Abstract
Introduction
Acetylic salicylic acid (aspirin) intolerance relates to altered generation and metabolism of arachidonic acid and eicosanoids, and prostaglandins and leukotrienes ingestion of salicylates or COX-inhibitors.
Methods
Selective review of literature in PubMed and the Cochrane Library.
Results
Rhinitis, asthma and nasal polyposis are typical presentations, but urticaria and gut inflammation are also described. The mechanism involves a specific reaction to COX inhibitor substances in analgesics, cosmetics or plants resulting in an abnormal pattern of eicosanoids (prostaglandins and leucotrienes). The diagnosis is based on symptoms occurring immediately following ingestion of these substances or on refractory polyp formation. Blood tests may be helpful in unclear cases. Avoidance of triggering agents is helpful. Corticosteroids are the mainstay of pharmacological treatment. Biological, desensitization treatment involving the administration of increasing amounts of acetylic salicylic acid may also be used.
Discussion
Asthma, rhinitis and nasal polyps, as well as chronic gastrointestinal irritation and urticaria following acetylic salicylic acid ingestion may suggest intolerance.
Keywords: salicylate intolerance, nasal polyps, asthma, allergies, desensitization
If a natural substance causes symptoms which do not involve the immune system, this is not an allergy or a side effect, but is known as intolerance. One example is the bronchial asthma and rhinitis which develops in some patients after administration of salicylates. Although salicylate intolerance has been known for more than 100 years, it is not adequately recognized in the relevant areas of medicine. The present article is intended to rectify this deficiency.
Methods
The literature search used the Medical Subject Heading (MeSH) and the Unified Medical Language System (UMLS) of the National Library of Medicine (NLM) for the period from 1970 to 2007. The key words were "eicosanoid," "leukotriene," "prostaglandin," "analgesic intolerance," "aspirin induced asthma," "nasal polyps," "inflammatory bowel disease," "urticaria," "adverse drug reaction," "allergy," and "tests."
The search was performed in the databases of the National Center for Biotechnology Information (NCBI), the Infosystem of Erlangen-Nuremberg University (DBIS), science direct, web info science, Scobus, Current Contents Medicine (CC-Med), PubMed, and SciSearch. As there are hardly any randomized controlled trials in this area, the selection of literature was somewhat subjective. The literature search concentrated on possible clinical relevance.
Etiology and Pathogenesis
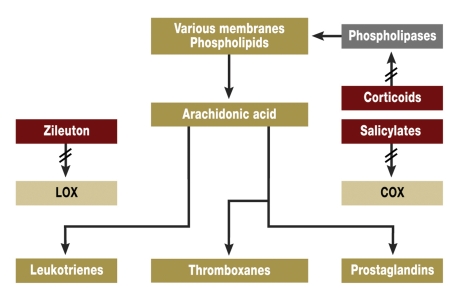
The focal point of the pathophysiology is the mechanism of arachidonic acid/eicosanoid metabolism, as clarified by the Nobel Prize winners Bergström (e1), Samuelson (e2), and Vane (e3). This is a system with complex elements (figure). Phospholipases break down phospholipids in cell membranes, giving arachidonic acid, the starting material for the eicosanoids. The main classes of eicosanoids – the leukotrienes (LT) and the prostanoids with the prostaglandins (PG) and thromboxanes (TX) – are predominantly formed by the lipoxygenases (LOX) and the cyclooxygenases (COX). They are important mediators of inflammation and hypersensitivity in almost all cells and organs.
Figure.
Eicosanoids: formation, breakdown, effects (greatly simplified from [3, e1, e2, e3])
Salicylates and other analgesics and anti-inflammatory drugs, particularly the non-steroidal anti-inflammatory drugs (NSAID) mainly used in rheumatology, inhibit cyclooxygenase, thus reducing prostaglandin synthesis (1). In intolerant individuals, there is also activation of basophiles and eosinophiles, macrophages, mast cells, platelets, and lymphocytes. These cells play an essential role in the symptoms (2, 3, 4, 5, 6, e4, e5, e6, e7, e8, e9).
This stimulation of basophiles and mast cells leads to the secretion of pharmacologically active substances. It is not immunological and may be designated as a "pseudoallergy" or a "pseudoimmunopathy", analogous to cold urticaria. It has also been suggested that the trigger might be infectious agents (e6). The deficiency in enzyme function caused by administration of COX inhibitors may be regarded as intolerance. Thus salicylate intolerance combines features of pseudoallergy and intolerance (2, 7, 8). The pattern of symptoms may depend on the relative numbers of the responsible cells. Studies on the tissues and blood of the same persons are consistent with the symptoms found (4, 5, 9–12).
The effects of salicylates in food from plants or in industrially produced COX inhibitors are in principle the same. COX-2 inhibitors (so-called coxibs) cause fewer gastrointestinal symptoms and effects than COX-1 inhibitors (such as indometacin or ibuprofen) (2, 10, 13, e10). In some cases, the most minor changes in chemical structure may cause substantial differences in the degree of intolerance (e4, 13).
Classical symptoms
The classical symptoms are in the respiratory tract. These were first described by Hirschberg in Germany in 1902 (e11), later in France by Widal ("Widal’s syndrome") (e12), and in the USA by Samter ("Samter’s triad") (e9). The manifestations are rhinosinusitis, nasal and sinus polyps, or bronchial asthma (2, 8, e4, e6, e9). When polyposis and asthma occur together with analgesic intolerance, this is known as the "triad." Up to 2.5% of the European population is affected and about 10% of intrinsic asthmatics. The rate of chronic sinusitis with nasal polyps is even higher (table 1) and there are assumed to be many unrecognized cases (2, 8, e4, e5, e6, e9). The symptoms from salicylates in food are also often misinterpreted as allergies. Moreover, salicylates sometimes only trigger symptoms in the presence of independent allergies, as a type of "augmentation phenomenon" (e13). For these reasons, figures on frequency cover a wide range.
Table 1. Symptoms of salicylate intolerance.
| Symptom | Frequency |
| Nasal polyps | 5% – 30% |
| Bronchial asthma | 10% |
| Polyps with asthma | 20% – 30% |
| Rhinitis | 5% – 10% |
| Chronic intestinal inflammation | 2% – 7% |
| Urticaria/Quincke edema | 5% – 10% |
Additional symptoms
The skin and the gastrointestinal tract may also be affected by salicylate intolerance. As the cause is not recognized and the triggering substances continue to be taken, this can lead to chronic processes, such as utricaria (14, e4, e6, e9), colitis (15, e14), or diarrhea (e15). On the other hand, effects on the circulation, or even anaphylactoid (not anaphylactic!) shock, are very rare (e12). Interestingly enough, the symptoms tend to manifest themselves at interfaces – the skin, mucous membranes or (to stretch a point) the vascular system. Salicylate intolerance shares this feature with genuine IgE-mediated allergies.
Anomalies in the eicosanoid complex, sometimes with another background, are also found in other diseases. These include familial intestinal polyposis (e16), malignant processes in the gastrointestinal tract (e17), and some gastroduodenal ulcers (e18, e19).
Diagnosis
Taking the history is of primary importance during the first visit to the doctor, who must attempt to establish the link between salicylate contact and the occurrence of the symptoms. This is only successful if they occur at close intervals. The doctor must therefore ask whether asthma, skin symptoms, swelling of the nasal mucous membrane, gastrointestinal symptoms or the (very rare) cardiovascular shock have occurred immediately after salicylate consumption. Polyps in the nose and nasal sinuses occur later and grow slowly; the decisive clue is then provided by their rapid and repeated recurrence after operative removal.
The accepted gold standard is the exposure or provocation test. However, these can only confirm or exclude the suspicion for rapid reactions such as asthma. Longterm developments such as polyps cannot be adequately followed.
Acetylsalicylic acid is normally administered orally or nasally (2, e4). This should only be done by persons familiar with these problems. Emergency precautions should be taken, as there may be violent reactions, such as asthma. This includes the possibility of observation in hospital and follow-up care. This diagnostic measure requires the proper equipment and is demanding for the personnel.
Fine tissue studies on biopsies can give valuable clues, particularly from eosinophilia (5, 9, 10, 11). This is an invasive approach, with the usual contraindications and risks.
Diagnosis can also be based on imaging techniques, including imaging procedures such as computed tomography (CT) for polyps and tests of lung functions to measure obstruction after exposure or provocation (2, 11, e6, e20). In individual cases, it may be necessary to perform endoscopy for local inspection and for the isolation of biopsies (9, 10, 11, 15, e14, e15, e17, e18).
Functional ex vivo tests are based on the detection of indicators in the patient’s tissues exposed to the test substances. Table 2 gives the sensitivity and specificity of functional in vitro tests and table 3 summarizes the positive and negative predictive values. This procedure is derived from the techniques and methods used for "genuine allergies." The following systems are currently commercially available:
Table 2. Sensitivity and specificity of functional in vitro tests.
| Test system | Released LT (17, 18, e7) | CD63 (6, 18, 19, e9) | FET (7, 14, 21, 22, e18, e19, e21, e22) | ||||||
| Parameter | SE | SP | (n) | SE | SP | (n) | SE | SP | (n) |
| Skin/respiratory tract | 63 | 99 | (88) | 63 | 93 | (90) | 96 | 83 | (465) |
| Respiratory tract | 68 | 97 | (66) | 60 | 93 | (50) | 96 | 89 | (407) |
| Skin | 50 | 99 | (72) | 65 | 93 | (70) | 96 | 97 | (58) |
| Stomach | – | – | – | – | – | – | 98 | 90 | (102) |
| Intestine | – | – | – | – | – | – | 64 | 89 | (132) |
| Respiratory tract/skin/intestine/stomach | – | – | – | – | – | – | 90 | 82 | (699) |
SE, sensitivity in %; SP, specificity in %; n, size of the test group; LT, leukotrienes; CD, cluster of differentiation; FET, functional eicosanoid test
Table 3. Positive and negative predictive values of functional in vitro tests.
| Test system | Released LT (17, 18, e7) | CD63 (6, 18, 19, e9) | FET (6, 14, 21, 22, e18, e19, e22) | ||||||
| Parameter | PPV | NPV | (n) | PPV | NPV | (n) | PPV | NPV | (n) |
| Skin/respiratory tract | 96 | 78 | (88) | 95 | 56 | (90) | 90 | 93 | (465 |
| Respiratory tract | 92 | 82 | (66) | 86 | 78 | (50) | 89 | 92 | (407) |
| Skin | 93 | 94 | (72) | 93 | 67 | (70) | 96 | 97 | (58) |
| Stomach | – | – | – | – | – | – | 70 | 98 | (102) |
| Intestine | – | – | – | – | – | – | 87 | 69 | (132) |
| Respiratory tract/skin/intestine/stomach | – | – | – | – | – | – | 87 | 87 | (699) |
PPV, positive predictive value in %; NPV, negative predictive value in %; n, size of the test group; LT, leukotrienes; CD, cluster of differentiation; FET, functional eicosanoid test
Measurement of the released quantity of LT from prepared basophiles (6, 16, e8). This corresponds to the LOX-dependent metabolic pathways.
Measurement of the lysozyme-associated membrane protein CD63 by flow cytometry. This has been reported to occur on degranulating basophiles (6, 16, 17, 18). The measurement of enriched basophiles is a less suitable approach to diagnose salicylate intolerance (e21). The activation marker CD203 is a transmembrane metalloenzyme. Its activation by CD63 can be used as a consistent marker. This can be detected by flow cytometry for allergy diagnosis (19, e9).
An extended functional eicosanoid test (FET) can be used to measure the eicosanoids LT and PG released after exposure to salicylates or other substances (4). This gives a more quantitative measurement of the metabolic pathways of LOX and COX under both normal conditions and conditions equivalent to the disease, together with the dependence on symptoms such as polyposis, rhinitis, and others. These values from blood samples are naturally exceeded by those from the affected tissues – which are only rarely demanded.
The FET is also capable of detecting other pathological features of eicosanoid metabolism, e.g., in gastroduodenal ulcer (e19), malignant processes in the intestinal tract (e17), sepsis, and "systemic inflammatory response syndrome" (SIRS) (e22). These are not cases of intolerance. Measurements are usually performed on blood samples (4, 7, 12, 14, 20, 21, 22, e17, e19, e22). As comparative studies have not been performed on the same patient, the value of the different tests cannot yet be rated. Only released substances, such as the eicosanoids, can be measured on tissues or biopsies (9, 10, 11). Flow cytometry cannot be used for this.
Value of diagnosis
These tests are limited by the technical possibilities and are mostly useful in recording the current situation. The newer functional tests are useful for unclear cases and when there is no close correlation in time between the symptoms and exposure or provocation. These tests are absolutely indispensable when exposure or provocation is unacceptable because of the circumstances and the patient’s expected reaction, or because of contraindications such as infection or bronchial asthma (2, 6, 23).
The doctor sends the patient’s blood for in vitro/ex vivo functional tests. Because of the biological character of these tests, sophisticated equipment and controls are necessary. Nevertheless, the analytical effort is justified and worthwhile, as the diagnosis is accelerated and improved, promising treatment can be initiated, and the stress and risks for the patient can be avoided. Prices range from 60 to 230 euros, depending on the clinical problem, the required sensitivity and specificity, whether the patient is an inpatient or an outpatient, and whether the insurance is private or not.
Therapy and prophylaxis
Interruption of treatment
The most reliable form of prophylaxis and therapy is to interrupt treatment. It is particularly important to avoid COX-1 inhibitors. However, some patients react with the same symptoms to very high dosages of paracetamol, used as a substitute (2, e4). In these cases, low-dose buprenorphine or tramadol must be prescribed. If highly sensitive patients interrupt treatment, they must also avoid cosmetics and food with high salicylate content, particularly spices and industrially processed food; 1 g curry may contain up to 2 mg salicylate (e23) (table 4). Advisory teams from university hospitals and other specialized facilities provide tables and diet recommendations for this purpose.
Table 4. Salicylate content (examples in mg/kg).
| Food | Salicylate content | Spices | Salicylate content |
| Sultanas | 78.0 | Curry | 2180 |
| Currants | 66.2 | Paprika | 2030 |
| Oranges | 23.0 | Oregano | 660 |
| Apples | 3.8 | Mustard | 260 |
| Pears | 2.7 | Cayenne | 176 |
| Potatoes | 1.2 | Pepper | 60 |
| Bananas | 0.1 | Garlic | 1 |
modified from (e22)
Surgery
An operation may be necessary if there is massive tissue growth in the upper respiratory tract and inhibition of the runoff of secretion. There are nevertheless frequent recurrences in patients with salicylate intolerance (2, 4, 6, 8, e4, e5, e6), which greatly reduces inactivation or desensitization (21, e20).
Drug treatment
The most active drugs are corticosteroids, as one of their activities is to inhibit the catalysis of the formation of arachidonic acid by phospholipases, the precursor of the responsible eicosanoids (figure). Steroids treatment can be topical or systemic (2, 6, 22, e5).
Biological methods
Inactivation or desensitization is a possible biological approach. The term "desensitization" is widely used in the USA, but is somewhat misleading, as it implies the specific suppression of immunological genuine allergies. The treatment is based on the administration of increasing quantities of acetylsalicylic acid. There is no fixed scheme. The first dose is usually 5 mg. The single doses are then increased up to 100 to 300 mg, which must then be taken once daily on a longterm basis. Depending on the procedure and the patient’s tolerance, this can last from a few days to two weeks (2, 22, 23). In about 80% of cases, improvements in nasal respiration, sense of smell and freedom from recurrent polyps are retained for two to three years (22, e20).
The effect can last for up to two weeks after salicylate administration has stopped. There are no consequences if a single dose is omitted or forgotten. If there are longer interruptions (as may be necessary before operations), the treatment must be restarted. As we know from first hand, this is always successful again.
It is better to perform the initial phase of treatment in hospital, as there is some risk of adverse reactions such as asthma or gastrointestinal symptoms, particularly in the phase of dose increase. This deactivation is only justified and permissible in patients with established salicylate intolerance.
The underlying principle is thought to be adaptive enzyme induction (2, 3, 4, 10). It is also possible that the irritable and partially responsible cells can be gradually shifted into a refractory state, in which the generation and metabolism of eicosanoids are arrested.
Other consequences of shifted eicosanoid patterns
Eicosanoid production may be abnormal even without exogenous factors. This influences vascular formation and apoptosis, as well as the formation and growth of tumors (e10, e11). In this context, familial accumulation of polypous and malignant processes in the gastrointestinal tract has long been known. This is reduced by COX inhibition, so that compounds of this group may be used for prophylactic therapy (e10, e24).
Interestingly enough, even nutrition can influence the eicosanoid complex. Because of the content of salicylates in plants, vegetarians exhibit increased serum concentrations (e24). This may be one of the reasons for their lower rate of cancer (e25). Increased consumption of unsaturated fatty acids leads to reduced de novo formation of arachidonic acid, coupled to decreased uptake from food. This inhibits inflammatory activity and also has an immunomodulatory effect (e26). This has been shown in gastroenterology (e27), rheumatology (e28), and neurology (e29). As however other triggering factors are important and other therapeutic approaches are available, diet alone cannot be more than an accompanying supportive measure.
Salicylate intolerance and the resulting eicosanoid shift is a specific systemic feature, accompanied by different individual manifestations and symptoms. It is necessary to think outside the box, e.g., by watching out for nasal polyps after protracted administration of COX inhibitors (24).
Perspectives
The systemic nature of the disturbance may hint at involvement in other diseases, such as Sudeck’s disease, eosinophilic organopathies or even the HELLP syndrome. Functional tests on blood or tissue would be capable of establishing this. They would also indicate whether, or to what extent, typical changes exist before the disease has broken out and who is at risk.
The eicosanoid complex and the actions of salicylates and fatty acids form a specific and interlocking theme in medicine. There is a wide variety of facets. Knowledge based on tests of function opens new approaches to diagnosis and therapy. Taken together with established options, these could help many more of those affected by these problems.
Acknowledgments
Translated from the original German by Rodney A. Yeates, M.A., Ph.D.
Footnotes
Conflict of interest statement
The author holds the patent to the test of eicosanoid function.
References
- 1.Willis AL, Smith DL. Metabolism of arachidonic acid. In: Cunningham FM, editor. The handbook of immunopharmacology and lipid mediators. London: Academic Press; 1994. pp. 1–32. [Google Scholar]
- 2.Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis and management. J Allergy Clin Immunol. 2003;111:913–920. doi: 10.1067/mai.2003.1487. [DOI] [PubMed] [Google Scholar]
- 3.Farooque S, Lee T. Mechanisms of aspirin-sensitive respiratory disease - as two component Model. Int Arch Allergy Immunol. 2007;142:59–63. doi: 10.1159/000095999. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer D. Testing and typing of eicosanoid-patterns. J Physiol Pharmacol. 2006;57:47–64. [PubMed] [Google Scholar]
- 5.Kaldenbach T, Schäfer D, Gosepath J, Bittinger F, Klimek L, Mann WJ. Die Bedeutung eosinophiler Granulozyten in Beziehung zu Allergie und Aspirin-Intoleranz bei Patienten mit Sinusitis polyposa. Laryngo-Rhino-Otol. 1999;17:429–434. doi: 10.1055/s-2007-996903. [DOI] [PubMed] [Google Scholar]
- 6.Sanz ML, Gamboa P, DeWeck A. A new combined test with flowcytometric basophile activation and determination of sulfidoleucotrienes is useful for in vitro diagnosis of hypersensitivity to Aspirin and other NSAID. Int Arch Allergy Immunol. 2005;136:58–72. doi: 10.1159/000082586. [DOI] [PubMed] [Google Scholar]
- 7.Schäfer D, Schmid M, Göde UC, Baenkler HW. Dynamics of eicosanoid in peripheral blood cells during bronchial, in: Aspirin-intolerant asthmatics. Eur Respir J. 1999;13:638–646. doi: 10.1183/09031936.99.13363899. [DOI] [PubMed] [Google Scholar]
- 8.Schiavino D, Nucera E, Milani A, Del Ninno M, Buenomo A, Sun J, Patriarca G. The aspirin disease. Thorax. 2000;55:66–69. doi: 10.1136/thorax.55.suppl_2.S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baenkler HW, Schäfer D, Hosemann W. Eicosanoid pattern in nasal polyps and nasal mucosa. Rhinology. 1996;34:166–170. [PubMed] [Google Scholar]
- 10.Schäfer D, Lindenthal U, Wagner M, Bölcskei PL, Baenkler HW. Effect of prostaglandine E2 on eicosanoid release by human bronchial biopsy specimens from normal and inflamed mucosa. Thorax. 1996;51:919–923. doi: 10.1136/thx.51.9.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid M, Göde U, Schäfer D, Wigand ME. Arachidonic acid metabolism in nasal tissue and peripheral blood cells in Aspirin-intolerant asthmatics. Acta Otolaryngol. 1999;119:227–230. doi: 10.1080/00016489950181819. [DOI] [PubMed] [Google Scholar]
- 12.Gosepath J, Hoffmann F, Schäfer D, Amedee RG, Mann WJ. Aspirin-intolerance in patients with chronic sinusitis. ORL. 1999;61:146–150. doi: 10.1159/000027660. [DOI] [PubMed] [Google Scholar]
- 13.Puhlmann U, Schäfer D, Ziemann C. Update on COX-2 inhibitor patents with focus on optimized formulation and therapeutic scope of drug combinations making use of COX-2 inhibitors. Expert Opin Ther Patents. 2006;16:403–430. [Google Scholar]
- 14.Velten FW, Bayerl C, Baenkler HW, Schaefer D. Functional eicosanoid test and typing (FET) in acetylsalicylic acid intolerant patients with urticaria. J Physiol Pharmacol. 2006;57:35–46. [PubMed] [Google Scholar]
- 15.Raithel M, Baenkler HW, Naegel A, Buchwald F, Schultis HW, Backhus B, Kimper S, Koch H, Mach E, Hahn EG, Konturek P. Significance of salicylate-intolerance in diseases of lower gastrointestinal tract. J Physiol Pharmacol. 2005;56:89–102. [PubMed] [Google Scholar]
- 16.May A, Weber A, Gall H, Kaufmann R, Zollner T. Means of increasing sensitivity of an in vitro diagnostic test for aspirin Intolerance. Clin Exp Allergy. 1999;29:1402–1411. doi: 10.1046/j.1365-2222.1999.00655.x. [DOI] [PubMed] [Google Scholar]
- 17.Ebo DG, Saint-Laudy A, Birdts CH, Mertens CH, Hagedorens MM, Schuerwegh AJ, De Clerck LS, Stevens WJ. Flow-assisted allergy-diagnosis: current application and future perspectives. Allergy. 2006;61:1028–1039. doi: 10.1111/j.1398-9995.2006.01039.x. [DOI] [PubMed] [Google Scholar]
- 18.Gamboa P, Sanz ML, Caballero MR, Urrutia I, Antepara I, Esparza R, et al. The flow-cytometric determination of basophile activation induced by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) is useful for in vitro diagnosis of the NSAID hypersensitivity syndrome. Clin Exp Allergy. 2004;34:1448–1457. doi: 10.1111/j.1365-2222.2004.02050.x. [DOI] [PubMed] [Google Scholar]
- 19.Buhring HJ, Streble A, Valent P. The basophile-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell. Activation and allergy diagnosis. Int Arch Allergy Immunol. 2004;133:317–329. doi: 10.1159/000077351. [DOI] [PubMed] [Google Scholar]
- 20.Hecksteden K, Schäfer D, Stuck BA, Klimek L, Hörmann K. Diagnostik des Analgetika-Intoleranz-Syndroms mittels funktioneller Zelltestung. Allergologie. 2003;26:263–271. [Google Scholar]
- 21.Gosepath J, Schäfer D, Amadee RG, Mann WJ. Individual monitoring of aspirin desensitization. Arch Otolaryngol Head Neck Surg. 2001;12:316–321. doi: 10.1001/archotol.127.3.316. [DOI] [PubMed] [Google Scholar]
- 22.Pfaar O, Klimek L. Aspirin desensitization in aspirin intolerance: update on current standards and recents improvements. Curr Opin Allergy Clin Immunol. 2006;6:161–166. doi: 10.1097/01.all.0000225153.45027.6a. [DOI] [PubMed] [Google Scholar]
- 23.Schapowal A, Schmitz-Schumann M. Provokationstests bei aspirinsensitivem Asthma und aspirinsensitiver Rhinosinusits. Allergologie. 1992;15:158–164. [Google Scholar]
- 24.Pearson DJ, Stones NA, Bentley SJ, Reid H. Proctocolitis induced by salicylate and associated with asthma and recurrent nasal polyps. Br Med J. 1983;287 doi: 10.1136/bmj.287.6406.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Bergström S. Lindsten Jan., editor. The prostaglandins: from the laboratory to the clinic. Nobel Lectures. Physiology or Medicine. Berichtszeitraum 1981-1990. 1982:93–112. [Google Scholar]
- e2.Samuelson B. Lindsten Jan., editor. From studies of biochemical mechanisms to novel biological mediators: prostaglandin endoperoxides, thromboxanes and leukotrienes. Nobel Lectures. Physiology or Medicine. Berichtszeitraum 1981-1990. 1982:117–138. doi: 10.1007/BF01133779. [DOI] [PubMed] [Google Scholar]
- e3.Vane JR. Lindsten Jan., editor. Adventures and excursions in bioassays: the stepping stones to prostacyclin. Nobel Lectures. Physiology or Medicine. Berichtszeitraum 1981-1990. 1982:145–170. [Google Scholar]
- e4.Settipane GA. Aspirin and allergic disease: a review. Am J Med. 1983;74:102–109. doi: 10.1016/0002-9343(83)90537-5. [DOI] [PubMed] [Google Scholar]
- e5.Szczeklik A, Sanak M, Nizankowski E, Kielbasa B. Aspirin-intolerance and cyclooxygenase-leukotriene pathways. Curr Opin Pulm Med. 2004;10:51–56. doi: 10.1097/00063198-200401000-00009. [DOI] [PubMed] [Google Scholar]
- e6.Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AJANE investigators European Network of aspirin-induced asthma. Eur Resp J. 2000;16:432–436. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- e7.May A, Zollner T, Weber A, Kaufmann R. Der Cellular Antigen Stimulations Test (CAST) in der Diagnostik Aspirin-sensitiver Rhinosinusitiden. Allergologie. 2000;23:103–109. [Google Scholar]
- e8.Kahlert H, Cromwell O, Fiebig H. Measurement of basophile-activating capacity of grass pollen allergens, Allergoids and hypoallergenic recombinant derivates by flow cytometry Using anti-CD203c. Int Arch Allergy Immunol. 2004;133:317–329. doi: 10.1046/j.1365-2222.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- e9.Samter M, Beers RF. Concerning the nature of intolerance to Aspirin. J Allergy. 1967;40:281–293. doi: 10.1016/0021-8707(67)90076-7. [DOI] [PubMed] [Google Scholar]
- e10.Becker JC, Domschke W, Pohle T. Current approaches to prevent NSAID-induced gastrophathy - COX selectivity and beyond. Br J Clin Pharmacol. 2004;58:586–600. doi: 10.1111/j.1365-2125.2004.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Hirschberg Mitteilung über einen Fall von Nebenwirkungen des Aspirin. DMW. 1902;28 [Google Scholar]
- e12.Widal MF, Abrani P, Lermoyez J. Anaphylaxie et idiosyncrasie. Presse Med. 1922;30:189–192. [Google Scholar]
- e13.Möller R, Paul E. Acetylsalicylsäure als Augmentationsfaktor bei Nahrungsmittelallergien. Hautarzt. 1996;47:281–283. doi: 10.1007/s001050050415. [DOI] [PubMed] [Google Scholar]
- e14.Chakroborty TK, Bhatia D, Heading RC, Ford MJ. Salicylate-induced exacerbation of ulcerative colitis. Gut. 1987;28:613–615. doi: 10.1136/gut.28.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Etienney I, Beaugerie L, Viboud C, Flahault A. Non-steroidal anti-inflammatory drugs as a risk factor for acute diarrhoea: A case crossover study. Gut. 2003;52:260–263. doi: 10.1136/gut.52.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e16.Higuchi T, Iwama T, Yoshinaga K, Toyooka M, Taketo MM, Sugihave K. A randomized, double blind, placebo controlled trial of the effects of Rolecoxib, a selective COX-2-inhibitor, on rectal polyps in familial adenomatous polyposis patients. Clin Cancer Res. 2003;9:4756–4760. [PubMed] [Google Scholar]
- e17.Baenkler HW, Schäfer D. Abnormal eicosanoid pattern of peripheral white blood cells in gastrointestinal cancer. J Physiol Pharmacol. 2005;56:119–128. [PubMed] [Google Scholar]
- e18.Konturek S, Piastucki I, Brzozowski T, Rodecki T, Dembinska-Kiec A, Zmuda A, Gryglewski R. Role of prostaglandins in the formation of Aspirin-induced gastric ulcer. Gastroenterology. 1981;80:4–9. [PubMed] [Google Scholar]
- e19.Baenkler HW, Zeus J, Schenk J, Schäfer D. Abnormal eicosanoid pattern by blood leukocytes in gastroduodenal ulcer. Med Science Monitor. 2004;10:447–456. [PubMed] [Google Scholar]
- e20.Gosepath J, Schäfer D, Mann WJ. Analgetika-Intoleranz: Langzeitergebnisse bis zu 3 Jahren bei adaptativer Desaktivierung mit einer täglichen Erhaltungsdosis von 100 mg Aspirin. Laryngo-Rhino-Otol. 2002;81:732–738. doi: 10.1055/s-2002-35002. [DOI] [PubMed] [Google Scholar]
- e21.Erdmann SM, Vetocilla S, Moll-Slodowy S, Sauer I, Merk HF. Basophilen-Aktivierungstest in der Diagnostik von Arzneimittelreaktionen. Hautarzt. 2005;56:38–43. doi: 10.1007/s00105-004-0871-8. [DOI] [PubMed] [Google Scholar]
- e22.Baenkler M, Leykauf M, John S. Functional analysis of eicosanoids from white blood cells in sepsis and SIRS. J Physiol Pharmacol. 2006;57:25–34. [PubMed] [Google Scholar]
- e23.Häberle M. Klinische und lebensmittelchemische Aspekte bei Unverträglichkeitsreaktionen auf Salizylat- und Additive-haltige Lebensmittel. Zbl Haut. 1987;153:75–95. [Google Scholar]
- e24.Thun MJ, Heuby SJ, Patrono C. NSAID as anticancer agents: mechanistic, pharmacologic and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- e25.Blacklock CJ, Lawrence JR, Wiles D, Malcolm EA, Gibson JH, Kelly CJ, Peterson JR. Salicylic acid in the serum of subjects not taking Aspirin. Comparison of salicylic acid concentrations in the serum of vegetarians, non-vegetarians and patients taking low dose Aspirin. J Clin Pathol. 2001;54:553–555. doi: 10.1136/jcp.54.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e26.Steinmetz KA, Potter JD. Vegetables, fruit and cancer. Epidemiology Cancer Causes Control. 1991;2:325–347. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- e27.Calder PC. Immunomodulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Proc Nutr Soc. 1996;95:727–734. doi: 10.1079/pns19960069. [DOI] [PubMed] [Google Scholar]
- e28.Rodgers JB. n-3 fatty acids in the treatment of ulcerative colitis. In: Kremer J, editor. Medical fatty acids in inflammation. Basel: Birkhäuser Verlag; 1998. pp. 91–101. [Google Scholar]
- e29.Cleland LG, James MJ, Proudman SM. The role of fish oils in the treatment of rheumatoid arthritis. Drug. 2003;63:845–853. doi: 10.2165/00003495-200363090-00001. [DOI] [PubMed] [Google Scholar]
- e30.Swank RL, Dugan BB. Effect of low saturated fat diet in early and late cases of multiple sclerosis. Lancet. 1990;336:37–39. doi: 10.1016/0140-6736(90)91533-g. [DOI] [PubMed] [Google Scholar]