Abstract
Introduction
Most hilar cholangiocarcinomas (Klatskin tumors) are diagnosed at an advanced stage. This article aims to review the literature of resection and palliative treatment in patients with hilar cholangiocarcinoma.
Methods
All studies with evidence levels I and II and relevant trials with evidence level III from 1996 to 04/2007 were included.
Results
The definition of resectability depends not only on tumor stage but also on operator experience. The best long-term results are achieved by hilar resection combined with extended liver resection. No clear clinical benefit has been demonstrated for neoadjuvant and adjuvant therapies. The role of liver transplantation requires redefinition in view of good long-term survival after neoadjuvant chemoradiation and the possibility of living-donor liver transplantation. Initial studies of a combination of biliary stenting and photodynamic therapy (PDT) for palliation have shown significantly prolonged survival times compared with stenting alone. There is no established standard palliative chemotherapy.
Discussion
The prognosis of patients with Klatskin tumors has been significantly improved by extended resection procedures. The combination of stenting and PDT is a useful palliative approach.
Keywords: Klatskin tumor, cancer treatment, therapeutic regimen, liver transplantation, liver resection, photodynamic therapy
Cancers of the intrahepatic and extrahepatic biliary ducts (hilar cholangiocarcinomas, Klatskin tumors) are the fifth most common tumors of the gastrointestinal tract. According to the Robert Koch-Institute (2006), about 1800 men and 4200 women in Germany develop gall bladder or extrahepatic cholangiocarcinomas every year. 40% to 60% of the cancers of the biliary ducts are Klatskin tumors. Gerald Klatskin, an American specialist in internal medicine, in 1965 described local, sclerosing adenocarcinomas of the hepatic fork as clinically independent tumor entities, which became known in the literature as so-called Klatskin tumors (synonyms: hilar, proximal, or central cholangiocarcinomas) (1). If a patient develops painless icterus, tumor disease is usually advanced. Only curative (R0) resection, which in older studies was done in less than 30% of patients, offers a chance of long-term survival and cure (2–9). In the past 5 to 10 years, increasingly radical surgery in the shape of extended hepatic resection achieved an improvement in resection rates of up to 50% to 60% and in five year survival rates of up to 35% to 45% (2, 6, 7, 10). Palliative therapeutic measures aim to improve quality of life and possibly extend the survival period. Because of the rare occurrence and anatomical complexity of Klatskin tumors, diagnosis and treatment will have to be delivered by an interdisciplinary team of surgical and gastroenterological experts.
This article describes current surgical and palliative therapeutical concepts and their results. The authors searched PubMed and analyzed review articles, and evaluated 131 publications of evidence levels I and II, and relevant studies of evidence level III for the time period 1996 to April 2007.
Specific diagnostics
If an extrahepatic cholangiocarcinoma is suspected, magnetic resonance imaging/cholangiography (MRI/MRC) is the gold standard to evaluate tumor status and plan treatment, which entails targeted biliary drainage, assessing the potential remaining liver volume before extended resection, and embolizing the portal vein. Location and extent of the tumors on the basis of the Bismuth-Corlette classification can be judged on the MRI scan with a sensitivity of 94% and a specificity of 100% (e1). In spite of the good quality of the MRI scan, most surgeons request cholangiography to assess the resectability of the tumor; endoscopic retrograde cholangiography (ERC) is used more often than the percutaneous transhepatic technique. In 40% to 70% of patients with suspected extrahepatic cholangiocarcinoma, the suspicion is confirmed by a combination of brush cytology and clamp biopsy (e2). Newer diagnostic procedures, such as positron emission tomography (PET) (e3), combined PET/computed tomography (CT), fluorescence in situ hybridization (FISH) (e6), and intraductal ultrasonography are currently not routine procedures.
Indications for surgery
In assessing the indication for resection, the patient’s general operability, but especially technical and functional resectability, will have to be assessed. The indication for surgery exists if distant metastases, cirrhosis of the liver, and an obviously locally advanced tumor that bilaterally affects bile duct segments and/or bilateral infiltration of the vessels have been excluded. Affected regional lymph nodes do not constitute a contraindication for surgery because long-term survival has been described in some patients with lymph node metastases (4, 8, 10) (e7). More than in almost any other tumor, resectability depends on the surgeon’s experience. The prominent role of the surgeon is reflected in a retrospective study that compared patient cohorts in a US and a Japanese hospital. The resectability rate was 25% in the US hospital and 79% in the Japanese hospital, although the Japanese patients had more advanced tumors (e8). Definite assessment of resectability still requires laparotomy in most patients, in spite of the vastly improved imaging methods.
Preoperative conditioning
Bile duct drainage
Liver function is impaired as a result of cholestasis, and this is an important risk factor in large liver resections. Most relevant publications describing the resection of Klatskin tumors recommend preoperative decompression of the biliary duct, aiming to reduce bilirubin measurements to below 2 mg/dL or 5 mg/dL (2, 7, 8, 10–12) (e9). Whether an endoscopic or percutaneous transhepatic approach is favored depends on the center. A disadvantage of the percutaneous technique is the risk of seeding tumor cells into the abdominal cavity (e10). Because of the risk of cholangitis, contrast medium imaging of the biliary passages that cannot be drained will have to be avoided. Regeneration of liver function after biliary drainage takes 2 to 4 weeks, depending on the severity and duration of cholestasis.
Induction of hypertrophy of the liver remnant
In liver resections where extensive loss of parenchyma can be predicted, with a calculated remaining liver volume below 350-400 mL – or less than 40% of the total liver mass – preoperative embolization of the part of the liver affected by the tumor will have to be considered (13). The aim of preoperative embolization procedures is perfusion-induced hypertrophy of the potential liver remnant on the one hand, and on the other hand, induction of atrophy of the part of the liver that is affected by the tumor. The most popular procedure is interventional, portal venous embolization via percutaneous transhepatic access.
Principles of oncosurgery
Surgical strategy
Fundamental principles of oncosurgery are R0 resection with wide longitudinal and lateral safety margins, monobloc resection of the tumor, and the no-touch technique – a method that avoids manipulation of the tumor. Exclusive resection of the hilum/hilus as a limited resection should be used only in patients whose general or functional operability is limited. In hilar resections with left sided partial liver resection, a dissection directly adjacent to the tumor is required, to prepare the right hepatic artery and right branch of the portal vein, so that only minimal safety margins are achieved. The most radical surgical result (6) is achieved by using right trisectionectomy (e11) – resection of segments 4 to 8 including segment 1 and portal vein resection. This is the surgical method of choice in patients with Klatskin tumors, if the extent of the tumor and surgical-technical and functional conditions permit this (figure 1). A study reported by Kitagawa et al. allows the conclusion that regional lymph node metastases do not constitute a contraindication for resection and that regional lymph nodes should be dissected (14).
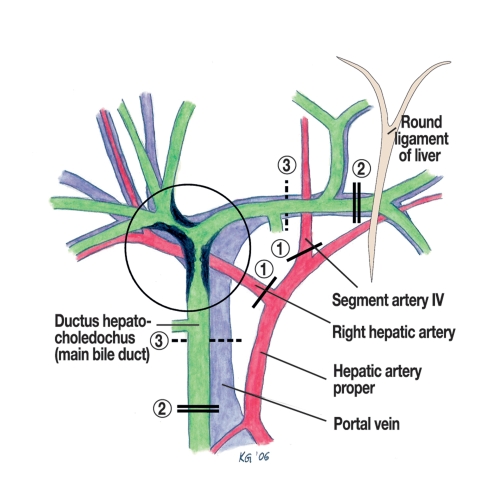
Figure 1.
Extent of dissection in the hepatic hilum in right trisectionectomy.
1 The right hepatic artery and the artery to segment IV are separated. 2 The ductus choledochus (main bile duct) is suprapancreatically cut and the left hepatic duct is separated in the umbilical fissure near the branch to segments 2 or 3. 3 Resecting the fork of the portal vein provides a wider safety margin to the tumor. The authors thank their colleague, Dr Gumpp, for preparing this graph.
Results after resection
Intraoperative mortality in studies with more than 40 patients, who were managed with extended resection, was between 7.5% and 18% (3–6, 10–12, 14–16, e7, e9, e12). Four newer publications have reported intraoperative death rates of 0% (7, 8, 17) and 1.3% (2). Liver failure is the most common cause of death.
As in other gastrointestinal cancers, R0 resection is indubitably the most important independent prognostic factor (2, 3, 5, 6, 10, 11, 15, 16). In most publications, grading is also an independent prognostic factor (4, 6, 8, 11, 12, 15, 16). Lymph node status correlates with the prognosis more obviously the higher the rate of positive lymph nodes and the more radical the lymphadenectomy (2, 4, 5, 7, 10). Five year survival in 11 relevant publications (including a minimum of 40 patients) with a study duration of at least 10 years, are 12.5% to 32.8% (table 1). These studies included many patients whose surgery did not conform to today’s radical principles. Newer studies with shorter investigative periods and results from patients who were exclusively radically operated and/or had R0 resection yielded five year survival rates of 35% to 44%, and even 72% (table 2). In spite of the different patient cohorts in these studies and administration of adjuvant radiochemotherapy in some, these more recent data hint at improved long-term results after resection of Klatskin tumors.
Table 1. Five year survival rates after resection, in studies of at least 10 years’ duration and including at least 40 patients *1.
| Authors | Year of Publication | Number of patients | Five year survival rate (in %) |
| Pichlmayr (3) | 1996 | 125 | 27.1 |
| Myazaki 9 | 1998 | 76 | 26 |
| Neuhaus (6) | 1999 | 95 | 22*2 |
| Kosuge (6) | 1999 | 65 | 32,8 |
| Launois (e37) | 1999 | 40 | 12.5*3 |
| Todoroki (11) | 2000 | 101 | 28*4 |
| Jarnagin (4) | 2001 | 80 | 27 |
| Ijiitsma (e12) | 2004 | 42 | 22 |
| Rea (21) | 2004 | 46 | 26 |
| Witzigmann (18) | 2006 | 60 | 22*5 |
| Baton (e13) | 2007 | 59 | 20*6 |
*1 Including operative mortality and R0, R1, and R2 resections;
*2 n = 15, liver transplantation with Kausch-Whipple operation
*3 n = 4, liver transplantation;
*4 postoperative radiotherapy in 28 patients;
*5 n = 1, liver transplantation
*6 n = 25, adjuvant therapy
Table 2. Five year survival rates after liver resection and/or R0 resection.
| Authors | Year of Publication | Number of patients | Five year survival rate (in %) |
| Neuhaus (19) | 2003 | 34 | 72*1 |
| Kawasaki (2) | 2003 | 79 | 40*2 |
| Seyama (7) | 2003 | 58 | 40*3 |
| Hemming (10) | 2005 | 53 | 35*4 |
| Sano (17) | 2006 | 102 | 44*5 |
| Lin (e38) | 2006 | 27 | 41 |
*1 in all patients, right trisectionectomy with resection of the portal vein and R0 resection, without intraoperative mortality;
*2 only patients with R0 resection;
*3 in all patients, extended hemihepatectomy right or left only one surgeon;
*4 postoperative radiochemotherapy after R1, R2 resection and in positive lymph nodes;
*5 n = 39, Intrahepatic cholangiocarcinoma with hilar infiltration
The role of liver transplantation
The oncological target variables are best achieved in liver transplantation by means of removing the entire bile duct system, the no-touch technique, and wide safety margins, Five year survival rates in these studies – which had small numbers of patients – were 17% to 36% and therefore no better than after resection (3, e14, e15). Even extended so-called cluster transplantations of the epigastric region and combined liver transplantation with partial pancreatoduodenectomy according to Kausch-Whipple did not yield more favorable results (6, e14). Over the past 10 years, the evaluation of liver transplantation in oncology has been possible to only a limited degree because of the lack of organs. Living organ donation between adults (20) and the excellent results of a neoadjuvant concept that is being realized at the Mayo Clinic in Rochester/USA (21) may result in an extended indication for liver transplantation in cholangiocarcinoma.
The so-called Mayo Clinic protocol entails external radiotherapy of up to 45 Gy plus bolus administration of 5-fluorouracil (5-FU), with subsequent transluminal iridium-192 brachytherapy at a dosis of 20 to 30 Gy. After radiochemotherapy has finished, staging laparotomy is undertaken. Patients without distant metastases or lymph node metastases are treated with 5-FU or capecitabine until they undergo transplantion. Using this therapeutic protocol has resulted in a five year survival rate of 82% in 38 patients (21). With regard to this study, the critical question is to what extent the excellent results are due to the particular patient selection and low tumor stages (all N0). In 2007, the German Medical Association decided a change in guidelines with regard to organ donations for liver transplantation for patients with cholangiocarcinoma according to article 16 of the transplantation law. This change allows liver transplantation in patients with cholangiocarcinoma whose tumor is technically non-resectable, the lesion has a diameter of less than 3 cm, no intrahepatic or extrahepatic distant metastases are found, and a biopsy or cytology has shown a neoplasm.
Neoadjuvant and adjuvant therapy
Radiotherapy, chemotherapy, radiochemotherapy
Because of the unsatisfactory long-term results even after radical surgery and because of the fact that 45% to 76% of recurrences develop locally and regionally (6, 8, 18, 21, e16), neoadjuvant and adjuvant therapeutic protocols are increasingly being developed. The so-called Mayo Clinic protocol with neoadjuvant radiochemotherapy before transplantation was discussed in the chapter on the role of liver transplantation. Radiochemotherapy before resection has been tested only in small case series (e17) and has not become established.
Two prospectively randomized studies with outdated protocols showed survival advantages neither for adjuvant radiotherapy alone (e18) nor for adjuvant chemotherapy (e19). By contrast, two retrospective analyses have shown a survival advantage for patients with adjuvant radiotherapy after resection compared with resection alone (11, e20). In summary, a clear clinical benefit for neoadjuvant and adjuvant therapeutic measures has thus far not been proved. However, patients with R1 or R2 resections and/or lymph node metastases should be included in adjuvant therapeutic studies.
Photodynamic therapy
In photodynamic therapy (PDT), an intravenously administered photosensitizing agent concentrates selectively in the tumor tissue. In Germany, two different fractions of hematoporphyrin derivatives (HpD) are licensed. Irradiation with non-thermic light of a low wavelength (630 nm) activates the photosensitizer, and the tumor tissue is selectively destroyed by apoptosis and necrosis (figures 2a and 2b). The authors have evaluated PDT as an adjuvant therapeutic measure in a prospective phase 2 study (22). Recurrence free one year survival was 83%. The hypothesis of this therapeutic concept is that selective destruction of subepithelial and fibromuscular tumor strands and dysplastic cells on the proximal margin will lower the rate of local recurrence. There are currently no long-term results regarding recurrence rates and survival of patients treated with neoadjuvant PDT and resection. Adjuvant PDT after R1/R2 resection is documented only in case reports (e21).
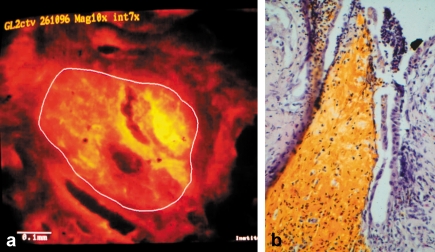
Figure 2.
Patient aged 72, with Klatskin tumor and neoadjuvant photodynamic therapy.
a) Fluorescence microscopy of a biliary duct biopsy after intravenous injection of the photosensitizing agent shows 2.4 times increased fluorescence of tumor cell nests compared with surrounding tissues (40 times enlarged). b) Representative histological section (hematoxylin-eosine staining; 40 times enlarged) of the cholangioma from resected tissue, 23 days after photodynamic therapy. Inflammatory cellular infiltrates without vital tumor cells are visible. The yellow-brown areas are residual degraded photofrin, which marks phototoxic tumor cell necrosis. The necrotic tumor cells are demarcated to a depth of 4 mm. From Berr F et al.: Neoadjuvant fotodynamic therapy before curative resection of proximal bile duct carcinoma J Hepatol 2000; 32: 352–7), with permission from Elsevier Publishers Philadelphia
Palliative treatment
The median survival of patients with non-resectable Klatskin tumors after palliative drainage is six to nine months. Most patients die from recurring bacterial cholangitis and/or liver failure. The aim of palliative treatment is an improvement in the patient’s quality of life by treating cholestasis and cholangitis, which secondarily prolongs survival.
Stenting
The palliative standard treatment is the implantation of biliary endoprostheses. Compared with the cheaper plastic stents, self expanding metal mesh stents have the advantage of a higher patency rate with lower subsequent rates of reintervention and rehospitalization (e22, e23). In patients whose life expectancy exceeds six months, metal stents are therefore recommended (e24). In most centers, endoscopic stent implantation is preferred to subcutaneous access. In difficult tumor stenoses, both methods can be combined in the so-called rendezvous method. Drainage of at least 25% of the liver volume is required to achieve a sufficient degree of palliation (e25). In unilateral drainage – in analogy to the preoperative situation – filling the non-drained biliary ducts with contrast medium must be avoided. Recent studies have shown that prior magnetic resonance cholangiography to enable targeted drainage of the most expanded biliary ducts can improve the results of stenting (e26, e27). In the literature, the differently defined success rates of endoscopic drainage range from 41% to 80% (e28–e31). The long-term success of stenting requires close monitoring of the patients, so as not to overlook latent biliary sepsis due to occlusion of the stent. In the context of patient monitoring, plastic prostheses should be routinely exchanged after three months (e24). There are no current randomized studies with regard to stent material, number, or placement.
Stenting and photodynamic therapy
A prospective, randomized study, in which the authors’ center is participating, has shown a significantly longer median survival (493 days versus 98 days) after combined PDT and stent implantation compared with stenting alone; further findings were more effective biliary drainage, and a better quality of life (23). A relevant criticism of this study is the lacking improvement in cholestasis after stenting alone and the associated shorter survival in the stent group. A significantly prolonged survival after PDT in non-resectable biliary duct cancers (21 months versus 7 months in the control group, p = 0.011) was shown in another prospective, randomized study (e32). Phototoxicity occurred in about 10% of patients (23). Implementing PDT into palliative management is an interesting research approach, which will need to be investigated in further phase 3 studies (figure 3). In England, a phase 3 study has been started in which German centers are participating (the Photostent-02 study). In future, improved efficacy of PDT is expected from using photosensitizers with a greater depth of penetration – for example, temoporphin.
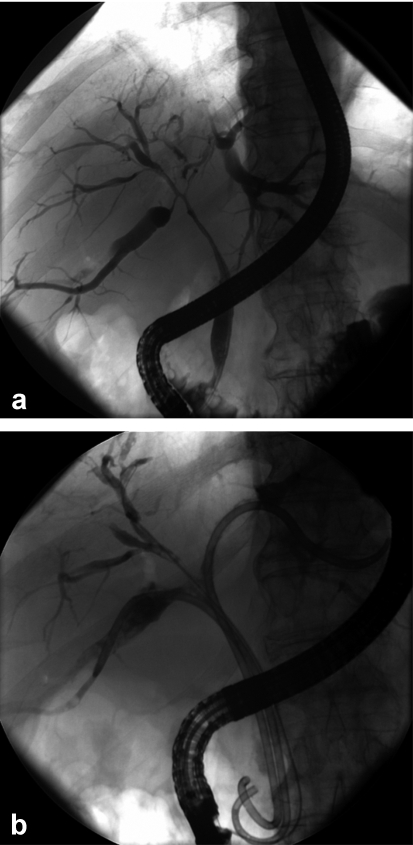
Figure 3.
Patient with inoperable hilar cholangiocarcionoma of the Bismuth-Corlette type IV.
a) Imaging of tumor extent with endoscopic retrograde cholangiography (ERC); b) reopened biliary ducts three months after photodynamic therapy (PDT) and stent implantation.
Surgical biliary drainage
If during exploratory laparotomy the tumor is found to be inoperable, intrahepatic cholangiojejunostomy using the segment III biliary duct or bilateral hepatojejunostomy may be considered in patients with insufficient endoscopic biliary drainage (7). However, because of the high success rate of endoscopic and percutaneous transhepatic stenting, these procedures are scarcely performed nowadays (3).
Radiotherapy, chemotherapy, radiochemotherapy
There are no relevant studies that document a survival advantage of external irradiation alone or of local brachytherapy with iridium-192. The situation is similar for palliative, combined radiochemotherapy, for which no indication exists owing to the small number of phase 1 and 2 studies with low patient numbers. Three prospective, randomized studies into chemotherapy in biliary duct cancers investigated combinations of 5-fluorouracil/leucovorin and etoposide (FELV), epirubicin, cisplatin and 5-FU (ECF), and 5-fluorouracil/folinic acid and cisplatin. Notable toxicity was found, and a tiny survival advantage (e33–e35). In several phase 2 studies, gemcitabine was found to be an effective monosubstance with regard to its efficacy and toxicity profile (24). Combinations of gemcitabine with cisplatin or oxaliplatin seem to be superior to monotherapy and are also the best tolerated treatment protocols (25) (e36). However, the low evidence level of the currently available data does not permit the recommendation of palliative chemotherapy outside studies.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241–256. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg. 1996;224:628–638. doi: 10.1097/00000658-199611000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. discussion 517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klempnauer J, Ridder GJ, von Wasielewski R, Werner M, Weimann A, Pichlmayr R. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol. 1997;15:947–954. doi: 10.1200/JCO.1997.15.3.947. [DOI] [PubMed] [Google Scholar]
- 6.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818. doi: 10.1097/00000658-199912000-00010. discussion 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo S, Hirano S, Ambo Y, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101. doi: 10.1097/01.sla.0000129491.43855.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klempnauer J, Ridder GJ, Werner M, Weimann A, Pichlmayr R. What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer. 1997;79:26–34. [PubMed] [Google Scholar]
- 10.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–699. doi: 10.1097/01.sla.0000160701.38945.82. discussion 699-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todoroki T, Kawamoto T, Koike N, et al. Radical resection of hilar bile duct carcinoma and predictors of survival. Br J Surg. 2000;87:306–313. doi: 10.1046/j.1365-2168.2000.01343.x. [DOI] [PubMed] [Google Scholar]
- 12.Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720–727. doi: 10.1097/01.sla.0000094437.68038.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitagawa Y, Nagino M, Kamiya J, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385–392. doi: 10.1097/00000658-200103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–671. doi: 10.1097/00000658-199911000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarnagin WR, Bowne W, Klimstra DS, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241:703–712. doi: 10.1097/01.sla.0000160817.94472.fd. discussion 712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240–247. doi: 10.1097/01.sla.0000217605.66519.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witzigmann H, Berr F, Ringel U, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to R1/R2 resection. Ann Surg. 2006;244:230–239. doi: 10.1097/01.sla.0000217639.10331.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuhaus P, Jonas S, Settmacher U, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg. 2003;388:194–200. doi: 10.1007/s00423-003-0383-5. [DOI] [PubMed] [Google Scholar]
- 20.Jonas S, Mittler J, Pascher A, et al. Extended indications in living-donor liver transplantation: bile duct cancer. Transplantation. 2005;80(1 Suppl):S101–S104. doi: 10.1097/01.tp.0000187106.29908.2b. [DOI] [PubMed] [Google Scholar]
- 21.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–458. doi: 10.1097/01.sla.0000179678.13285.fa. discussion 458-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiedmann M, Caca K, Berr F, et al. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer. 2003;97:2783–2790. doi: 10.1002/cncr.11401. [DOI] [PubMed] [Google Scholar]
- 23.Ortner MEJ, Caca K, Berr, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Kubicka S, Rudolph KL, Tietze MK, Lorenz M, Manns M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology. 2001;48:783–789. [PubMed] [Google Scholar]
- 25.Kim ST, Park JO, Lee J, et al. A phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106:1339–1346. doi: 10.1002/cncr.21741. [DOI] [PubMed] [Google Scholar]
- e1.Vogl TJ, Schwarz WO, Heller M, et al. Staging of Klatskin tumours (hilar cholangiocarcinomas): comparison of MR cholangiography, MR imaging, and endoscopic retrograde cholangiography. Eur Radiol. 2006;16:2317–2325. doi: 10.1007/s00330-005-0139-4. [DOI] [PubMed] [Google Scholar]
- e2.Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):VI1–VI9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e3.Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90–97. doi: 10.1016/j.gassur.2003.10.003. [DOI] [PubMed] [Google Scholar]
- e4.Reinhardt MJ, Strunk H, Gerhardt T, et al. Detection of Klatskin’s tumor in extrahepatic bile duct strictures using delayed 18F-FDG PET/CT: preliminary results for 22 patient studies. J Nucl Med. 2005;46:1158–1163. [PubMed] [Google Scholar]
- e5.Petrowsky H, Wildbrett P, Husarik DB, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- e6.Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675–1681. doi: 10.1111/j.1572-0241.2004.30281.x. [DOI] [PubMed] [Google Scholar]
- e7.Capussotti L, Muratore A, Polastri R, Ferrero A, Massucco P. Liver resection for hilar cholangiocarcinoma: in-hospital mortality and longterm survival. J Am Coll Surg. 2002;195:641–647. doi: 10.1016/s1072-7515(02)01481-3. [DOI] [PubMed] [Google Scholar]
- e8.Tsao JI, Nimura Y, Kamiya J, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232:166–174. doi: 10.1097/00000658-200008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Miyazaki M, Ito H, Nakagawa K, et al. Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery. 1998;123:131–136. [PubMed] [Google Scholar]
- e10.Sakamoto E, Nimura Y, Hayakawa N, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg. 1998;227:405–411. doi: 10.1097/00000658-199803000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Belghiti J, Clavien PA, Gadzijev E, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2:333–339. doi: 10.1080/136518202760378489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.IJitsma A, Appeltans BM, de Jong KP, Porte RJ, Peeters PM, Slooff MJ. Extrahepatic bile duct resection in combination with liver resection for hilar cholangiocarcinoma: a report of 42 cases. J Gastrointest Surg. 2004;8:686–694. doi: 10.1016/j.gassur.2004.04.006. [DOI] [PubMed] [Google Scholar]
- e13.Baton O, Azoulay D, Adam DV, Castaing D. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg. 2007;204:250–260. doi: 10.1016/j.jamcollsurg.2006.10.028. [DOI] [PubMed] [Google Scholar]
- e14.Iwatsuki S, Todo S, Marsh JW, et al. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187:358–364. doi: 10.1016/s1072-7515(98)00207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Robles R, Figueras J, Turrion VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–271. doi: 10.1097/01.sla.0000108702.45715.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e16.Mittal B, Deutsch M, Iwatsuki S. Primary cancers of extrahepatic biliary passages. Int J Radiat Oncol Biol Phys. 1985;11:849–854. doi: 10.1016/0360-3016(85)90320-7. [DOI] [PubMed] [Google Scholar]
- e17.McMasters KM, Tuttle TM, Leach SD, et al. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg. 1997;174:605–608. doi: 10.1016/s0002-9610(97)00203-1. discussion 608-9. [DOI] [PubMed] [Google Scholar]
- e18.Pitt HA, Nakeeb A, Abrams RA, et al. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995;221:788–797. doi: 10.1097/00000658-199506000-00017. discussion 797-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e19.Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- e20.Gerhards MF, van Gulik TM, Gonzalez Gonzalez D, Rauws EA, Gouma DJ. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27:173–179. doi: 10.1007/s00268-002-6434-1. [DOI] [PubMed] [Google Scholar]
- e21.Nanashima A, Yamaguchi H, Shibasaki S, et al. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: a preliminary study. J Gastroenterol. 2004;39:1095–1101. doi: 10.1007/s00535-004-1449-z. [DOI] [PubMed] [Google Scholar]
- e22.Deviere J, Baize M, de Toeuf J, Cremer M. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc. 1988;34:95–101. doi: 10.1016/s0016-5107(88)71271-7. [DOI] [PubMed] [Google Scholar]
- e23.Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy. 1993;25:213–218. doi: 10.1055/s-2007-1010295. [DOI] [PubMed] [Google Scholar]
- e24.Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1–7. doi: 10.1016/s0016-5107(98)70291-3. [DOI] [PubMed] [Google Scholar]
- e25.Dowsett JF, Vaira D, Hatfield AR, et al. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology. 1989;96:1180–1186. doi: 10.1016/0016-5085(89)91639-9. [DOI] [PubMed] [Google Scholar]
- e26.Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self-expanding metallic stents. Gastrointest Endosc. 2003;58:41–49. doi: 10.1067/mge.2003.292. [DOI] [PubMed] [Google Scholar]
- e27.Hintze RE, Abou-Rebyeh H, Adler A, Veltzke-Schlieker W, Felix R, Wiedenmann B. Magnetic resonance cholangiopancreatography-guided unilateral endoscopic stent placement for Klatskin tumors. Gastrointest Endosc. 2001;53:40–46. doi: 10.1067/mge.2001.111388. [DOI] [PubMed] [Google Scholar]
- e28.Liu CL, Lo CM, Lai EC, Fan ST. Endoscopic retrograde cholangiopancreatography and endoscopic endoprosthesis insertion in patients with Klatskin tumors. Arch Surg. 1998;133:293–296. doi: 10.1001/archsurg.133.3.293. [DOI] [PubMed] [Google Scholar]
- e29.Ducreux M, Liguory C, Lefebvre JF, et al. Management of malignant hilar biliary obstruction by endoscopy. Results and prognostic factors. Dig Dis Sci. 1992;37:778–783. doi: 10.1007/BF01296439. [DOI] [PubMed] [Google Scholar]
- e30.Polydorou AA, Chisholm EM, Romanos AA, et al. A comparison of right versus left hepatic duct endoprosthesis insertion in malignant hilar biliary obstruction. Endoscopy. 1989;21:266–271. doi: 10.1055/s-2007-1012966. [DOI] [PubMed] [Google Scholar]
- e31.Polydorou AA, Cairns SR, Dowsett JF, et al. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32:685–689. doi: 10.1136/gut.32.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e32.Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426–2430. doi: 10.1111/j.1572-0241.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- e33.Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- e34.Rao S, Cunningham D, Hawkins RE, et al. Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br J Cancer. 2005;92:1650–1654. doi: 10.1038/sj.bjc.6602576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e35.Ducreux M, Van Cutsem E, Van Laethem JL, et al. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer. 2005;41:398–403. doi: 10.1016/j.ejca.2004.10.026. [DOI] [PubMed] [Google Scholar]
- e36.André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- e37.Launois B, Terblanche J, Lakehal M, et al. Proximal bile duct cancer: high resectability rate and 5-year survival. Ann Surg. 1999;230:266–275. doi: 10.1097/00000658-199908000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e38.Liu CL, Fan ST, Lo CM, Tso WK, Lam CM, Wong J. Improved operative and survival outcomes of surgical treatment for hilar cholangiocarcinoma. Br J Surg. 2006;93:1488–1494. doi: 10.1002/bjs.5482. [DOI] [PubMed] [Google Scholar]