. Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference. Acta Radiol. 1989;30:225–232. [PubMed] [Google Scholar]
- e26.Ogawa T, Shishido F, Kanno I, et al. Cerebral glioma: evaluation with methionine PET. Radiology. 1993;186:45–53. doi: 10.1148/radiology.186.1.8380108. [DOI] [PubMed] [Google Scholar]
- e27.Goldman S, Levivier M, Pirotte B, et al. Regional methionine and glucose uptake in high-grade gliomas: a comparative study on PET-guided stereotactic biopsy. J Nucl Med. 1997;38:1459–1462. [PubMed] [Google Scholar]
- e28.Pirotte B, Goldman S, Massager N, et al. Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J Nucl Med. 2004;45:1293–1298. [PubMed] [Google Scholar]
- e29.Pauleit D, Floeth FW, Tellmann L, et al. Comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET) PET and 3-[123I]iodo-α-methyl-L-tyrosine (IMT) SPECT in brain tumors. J Nucl Med. 2004;45:374–381. [PubMed] [Google Scholar]
- e30.Dethy S, Goldman S, Blecic S, Luxen A, Levivier M, Hildebrand J. Carbon-11-methionine and fluorine-18-FDG PET study in brain hematoma. J Nucl Med. 1994;35:1162–1166. [PubMed] [Google Scholar]
- e31.Jacobs A. Amino acid uptake in ischemically compromized brain tissue. Stroke. 1995;26:1859–1866. doi: 10.1161/01.str.26.10.1859. [DOI] [PubMed] [Google Scholar]
- e32.Nakagawa M, Kuwabara Y, Sasaki M, Koga H, Chen T, Kaneko O, et al. 11C-methionine uptake in cerebrovascular disease: a comparison with 18F-FDG PET and 99mTc-HMPAO SPECT. Ann Nucl Med. 2002;16:207–211. doi: 10.1007/BF02996302. [DOI] [PubMed] [Google Scholar]
- e33.Ishii K, Ogawa T, Hatazawa J, et al. High L-methyl-[11C]methionine uptake in brain abscess: a PET study. J Comput Assist Tomogr. 1993;17:660–661. doi: 10.1097/00004728-199307000-00029. [DOI] [PubMed] [Google Scholar]
- e34.Floeth FW, Pauleit D, Sabel M, et al. Differentiation of tumorous and non-tumorous ring enhancing lesions (REL) with FET PET. J Nucl Med. 2006;47:776–782. [PubMed] [Google Scholar]
- e35.Derlon JM, Bourdet C, Bustany P, et al. [11C]L-methionine uptake in gliomas. Neurosurgery. 1989;25:720–728. doi: 10.1097/00006123-198911000-00006. [DOI] [PubMed] [Google Scholar]
- e36.Woesler B, Kuwert T, Morgenroth C, et al. Non-invasive grading of primary brain tumors: results of a comparative study between SPECT with 123I-α-methyltyrosine and PET with 18F-deoxyglucose. Eur J Nucl Med. 1997;24:428–434. doi: 10.1007/BF00881816. [DOI] [PubMed] [Google Scholar]
- e37.Kaschten B, Stevenaert A, Sadzot B, Deprez M, Degueldre C, Del Fiore G, et al. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med. 1998;39:778–785. [PubMed] [Google Scholar]
- e38.Schmidt D, Gottwald U, Langen KJ, et al. 3-[123I]iodo-α-methyl-L-tyrosine uptake in cerebral gliomas: relationship to histopathological grading and prognosis. Eur J Nucl Med. 2001;28:855–861. doi: 10.1007/s002590100553. [DOI] [PubMed] [Google Scholar]
- e39.Van Laere K, Ceyssens S, Van Calenbergh F, et al. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, interobserver variability and prognostic value. Eur J Nucl Med Mol Imaging. 2005;32:39–51. doi: 10.1007/s00259-004-1564-3. [DOI] [PubMed] [Google Scholar]
- e40.Tsuyuguchi N, Takami T, Sunada I, et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery in malignant glioma. Ann Nucl Med. 2004;18:291–296. doi: 10.1007/BF02984466. [DOI] [PubMed] [Google Scholar]
- e41.Bader JB, Samnick S, Moringlane JR, et al. Evaluation of L-3-[123I]iodo-α-methyltyrosine SPECT and [18F]fluorodeoxyglucose PET in the detection and grading of recurrences in patients pretreated for gliomas at follow-up: a comparative study with stereotactic biopsy. Eur. J Nucl Med. 1999;26:144–151. doi: 10.1007/s002590050370. [DOI] [PubMed] [Google Scholar]
- e42.Guth-Tougelidis B, Muller S, Mehdorn MM, Knust EJ, Dutschka K, Reiners C. Uptake of DL-3-[123I]iodo-α-methyltyrosine in recurrent brain tumors. Nuklearmedizin. 1995;34:71–75. [PubMed] [Google Scholar]
- e43.Henze M, Mohammed A, Schlemmer H, et al. Detection of tumor progression in the follow-up of irradiated low-grade astrocytomas: comparison of 3-[123I]iodo-α-methyl-L-tyrosine and 99mTc-MIBI SPET. Eur J Nucl Med Mol Imaging. 2002;29:1455–1461. doi: 10.1007/s00259-002-0896-0. [DOI] [PubMed] [Google Scholar]
- e44.Lichy MP, Henze M, Plathow C, Bachert P, Kauczor HU, Schlemmer HP. Metabolic imaging to follow stereotactic radiation of gliomas - the role of 1H MR spectroscopy in comparison to FDG-PET and IMT-SPECT. Röfo. 2004;176:1114–1121. doi: 10.1055/s-2004-813194. [DOI] [PubMed] [Google Scholar]
- e45.Würker M, Herholz K, Voges J, et al. Glucose consumption and methionine uptake in low-grade gliomas after iodine-125 brachytherapy. Eur J Nucl Med. 1996;23:583–586. doi: 10.1007/BF00833397. [DOI] [PubMed] [Google Scholar]
- e46.Molenkamp G, Riemann B, Kuwert T, et al. Monitoring tumor activity in low-grade glioma of childhood. Klin Padiatr. 1998;210:239–242. doi: 10.1055/s-2008-1043885. [DOI] [PubMed] [Google Scholar]
- e47.Nariai T, Tanaka Y, Wakimoto H, et al. Usefulness of L-[methyl-11C] methionine positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg. 2005;103:498–507. doi: 10.3171/jns.2005.103.3.0498. [DOI] [PubMed] [Google Scholar]
- e48.Herholz K, Kracht LW, Heiss WD. Monitoring the effect of chemotherapy in a mixed glioma by C-11-methionine PET. J Neuroimaging. 2003;13:269–271. [PubMed] [Google Scholar]
- e49.Pöpperl G, Götz C, Rachinger W, et al. Serial O-(2-[(18)F]fluoroethyl)-L-tyrosine PET for monitoring the effects of intracavitary radioimmunotherapy in patients with malignant glioma. Eur J Nucl Med Mol Imaging. 2006;33:792–800. doi: 10.1007/s00259-005-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e50.Floeth FW, Pauleit D, Sabel M, et al. Prognostic value of O-(2-[18F]fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma patients. J Nucl Med. 2007;48:519–527. doi: 10.2967/jnumed.106.037895. [DOI] [PubMed] [Google Scholar]
- e51.Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP. Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? Am J Neuroradiol. 1998;19:407–413. [PMC free article] [PubMed] [Google Scholar]
- e52.Sorensen J, Savitcheva II, Engler H, Langstrom B. Utility of PET and 11C-methionine in the Paediatric Brain Tumors. Clin Positron Imaging. 2000;3:157. doi: 10.1016/s1095-0397(00)00069-8. [DOI] [PubMed] [Google Scholar]
- e53.Lang K, Kloska S, Straeter R, et al. Clinical value of amino acid imaging in paediatric brain tumors. comparison with MRI. Nuklearmedizin. 2005;44:131–136. doi: 10.1267/nukl05040131. [DOI] [PubMed] [Google Scholar]
- e54.Utriainen M, Metsahonkala L, Salmi TT, et al. Metabolic characterization of childhood brain tumors: comparison of 18F-fluorodeoxyglucose and 11C-methionine positron emission tomography. Cancer. 2002;95:1376–1386. doi: 10.1002/cncr.10798. [DOI] [PubMed] [Google Scholar]
- e55.Messing-Jünger AM, Floeth FW, Pauleit D, et al. Multimodal target point assessment for stereotactic biopsy in children with diffuse bithalamic astrocytomas. Childs Nerv Syst. 2002;18:445–449. doi: 10.1007/s00381-002-0644-6. [DOI] [PubMed] [Google Scholar]
- e56.Moser E. Konsensus - Neuro-PET. Nuklearmedizin. 1997;36:46–47. [Google Scholar]
- e57.Langen KJ, Floeth F, Stoffels G, Hamacher K, Coenen HH, Pauleit D. Verbesserte Diagnostik von zerebralen Gliomen mit der FET-PET. Zeitschr Med Physik. 2007;17 doi: 10.1016/j.zemedi.2006.08.001. [DOI] [PubMed] [Google Scholar]