Abstract
The hypoxia-mediated response of tumours is a major determining factor in growth and metastasis. Understanding tumour biology under hypoxic conditions is crucial for the development of antiangiogenic therapy. Using one of the largest cohorts of rectal adenocarcinomas to date, this study investigated hypoxia-inducible factor-1α (HIF-1α) and HIF-2α protein expression in relation to rectal cancer recurrence and cancer-specific survival. Patients (n=90) who had undergone surgery for rectal adenocarcinoma, with no prior neoadjuvant therapy or metastatic disease, and for whom adequate follow-up data were available were selected. Microvessel density (MVD), HIF-1α and HIF-2α expressions were assessed immunohistologically with the CD34 antibody for vessel identification and the NB100-131B and NB100-132D3 antibodies for HIF-1α and HIF-2α, respectively. In a multifactorial analysis, results were correlated with tumour stage, recurrence rate and long-term survival. Microvessel density was higher across T and N stages (P<0.001) and associated with poor survival (hazard ratio (HR)=8.7, P<0.005) and decreased disease-free survival (HR=4.7, P<0.005). hypoxia-inducible factor-1α and -2α were expressed in >50% of rectal cancers (HIF-1α, 54%, 48/90; HIF-2α, 64%, 58/90). HIF-1α positivity was associated with both TNM stage (P<0.05) and vascular invasion (P<0.005). In contrast, no associations were shown between HIF-2α expression and any pathological features, and HIF-1α positivity had no effect on outcome. The study showed an independent association between HIF-1α expression and advanced TNM stage with poor outcome. Our results indicate that HIF-1α, but not HIF-2α, might be used as a marker of prognosis, in addition to methods currently used, to enhance patient management.
Keywords: rectal cancer, angiogenesis, hypoxia, HIF-1α and HIF-2α, microvessel density
The UK incidence of colorectal carcinoma is approximately 34 000 of which a third are rectal cancers (Kmietowicz, 2004). In the United States, colorectal cancer is the second most common cancer in women and the third commonest in men with over 148 000 new cases each year (American Cancer Society, 2006). After potentially curative surgical resection, local recurrence rates of rectal cancer have been reported from 4 to 32% (Sagar and Pemberton, 1996) with overall 5 year survival of less than 40% (Lindmark et al, 1994; Dahlberg et al, 1998).
Hypoxia is one of the key stimuli for the release of angiogenic factors necessary for angiogenesis and tumour growth. Tumours outgrow their local blood supply resulting in a hypoxic microenvironment. Although lesions 1–2 mm in diameter receive nutrients by cellular diffusion, an increase in tumour size beyond this requires rapid adaptation to hypoxia to prevent cessation of growth and necrosis (Pugh and Ratcliffe, 2003). A component of this adaptation, increased microvessel density (MVD), has been shown in polyps and colorectal cancers (Bossi et al, 1995) and linked to increasing transmural tumour extension (Choi et al, 1998; Rasheed et al, 2009). High colorectal cancer MVD has also been associated with an increased incidence of haematogenous metastases and poor prognosis (Tomisaki et al, 1996; Gulubova and Vlaykova, 2007; Rasheed et al, 2008).
Hypoxia-inducible factor (HIF) is a heterodimeric basic helix-loop-helix transcription factor involved in the regulation of cellular adaptation to hypoxia by upregulating genes directly responsible for angiogenesis. HIF consists of an α- and β-subunit (HIF-α and HIF-β/ARNT; Conway et al, 2001), which can be further subdivided into distinct isoforms, including HIF-1, HIF-2 and HIF-3. The first two of these exhibit conserved amino-acid sequences, but different mRNA expression patterns (Conway et al, 2001; Aprelikova et al, 2004). The HIF-β/ARNT subunit is constitutively expressed and involved in a number of non-hypoxia-related processes, whereas the α-subunit is the hypoxia-regulating component (Carmeliet et al, 1998; Talks et al, 2000). Both HIF-1α and HIF-2α are subject to post-transcriptional regulation mediated by the von Hippel–Lindau protein (VHL), but only HIF-1α regulates the induction of the glycolytic enzymes essential for cell proliferation and survival under hypoxic stress (Aprelikova et al, 2004). Under physiological normoxic conditions, HIF-α is propyl hydroxylated after activation of VHL and then prepared for degradation through mitochondrial ubiquitination. However, under hypoxic conditions, this degradation cannot take place due to a reduction in propyl hydroxylation, which leads to HIF-1α accumulation; levels of HIF-1α correlate with the oxygen status of the cell (Iyer et al, 1998; Blancher et al, 2000). HIF-α upregulation causes activation of target genes and expression of various growth factors, including VEGF, which induce endothelial cell proliferation and migration resulting in new vessel growth (Ratcliffe et al, 1997; Talks et al, 2000; Giatromanolaki and Harris, 2001).
Patients with mutations in the VHL tumour suppressor gene develop highly vascular tumours, including renal cell carcinomas, phaeochromocytomas and retinal haemangioblastomas (Semenza, 2001; Wykoff et al, 2001). Hypoxia-inducible factor has been shown to play a major role in the angiogenesis and growth of various tumours, including breast (Leek et al, 2002), bladder (Palit et al, 2005), renal (Klatte et al, 2007), pancreatic (Shibaji et al, 2003) and cervical (Birner et al, 2000) cancers. Indeed, overexpression of, in particular, HIF-1α, has been correlated with unfavourable prognosis in a number of malignancies (Theodoropoulos et al, 2005; Maynard and Ohh, 2007; Trastour et al, 2007).
In colorectal cancer, Jiang et al (2003a, 2003b) showed that HIF-1α mRNA was present in a significant number of colorectal adenoma and carcinoma specimens. They also found an increase in HIF-1α expression concordant with more advanced Dukes’ stage. Kuwai et al (2003) associated HIF-1α expression with tumour invasion, venous invasion, liver metastasis and vascular endothelial growth factor (VEGF) expression. Similarly, Lu et al (2006) found a strong association between HIF-1α expression, high VEGF expression, nodal metastasis and Dukes’ stage in rectal cancer and, in contrast to Kuwai et al (2003) showed an overall reduction in survival in patients with high HIF-1α expression. These findings were supported by an investigation in patients with locally advanced rectal cancer where an association was shown between HIF-1α expression, lymph node metastasis and poor outcome. Antiangiogenic drugs have been shown to be effective both experimentally and in clinical trials in various malignancies, including colorectal cancer. The monoclonal IgG1 antibody, Bevacizumab (Avastin), which binds VEGF preventing receptor binding, has been evaluated in the first- and second-line treatment in patients with colorectal cancer (Willett et al, 2004, 2006; Chua and Cunningham, 2006; Rasheed et al, 2008). Interestingly, novel antiangiogenic compounds have been developed that decrease HIF-1α and other HIFs (Mackay et al, 2005; Zhu et al, 2007; Singh et al, 2008).
We are evaluating whether detection of hypoxic factor expression in rectal cancer can be used to identify patient subgroups at increased risk of recurrences and poorer outcome. Recognising positive factor expression will identify patients most likely to benefit from specific antiangiogenic therapies, including HIF inhibitors. In this study, we investigate one of the largest cohorts of patients to date with rectal adenocarcinoma across all stages for HIF-1α and HIF-2α expressions and have appraised associations between expression and a number of clinicopathological variables.
Materials and methods
Patients with rectal adenocarcinoma operated on between 1991 and 1997 at St Mark's Hospital were selected for this study provided they had curable, local disease before surgery. Those with metastatic disease and/or had received neoadjuvant chemoradiotherapy were excluded. Complete follow-up data and sufficient paraffin-embedded tissue to stain with antibodies for HIF-1α, HIF-2α and CD34 (for MVD) were available in 90 cases. Normal proximal adjacent bowel obtained from 25 randomly selected patients was used as internal control tissue.
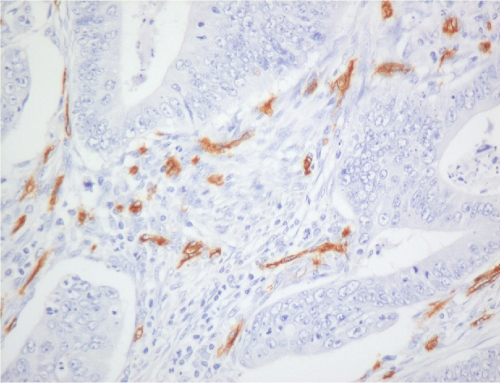
Tissue sections (4 μm) of archival paraffin-embedded block specimens were mounted onto slides. The antibodies used were for identification of CD34 blood vessels (Novus Biologicals, LLC, Littleton, CO, USA, QBend10, dilution 1 : 100, no pre-treatment), HIF-1α (NB 100-131B, Novus Biologicals, dilution 1 : 500, pressure cooked for 2 min at full pressure, pre-treated with citrate buffer, pH 6.0) and HIF-2α (NB100-132D3, Novus Biologicals, dilution 1 : 100, pressure cooked for 2 min at full pressure, pre-treated with citrate buffer, pH 6.0; Talks et al, 2000; Koukourakis et al, 2002; Leek et al, 2002). Immunohistochemistry was undertaken in a DAKO Immuno Autostainer Plus (Glostrup, Denmark) with ‘Chemmate Envision’ after standard dewaxing with xylene and ethanol and pre-treatment. Microvessel density was assessed by counting vessels per power field (23-mm eye piece width) at × 200 magnification following identification of the three most vessel-dense ‘hotspots’ at × 40 magnification (Figures 1 and 2; Bossi et al, 1995). Vessels were counted in the three most vessel-dense areas within the central part of the tumour and at the invasive tumour front. A mean score of all areas was calculated. The mean value of 60 vessels per high power field was used as the cutsoff between high and low MVDs.
Figure 1.
Microvessels stained with CD34 monoclonal antibody within rectal adenocarcinoma showing high vessel density ( × 200).
Figure 2.
Microvessels stained with CD34 monoclonal antibody within rectal adenocarcinoma showing low vessel density ( × 200).
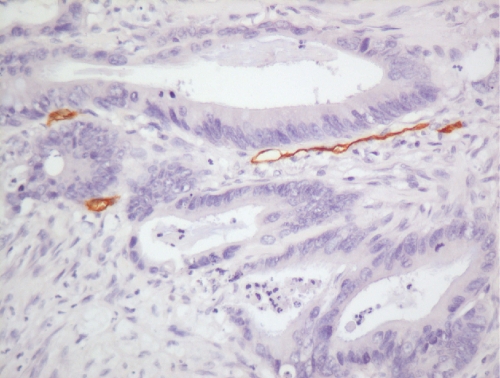
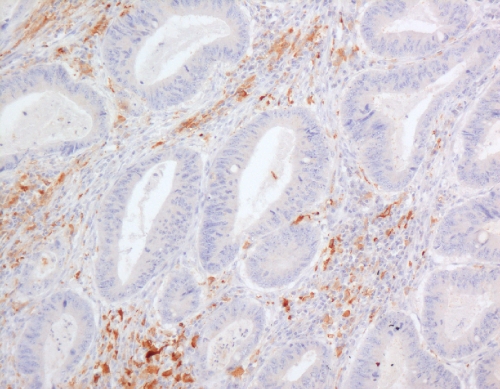
Hypoxia-inducible factor-1α and -2α (nuclear and cytoplasmic) stainings (Figures 3, 4 and 5) were reviewed by two experienced assessors (SR and TG) in terms of percentage of positive tumour cells. The predominant site of distribution, for example, intratumoral, invasive edge of tumour was noted. The following clinicopathological factors were analysed: age at the time of surgery; gender; surgical procedure categorised as abdominoperineal excision of rectum or anterior resection of rectum; tumour grading; staging according to the Dukes’ and tumour node metastasis (TNM) classifications; venous invasion (intramural and extramural); perineural invasion, number of regional lymph nodes and number of involved lymph nodes.
Figure 3.
Hypoxia-inducible factor-1α nuclear staining in rectal adenocarcinoma ( × 200).
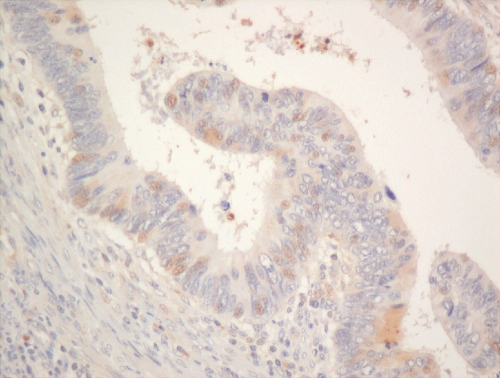
Figure 4.
Cancer and macrophage cytoplasmic staining for HIF-2α ( × 200).
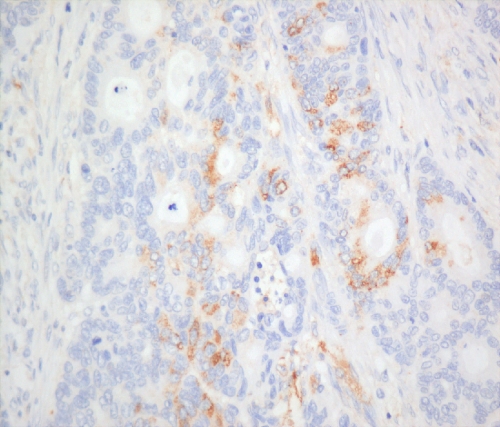
Figure 5.
Predominant macrophage staining for HIF-2α ( × 100).
Data sources and study approval
The study was approved by the North West London Hospitals’ Research and Development Committee and the Harrow Research and Ethics Committee.
Statistical analysis
Statistical analysis was carried out using SPSS for Windows version 14 (SPSS Inc, Chicago, IL, USA). The Fisher's exact test or Yates continuity corrected χ2-test was used for testing relationships between categorical variables where appropriate. Kaplan–Meier survival curves were constructed, and the log-rank test was used to determine statistical differences between the groups. A Cox proportional hazard model was used to assess the effects of patient and tumour variables on survival with P<0.05 taken as significant. Hazard ratios (HRs) were calculated to assess the independent relationship of variables with cancer-specific and disease-free survivals.
Results
Patients and clinicopathogical characteristics
A total of 90 patients comprising 56 males (62%), 34 females (38%) were studied with a mean age of 59 (standard deviation, s.d.±12) years and a median follow-up of 78 months (range: 2–228 months). There was no significant difference between male and female patients in terms of mean age or follow-up. Clinicopathological characteristics are described in Table 1.
Table 1. Clinicopathological features of a cohort of rectal cancer patients.
| Clinicopathological variables | N (%) |
|---|---|
| T stage | |
| T1 | 2 (2) |
| T2 | 16 (18) |
| T2 | 68 (76) |
| T3 | 4 (4) |
| T4 | 68 (76) |
| N stage | |
| N0 | 57 (63) |
| N1 | 21 (23) |
| N2 | 12 (13) |
| Operation | |
| Anterior resection | 75 (83) |
| Abdominoperineal excision | 15 (17 |
| Histogical grade | |
| Well differentiated | 4 (4) |
| Moderately differentiated | 73 (81) |
| Poorly differentiated | 13 (14) |
| Dukes’ stage | |
| Dukes’ A | 17 (19) |
| Dukes’ B | 40 (44) |
| Dukes’ C1 | 28 (31) |
| Dukes’ C2 | 5 (6) |
| TNM stage | |
| Stage I | 17 (19) |
| Stage II | 40 (44) |
| Stage III | 33 (37) |
| Vascular invasion | |
| Not seen | 63 (70) |
| Intramural | 11 (12) |
| Extramural | 16 (18) |
TNM=tumour node metastasis.
Microvessel density differs between tumour and normal tissue, across T and N stages and is associated with poor survival
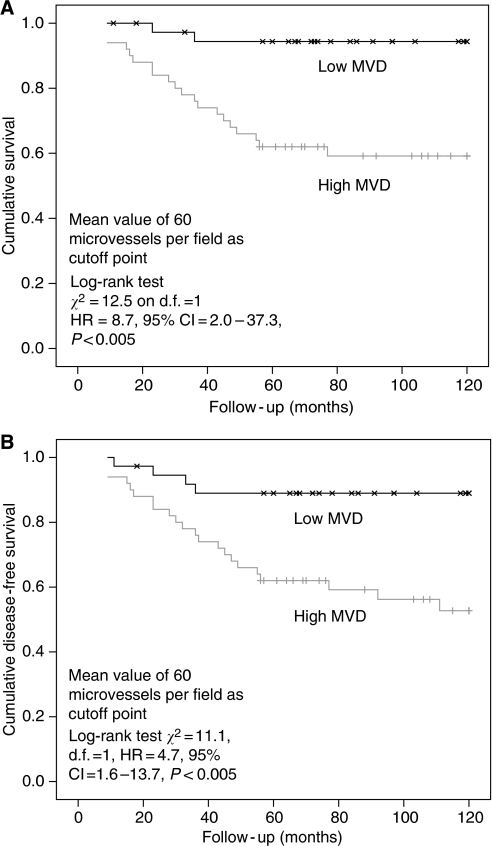
For the cases (n=90), the mean MVD was 63 vessels per high-power field (HPF; s.d.±20) and for controls (n=23), the MVD was 22 vessels per HPF. The MVD was significantly higher in tumour versus non-neoplastic mucosa (63 vs 22; P<0.01, Fisher's exact) and across the various T stages: T1=44 (s.d.±3); T2=45 (s.d.±13); T3=67 (s.d.±21) and T4=68 (s.d.±9; post hoc, ANOVA, P<0.001); and N stages: N0=56 (s.d.±16), N1=74 (s.d.±17), N2=76 (s.d.±26; post hoc, ANOVA, P<0.001). Survival analysis showed a significant difference in cancer-specific survival in the high versus low MVD groups divided by the median MVD of 60 vessels per HPF (Figure 6A; log-rank test χ2=12.5, d.f.=1 (degree of freedom); HR=8.7, 95% confidence interval (CI:) 2.0–37.3, P<0.005) and disease-free survival (Figure 6B; log-rank test χ2=11.1, d.f.=1, HR=4.7, 95% CI: 1.6–13.7, P<0.005).
Figure 6.
(A) Kaplan–Meier cancer-specific survival curve of patients grouped by high and low microvessel density (MVD). (B) Kaplan–Meier disease-free survival curve of patients grouped by high and low (MVD).
HIF-1α but not HIF-2α protein expression is associated with advanced pathological features and poor survival
Hypoxia-inducible factor-1α and -2α stains were present within the epithelial and stromal compartments in over half of rectal cancers (54%, 48/90 and 64%, 58/90, respectively). In contrast, HIF-1α stain was only present in a small fraction of control sections (8%, 2/25; HIF-1α staining in cases vs controls, P<0.005) and HIF-2α stain was not seen in any of the control sections. The following features were observed to be associated with HIF-1α positivity (Table 2): lymph node stage (P<0.02); TNM stage (P<0.05); and vascular invasion (P<0.005).
Table 2. Patient and tumour characteristics and HIF-1α staining.
|
HIF-1α, number of patients (%)
|
|||
|---|---|---|---|
| Negative | Positive | P-value | |
| Gender | P=0.35 | ||
| Male | 24 (43) | 32 (57) | |
| Female | 18 (53) | 16 (47) | |
| T stage | P=0.48 | ||
| T1/2 | 9 (50) | 9 (50) | |
| T3/4 | 33 (46) | 39 (54) | |
| N stage | P<0.02* | ||
| N0 | 32 (56) | 25 (44) | |
| N1 | 6 (29) | 15 (71) | |
| N2 | 4 (33) | 8 (67) | |
| Differentiation | P=0.58 | ||
| Well | 3 (75) | 1 (25) | |
| Moderate | 33 (45) | 40 (55) | |
| Poor | 6 (46) | 7 (54) | |
| Dukes’ stage | P=0.05 | ||
| Dukes’ A | 9 (53) | 8 (47) | |
| Dukes’ B | 23 (58) | 17 (42) | |
| Dukes C1 | 8 (29) | 20 (71) | |
| Dukes’ C2 | 2 (40) | 3 (60) | |
| Dukes’ stages combined | P<0.02* | ||
| A/B | 32 | 25 | |
| C | 10 | 23 | |
| TNM stage | P<0.05* | ||
| Stage I | 9 (53) | 8 (47) | |
| Stage II | 23 (58) | 17 (42) | |
| Stage III | 10 (30) | 23 (70) | |
| Vascular invasion | P<0.005* | ||
| Absent | 35 (56) | 28 (44) | |
| Intramural | 4 (36) | 7 (64) | |
| Extramural | 3 (19) | 13 (81) | |
| Microvessel density^ | P=0.32 | ||
| <60 vessels | 20 (53) | 18 (47) | |
| ⩾60 vessels | 21 (42) | 29 (58) | |
HIF=hypoxia-inducible factor; TNM=tumour node metastasis.
*Statistically significant at 5% level.
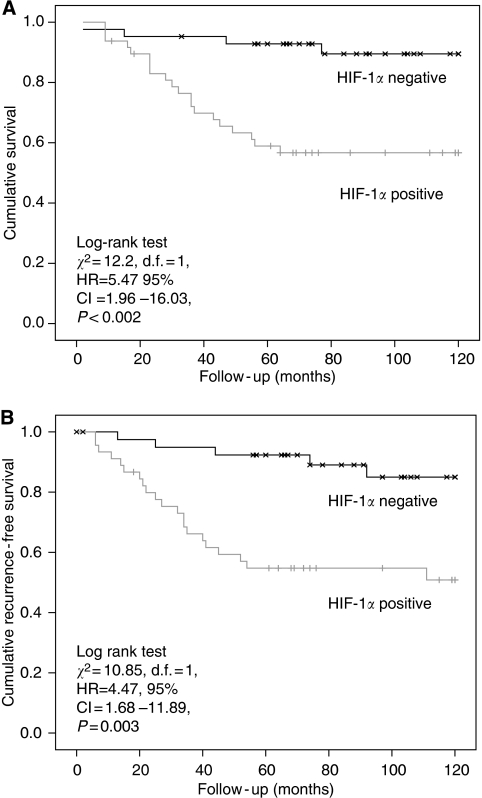
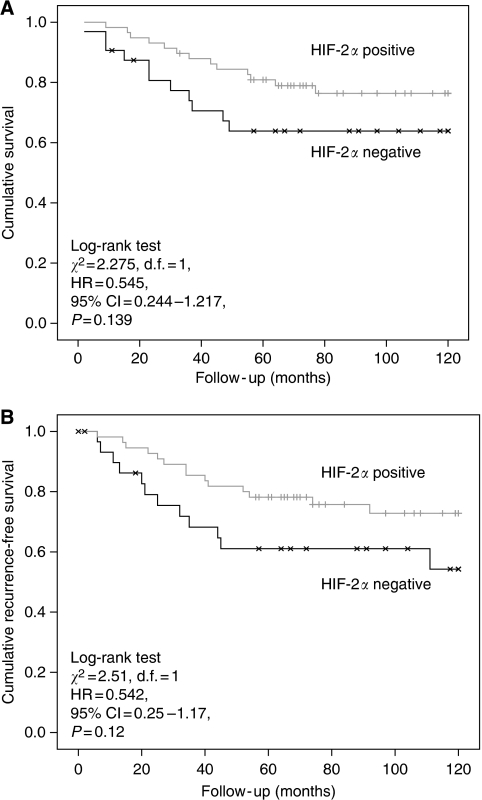
The association between Dukes’ stage and HIF-1α positivity almost reached statistical significance (P=0.05), although combining Dukes’ A and B cancers together and C1 with C2 cancers resulted in a statistically significant difference in HIF-1α positivity between Dukes’ A/B and C (Dukes’ A/B, 44% positive vs 56% negative; Dukes’ C, 70% positive vs 30% negative; P<0.02). In contrast to results for HIF-1α, there were no observed associations between HIF-2α positivity and any of the pathological features under investigation (Table 3). Cox regression univariate analysis revealed a significant effect of HIF-1α positivity on cancer-specific survival (Figure 7A; log-rank test χ2=12.2, d.f.=1, HR=5.47 95% CI: 1.96–16.03, P<0.002) and disease-free survival (Figure 7B; log-rank test χ2=10.85, d.f.=1, HR=4.47, 95% CI: 1.68–11.89, P=0.003). On multivariate analysis, HIF-1α positivity retained a significant effect on cancer-specific survival independent of TNM stage and vascular invasion (HR=4.11, 95% CI=1.37–12.35, P=0.012; Table 4). Again, in direct contrast, there was no effect of HIF-2α positivity on cancer-specific survival (log-rank test χ2=2.275, d.f.=1, HR=0.545, 95% CI: 0.244–1.217, P=0.139; Figure 8A), although, contrary to HIF-1α, a trend towards decreased recurrence (log-rank test χ2=2.51, d.f.=1, HR=0.542, 95% CI=0.25–1.17, P=0.12) was observed for HIF-2α positivity (Figure 8B).
Table 3. Patient and tumour characteristics and HIF-2α staining.
|
HIF-2α, number of patients (%)
|
|||
|---|---|---|---|
| Negative | Positive | P-value | |
| Gender | P=0.68 | ||
| Male | 19 (34) | 37 (66) | |
| Female | 13 (38) | 21 (62) | |
| T stage | P=0.53 | ||
| T1/2 | 6 (33) | 12 (67) | |
| T3/4 | 26 (36) | 46 (64) | |
| N stage | P=0.10 | ||
| N0 | 17 (30) | 40 (70) | |
| N1 | 8 (38) | 13 (62) | |
| N2 | 7 (58) | 5 (42) | |
| Differentiation | P=0.94 | ||
| Well | 2 (50) | 2 (50) | |
| Moderate | 25 (34) | 48 (66) | |
| Poor | 5 (39) | 8 (61) | |
| Dukes’ stage | P=0.25 | ||
| Dukes’ A | 6 (35) | 11 (65) | |
| Dukes’ B | 11 (28) | 29 (72) | |
| Dukes C1 | 12 (43) | 16 (57) | |
| Dukes’ C2 | 3 (60) | 2 (40) | |
| TNM stage | P=0.28 | ||
| Stage I | 6 (35) | 11 (65) | |
| Stage II | 11 (28) | 29 (72) | |
| Stage III | 15 (46) | 18 (54) | |
| Vascular invasion | P=0.74 | ||
| Absent | 22 (35) | 41 (65) | |
| Intramural | 3 (27) | 8 (73) | |
| Extramural | 7 (44) | 9 (56) | |
| Microvessel density | P=0.86 | ||
| <60 vessels | 13 (34) | 25 (66) | |
| ⩾60 vessels | 18 (36) | 32 (64) | |
HIF=hypoxia-inducible factor; TNM=tumour node metastasis.
Figure 7.
(A) Kaplan–Meier cancer-specific survival curve in rectal cancer patients grouped by HIF-1α positivity. (B) Kaplan–Meier disease-free survival curve in rectal cancer patients grouped by HIF-1α positivity.
Table 4. Multivariate analysis of survival for HIF-1α.
| HR | 95% CI | P-value | |
|---|---|---|---|
| HIF-1α | |||
| Noa | 1 | ||
| Yes | 4.108 | 1.366-12.352 | 0.012 |
| Vascular invasion | |||
| Noa | 1 | ||
| Intramural | 1.483 | 0.400-5.495 | 0.555 |
| Extramural | 2.284 | 0.917-5.688 | 0.016 |
| TNM stage | |||
| Ia | 1 | ||
| II | 1.724 | 0.197-15.069 | 0.622 |
| III | 10.048 | 1.302-77.553 | 0.027 |
CI=confidence interval; HIF=hypoxia-inducible factor; HR=hazard ratio; TNM=tumour node metastasis.
Reference values.
Figure 8.
(A) Kaplan–Meier cancer-specific survival curve in rectal cancer patients grouped by HIF-2α positivity. (B) Kaplan–Meier recurrence-free survival curve in rectal cancer patients grouped by HIF-2α positivity.
Discussion
Our study comprises one of the largest groups of rectal cancer cases across all stages and we have determined that both HIF-1α and HIF-2α were widely expressed in rectal cancer compared with normal large bowel mucosa (controls). Over half of all cases showed HIF-1α expression (54%) and nearly two-thirds were found to have positive HIF-2α expression (64%). As discussed earlier by Talks et al (2000), as HIF-α-subunits are affected by cellular oxygenation, it is uncertain as to whether the findings in paraffin-embedded-fixed tissue accurately reflect cellular status in vivo. However, our observed findings do show clear differences between the two groups and, as cases and controls have been processed in a consistent manner, this does represent a genuine finding of increased hypoxic factor expression in rectal cancer cases when compared with normal rectal tissue.
We have shown an association between increasing depth of tumour invasion and MVD and have shown a relationship between increased MVD and poor prognosis, a finding consistent with earlier studies investigating colorectal cancers (Tomisaki et al, 1996; Choi et al, 1998). In a large series of 97 rectal cancer-specific patients, Chen et al (2004) reported that cases with a higher MVD were more likely to develop tumour recurrence or metastasis. Tomisaki et al (1996), in assessing an association correlation between MVD and liver metastasis, found a mean MVD of 64 in colorectal cancer patients with hepatic metastasis compared with a mean MVD of 52 in patients without metastasis (P=0.001). In addition, the relationship in colorectal cancer between MVD and prognosis has also been described in most studies indicating a positive correlation between high MVD and poor outcome (Vermeulen et al, 1999; Li et al, 2003). We have shown a direct relationship between increasing MVD and cancer-specific survival. In our study, an MVD of above the cutoff of 60 microvessels per HPF was a clear determinant of poor outcome. As such, we would propose that high MVD (above 60 HPF) can be used as a factor to determine surveillance and adjuvant therapy. As yet, to our knowledge, the effect of specific chemotherapeutic or antiangiogenic agents has not been assessed in a randomised controlled trial with groups determined by MVD.
Our data show an association between HIF-1α expression and TNM stage, nodal stage, vascular invasion and Dukes’ stage, on subdivision between node-positive and -negative cases, suggesting a direct role for HIF-1α in disease progression. Interestingly, we found no association between HIF-1α and MVD in our study, and either more numbers may be needed to show an association or it may suggest an alternative mechanism of action for this hypoxic-inducible factor. We have also shown a strong correlation between HIF-1α expression and cancer-specific mortality and tumour recurrence suggesting that this factor is an integral component of rectal cancer growth. Perhaps HIF is regulated by oncogenes in addition to acting through the pathway regulated by hypoxia. For example, interactions of HIF1 with β-catenin in colon cancer cell lines have been reported (Kaidi et al, 2007). Also, it is possible that as both mature and immature vessels are being assessed, this may reflect effects of other growth factors and vascular differentiation.
Curiously, although HIF-2α is considered to act as an oncogene, we did not find any association between the various pathological factors studied and protein expression of this gene, nor did we find an association between HIF-2α expression and cancer-specific survival or recurrence. We did not show a relationship between HIF-2α and MVD, which is also surprising. There did appear to be a trend towards a reduction in tumour recurrence with HIF-2α expression, although this did not reach statistical significance. Both Jiang et al (2003b), in colorectal cancer, and Lu et al (2006), in rectal cancer, showed increased positivity for HIF-1α expression with increasing Dukes’ stage. The latter group also found an overall reduction in survival in patients with high HIF-1α expression. Theodoropoulos et al (2006) showed a significant association between HIF-1α and lymph node metastasis, low rectal location and advanced tumour grade. Although HIF-1α expression, infiltrative tumour growth pattern, positive lymph node status and VEGF upregulation were associated with decreased disease-free and overall survival, multivariate analysis only revealed high HIF-1α reactivity and lymph node positivity as predictors of poor outcome. This is the more notable of the earlier rectal cancer studies due to the higher numbers, and it is interesting to note the similarities in terms of percentage of expression in these studies to our own.
It is quite likely that numerous factors act separately to cause tumour growth and spread, and pathways may interact in an as yet undefined manner. A number of these factors have been described to be hypoxia and non-hypoxia dependent, including VHL, COX-2, CA-9, CHOP and ATF4, among many others (Yoshimura et al, 2004; Kivela et al, 2005; Carracedo et al, 2006; Cleven et al, 2007).
References
- American Cancer Society (2006) Cancer Facts and Figures. American Cancer Society: Atlanta [Google Scholar]
- Aprelikova O, Chandramouli GV, Wood M, Vasselli JR, Riss J, Maranchie JK, Linehan WM, Barrett JC (2004) Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J Cell Biochem 92: 491–501 [DOI] [PubMed] [Google Scholar]
- Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G (2000) Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res 60: 4693–4696 [PubMed] [Google Scholar]
- Blancher C, Moore JW, Talks KL, Houlbrook S, Harris AL (2000) Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res 60: 7106–7113 [PubMed] [Google Scholar]
- Bossi P, Viale G, Lee AK, Alfano R, Coggi G, Bosari S (1995) Angiogenesis in colorectal tumors: microvessel quantitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res 55: 5049–5053 [PubMed] [Google Scholar]
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E, Keshet E (1998) Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394: 485–490 [DOI] [PubMed] [Google Scholar]
- Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, Malicet C, Villuendas R, Gironella M, Gonzalez-Feria L, Piris MA, Iovanna JL, Guzman M, Velasco G (2006) The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell 9: 301–312 [DOI] [PubMed] [Google Scholar]
- Chen YB, Wan DS, Zhan YQ, Zhou ZW, Li W, Chen G (2004) Correlation of tumor microvessel density to metastasis and recurrence of rectal cancer. Ai Zheng 23: 1203–1206 [PubMed] [Google Scholar]
- Choi HJ, Hyun MS, Jung GJ, Kim SS, Hong SH (1998) Tumor angiogenesis as a prognostic predictor in colorectal carcinoma with special reference to mode of metastasis and recurrence. Oncology 55: 575–581 [DOI] [PubMed] [Google Scholar]
- Chua YJ, Cunningham D (2006) Emerging therapies for rectal cancer. Colorectal Dis 8(Suppl 3): 18–20 [DOI] [PubMed] [Google Scholar]
- Cleven AH, van Engeland M, Wouters BG, de Bruine AP (2007) Stromal expression of hypoxia-regulated proteins is an adverse prognostic factor in colorectal carcinomas. Cell Oncol 29: 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway EM, Collen D, Carmeliet P (2001) Molecular mechanisms of blood vessel growth. Cardiovasc Res 49: 507–521 [DOI] [PubMed] [Google Scholar]
- Dahlberg M, Pahlman L, Bergstrom R, Glimelius B (1998) Improved survival in patients with rectal cancer: a population-based register study. Br J Surg 85: 515–520 [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Harris AL (2001) Tumour hypoxia, hypoxia signaling pathways and hypoxia inducible factor expression in human cancer. Anticancer Res 21: 4317–4324 [PubMed] [Google Scholar]
- Gulubova M, Vlaykova T (2007) Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol [E-pub ahead of print July 2007] [DOI] [PubMed]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12: 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CQ, Liu ZS, Qian Q, He YM, Yuan YF, Ai ZL (2003a) [Relationship of hypoxia-inducible factor 1 alpha (HIF-1alpha) gene expression with vascular endothelial growth factor (VEGF) and microvessel density (MVD) in human colorectal adenoma and adenocarcinoma]. Ai Zheng 22: 1170–1174 [PubMed] [Google Scholar]
- Jiang YA, Fan LF, Jiang CQ, Zhang YY, Luo HS, Tang ZJ, Xia D, Wang M (2003b) Expression and significance of PTEN, hypoxia-inducible factor-1 alpha in colorectal adenoma and adenocarcinoma. World J Gastroenterol 9: 491–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidi A, Williams AC, Paraskeva C (2007) Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol 9: 210–217 [DOI] [PubMed] [Google Scholar]
- Kivela AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Bartosova M, Mucha V, Novak M, Waheed A, Sly WS, Rajaniemi H, Pastorekova S, Pastorek J (2005) Expression of von Hippel-Lindau tumor suppressor and tumor-associated carbonic anhydrases IX and XII in normal and neoplastic colorectal mucosa. World J Gastroenterol 11: 2616–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatte T, Seligson DB, Riggs SB, Leppert JT, Berkman MK, Kleid MD, Yu H, Kabbinavar FF, Pantuck AJ, Belldegrun AS (2007) Hypoxia-inducible factor 1 alpha in clear cell renal cell carcinoma. Clin Cancer Res 13: 7388–7393 [DOI] [PubMed] [Google Scholar]
- Kmietowicz Z (2004) British cancer death rates fell by 12% between 1972 and 2002. Bmj 328: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Simopoulos C, Turley H, Talks K, Gatter KC, Harris AL (2002) Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys 53: 1192–1202 [DOI] [PubMed] [Google Scholar]
- Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, Ito M, Chayama K (2003) Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer 105: 176–181 [DOI] [PubMed] [Google Scholar]
- Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC, Harris AL (2002) Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in Human breast cancer. Cancer Res 62: 1326–1329 [PubMed] [Google Scholar]
- Li C, Gardy R, Seon BK, Duff SE, Abdalla S, Renehan A, O’Dwyer ST, Haboubi N, Kumar S (2003) Both high intratumoral microvessel density determined using CD105 antibody and elevated plasma levels of CD105 in colorectal cancer patients correlate with poor prognosis. Br J Cancer 88: 1424–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark G, Gerdin B, Pahlman L, Bergstrom R, Glimelius B (1994) Prognostic predictors in colorectal cancer. Dis Colon Rectum 37: 1219–1227 [DOI] [PubMed] [Google Scholar]
- Lu XG, Xing CG, Feng YZ, Chen J, Deng C (2006) Clinical significance of immunohistochemical expression of hypoxia-inducible factor-1alpha as a prognostic marker in rectal adenocarcinoma. Clin Colorectal Cancer 5: 350–353 [DOI] [PubMed] [Google Scholar]
- Mackay H, Hedley D, Major P, Townsley C, Mackenzie M, Vincent M, Degendorfer P, Tsao MS, Nicklee T, Birle D, Wright J, Siu L, Moore M, Oza A (2005) A phase II trial with pharmacodynamic endpoints of the proteasome inhibitor bortezomib in patients with metastatic colorectal cancer. Clin Cancer Res 11: 5526–5533 [DOI] [PubMed] [Google Scholar]
- Maynard MA, Ohh M (2007) The role of hypoxia-inducible factors in cancer. Cell Mol Life Sci 64: 2170–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palit V, Phillips RM, Puri R, Shah T, Bibby MC (2005) Expression of HIF-1alpha and Glut-1 in human bladder cancer. Oncol Rep 14: 909–913 [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9: 677–684 [DOI] [PubMed] [Google Scholar]
- Rasheed S, Harris AL, Tekkis PP, Turley H, Silver A, McDonald PJ, Talbot IC, Glynne-Jones R, Northover JM, Guenther T (2009) Assessment of microvessel density and carbonic anhydrase-9 (CA-9) expression in rectal cancer. Pathol Res Pract 205(1): 1–9 [DOI] [PubMed] [Google Scholar]
- Rasheed S, McDonald PJ, Northover JM, Guenther T (2008) Angiogenesis and hypoxic factors in colorectal cancer. Pathol Res Pract 204: 501–510 [DOI] [PubMed] [Google Scholar]
- Ratcliffe PJ, Ebert BL, Firth JD, Gleadle JM, Maxwell PH, Nagao M, O’Rourke JF, Pugh CW, Wood SM (1997) Oxygen regulated gene expression: erythropoietin as a model system. Kidney Int 51: 514–526 [DOI] [PubMed] [Google Scholar]
- Sagar P, Pemberton J (1996) Surgical management of locally recurrent rectal cancer. Br J Surg 83: 293–304 [DOI] [PubMed] [Google Scholar]
- Semenza GL (2001) Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr Res 49: 614–617 [DOI] [PubMed] [Google Scholar]
- Shibaji T, Nagao M, Ikeda N, Kanehiro H, Hisanaga M, Ko S, Fukumoto A, Nakajima Y (2003) Prognostic significance of HIF-1 alpha overexpression in human pancreatic cancer. Anticancer Res 23: 4721–4727 [PubMed] [Google Scholar]
- Singh RP, Gu M, Agarwal R (2008) Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res 68: 2043–2050 [DOI] [PubMed] [Google Scholar]
- Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 157: 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoropoulos GE, Lazaris AC, Theodoropoulos VE, Papatheodosiou K, Gazouli M, Bramis J, Patsouris E, Panoussopoulos D (2006) Hypoxia, angiogenesis and apoptosis markers in locally advanced rectal cancer. Int J Colorectal Dis 21: 248–257 [DOI] [PubMed] [Google Scholar]
- Theodoropoulos VE, Lazaris AC, Kastriotis I, Spiliadi C, Theodoropoulos GE, Tsoukala V, Patsouris E, Sofras F (2005) Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU Int 95: 425–431 [DOI] [PubMed] [Google Scholar]
- Tomisaki S, Ohno S, Ichiyoshi Y, Kuwano H, Maehara Y, Sugimachi K (1996) Microvessel quantification and its possible relation with liver metastasis in colorectal cancer. Cancer 77: 1722–1728 [DOI] [PubMed] [Google Scholar]
- Trastour C, Benizri E, Ettore F, Ramaioli A, Chamorey E, Pouyssegur J, Berra E (2007) HIF-1alpha and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int J Cancer 120: 1451–1458 [DOI] [PubMed] [Google Scholar]
- Vermeulen PB, Van den Eynden GG, Huget P, Goovaerts G, Weyler J, Lardon F, Van Marck E, Hubens G, Dirix LY (1999) Prospective study of intratumoral microvessel density, p53 expression and survival in colorectal cancer. Br J Cancer 79: 316–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10: 145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett CG, Kozin SV, Duda DG, di Tomaso E, Kozak KR, Boucher Y, Jain RK (2006) Combined vascular endothelial growth factor-targeted therapy and radiotherapy for rectal cancer: theory and clinical practice. Semin Oncol 33: S35–S40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff CC, Pugh CW, Harris AL, Maxwell PH, Ratcliffe PJ (2001) The HIF pathway: implications for patterns of gene expression in cancer. Novartis Found Symp 240: 212–225; discussion 225–231) [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Dhar DK, Kohno H, Kubota H, Fujii T, Ueda S, Kinugasa S, Tachibana M, Nagasue N (2004) Prognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res 10: 8554–8560 [DOI] [PubMed] [Google Scholar]
- Zhu B, Lu L, Cai W, Yang X, Li C, Yang Z, Zhan W, Ma JX, Gao G (2007) Kallikrein-binding protein inhibits growth of gastric carcinoma by reducing vascular endothelial growth factor production and angiogenesis. Mol Cancer Ther 6: 3297–3306 [DOI] [PubMed] [Google Scholar]