Abstract
Introduction
The choice of type of heart valve prosthesis is determined by the patient’s age since bioprostheses have a limited lifespan. This article reviews current recommendations and the literature on cardiac valve replacement.
Methods
Selective literature search in Medline/PubMed back to 1996 and review of current national and international recommendations from specialist societies.
Results
The recommendations guiding the type of heart valve replacement have been revised in recent years. Of particular interest are the new generation of biological prostheses with extended durability, a growing use of stentless bioprostheses, a decrease in mortality of reoperation and an increase in life expectancy. Comorbidities such as chronic renal insufficiency or chronic atrial fibrillation are no longer contraindications to bioprosthesis.
The number of heart valve replacements in recent years rose despite a concomitant increase in valve repairs. Aortic valves are being increasingly replaced by bioprostheses.
Discussion
The choice of heart valve prosthesis should be tailored to each patient taking into account the patient’s age, life expectancy, comorbidities, and life style. Different decisions may be made now than those based on earlier recommendations resulting in an individualized treatment, in patients over the age of 65 or 70.
Keywords: cardiac valve replacement, biomaterial, cardiac surgery, comorbidity, guideline, indication
About one-third of the nearly 100 000 operations that were performed on a heart-lung machine in Germany in 2005 were cardiac valvular procedures, either alone or in combination with coronary artery surgery. The valvular procedures that were performed without coronary artery surgery involved the aortic valve in 65% of cases and the mitral valve in 25%. Biological prostheses are the main type of prosthetic valve used for aortic valve replacement: in the last 10 years, the percentage of mechanical aortic prostheses implanted has declined from 70% to 30%. In the mitral position, in contrast, every second procedure is a reconstruction (1). The good results that have been obtained with this technique so far imply that it will be performed even more frequently in the near future.
On the national level, the German Society for Cardiovascular Research (Deutsche Gesellschaft für Herz- und Kreislaufforschung) has published updated guidelines for the treatment of cardiac valvular diseases in which the choice of a prosthesis is only briefly discussed (2). On the other hand, the updated recommendations of the American specialty societies on the treatment of valvular diseases, which were published in 2006, include comprehensive guidelines on this matter, based on an analysis of more than 250 different studies (3). The long-term results of the first bioprostheses to be implanted are now available. As for the second generation of bioprostheses, which differs from the first in both construction and preservation, the currently available results extend to 10 years of follow-up. Shorter longitudinal observations have been made to date with the most recent (third) generation of stented and unstented prostheses.
The high rate of degeneration of the first bioprostheses that were implanted in younger patients led to a renewed preference for mechanical heart valves (1, 2, 3). Comorbidities such as atrial fibrillation, which necessitates permanent anticoagulation, or chronic renal failure were at one time considered relative contraindications to the implantation of biological prostheses. These strict criteria for the choice of prosthesis are now increasingly being relaxed, and the choice is now commonly tailored to the individual patient. The increased use of biological cardiac valvular prostheses is justified by current state of the data on biological prostheses of the most recent generation, which are proving to be more durable than previous types, and particularly on stentless prostheses, as well as by the decreasing mortality of repeated procedures. This article contains the recommendations of the specialty societies while taking account of the current state of knowledge as reflected by our selective review of the literature, which encompassed prospective, randomized trials, cohort studies, and review articles that have been published in the last 10 years. A literature search was performed on the terms "bioprostheses," "freedom from degeneration," "mid and long term results," "comorbidities," "mortality," and "complications." The currently available types of biological prosthesis are discussed in what follows.
Types of prosthesis
There are important differences between biological valvular prostheses of animal origin and mechanical valvular prostheses. Mechanical prostheses are highly durable but necessitate lifelong anticoagulation (table 1). They are often implanted in younger patients in order to obviate the need for a second valve replacement procedure. Their use also seems reasonable when the patient already must be anticoagulated for life for other medical reasons. Biological and mechanical prostheses have comparable hemodynamic properties. In some cases, a mechanical prosthesis is preferred when the patient has a very narrow aortic base, because its effective opening area is somewhat larger than that of a bioprosthesis. The risk of prosthesis endocarditis is equally high in the two groups.
Table 1. Biological and mechanical heart valves: a comparison.
| Advantages | Disadvantages | |
| Biological valves | No need for lifelong anticoagulation | Limited durability |
| Mechanical valves | Practically unlimited durability | Lifelong anticoagulation |
The ideal cardiac valvular prosthesis has yet to be built. Such a valve would have the same biological and hemodynamic properties as a normal valve and, furthermore, would not undergo degeneration, give rise to thrombi, or elevate the risk of endocarditis. None of the currently available biological or mechanical valvular prostheses meet these specifications.
Biological prostheses
Biological valvular prostheses are classified into a number of subtypes (table 2). A human heart valve that is harvested from, and implanted into, the same person is called an autograft: in the Ross procedure, for example, the patient’s pulmonic valve is transferred to the aortic position. These pulmonic autografts have excellent hemodynamic properties as well as low rates of thrombosis, degeneration, and endocarditis. The Ross procedure is suitable for children and young adults because it is compatible with further growth of the aortic root. Its long-term success rate is not yet adequately known, however, and a few cases of dilatation of the autograft have been reported (4).
Table 2. Overview of valvular bioprostheses.
| Type of prosthesis | Definition |
| Autograft | Heart valve from the same individual |
| Homograft/allograft | Human heart valve removed post mortem |
| Xenograft | Artificial or mechanical heart valve |
| Heterograft | Valve from a non-human species (porcine, bovine) |
A homograft, in contrast, is a human heart valve that has been cryopreserved and treated with antibiotics. Homografts are often used in patients with extensive evidence of endocarditis, but their availability is limited and they tend to degenerate (5).
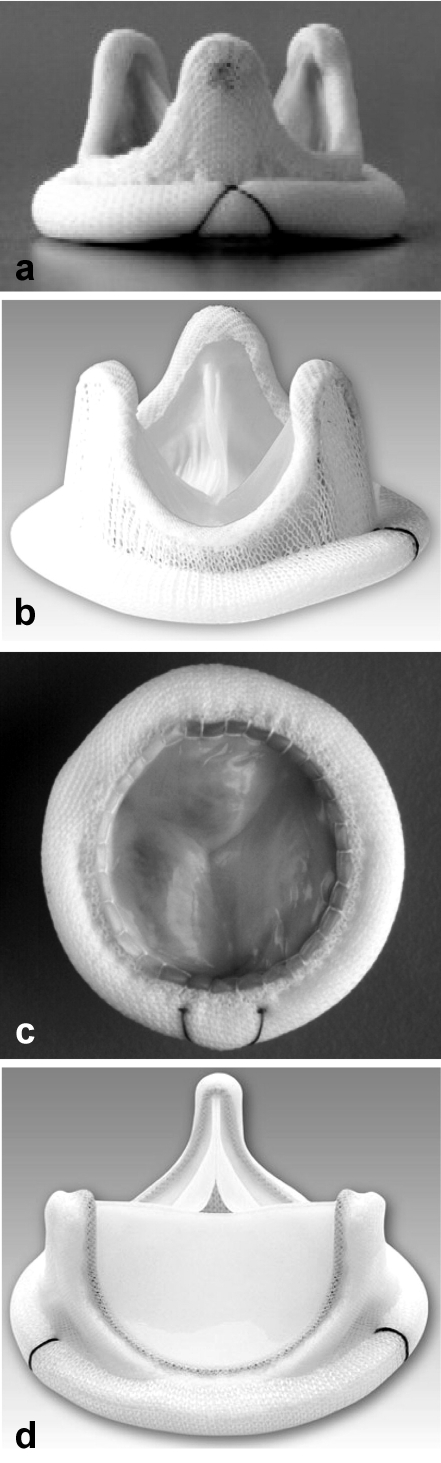
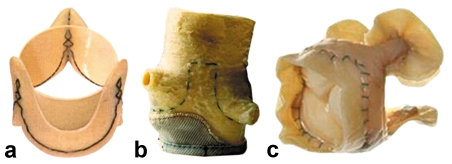
Xenografts are of porcine or bovine origin and are often reinforced with a scaffolding ("stent") that may be composed of various types of material and serves to fix the valvular tissue in its natural, anatomical-functional position (stented bioprostheses) (figure 1). Xenograft prostheses are made of porcine aortic valves or bovine pericardium. The commonly used preservative glutaraldehyde stabilizes the collagen scaffolding and lessens its antigenicity. A number of techniques have been developed to slow the processes of calcification and degeneration. Stentless bioprostheses have also become available in the last few years (figure 2). These prostheses of animal origin do not contain a stabilizing metallic scaffolding and therefore offer a larger opening surface and more favorable hemodynamic properties than stented prostheses. The clinical significance of these rheological properties in the long term is currently under study by many different research teams and is a subject of active debate. The implantation of a stentless valvular prosthesis often requires a more complicated surgical technique than that of a stented one (6).
Figure 1.
A selection of stented valvular prostheses:
a) Hancock IITM,
b) Carpentier-Edwards,
c) Mosaic,
d) Perimonnt.
From: Edmunds L, Cohn L: Cardiac surgery in the adult, 2003, with kind permission from McGraw-Hill, New York
Figure 2.
A selection of stentless valvular bioprostheses: a) Freedom Solo, Sorin Group;
b) Prima Plus, Edwards; c) NR200, Shelhigh. With kind permission from the Sorin Group, Edwards, and Shelhigh companies.
Results
Stented bioprosthetic aortic valves
The perioperative mortality of aortic valve replacement with a stented bioprosthesis is roughly 4% (1, 7). About 40% of porcine valves of the type that was initially used were free of degeneration at 18 years (7, 8, 9). Younger patients, particularly those less than 40 years old, were found to have a markedly elevated rate of premature degeneration of their valvular prostheses. The degeneration rate in this age group at 10 years was already over 40%, compared to 15% in patients aged 60 to 70, and 10% in patients over 70 (8, 9, 10) (table 3). This is explicable as being due to the lesser hemodynamic demand placed on the prosthesis in an elderly patient.
Table 3. The durability of bioprosthetic valves in the aortic position as a function of the age of the patient (10).
| Age group | 5 years (%) | 10 years (%) | 15 years (%) |
| ≤ 35 | 79 | 51 | |
| 36–50 | 99 | 68 | 48 |
| 51–64 | 98 | 72 | 42 |
| 65–69 | 98 | 74 | 64 |
| ≥ 70 | 100 | 90 | 90 |
Valves derived from pericardium have a comparable or slightly lower degeneration rate: 77% of surviving patients still have a properly functioning prosthesis 15 years after implantation. Fewer than 10% of patients over age 65 need a second valve replacement procedure (11).
Stentless bioprosthetic aortic valves
Interim results at 5 and 10 years are now available for stentless bioprostheses. The early mortality of 5% is comparable to that of stented bioprostheses. The rates of early reoperation, thromboembolic events, and endocarditis are currently lower than the corresponding rates for stented bioprostheses (12). The patients have also been found to have a better NYHA (New York Heart Association) stage in the first 5 to 10 years after implantation, presumably because of the more favorable hemodynamic properties of stentless prostheses (13). These may be particularly important in patients with a large body surface area, for whom a smaller prosthesis may not be large enough. Studies have shown a survival advantage in the first few years after surgery compared to patients that have received stented bioprosthetic valves (14).
Mitral valve replacement
The perioperative mortality in the first 30 days after mitral valve replacement has markedly declined over the last 20 years to 4% to 6%. The 10-year survival rate is currently 50% to 60%, regardless of whether a mechanical or a biological valve is used (15). The early mortality after repeated mitral valve replacement is currently less than 10%. In contrast to aortic valve replacement, mitral valve replacement still more commonly involves a mechanical than a biological prosthesis (1). Bioprostheses in the mitral position degenerate more frequently than in the aortic position (table 4), presumably because of the greater hemodynamic demand. The degeneration rate of bioprosthetic mitral valves, like that of bioprosthetic aortic valves, strongly depends on the age of the patient: the degeneration rate at 10 years in patients under 40 years of age is 20%, four times higher than the corresponding figure for patients over 60 (10). The overall reoperation rate for bioprosthetic mitral valve replacement is 50%, compared to 29% when a mechanical prosthesis is used (9).
Table 4. The durability of bioprosthetic valves in the mitral position.
Comparison of biological and mechanical prostheses in the aortic and mitral positions
There are only a few current randomized trials comparing the long-term results of biological and mechanical valves. A large-scale review revealed no difference in survival rates at 10 years and a slightly higher survival rate at 15 years for patients with mechanical prostheses (18). The bioprostheses had higher rates of degeneration and reoperation. The reoperation rate for mechanical valves in the aortic position is less than 5% at 10 years and less than 10% at 15 years, while the corresponding figures for bioprostheses are 10% and 30%, respectively. Hemorrhagic complications are significantly more common in patients with mechanical valves because of anticoagulation (19).
Recommendations
The currently available bioprostheses show satisfactory long-term results in the aortic and mitral positions. In patients aged 65 or higher, the rate of prosthetic valve degeneration at 15 years is 10% to 36% (3, 7, 8, 9). If a repeated valve replacement procedure is necessary, the operative mortality is 4% to 6% (3). The degeneration rate of bioprostheses is inversely related to the age of the patient at implantation; thus, biological valvular prostheses can be generally recommended for patients aged 65 or older. The choice of prosthesis is also influenced by the opportunity that has recently become available of correcting a cardiac arrhythmia by surgical means during the valve replacement procedure. Thus, the fact that the patient is anticoagulated because of chronic atrial fibrillation no longer necessarily implies that a mechanical prosthesis should be chosen.
The choice of an aortic valvular prosthesis
For a number of reasons, the last few years have seen a trend toward the implantation of bioprostheses in the aortic position. Current studies reveal that bioprostheses of the most recent generation last longer than earlier types. Furthermore, reoperation rates have declined, mainly because the recommended age limits have been respected. Because life expectancies in general have risen, more and more elderly patients are presenting for valve replacement, and for these patients a bioprosthesis is usually chosen.
Some younger patients are averse to oral anticoagulation and therefore prefer a biological valvular prosthesis, despite the known risk of degeneration and reoperation in persons under 60 years of age. The operative risk of a second valve replacement has significantly decreased, however, mainly because of advances in cardioprotection. Thus, younger patients opting for a bioprosthesis can enjoy a normal quality of life without anticoagulation for many years but may need to undergo a second valve replacement procedure with an acceptable degree of risk. Persons suffering from coronary heart disease in addition to their valvular disease have a lower life expectancy, so that bioprostheses can be chosen more frequently for patients in this group (20) (box 1).
Box 1. Recommendations for the choice of a prosthesis in the aortic position (3).
Class I (recommended choices)
A mechanical prosthesis for a patient who already has a mechanical prosthesis in the mitral or tricuspid position (level C)
A bioprosthesis for a patient of any age who is averse to taking oral anticoagulants or for whom oral anticoagulation is absolutely contraindicated (level C)
Class IIa (reasonable choices)
A mechanical prosthesis for a patient under age 65 for whom oral anticoagulation is not contraindicated
A biological prosthesis for a patient under age 65 who has taken a personal decision not to have a mechanical prosthesis implanted in view of his or her lifestyle, after thorough discussion of the risks of anticoagulation as well as of the likelihood that a second valve replacement procedure will be necessary (level C)
A bioprosthesis for a patient aged 65 or older who is not at elevated risk of thromboembolism (level C)
A second valve replacement procedure with a homograft is reasonable for a patient with active prosthesis endocarditis (level C)
Class IIb (choices worth considering)
A bioprosthesis can be considered for a female patient of childbearing age who desires to have children (level C)
Recommendation classes of the Task Force on Practice Guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA):
| Class I: | Conditions for which there is evidence and/or general agreement that a given procedure or treatment is useful and effective. |
| Class II: | Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a procedure or treatment. |
| a: | Weight of evidence/opinion is in favor of usefulness/efficacy |
| b: | Usefulness/efficacy is less well established by evidence/opinion. |
| Class III: | Conditions for which there is evidence and/or general agreement that the procedure/treatment is not useful/effective, and in some cases may be harmful. |
| ACC/AHA definitions of levels of evidence: | |
| Level A: | Data derived from multiple randomized clinical trials |
| Level B: | Data derived from a single randomized trial, or non-randomized studies |
| Level C: | Consensus opinion of experts |
In view of the heterogeneous data currently available from clinical trials, the surgeon performing valvular replacement must still rely to some extent on personal experience and subjective assessment when choosing the type of prosthesis and the particular operative method to be used. This is particularly the case for patients with difficult anatomy and/or significant comorbidity. Stented bioprostheses are used more commonly than stentless ones because of their relative ease of implantation, their extensively documented long-term results, and the low risk associated with reoperation. The observations that have been made to date show stentless valves to have certain clinical advantages, because of their more favorable hemodynamic properties; no definitive judgment can be made as to whether these advantages will persist in the long term. The initial results of 10-year follow-up indicate a degeneration rate of about 20%, which is comparable to that of conventional, stented prostheses (12, 13). It seems reasonable to predict that patients with a narrow aortic root and a high risk of disproportion between the prosthesis size and the body surface area stand to benefit from a stentless prosthesis. The original expectation that homografts would be associated with a lower rate of degeneration has been found to be incorrect in long-term follow-up studies.
The choice of a mitral valvular prosthesis and ablative surgery in atrial fibrillation
In principle, mitral valvular prostheses are chosen according to nearly the same criteria as aortic valvular prostheses. It must be borne in mind, however, that the rate of degeneration of bioprostheses is higher in the mitral than in the aortic position. Thus, mitral valve bioprostheses should not be implanted in patients less than 65 years of age unless there are compelling reasons to do so, as discussed in box 2.
Box2. Recommendations for the choice of a prosthesis in the mitral position (3).
Class I (recommended choices)
A bioprosthesis for a patient who is not taking oral anticoagulants or for whom these are clearly contraindicated (level C)
Class IIa (reasonable choices)
A mechanical prosthesis for a patient under age 65 with longstanding atrial fibrillation (level C)
A bioprosthesis for a patient aged 65 or older (level C)
A bioprosthesis for a patient under age 65 in sinus rhythm who has taken a personal decision not to have a mechanical prosthesis implanted in view of his or her lifestyle, after thorough discussion of the risks of anticoagulation as well as of the likelihood that a second valve replacement procedure will be necessary (level C)
For patients suffering from atrial fibrillation in addition to valvular heart disease, the current trend is toward performing ablative techniques to achieve sinus rhythm at the same time as the valvular procedure. If the valve can be reconstructed or a bioprosthesis is implanted, oral anticoagulation can be avoided. Previously, such patients were more commonly given mechanical heart valves, because they would need anticoagulation postoperatively in any case. 75% to 90% of patients undergoing rhythm surgery at the same time as a mitral valve procedure are still in sinus rhythm six months afterward. The success rate of ablative surgery is higher if atrial fibrillation has not been present for longer than one year. Ablative surgery also reduces the incidence of stroke. Even patients who cannot be permanently converted to sinus rhythm and therefore still require oral anticoagulation will benefit from bioprosthetic valve implantation, because they require a lower target INR (2 to 3) than they would have required if they had received a mechanical prosthesis (2.5 to 3.5) (21).
The choice of valvular prosthesis for a patient with end-stage renal failure
In earlier years, patients with dialysis-dependent renal failure who required cardiac valvular prostheses were usually given mechanical ones, because it was feared that their altered metabolic situation would lead to more rapid degeneration of a bioprosthetic valve. It has been found, however, that these patients’ life expectancy is already curtailed to such an extent that bioprosthesis degeneration often does not occur in their remaining lifetime (22). The supposed advantage of the longer durability of a mechanical valve is also offset by the potential complications of oral anticoagulation, especially because anticoagulation is more difficult to manage in dialysis patients than in others. Accordingly, there has been a move away from the recommendations found in the 1998 guidelines of the American College of Cardiology and the American Heart Association. The current guidelines no longer recommend any particular type of prosthesis for patients with end-stage renal failure, but they do warn about the risks of systemic anticoagulation in this patient group (3).
The choice of valvular prosthesis for a woman desiring to have children
At present, there is still no optimal type of valvular prosthesis for women desiring to have children. Regardless of the type of prosthetic valve, pregnant women with artificial valves are at elevated risk for heart failure, arrhythmia, or (maternal) endocarditis. Pregnancy shortens the life span of bioprosthetic valves; therefore, a female patient wishing to have children should try to become pregnant within five years after the implantation of the prosthesis. On the other hand, pregnant women with mechanical valvular prostheses have the highest rate of maternal and fetal complications. The miscarriage rate under treatment with phenprocoumone is as high as 70%; if oral anticoagulation is switched to heparin, it is still approximately 20%. The rate of serious cardiac complications in pregnant women with mechanical valvular prostheses is 20%, which is about twice as high as the rate with biological prostheses (23, 24, 25).
Summary and perspectives
The choice of a valvular prosthesis should be taken jointly by the patient and the surgeon after consideration of the advantages and disadvantages of each type of prosthesis. For patients aged 70 or older, it is clear that a biological valve should be recommended; likewise, a biological valve should be recommended for patients aged 65 to 70 whose life expectancy is reduced by comorbidity. For patients under age 65, a mechanical valve is to be preferred, at least in the mitral position. If a patient in this age group is averse to anticoagulation, it is proper to implant a bioprosthesis as long as the patient has been fully informed about the long-term implications, because reoperation, if it should become necessary, can be performed with an acceptably low risk.
Patients with longstanding atrial fibrillation are unlikely to be permanently converted to sinus rhythm by an ablative procedure. For those who have had atrial fibrillation for a shorter time, permanent oral anticoagulation can be avoided by the implantation of a biological valvular prosthesis in combination with ablative surgery. When a bioprosthetic valve is to be implanted in a patient with a narrow aortic root, it seems to be advantageous over the long term to implant a stentless bioprosthesis, even though the technique of implantation is more difficult. A bioprosthetic valve remains the recommended type to implant in the presence of endocarditis. For patients with prosthesis endocarditis, a stented or stentless bioprosthesis is recommended (also as an aortic root replacement, if necessary).
In the coming years, the durability of stented bioprosthetic valves is likely to improve, because of further advances in methods of bioprosthesis construction and preservation. In the near future, the first long-term results of the third generation of bioprosthetic valves and stentless valves will be published. We can also expect advances and data from clinical trials in the fields of percutaneous catheter procedures, minimally invasive techniques, and the construction of valvular prostheses by means of tissue engineering.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors state that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Gummert JF, Funkat A, Beckmann A, Hekmat K, Ernst M, Krian A. Cardiac surgery in Germany during 2005: a report on behalf of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg. 2006;54:362–371. doi: 10.1055/s-2006-924405. [DOI] [PubMed] [Google Scholar]
- 2.Daniel WG, Baumgartner H, Gohlke-Bärwolf C, et al. Klappenvitien im Erwachsenenalter. Clin Res Cardiol. 2006;95:620–641. doi: 10.1007/s00392-006-0458-8. [DOI] [PubMed] [Google Scholar]
- 3.ACC/AHA 2006 guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/ American Heart Association on practice guidelines. Circulation. 2006;114 [Google Scholar]
- 4.Sievers H, Stierle U, Hanke T, et al. Die Ross-Operation - eine Therapieoption bei Aortenklappenerkrankungen. Dtsch Arztebl. 2005;102(30):A 2090–A 2097. [Google Scholar]
- 5.Nägele H, Döring V, Rödiger W, Kalmár P. Aortic valve replacement with homografts. An overview. Herz. 2000;25:651–658. doi: 10.1007/pl00001979. [DOI] [PubMed] [Google Scholar]
- 6.de Kerchove L, Glineur D, El Khoury G, Noirhomme P. Stentless valves for aortic valve replacement: where do we stand? Curr Opin Cardiol. 2007;22:96–103. doi: 10.1097/HCO.0b013e328014670a. [DOI] [PubMed] [Google Scholar]
- 7.Vongpatanasin W, Hilis LD, Lange RA, et al. Prosthetic heart valves. N Engl J Med. 1996;335:407–416. doi: 10.1056/NEJM199608083350607. [DOI] [PubMed] [Google Scholar]
- 8.Borger MA, Ivanov J, Armstrong S, Christie-Hrybinski D, Feindel CM, David TE. Twenty-year result of the Hancock II bioprosthesis. J Heart Valve Dis. 2006;15:49–55. [PubMed] [Google Scholar]
- 9.Jamieson WR, Burr LH, Miyagishima RT, et al. Carpentier-Edwards supra-annular aortic porcine bioprosthesis: clinical performance over 20 years. J Thorac Cardiovasc Surg. 2005;130:994–1000. doi: 10.1016/j.jtcvs.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 10.Jamieson WR, Ling H, Burr LH, et al. Carpentier-Edwards supraannular bioprosthesis evaluation over 15 years. Ann Thorac Surg. 1998;66(6 suppl):S49–S52. doi: 10.1016/s0003-4975(98)01127-8. [DOI] [PubMed] [Google Scholar]
- 11.Aupart MR, Mirza A, Meurisse YA, Sirinelli AL, Neville PH, Marchand MA. Perimount pericardial bioprosthesis for aortic calcified stenosis: 18-year experience with 1133 patients. J Heart Valve Dis. 2006;15:768–775. [PubMed] [Google Scholar]
- 12.Cohen G, Christakis GT, Joyner CD, et al. Are stentless valves superior to stented valves? A prospective randomized trial. Ann Thorac Surg. 2002;73:767–778. doi: 10.1016/s0003-4975(01)03338-0. [DOI] [PubMed] [Google Scholar]
- 13.Rajappan K, Melina G, Bellenger NG, et al. Evaluation of left ventricular function and mass after metronic freestyle versus homograft aortic root replacement using cardiovascular magnetic resonance. J Heart Valve Dis. 2002;11:S60–S65. [PubMed] [Google Scholar]
- 14.Pibarot P, Dumesnil J. Prosthesis-patient-mismatch: definition, clinical impact, and prevention. Heart. 2006;92:1022–1029. doi: 10.1136/hrt.2005.067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidhu P, O’Kane H, Ali N, et al. Mechanical or bioprosthetic valves in the elderly: a 20-year comparison. Ann Thorac Surg. 2001;71:S257–S260. doi: 10.1016/s0003-4975(01)02522-x. [DOI] [PubMed] [Google Scholar]
- 16.David TE, Ivanov J, Armstrong S, Feindel CM, Cohen G. Late results of heart valve replacement with the Hancock II bioprosthesis. J Thorac Cardiovasc Surg. 2001;121:268–277. doi: 10.1067/mtc.2001.112208. [DOI] [PubMed] [Google Scholar]
- 17.Neville PH, Aupart MR, Diemont FF, Sirinelli AL, Lemoine EM, Marchand MA. Carpentier-Edwards pericardial bioprosthesis in aortic or mitral position: a 12-year experience. Ann Thorac Surg. 1998;66:143–147. doi: 10.1016/s0003-4975(98)01122-9. [DOI] [PubMed] [Google Scholar]
- 18.Grunkemeier GL, Li HH, Naftel DC, et al. Long-term perfomance of heart valve prostheses. Current Probl Cardiol. 2000;25:75–154. doi: 10.1053/cd.2000.v25.a103682. [DOI] [PubMed] [Google Scholar]
- 19.Hammermeister K, Sethi GK, Henderson WG, et al. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–1158. doi: 10.1016/s0735-1097(00)00834-2. [DOI] [PubMed] [Google Scholar]
- 20.Stassano P, DiTommaso L, Vitale DF, et al. Aortic valve replacement and coronary artery surgery: determinants affecting early and long-term results. Thorac Cardiovasc Surg. 2006;54:521–527. doi: 10.1055/s-2006-924467. [DOI] [PubMed] [Google Scholar]
- 21.Cox JL, Ad N, Pallazzo T. Impact of the Maze procedure on the stroke rate in patient with atrial fibrillation. J Thorac Cardiovasc Surg. 1999;118:883–840. doi: 10.1016/s0022-5223(99)70052-8. [DOI] [PubMed] [Google Scholar]
- 22.Chan V, Jamieson WR, Fleisher AG, Denmark D, Chan F, Germann E. Valve replacement in end-stage renal failure: mechanical versus bioprostheses. Ann Thorac Surg. 2006;81:857–862. doi: 10.1016/j.athoracsur.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.The task force on the management of cardiovascular diseases during pregnancy of the European society of cardiology expert consensus document on management of cardiovascular diseases during pregnancy. Eur Heart J. 2003;24:761–781. doi: 10.1016/s0195-668x(03)00098-8. [DOI] [PubMed] [Google Scholar]
- 24.Mihaljevic T, Paul S, Leacche M, Rawn JD, Cohn LH, Byrne JG. Valve replacement in women of childbearing age: influences on mother, fetus and neonate. J Heart Valve Dis. 2005;14:151–157. [PubMed] [Google Scholar]
- 25.Sadler L, McCowan L, White H, Stewart A, Bracken M, North R. Pregnancy outcomes and cardiac complications in women with mechanical, bioprothetic and homograft valves. BJOG. 2000;107:245–253. doi: 10.1111/j.1471-0528.2000.tb11696.x. [DOI] [PubMed] [Google Scholar]