Abstract
Introduction
Polycythemias are characterized by an increased concentration of red blood cells. Because blood cell counts are a routine investigation, these disorders present to non-hematologic physicians. Polycythemia vera (PV), an acquired stem cell disease, is the most important variant.
Methods
Selective literature review and the authors’ own clinical experiences.
Results and Discussion
Erythropoietin, which is produced in the kidneys, and its receptor system in the bone marrow, are of critical importance in polycythemia. Congenital polycythemias are caused by mutations of the Erythropoietin-receptor gene, hemoglobin variants, 2,3-bisphosphoglycerate mutase deficiency or by disturbances of renal oxygen sensing. Acquired polycythemias can occur secondary to hypoxia at high altitudes, or primarily through acquired mutations in the EPO-receptor signaling system (JAK2 mutations). Alternatively they may be caused by pulmonary or renal disease. An artificial erythrocytosis is induced by athletes through doping. Differential diagnosis comprises erythropoietin determination, JAK2 mutation analysis and if necessary hemoglobin electrophoresis. Only PV requires immediate treatment, because of a high thromboembolic risk. Epidemiological studies on polycythemias in German speaking countries are urgently needed.
Keywords: polycythemia vera, JAK2 mutation, erythropoietin, doping, aquagenic pruritus
At the Olympic Winter Games in 2006, Evi Sachenbacher-Stehle had an elevated hemoglobin reading of 16.4 g/dL in a doping sample and was suspended for five days. In the summer of 2006, more than 50 cyclists were involved in the scandal surrounding the team physician Fuentes in the run-up to the Tour de France (1). Investigators found stored blood units intended to artificially increase hematocrit and evidence of orders for erythropoietin. Professional cyclist Jörg Raschke recently confirmed that erythropoietin doping is normal practice in the cycling world (1). Hematocrit can be regarded as a symbol of manipulation in endurance sports: it can be increased legally by high altitude training or illegally with erythropoietin (EPO), androgens, and autologous blood transfusions. But not every elevated hematocrit is attributable to high altitude training or doping. The Finnish cross-country skier Eero Mäntyranta was known since youth to have high hemoglobin levels of above 20 g dL (hematocrit above 60%). Examinations performed on the several times Olympic champion revealed increased sensitivity of erythropoietin precursors in the bone marrow to erythropoietin. Causally responsible is a hereditary point mutation in the erythropoietin receptor gene which leads to permanent activation of the EPO receptor system and thus to erythrocytosis. Unlike congenital forms of erythrocytosis, the commonest form – polycythemia vera (PV) – is acquired. Although the term polycythemia originally denoted "too many" cells in the peripheral blood, it is now used as a synonym for erythrocytosis. In polycythemia vera (PV) not the erythropoietin receptor, but the signal cascade associated with it is changed. A basic distinction can be made between relatively rare congenital and the more common acquired polycythemias. The following overview of polycythemias is based on a selective literature review and the authors’ own clinical experiences.
Congenital polycythemias
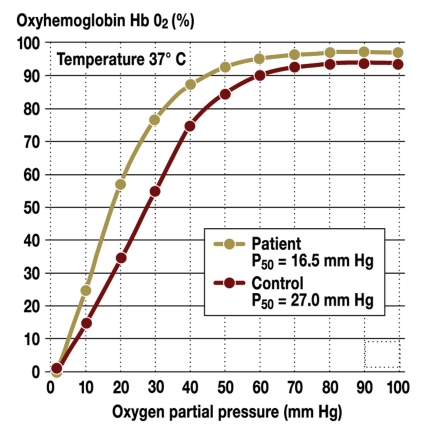
Certain mutations in the alpha and beta chains of hemoglobin can lead to high affinity hemoglobins which release oxygen to a reduced extent in peripheral tissue. At an O2 partial pressure of 20 mm Hg in the capillaries, for example, 35% oxyhemoglobin is present, whereas with high oxygen affinity hemoglobin Johnstown the oxygenation level is still 60% (figure 1). The resulting decrease in oxygen release in peripheral tissue leads to a compensatory increase in the hemoglobin concentration. In addition, a decrease in the intraerythrocytic 2,3-bisphosphoglycerate level due to 2,3-bisphosphoglycerate mutase deficiency can lead to increased oxygen affinity of hemoglobin.
Figure 1.
Oxygen binding curve of high oxygen affinity hemoglobin Johnstown compared to the hemoglobin molecule of a control person; P50 = pressure at which 50% of the hemoglobin is loaded with oxygen
Congenital erythropoietin (EPO) receptor mutations result in a decrease in intracellular receptor protein (2). As a result, negative regulators can no longer bind, resulting in constitutive activation of the receptor. In contrast to PV, the persons affected do not have an increased risk of thrombosis and bleeding, which suggests that erythrocytosis alone is not responsible for this situation.
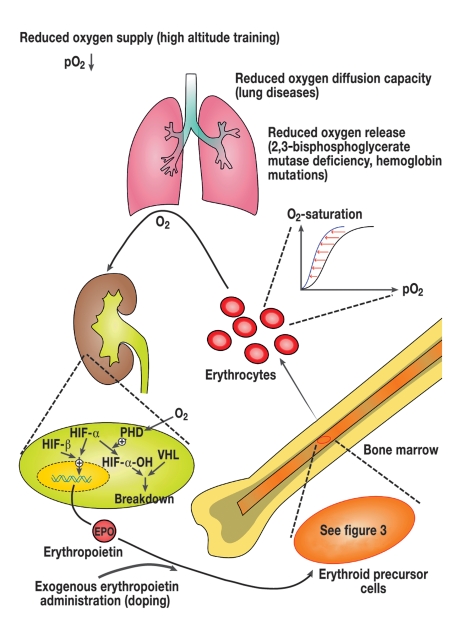
Initially in Eastern Russia and later in Central Europe, a special form of congenital polycythemia was identified which is based on an autosomal recessive inherited mutation in the von Hippel-Lindau (VHL) gene. The VHL protein regulates the breakdown of hypoxia inducible factor (HIF1) in the peritubular fibroblasts of the kidney (figure 2). HIF 1 consists of two subunits alpha and beta and mediates oxygen measurement in the kidneys. In the presence of oxygen the alpha chains are degraded with involvement of the VHL gene product (3). A homozygotic mutation of the VHL gene results in the formation of a VHL protein with reduced activity, so that the alpha chains are not degraded even in normoxemic conditions. The resulting increase in erythropoietin release leads to a marked increase in the hemoglobin level. The persons affected develop an increased incidence of thromboses and bleeding. Therapeutic bloodletting does not reduce the rate of complications. This suggests the presence of other causes, such as the observed increased production of vascular endothelial growth factor (VEGF) which is also regulated by HIF (3).
Figure 2.
Erythropoiesis feedback system regulated by erythropoietin: when the oxygen supply is reduced, a decrease in oxygen is registered in the kidneys; the breakdown of HIF a is in-hibited by the hypoxia. This inhibition comes about by hydroxylation of HIF a in the peri-tubular fibroblasts by oxygen dependent proline hydrolase. The hydroxylated HIF a is de-graded in binding to the VHL protein. As a result, more erythropoietin is produced, leading to increased erythrocyte production in the bone marrow. The same conditions can be achieved by exogenous supply of erythropoietin. The system is regulated by the resulting negative feedback (PHD, prolyl hydroxylase; HIF, hypoxia inducible factor; VHL, von Hippel-Lindau).
Various heart defects associated with cyanosis (such as septal defects, left-right shunt) lead to chronic hypoxemia and thus, via an increase in erythropoietin, to compensatory erythrocytosis. These secondary polycythemias are a physiological response to the developing tissue hypoxia. Bloodletting is therefore only indicated in exceptional cases (4).
Acquired polycythemias
Polycythemias secondary to hypoxemia
Reactive polycythemia can also develop as a physiological compensatory reaction in persisting hypoxemic states, such as smoker’s polycythemia, chronic obstructive pulmonary disease (COPD) or sleep apnea. While no erythropoietin elevation is generally observed in sleep apnea, it does occur in COPD when oxygen partial pressure falls below 67 mm Hg. Competitive athletes use this fact to their advantage in high altitude training.
Polycythemias not caused by hypoxemia
Various renal function impairments can also lead to erythrocytosis without hypoxic stimuli. Examples include Wilms’ tumor, polycystic kidney disease, renal cell cancer, and post-transplantation erythrocytosis. The administration of erythropoietin, e.g., for the treatment of malignant or renal anemia, causes an increase in erythrocytes. The level to which hemoglobin should be raised is currently a subject of debate, because in some studies excessive stimulation was found to be associated with a declining survival rate (5).
Polycythemias caused by mutation of a bone marrow stem cell
The commonest form is polycythemia vera. Together with primary thrombocythemia (6) and primary myelofibrosis, it is one of the Philadelphia chromosome negative chronic myeloproliferative diseases. It is associated with an increase in erythrocytes and in some cases granulocytes and/or platelets. The resulting rise in hematocrit increases the risk of thromboembolic complications that can occur throughout the vascular system (table 1). Recent findings suggest that the risk of thrombosis is promoted by JAK2-induced changes (of a disease associated mutation) in surface proteins of the PV erythrocytes (7). In addition, platelet abnormalities can lead to bleeding and thrombosis (8). More than half of these patients have the clinical symptoms splenomegaly (9) and frequently severe aquagenic pruritus, i.e. itching induced by contact with water (10) (table 2) as well as a number of unspecific symptoms (box 1). In Sweden, the incidence of PV is 2.8 per 100 000 population (11). Unfortunately, no epidemiological data are available for Germany. Extrapolating these values, therefore, 2200 new cases of disease per year are to be expected. The condition is generally diagnosed after the age of 60 years, and nothing is known about the precipitating mutagens. Long-term risks of the disease include transition to acute leukemia or post-polycythemic myelofibrosis (12). The mean leukemic risk is 7.4%. The risk in-creases from 2.4% (with bloodletting, anagrelide, interferon alpha) to 16.7% in therapy with at least two cytotoxic medications.
Table 1. Thromboembolic and bleeding complications in 1638 patients with polycythemia vera (25).
| Type | Incidence (%) |
| Arterial thromboses | 28.7 |
|
10.3 |
| 8.9 | |
| 8.9 | |
| 5.5 | |
| Venous thromboses | 13.7 |
|
8.2 |
| 6.1 | |
| 2.4 | |
| Erythromelalgia (painful reddening of the hands or feet) | 5.3 |
| Intermittent claudication | 4.7 |
| Hemorrhage | 8.1 |
Table 2. Complications of polycythemia vera.
| Complication | Cause |
| Thrombosis | Elevated hematocrit |
| Hemorrhage | Platelet dysfunction |
| Hepato/splenomegaly | Increased cell production or extramedullary hematopoiesis |
| Aquagenic pruritus | Inflammatory mediators and/or elevated hematocrit |
| Hyperuricemia, gout, kidney stones | Increased cell turnover |
| Erythromelalgia or visual disorders | Thrombocythemia and/or platelet dysfunction |
| Myelofibrosis | Reaction to the neoplastic clone |
| Acute leukemia | Iatrogenic or clonal evolution |
Box 1. Unspecific symptoms.
Headache
Asthenia
Tendency to sweating
Paresthesias
Upper abdominal discomfort
Weight loss
Lightheadedness
Pathogenesis of PV
Bone marrow erythrocyte precursors of patients with PV proliferate spontaneously in vitro without addition of erythropoietin. At the same time, the EPO level is lowered in most patients. This suggests acquired mutations in the erythropoietin receptor and the associated signal cascade genes.
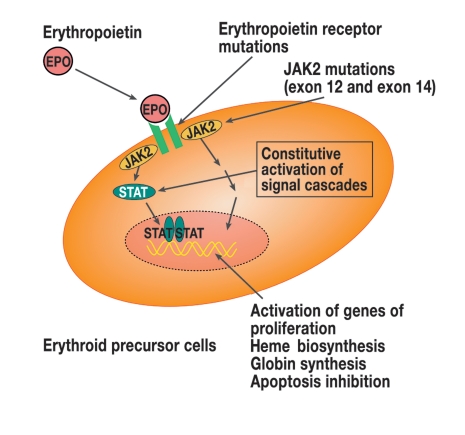
In 2005, five research groups working independently of each other discovered the JAK2V617F mutation in exon 14 of the JAK2 gene (14). JAK2 (Janus kinase 2) is a cytoplasmic tyrosine kinase involved in signal transduction of various cytokines (including at the EPO receptor). The mutation intensifies the activity of JAK2 and thus leads to EPO-independent growth (figure 3). This mutation is acquired because it is not detectable in the germ line. Since erythrocytes no longer have a nucleus, this JAK2V617F mutation is detected in peripheral blood via granulocyte DNA.
Figure 3.
Simplified presentation of the erythropoietin receptor signal cascade via STAT-dependent and STAT-independent signal pathways and their consequences (EPO, erythropoietin; JAK2, Janus kinase 2; STAT, signal transducer and activator of transcription)
95% of all patients with PV have this mutation, and the majority of patients are homozygotic. In the search for mutations in JAK2V617F negative patients, changes in the JAK2 gene have recently been identified in exon 12 (amino acids 537 to 543) (figure 3). In contrast to the JAK2V617F mutation, which can also occur in patients with primary thrombocythemia or primary myelofibrosis, however, these mutations are specific for PV (box 2). Patients with exon 12 mutations have higher erythrocyte but lower leukocyte and platelet levels than patients with the exon 14 mutation (15). Since the mutations in exon 12 and 14 of the JAK2 gene occur in almost 100% of patients with PV, mutation analysis is a useful diagnostic criterion.
Box 2. The newly discovered JAK2 mutations.
JAK2F537-K539delinsL
JAK2H538QK539L
JAK2N542-E543del
JAK2K539L
Differential diagnosis of polycythemia
The discovery of the mutations in the exons 12 and 14 of JAK2 kinase has revolutionized the diagnosis of polycythemia. The WHO classification of PV, which is based only on non-molecular principles, is therefore currently being revised and will not be discussed further here.
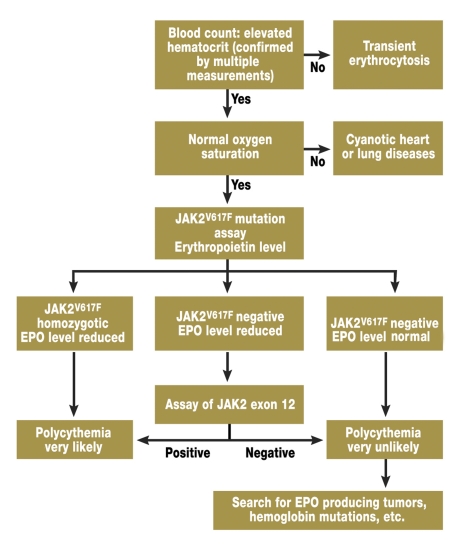
Instead, a diagnostic algorithm is presented (figure 4) which is presently under discussion (16): at a permanently elevated hematocrit (52% in men, 48% in women) with normal oxygen saturation, an erythropoietin assay and a JAK2V617F mutation analysis are performed. If the mutation is homozygotically present and the erythropoietin level lowered, the diagnosis of PV is conclusive.
Figure 4.
Diagnostic algorithm for polycythemias. A search is first conducted for the more common JAK2V617F mutation and only if the result is negative for the more rare, newly discovered mutations in exon 12.
If the JAK2V617F mutation is not present and the EPO level lowered, exon 12 should then be examined for mutations. If one of the mutations is detected, PV is also present in this case.
If the JAK2 specific mutations are not detected and if the EPO level is normal or elevated, PV is unlikely. In this case a search should be conducted for tumors that can cause EPO elevation and primary polycythemia should be ruled out.
In our view, the presence of aquagenic pruritus accompanied by a hematocrit elevation is a definite sign of PV because about 40% of all PV patients already suffer from water-induced itching before or at diagnosis (10).
Treatment
Since the congenital polycythemias and secondary polycythemia associated with cyanotic congenital heart diseases rarely require hematological management, only the treatment of PV will be presented below. The goal is to reduce the PV-associated potentially life-threatening thromboembolic and hemorrhagic complications as well as the unspecific symptoms.
Reduction of complication rate
Phlebotomy – Since blood viscosity and thus the risk of thrombosis greatly increase with rising hematocrit, the primary goal is to permanently reduce hematocrit to <45% in men and <40% in women. However, this goal is frequently not attained because of inconsistent treatment.
This goal can be achieved with cytoreductive therapy with phosphorus-32, chlorambucil, busulfan, hydroxyurea, or by phlebotomy. It was shown in the PVSG-01 study that although the now obsolete cytostatic therapy with chlorambucil and phosphorus-32 reduces thrombotic risk to a greater extent than phlebotomy, it is associated with a distinctly higher risk of leukemia (17). Whether the likelihood of leukemia is also increased with hydroxyurea alone is now being debated. This important question can only be resolved by long-term analyses.
Phlebotomy, a method practiced since the time of Hippocrates, is the treatment of choice. Each bloodletting involves aspirating 500 mL of blood into a vacuum bottle. The fluid loss can be compensated by oral or intravenous replacement. Because of the lifetime of erythrocytes of 120 days only these cells are reduced over a prolonged period, while granulocytes and platelets are rapidly regenerated again because of their much shorter lifetime of 8 days. Although phlebotomy can effectively lower blood viscosity, the platelet count can transiently increase due to overcompensation (18). The removal of iron with the erythrocytes causes secondary iron deficiency which limits erythropoiesis and can result in fatigue and decreased performance.
Antiplatelet therapy – Platelets can also be implicated in the development of thromboses in PV patients. The results of the ECLAP study (19) – a randomized, placebo-controlled double-blind study in 518 patients – showed that patients who take 50 to 100 mg acetylsalicylic acid (ASA) daily have a lower thrombotic risk. The daily intake of 100 mg aspirin is therefore recommended. Critics of the study object that individual patients in this study only required aspirin therapy because their hematocrit was insufficiently lowered (20). Since individual PV patients have an increased bleeding risk, physicians should be alert for bleeding complications during aspirin therapy.
Cytoreductive therapy – Medicinal therapy is only indicated if an adequate hematocrit reduction cannot be achieved by bloodletting or if a thromboembolic complication has occurred despite low-dose aspirin with normalized hematocrit. This may be due to leukocyte and platelet elevations. Further indications include increasing splenomegaly or water-induced pruritus refractory to bloodletting. In some patients, the iron deficiency secondary to regular bloodletting causes fatigue or concentration difficulties, prompting them to request a changeover from iron depleting to cytoreductive therapy.
Today, only hydroxyurea and interferon alpha should be used for therapy: with hydroxyurea, erythrocytes, granulocytes, and platelets are reduced (500 to 2500 mg daily). The commonest adverse effects are mucosal irritations and cutaneous tumors (spinocellular carcinoma). Sporadically, marked oscillations in platelet levels are observed. Since a leukemogenic activity has not so far been ruled out, caution is advised during long-term use (>10 years) (21).
Conventional interferon alpha and pegylated interferon alpha (interferon alpha covalently bound to a polyethylene residue) combine high efficacy with an absence of leukemogenic and tumorigenic potential. In a weekly subcutaneous dosage between 3 x 3 and 5 x 5 million IU interferon alpha (or 40 µg pegylated interferon alpha), it reduces erythrocytosis, splenomegaly, and pruritus. In many cases, however, the therapy has to be discontinued due to influenza-like symptoms, alopecia and/or psychiatric side effects (22).
Because of its oral availability, better tolerability, low cost, and regulatory approval, hydroxyurea is generally preferred to interferon alpha.
If platelet reduction is the sole concern, anagrelide is the first line medication (mean dosage 2 mg daily). This product acts selectively on megakaryocytes and is non-leukemogenic. Adverse effects may include headache and palpitations (23) which are generally reversible within four weeks. The platelet level at which a reduction should be considered is uncertain.
There is unfortunately a lack of randomized studies on the management of PV with interferon alpha and anagrelide (table 3). Differing therapeutic strategies are employed for the management of hematocrit and thrombocytosis in the USA (24). Nothing is so far known about the quality of therapy in Germany, which is one of the reasons why a national register is urgently required. The observation that close to 100% of PV patients have a JAK2 mutation is the starting point for the development of JAK2 inhibitors. So far, these agents have only been studied for PV in murine models, while phase 1 studies have already produced encouraging results for myelofibrosis (ASH 12/2007).
Table 3. Medicinal treatment options for polycythemia vera (PV).
| Active substance | Undesirable effects | Prospective randomized studies | Approved in Germany for treatment of PV |
| Hydroxyurea | Mucosal intolerance, drug fever, skin tumors (spinocellular cancer), leukemogenic activity not ruled out so far | Yes | Approved |
| Interferon α | Influenza like symptoms, psychiatric side effects or even psychosis | No | Not approved |
| Anagrelide | Palpitations, diarrhea | No | Not approved |
Management of aquagenic pruritus
Water-induced itching is the chronic symptom of PV which most severely impairs quality of life. It affects more than 60% of patients and is induced by water of differing quality and temperature, and in some cases even by heavy perspiration or hand washing. Many patients find it impossible to take a bath. Aquagenic pruritus is most effectively treated by consistent management of the PV (bloodletting or cytoreductive therapy). For persisting pruritus, the authors recommend adding bicarbonate or starch to the bath water, although it is uncertain how the effect is produced. If these approaches fail, antihistamines, serotonin reuptake inhibitors (such as fluoxetine, paroxetine), or topical application of a capsaicin cream may be attempted. Severely refractory pruritus may respond to phototherapy, although a carcinogenic potential has not been ruled out for this therapeutic modality (10).
Prognosis of polycythemia vera
An Italian retrospective study in 70 under-50-year-old patients estimates mean life expectancy as over 23 years. 73% of these subjects had received pipobroman, which is now no longer recommended because of its leukemogenic risk. The 20-year risk of transition to acute leukemia is 15%, and the earliest onset was observed after 9 years. The use of cytoreductive medications with a leukemogenic risk profile should be avoided especially in young patients. The 20-year risk of transition to postpolycythemic myelofibrosis, i.e., fibrotic degeneration of the bone marrow with increasing diversion of hematopoiesis to the spleen and liver, reached a level of 10% in this study (12).
Pregnancy in PV patients
The presence of PV does not preclude pregnancy. Because of the increased thrombotic risk for the patient and the fetus (elevated rate of spontaneous abortions, placental infarction/insufficiency), however, close interdisciplinary management is required which can result in viable newborns in a good 50% of cases.
Patient support groups
Several patient support groups have been founded in Germany with the aim of improving the care of PV patients (www.mpd-netzwerk.de, www.cmpe.de, www.polyzythaemie.de).
Acknowledgments
Translated from the original German by mt-g.
Footnotes
Conflict of interest statement
Prof. Dr. Petrides has received lecture fees from the companies Shire and AOP. Mr. Siegel declares that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Gorris L, Hacke D, Ludwig U. Bellas Blut. Der Spiegel. 2007;27:64. [Google Scholar]
- 2.Rives S, Pahl HL, Florensa L, et al. Molecular genetic analyses in familiar and sporadic congenital primary erythrocytosis. Haematologica. 2007;92:674–677. doi: 10.3324/haematol.10787. [DOI] [PubMed] [Google Scholar]
- 3.Gordeuk VR, Prchal JT. Vascular complications in Chuvash polycythemia. Semin Thromb Hemost. 2006;32:289–294. doi: 10.1055/s-2006-939441. [DOI] [PubMed] [Google Scholar]
- 4.Thorne SA. Management of polycythaemia in adults with cyanotic congenital heart disease. Heart. 1998;79:315–316. doi: 10.1136/hrt.79.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Los Santos JF, Thomas GM. Anemia correction in malignancy management: Threat or opportunity? Gyn Onc. 2007;105:517–529. doi: 10.1016/j.ygyno.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Petrides PE. Primäre Thrombozythämie: Diagnose und Therapie. Med Klin. 2006;101:624–634. doi: 10.1007/s00063-006-1092-y. [DOI] [PubMed] [Google Scholar]
- 7.Wautier MP, El NW, Gane P, et al. Increased adhesion to endothelial cells of erythrocytes from patients with Polycythemia Vera is mediated by laminin-alpha5-chain and Lu/BCAM. Blood. 2007;110:894–901. doi: 10.1182/blood-2006-10-048298. [DOI] [PubMed] [Google Scholar]
- 8.Wehmeier A, Sudhoff T, Meierkord F. Relation of platelet abnormalities to thrombosis and hemorrhage in chronic myeloproliferative disorders. Semin Thromb Hemost. 1997;23:391–402. doi: 10.1055/s-2007-996114. [DOI] [PubMed] [Google Scholar]
- 9.Anger B, Haug U, Seidler R, Heimpel H. Polycythemia vera. A clinical study of 141 patients. Blut. 1989;59:493–500. doi: 10.1007/BF00329494. [DOI] [PubMed] [Google Scholar]
- 10.Siegel F, Petrides PE. Aquagener Pruritus: Diagnose und Therapie. Arzneimitteltherapie. 2007;25:9–15. [Google Scholar]
- 11.Kutti J, Ridell B. Epidemiology of the myeloproliferative disorders: essential thrombocythaemia, polycythaemia vera and idiopathic myelofibrosis. Pathol Biol. 2001;49:164–166. doi: 10.1016/s0369-8114(00)00023-7. [DOI] [PubMed] [Google Scholar]
- 12.Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Gangat N, Strand J, Li CY, et al. Leucocytosis in polycythemia vera predicts both inferior survival and leucaemic transformation. Br J Haemat. 2007;138:354–358. doi: 10.1111/j.1365-2141.2007.06674.x. [DOI] [PubMed] [Google Scholar]
- 14.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–2466. doi: 10.1056/NEJMra063728. [DOI] [PubMed] [Google Scholar]
- 15.Scott LM, Tong W, Levine RL, et al. JAK2-exon-12-mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tefferi A. JAK2-mutations in polycythemia vera. Molecular mechanisms and clinical applications. N Engl J Med. 2007;356:444–445. doi: 10.1056/NEJMp068293. [DOI] [PubMed] [Google Scholar]
- 17.Berk PD, Goldberg JD, Silverstein MN, et al. Increased incidence if acute leukemia in Polycythemia Vera associated with chlorambucil therapy. N Engl J Med. 1981;304:441–447. doi: 10.1056/NEJM198102193040801. [DOI] [PubMed] [Google Scholar]
- 18.Boughton BJ. Chronic myeloproliferative disorders: improved platelet aggregation following venesection. Br J Haematol. 1978;39:589–598. doi: 10.1111/j.1365-2141.1978.tb03629.x. [DOI] [PubMed] [Google Scholar]
- 19.Landolfi R, Marchioli R, Kutti J, et al. ECLAP (European Collaboration on Low Dose Aspirin in Polycythemia Vera): efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114–124. doi: 10.1056/NEJMoa035572. [DOI] [PubMed] [Google Scholar]
- 20.Spivak J. Daily aspirin-only half the answer. N Engl J Med. 2004;350:99–101. doi: 10.1056/NEJMp038177. [DOI] [PubMed] [Google Scholar]
- 21.McMullin MF. A review of the therapeutic agents used in the management of polycythaemia vera. Hematol Oncol. 2007;25:58–65. doi: 10.1002/hon.809. [DOI] [PubMed] [Google Scholar]
- 22.Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biological effects, and impact on quality of life. Cancer. 2006;106:2397–2405. doi: 10.1002/cncr.21900. [DOI] [PubMed] [Google Scholar]
- 23.Petrides PE. Anagrelid zur Behandlung der primären Thrombozythämie. Arzneimitteltherapie. 2005;22:225–235. [Google Scholar]
- 24.Streiff MB, Smith B, Spivak JL. The diagnosis and management of polycythemia vera in the era since the Polycythemia Vera Study Group: a survey of American Society of Hematology members’ practice patterns. Blood. 2002;99:1144–1149. doi: 10.1182/blood.v99.4.1144. [DOI] [PubMed] [Google Scholar]
- 25.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–2232. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]