Summary
Leukocyte activity is controlled by numerous interactions between membrane receptors and ligands on the cell surface. These interactions are of low affinity making detection difficult. We developed a sensitive assay that could readily detect extremely weak interactions such as that between CD200 and the activating receptor CD200RLa (Kd>500μM) at the protein level. We used the new technology to screen for interaction of inhibitory receptors for collagens. We confirmed that both human and mouse leukocyte-associated immunoglobulin-like receptor (LAIR-1) and in addition the related inhibitory leukocyte immunoglobulin-like receptor subfamily B member 4 (Lilrb4, CD85K, Gp49B), bound collagen specifically whereas other cell surface proteins gave no binding. The monomeric affinities of the interactions were then determined to allow comparison with other leukocyte interactions and indicate conditions when this interaction might lead to inhibitory signals.
Keywords: inhibitory receptor, collagen, LAIR-1, surface interaction and immunoregulation
Introduction
Leukocyte activity is controlled by the interactions of its surface proteins with both soluble factors such as cytokines and chemokines, and ligands on other cells. Thus identification of ligands is essential in understanding the role of the receptors in regulation. However, the interactions of cell surface proteins with other cell surfaces tend to be of very low affinity when determined using isolated recombinant proteins corresponding to the extracellular regions of the proteins. For instance typical affinities for such interactions are around 2 μM (CTLA4/CD80 Kd = 2 μM, SIRPα/CD47 Kd = 2 μM, CD2/CD58 Kd = 10 μM, CD200/CD200R Kd = 2 μM) [1-6] with fast off rates usually giving half lives of the order of one second. Some highly functionally significant interactions have even lower affinities such as for CD8αα to MHC class I molecule HLA-A2 (Kd ~ 200 μm) [3]. Thus in order to detect binding to cells, recombinant proteins corresponding to the extracellular regions are often made multivalent for example by fusing to the Fc regions of IgG giving divalent protein, the Fc from IgM giving decavalent protein or by attaching the protein onto beads making a very avid binding reagent (discussed in [7]). These proteins, of various valencies, could be adapted and applied to screen for cells expressing receptors by flow cytometry and protein microarray [5, 8, 9]. With the ease of producing panels of recombinant proteins a powerful way of identifying new interactions is by screening at the protein level as illustrated by Eaton and co-workers using libraries of Fc fusion proteins [10].
In the present study we developed a highly sensitive bead binding assay capable of detecting exceptionally weak interactions using immobilised proteins and surface plasmon resonance (SPR). This is the combination of a conventional binding assay using SPR with the screening reagent – multivalent nanoparticles – taking advantage of the avidity of both reactants. Using the new assay we were able to examine the weak interaction between collagen and leukocyte membrane proteins. The recent identification of a motif in collagen as a ligand for the inhibitory leukocyte-associated immunoglobulin-like receptor (LAIR-1) is intriguing as it suggests that collagens could have immunoregulatory roles by interacting with this inhibitory receptor [11, 12]. LAIR-1 is composed of a single immunoglobulin superfamily (IgSF) extracellular domain, a transmembrane region and a cytoplasmic region containing ITIM (immuno-receptor tyrosine-based inhibitory motifs). LAIR-1 is expressed on the majority of peripheral blood mononuclear cells and thymocytes in mice and humans [13]. Collagens are the most abundant proteins in the body with major roles in the extracellular matrix of mammalian cells, either as network-forming or fibrillar molecules. They are intimately involved in cell adhesion, migration and differentiation [14, 15]. In humans, numerous diseases result from mutations in collagen genes [16]. In addition to structural roles, collagens interact with many cell membrane proteins, such as during cell adhesion to collagen in tissues, cell migration through collagen-rich stroma in normal differentiation and abnormal metastasis and in cell interactions in hemostasis [17, 18]. Fewer interactions have been reported between leukocytes and collagen as might be expected given the requirement of leukocytes to respond to the invasion by pathogens such as bacteria and viruses. In this paper, we examine in detail the interaction of mouse and human LAIR-1 with collagen and test the possibility that other ligands might exist for LAIR-1 and be present in the family of CD85 proteins using the new assay.
Results
Detection of low affinity interactions with a new assay using multivalent beads and BIAcore™ SPR analysis
In order to increase the sensitivity of detection of weak interactions such as those between leukocyte membrane proteins, the previously used bead assay was modified [7]. Recombinant proteins corresponding to the extracellular regions of the leukocyte membrane proteins were transiently expressed and immobilized via a tag [19] that can be biotinylated, to beads coated with avidin to provide a highly avid binding reagent that enabled the recognition of new interactions by binding to cells [9, 20]. We now combined these beads with SPR detection to identify interactions with leukocyte recombinant proteins. The assay was established using a known interaction at the limit of detection using monomeric recombinant proteins namely the interaction between mouse CD200 and CD200RLa (CD200R4) that has a Kd greater than 500 μM [21]. These and subsequent experiments were carried out at 37°C to exclude any effects due to the use of non-physiological temperature. Fig. 1 shows mCD200/rCD4d3+4 coupled beads passed over mCD200R (moderate affinity), mCD200RLa (very low affinity) and mCD200RLb (no binding) immobilised on the chip. Strong binding and roughly equivalent binding was obtained from both mCD200R (Kd ~ 4 μM) and mCD200RLa (Kd >500 μM) with minimal binding to control mCD200RLb/rCD4d3+4 proteins. This contrasts with the good binding of monomeric mCD200 to mCD200R but barely detectable binding of CD200 to mCD200RLa as studied previously [21]. In addition the dimeric mCD200-Fc fusion proteins have failed to detect mCD200RLa on cells and in the BIAcore [21]. Thus the use of the multivalent binding reagent to multivalent immobilised proteins clearly provides a highly sensitive assay capable of detecting extremely weak interactions – for comparison the monomeric affinity of MHC Class I for CD8 is in the region of 200 μM [6]. The almost complete lack of dissociation at 37°C even for the beads detecting the extremely low affinity mCD200-mCD200RLa binding (Fig. 1) indicates that the interaction must be highly polyvalent. Whilst not providing a quantitative assay it allows ready detection of weak interactions prior to more quantitative analysis. The use of transiently expressed protein coupled directly to avidin coated beads obviates the need for low pH elution steps in affinity purification steps minimising protein denaturation that can give anomalous binding in these types of assays.
Figure 1.

Sensitive assay to detect low affinity interactions using beads expressing multivalent ligands to immobilised proteins detected by SPR. Three flow cells were coated with ~2000 RU mCD200R, mCD200RLa and mCD200RLb, which respectively have moderate, very low and no affinity for monomeric mCD200 [21]. When the multivalent mCD200 coated beads were injected at 2 μl/min at 37°C, mCD200RLa gave as strong binding to mCD200 as mCD200R (>3000 RU). No binding of mCD200RLb to mCD200 was detected and only the signal due to the analyte itself is seen.
Detection of the interaction of LAIR-1 with collagen I using the new assay
The reported interaction between LAIR-1 and a motif present in collagens was surprising as collagens are so widely distributed and LAIR-1 is an inhibitory receptor [12]. To confirm the LAIR-1/collagen interaction, the new bead/BIAcore™ assay described above was used. Human collagen type I (collagen I) was directly immobilized on the chip and beads coated with hLAIR-1/rCD4d3+4 were applied (Fig. 2). Even at a concentration as low as 0.004% w/v in the presence of 1% BSA in HBS running buffer, these beads bound strongly to the collagen I (solid line – 2700 RU responses) compared to negligible binding to control rCD4d3+4 and slight binding to LAIR-1 itself (see below). Note there is a strong bulk effect during the injection due to the signal from the BSA included to minimise any possible non-specific effects reflected by a constant signal during the injection. This confirms the previous report identifying collagen I as a ligand for LAIR-1 from cDNA library screening [12].
Figure 2.

hLAIR1/rCD4d3+4 beads bound to collagen but not to control protein. Collagen (1000 RU), hLAIR1/rCD4d3+4 (2000 RU) and rCD4d3+4 (1500 RU, negative control) were immobilised, and hLAIR-1/rCD4d3+4 beads passed over. The figure shows responses from the three flow cells superimposed.
Although it is hard to calculate precisely the coverage of hLAIR-1/rCD4d3+4 on the bead, that is, the valency of the beads, the average concentration of hLAIR-1/rCD4d3+4 protein in the flow could be estimated to be 1.5 nM by inhibition ELISA standardized with rCD4d3+4 mAb OX68. The use of the multivalent bead is advantageous in amplifying such specific interactions with a requirement for minimal amount of protein.
Monomeric interactions of leukocyte membrane proteins with collagen I
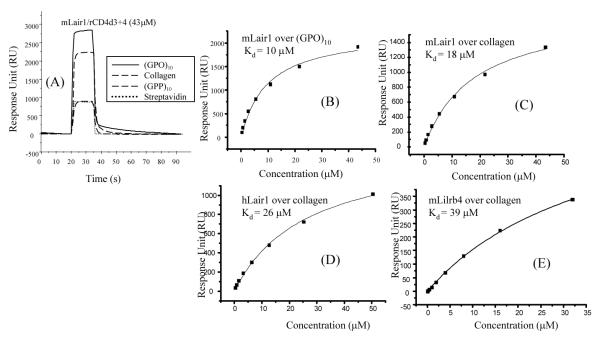
The interaction between LAIR-1 and collagen has been described as high affinity [11, 12], but the measurements were made with dimeric protein and not directly comparable to the affinities measured for other interactions measured with monomeric proteins. Recombinant monomeric human and mouse LAIR-1 proteins were produced as monomeric protein with domain 3+4 of rat CD4 as a tag as used in other studies [7]. The affinity of LAIR-1 to collagen I was measured by SPR and in addition a synthetic collagen repeat previously shown to bind LAIR-1 was tested together with control non binding peptide [22]. Clear binding was obtained between mLAIR-1 and both (GPO)10 and collagen I but minimal to the control peptide (GPP)10 and also streptavidin (Fig. 3A). The dissociation constants Kd for mouse LAIR-1 binding to (GPO)10 and collagen I were calculated to be 10 and 18 μM respectively (Fig. 3B and C). The similarity in affinities between collagen I and the synthetic peptide indicates that the trimeric repeat is an excellent structural mimic of the native collagen and compatible with the finding that LAIR-1 can bind several different kinds of collagen in its family, such as human collagen type III [11, 12, 22]. The human LAIR-1 gave a similar affinity (Kd = 26 μM) with collagen as the mouse LAIR-1 (Fig. 3D).
Figure 3.
Binding of LAIR-1 and Lilrb4 to collagen or collagen related peptide. (A) Soluble monomeric mLAIR-1/rCD4d3+4 (43 μM) was applied to collagen (2000 RU), collagen related peptide trimer, (GPO)10 (2000 RU), control peptide trimer (GPP)10 (2000 RU) and negative control streptavidin (1000 RU) coated flow cells. (B, C) Binding curves for experiments as in (A) but varying the concentration of purified mLAIR-1/rCD4d3+4. Kd of 10 and 18 μM were calculated by nonlinear curve fitting of the Langmuir isotherm (line) to the binding data of monomeric mLAIR-1/rCD4d3+4 to (GPP)10 and collagen with the values for the negative control (GPP)10 subtracted. (D) and (E) Comparable experiment to (B) and (C) showing hLAIR-1/rCD4d3+4 and mLilrb4/rCD4d3+4 bound to collagen with Kd = 26 and 39 μM respectively.
As LAIR-1 is present in a gene cluster on human chromosome 19q3.3 including several other receptors that are structurally related - the CD85 proteins, it seemed possible that additional collagen binding proteins might be found in the CD85 family. Fig. 2E shows that monomeric mLilrb4/rCD4d3+4 also bound to collagen I coated flow cells although the interaction was weaker (Kd = 39 μM) than that of LAIR-1. Similarly, the affinity of mLilrb4/rCD4d3+4 to (GPO)10 was Kd = 33 μM (data not shown).
The possibility that collagens could interact with many surface proteins and that the interaction was some type of non-specific effect was tested by screening other cell surface proteins for binding to collagen I. Two other membrane proteins containing IgSF domains were tested in the same way with recombinant proteins. mCD200 and hSIRPα gave no detectable binding to collagen I or collagen related peptides (Fig. 4A and B). Other proteins such as human and rat CD200 also failed to give binding (data not shown) indicating that the collagen binding is not a broad non-specific effect common to many proteins with IgSF domains or due to the tag (note these experiments used either HIS tag or a CD4d3+4 tag in contrast to the published data on LAIR-1 binding that used Fc fusion proteins [11, 12]). The interaction of LAIR-1 with collagen I could also be detected in the presence of human serum (data not shown), suggesting that the interaction could occur in body fluids. Thus the LAIR-1 and Lilrb4 clearly bind specifically to collagen I although the latter binding is weaker.
Figure 4.

CD200 and SIRPα do not bind collagen. Soluble monomeric mCD200/rCD4d3+4 (9.3 μM) (A) and hSIRPα (26 μM) (B) were applied to the collagen related peptide, (GPO)10, collagen, control peptide (GPP)10 and negative control streptavidin coated flow cells. The X-axis indicates the duration of injection of ligand in seconds. The binding was indistinguishable to the control with only the bulk effect due to the high concentrations of protein in the analyte.
Specific binding between LAIR-1 to Lilrb4
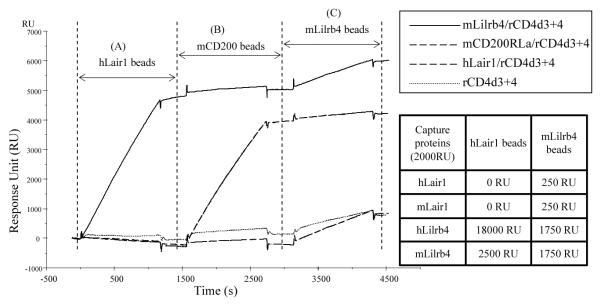
Because LAIR-1 and Lilrb4 are structurally related and members of a group of linked genes, it seemed possible that other interactions might occur between members of the CD85 gene cluster. The paradigm for this is the CD2/SLAM gene cluster of IgSF membrane proteins where several members interact with each other or homophilically [23, 24]. Weak binding between LAIR-1 and Lilrb4 was detected and characterised in both mouse and human by the qualitative multivalent SPR screening assay (Fig. 5). hLAIR-1/rCD4d3+4, mLilrb4/rCD4d3+4, mCD200RLa/rCD4d3+4 and rCD4d3+4 were immobilized on the four flow cell surfaces via biotin-streptavidin. Three types of multivalent beads coated with hLAIR-1, mCD200 and mLilrb4, were injected over the four flow cells. The hLAIR-1 coated beads gave significantly stronger binding to mLilrb4/rCD4d3+4 than to the other three flow cells. However the binding is weak when compared to the control weak interaction between mCD200 and mCD200RLa (Kd > 500 μM). The experiment was repeated in the reverse orientation with multivalent mLilrb4 beads binding to both Lilrb4 and LAIR-1 coated surfaces. The binding of mLilrb4 beads to hLAIR-1 (250 RU) was not as strong as the reverse interaction between hLAIR-1 beads and mLilrb4 (2500 RU). We attribute this to the different properties of the beads. For example, the mLirb4 protein has larger dimensions than hLAIR-1 and is more glycosylated which may result in less molar coverage of the mLilrb4 coated particles. In addition mLilrb4 beads tend to bind more to hLilrb4 or mLilrb4 coated surface (1750 RU) than to hLAIR-1 or mLAIR-1 (250 RU), as shown in the inset table of Fig. 5. This suggested that Lilrb4 could be homophilic across species. Further experiments compared the binding of hLAIR-1 to all the other members of CD85 family (CD85, CD85D, CD85E), which confirmed that Lilrb4 has the highest binding to human and mouse LAIR-1 (data not shown).
Figure 5.
hLAIR-1 gives comparable bead binding to mLilrb4 as that between mCD200 and mCD200RLa. mLilrb4/rCD4d3+4, mCD200RLa/rCD4d3+4, hLAIR1/rCD4d3+4 and rCD4d3+4 were immobilized on four flow cells and then three conjugated sets of beads were injected sequentially. (A) hLAIR-1 beads gave strong binding to mLilrb4 but not mCD200RLa or control rCD4d3+4 with traces on hLAIR-1. (B) mCD200RLa beads gave strong binding to its ligand CD200 but not the other proteins. (C) mLilrb4 beads gave minimal binding to mCD200 but very weak binding to the other proteins including the control protein. Inset table: the amount of multivalent beads coated with hLair1 and mLilrb4 that bound to surfaces with immobilized hLair1, mLair1, hLilrb4 and mLilrb4 from another experiment are listed.
The affinity of LAIR-1 binding to Lilrb4 was determined by SPR. A series of concentrations of recombinant monomeric hLAIR-1/rCD4d3+4 was passed over hLilrb4/rCD4d3+4 and hLAIR-1/rCD4d3+4 (negative control) immobilized on the BIAcore™ (Fig. 6). Only a weak interaction between hLAIR-1 to hLilrb4 at the limit of the assay was found and a dissociation constant Kd > 200μM was estimated (Fig. 6), consistent with the result with the beads in Fig. 5.
Figure 6.

The interaction between hLAIR-1 and hLilrb4 is very weak. Y-axis indicates the equilibrium binding of soluble monomeric hLAIR1/rCD4d3+4 to immobilized hLilrb4/rCD4d3+4 (2500 RU), as set up in Fig. 3. Despite the high concentration of protein used only minimal binding was observed. Thus only an approximate dissociation constant Kd of > 200μM could be estimated from the nonlinear curve fitting shown by the dotted line.
Discussion
Several transmembrane receptors that recognise the typical collagen triple helix have the potential to signal. These include integrins, discoidin domain receptors (DDR1 and DDR2), Glycoprotein VI (GPVI) and LAIR-1 (reviewed in [14]). Collagen binds to preformed DDR dimers on the cell membrane, and according to biophysical assays it binds only to dimeric DDRs with high affinity but not the monomeric DDR proteins [25]. Recently the binding motifs for DDR2 in collagen triple helix were identified [26]. The GPVI-collagen interaction is much weaker than the integrin-collagen interaction. Dimeric GPVI-Fc protein bound collagen and (GPO)10 with dissociation constants Kd of 0.6 and 5 μM respectively, whilst monomeric GPVI protein displayed almost no binding to collagen by SPR although some binding was found by plate binding assay [27-29]. Recently, it was found that LAIR-1 is also a receptor for collagen and that the dimeric LAIR-1-Fc protein binds collagen and (GPO)10 with nM affinity [12]. So far most of the quantitative biophysical assays on collagens as above were detected with its receptors in the form of dimeric Fc fusion proteins. In this study we measured the affinity of monomeric LAIR-1 to collagen I showing it to be relatively weak but not dissimilar to many other cell-cell interactions [1-6]. It is significantly stronger than the GPVI interaction that is undetectable with monomeric protein. Thus this affinity is sufficient to explain the functional data on LAIR-1/collagen. These include showing that LAIR-1 transfected cells associated with collagen XVII transfected cells, that the binding of LAIR-1 Fc fusion protein to collagen expressing cells could be blocked by collagenase and purified collagen and that collagen could induce the inhibitory signal in LAIR-1 transfected reporter cells in both human and mouse [11, 12]. The biochemical studies described above support the immunological importance of the weak affinity ligand-receptor binding pair between collagen and LAIR-1.
We also identified a second member of the CD85 cluster, Lilrb4 (CD85K), that can bind collagen I albeit weaker than LAIR-1. The finding that other cell surface proteins did not bind collagen I under these conditions suggests that the phenomenon is not due to non-specific stickiness. Lilrb4 also showed some weak binding to LAIR-1 in both human and mouse species, but the significance of this interaction is difficult to evaluate because the Lilrb4 proteins also showed some tendency to bind to themselves. The binding of Lilrb4 and LAIR-1 to collagen distinguished these receptors from the other members of CD85 family, such as CD85D and CD85, which have been reported to bind HLA-G and classical MHC class I molecules [30, 31].
Lilrb4 has been shown to interact with the integrin αvβ3 although the interaction has not been established using isolated proteins [32]. It is possible that this interaction was mediated by collagen as the collagen was found to bind many members of integrin family (α1β1, α2β1, α10β1 and α11β1) [33-35] and in some in vitro assays, αvβ3 also appears to be able to interact with collagen although this interaction may involve RGD motifs in denatured or partially unfolded collagen chains [36-38]. LAIR-1 has a higher affinity for collagen I (Kd = 26 μM for hLAIR-1 and 18 μM for mLAIR-1) than Lilrb4 (Kd = 38 μM). In the platelet adhesion scenario there was a similar situation where both GPVI and integrin bind collagen with different affinities [39-41]. Later it was found that GPVI and integrin act in a highly cooperative and integrated fashion to mediate the firm cell adhesion at the site of platelet-collagen contact [42, 43]. This suggests that collagens may interact with a variety of receptors including inhibitory receptors on the membrane surface.
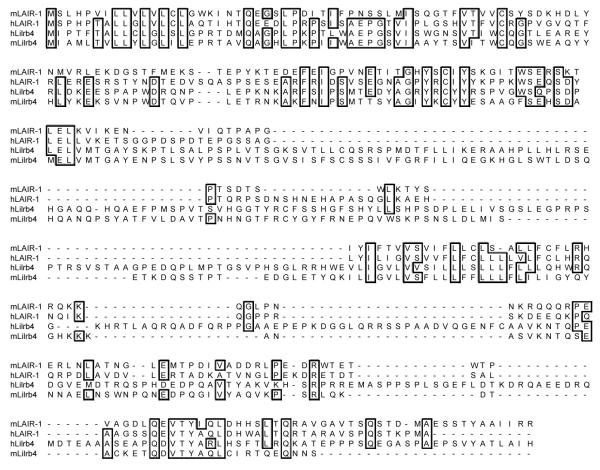
The results in Fig. 5 implied that there could be specific interaction between LAIR-1 and Lilrb4 and the monomeric binding assay in Fig. 6 indicated a Kd greater than 200 μM. It seems reasonable to consider these proteins together as closely related IgSF members can interact among themselves with the best example in the CD2/SLAM family of receptors that interact with each other and probably evolved from a primordial homophilic interaction [44]. Both LAIR-1 and Lirb4 are present in a gene cluster on human chromosome 19q3.3 and are structurally related as shown in the sequence alignments in Fig. 7.
Figure 7.
Comparison of the sequences of hLAIR-1, mLAIR-1, hLilrb4 and mLilr4. Residues identical in 3 or 4 sequences are boxed.
In conclusion, a new screening assay has been developed combining the advantage of surface plasmon resonance (SPR) with the innovative multivalent beads, onto which the relevant recombinant proteins are attached through different tags. One important aspect of the assay is that the proteins are immobilised directly from transient expression with no purification step involving denaturation that could give some denatured protein that may give anomalous binding. This assay enabled us to identify the weak interactions on leukocyte membrane surface whose affinity was too low to detect previously. Through this method, it was found that both the leukocyte membrane inhibitory receptors, LAIR-1 and Lilrb4 (CD85K), show specific binding to collagen with different affinities, in addition to their weak affinity to each other. Although weak, the interaction may have significant functional effect in the regulation of immunological responses [12]. This interaction with inhibitory receptors also complements those characterised between collagen and both integrins and GPVI. One feature that distinguishes the LAIR-1 interaction from the well characterised interactions of collagen with integrins is that LAIR-1 is a very small cell surface protein with a single IgSF domain and a short hinge region, so interactions with collagen will require much closer proximity of the cell with collagen than for example with integrins. It is possible that any inhibition through LAIR-1 will depend on rearrangement of cell surface proteins in synapse-like regions and collagen inhibition of leukocyte activity may be only occur in restricted circumstances.
Materials and methods
Construction, expression and purification of soluble recombinant proteins
Constructs for the human and mouse LAIR-1 and Lilrb4 were prepared from cDNA clones from Geneservice Ltd (UK) by inserting the sequence for the extracellular domains into the pEF-BOS vector [45] together with the sequence for domains 3 and 4 of rat CD4 (rCD4d3+4) and sequence to permit biotinylation by biotin ligase BirA (Avidity LLC, Colorado, US) near the C terminus [9]. The boundary between the hLAIR1 part and CD4 was AEGAPSTSIT, for mLAIR1 SDTSWSTSIT, for hLilrb4 SGLRRSTSIT and for mLilrb4 ETKDQSTSIT (CD4 linker is underlined). The pEF-BOS constructs were transfected into HEK 293T cells with reagent PEI (polyethylenimine, linear MW ~25,000, Polysciences, Inc.) at the DNA:PEI ratio of 1:10 [46]. The cells were incubated in serum free Xvivo10 medium (BioWhittaker, Walkersville, MD, USA) for 5 days at 37°C, the supernatants were collected and concentrated, assayed by an inhibition ELISA using OX68 mAb (specific for the rCD4d3+4 tag) to measure the expression level of recombinant proteins [21]. The yield of the protein was 5 - 20 μg/ml. The protein was biotinylated, dialyzed in PBS and coupled to avidin coated yellow fluorescent beads (SPHERO™, 0.46 μm diameter, Spherotech Inc. Libertyville, IL) as described previously [5, 8].
For monomeric binding assays where larger amounts of protein were needed, stable cell lines were produced using the pEE14 vector (Lonza Biologics, Slough, UK) and Chinese hamster ovary (CHO) cells as described. The recombinant protein was purified by affinity chromatography with rat CD4d3+4 mAb, OX68 Sepharose 4B column, eluted with 0.1 M glycine-HCl buffer (pH 2.5) and subsequent gel filtration through a Superdex S-200 HR 10/30 column (Pharmacia, Uppsala, Sweden) to exclude the large aggregates. The yield of purified proteins was between 0.2 and 1 mg/l.
Detection of low affinity interactions and measurement of affinities using surface plasmon resonance (SPR)
Affinity and kinetic data were collected using a BIAcore 2000 (Biacore™) at 37°C as previously described [21]. Streptavidin (~2000 Response Units (RU)) was coupled to a CM5 research grade chip using amine coupling. Biotinylated recombinant proteins and control rCD4d3+4 were each immobilized at about 2000 RU. For kinetic analysis of monomeric binding, serially diluted monomeric purified proteins, such as hLAIR-1/rCD4d3+4, mLAIR-1/rCD4d3+4, hLilrb4/rCD4d3+4 or mLilrb4/rCD4d3+4 were injected at the indicated concentrations over all four flow cells connected in series. The theoretical molecular extinction coefficients of 38,010, 49,390, 64,850 and 78,100 M-1cm-1 respectively were calculated. Dissociation constant Kd values were obtained by nonlinear curve fitting to the equilibrium binding data. For the qualitative binding assay of multivalent beads to immobilized proteins, fresh samples were prepared before each experiment. 10 μl 0.1%w/v (in PBS buffer) avidin coated beads were incubated with dialyzed biotinylated recombinant proteins for 3 hours, followed by centrifuging (10 min at 13,000rpm), removal of supernatant and resuspension of the beads in 250 μl HBS buffer (0.01 M HEPES, pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% surfactant P20) with 1% bovine serum albumin (BSA). 10μl diluted bead suspension was injected over the flow cells at 1-2 μl/min with HBS pH 7.4 as running buffer at 37°C. The results shown are representative of at least three experiments.
Acid-soluble human collagen type I (from human placenta, C7774, Sigma, UK) was immobilized (500–2000 RU) on a CM5 biosensor chip using amine coupling. Immobilized triple-helical peptides composed of GCO(GPO)10GCOG-NH2 ((GPO)10, also known as collagen-related peptide or CRP) and GCP(GPP)10GCPG-NH2 ((GPP)10) [22] were immobilized (~2000 RU) using a cysteine coupling kit, according to the manufacturer’s instructions [47].
Acknowledgments
We thank Richard W. Farndale (University of Cambridge, UK) for providing the collagen related peptides, Deborah Hatherley for purified CD200 and SIRP proteins and discussion and Marion H. Brown for advice. This research was funded by the Medical Research Council (MRC).
Abbreviations
- LAIR-1
leukocyte-associated immunoglobulin-like receptor 1
- SPR
surface plasmon resonance
- IgSF
immunoglobulin superfamily
- Lilrb4
leukocyte immunoglobulin-like receptor subfamily B member 4
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.van der Merwe PA, Barclay AN, Mason DW, Davies EA, Morgan BP, Tone M, Krishnam AK, et al. Human cell-adhesion molecule CD2 binds CD58 (LFA-3) with a very low affinity and an extremely fast dissociation rate but does not bind CD48 or CD59. Biochemistry. 1994;33:10149–10160. doi: 10.1021/bi00199a043. [DOI] [PubMed] [Google Scholar]
- 2.van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J. Exp. Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu. Rev. Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 4.Brooke G, Holbrook JD, Brown MH, Barclay AN. Human Lymphocytes Interact Directly with CD47 through a Novel Member of the Signal Regulatory Protein (SIRP) Family. J. Immunol. 2004;173:2562–2570. doi: 10.4049/jimmunol.173.4.2562. [DOI] [PubMed] [Google Scholar]
- 5.Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, Barclay AN. Lymphoid/Neuronal Cell Surface OX2 Glycoprotein Recognizes a Novel Receptor on Macrophages Implicated in the Control of Their Function. Immunity. 2000;13:233–242. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 6.Wyer JR, Willcox BE, Gao GF, Gerth UC, Davis SJ, Bell JI, van der Merwe PA, et al. T cell receptor and coreceptor CD8 alphaalpha bind peptide-MHC independently and with distinct kinetics. Immunity. 1999;10:219–225. doi: 10.1016/s1074-7613(00)80022-9. [DOI] [PubMed] [Google Scholar]
- 7.Voulgaraki D, Mitnacht-Kraus R, Letarte M, Foster-Cuevas M, Brown MH, Barclay AN. Multivalent recombinant proteins for probing functions of leucocyte surface proteins such as the CD200 receptor. Immunology. 2005;115:337–346. doi: 10.1111/j.1365-2567.2005.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letarte M, Voulgaraki D, Hatherley D, Foster-Cuevas M, Saunders NJ, Barclay AN. Analysis of leukocyte membrane protein interactions using protein microarrays. BMC Biochem. 2005;6:2. doi: 10.1186/1471-2091-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J. Exp. Med. 1998;188:2083–2090. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez LC, Loyet KM, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, Eaton DL. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc. Natl. Acad. Sci. USA. 2005;102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebbink RJ, de Ruiter T, Kaptijn GJ, Bihan DG, Jansen CA, Lenting PJ, Meyaard L. Mouse leukocyte-associated Ig-like receptor-1 (mLAIR-1) functions as an inhibitory collagen-binding receptor on immune cells. Int. Immunol. 2007;19:1011–1019. doi: 10.1093/intimm/dxm071. [DOI] [PubMed] [Google Scholar]
- 12.Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 2006;203:1419–1425. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyaard L. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7:283–290. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 14.Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Wess TJ. Collagen fibril form and function. Adv. Protein Chem. 2005;70:341–374. doi: 10.1016/S0065-3233(05)70010-3. [DOI] [PubMed] [Google Scholar]
- 16.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Ortega N, Werb Z. New functional roles for non-collagenous domains of basement membrane collagens. J. Cell Sci. 2002;115:4201–4214. doi: 10.1242/jcs.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant MA, Kalluri R. Structural basis for the functions of endogenous angiogenesis inhibitors. Cold Spring Harb. Symp. Quant. Biol. 2005;70:399–410. doi: 10.1101/sqb.2005.70.017. [DOI] [PubMed] [Google Scholar]
- 19.Schatz PJ. Use of Peptide Libraries to Map the Substrate Specificity of a Peptide-Modifying Enzyme: A 13 Residue Consensus Peptide Specifies Biotinylation in Escherichia coli. Nat. Biotech. 1993;11:1138–1143. doi: 10.1038/nbt1093-1138. [DOI] [PubMed] [Google Scholar]
- 20.Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102:173–179. doi: 10.1046/j.1365-2567.2001.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatherley D, Cherwinski HM, Moshref M, Barclay AN. Recombinant CD200 Protein Does Not Bind Activating Proteins Closely Related to CD200 Receptor. J. Immunol. 2005;175:2469–2474. doi: 10.4049/jimmunol.175.4.2469. [DOI] [PubMed] [Google Scholar]
- 22.Knight CG, Morton LF, Onley DJ, Peachey AR, Ichinohe T, Okuma M, Farndale RW, et al. Collagen-platelet interaction: Gly-Pro-Hyp is uniquely specific for platelet Gp VI and mediates platelet activation by collagen. Cardiovasc. Res. 1999;41:450–457. doi: 10.1016/s0008-6363(98)00306-x. [DOI] [PubMed] [Google Scholar]
- 23.Tangye SG, Phillips JH, Lanier LL. The CD2-subset of the Ig superfamily of cell surface molecules: receptor-ligand pairs expressed by NK cells and other immune cells. Semin. Immunol. 2000;12:149–157. doi: 10.1006/smim.2000.0217. [DOI] [PubMed] [Google Scholar]
- 24.van der Merwe PA, McNamee PN, Davies EA, Barclay AN, Davis SJ. Topology of the CD2-CD48 cell-adhesion molecule complex: implications for antigen recognition by T cells. Curr. Biol. 1995;5:74–84. doi: 10.1016/s0960-9822(95)00019-4. [DOI] [PubMed] [Google Scholar]
- 25.Noordeen NA, Carafoli F, Hohenester E, Horton MA, Leitinger B. A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase DDR1. J. Biol. Chem. 2006;281:22744–22751. doi: 10.1074/jbc.M603233200. [DOI] [PubMed] [Google Scholar]
- 26.Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen. J. Biol. Chem. 2008;283:6861–6868. doi: 10.1074/jbc.M709290200. [DOI] [PubMed] [Google Scholar]
- 27.Miura Y, Takahashi T, Jung SM, Moroi M. Analysis of the interaction of platelet collagen receptor glycoprotein VI (GPVI) with collagen. A dimeric form of GPVI, but not the monomeric form, shows affinity to fibrous collagen. J. Biol. Chem. 2002;277:46197–46204. doi: 10.1074/jbc.M204029200. [DOI] [PubMed] [Google Scholar]
- 28.Smethurst PA, Joutsi-Korhonen L, O’Connor MN, Wilson E, Jennings NS, Garner SF, Zhang YJ, et al. Identification of the primary collagen-binding surface on human glycoprotein VI by site-directed mutagenesis and by a blocking phage antibody. Blood. 2004;103:903–911. doi: 10.1182/blood-2003-01-0308. [DOI] [PubMed] [Google Scholar]
- 29.Smethurst PA, Onley DJ, Jarvis GE, O’Connor MN, Knight CG, Herr AB, Ouwehand WH, et al. Structural basis for the platelet-collagen interaction: the smallest motif within collagen that recognizes and activates platelet Glycoprotein VI contains two glycine-proline-hydroxyproline triplets. J. Biol. Chem. 2007;282:1296–1304. doi: 10.1074/jbc.M606479200. [DOI] [PubMed] [Google Scholar]
- 30.Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, Allan DS, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc. Natl. Acad. Sci. USA. 2003;100:8856–8861. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naji A, Durrbach A, Carosella ED, Rouas-Freiss N. Soluble HLA-G and HLA-G1 expressing antigen-presenting cells inhibit T-cell alloproliferation through ILT-2/ILT-4/FasL-mediated pathways. Hum. Immunol. 2007;68:233–239. doi: 10.1016/j.humimm.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Castells MC, Klickstein LB, Hassani K, Cumplido JA, Lacouture ME, Austen KF, Katz HR. gp49B1-alpha(v)beta3 interaction inhibits antigen-induced mast cell activation. Nat. Immunol. 2001;2:436–442. doi: 10.1038/87749. [DOI] [PubMed] [Google Scholar]
- 33.Camper L, Hellman U, Lundgren-Akerlund E. Isolation, cloning, and sequence analysis of the integrin subunit alpha10, a beta1-associated collagen binding integrin expressed on chondrocytes. J. Biol. Chem. 1998;273:20383–20389. doi: 10.1074/jbc.273.32.20383. [DOI] [PubMed] [Google Scholar]
- 34.Velling T, Kusche-Gullberg M, Sejersen T, Gullberg D. cDNA cloning and chromosomal localization of human alpha(11) integrin. A collagen-binding, I domain-containing, beta(1)-associated integrin alpha-chain present in muscle tissues. J. Biol. Chem. 1999;274:25735–25742. doi: 10.1074/jbc.274.36.25735. [DOI] [PubMed] [Google Scholar]
- 35.Zhang WM, Kapyla J, Puranen JS, Knight CG, Tiger CF, Pentikainen OT, Johnson MS, et al. alpha 11beta 1 integrin recognizes the GFOGER sequence in interstitial collagens. J. Biol. Chem. 2003;278:7270–7277. doi: 10.1074/jbc.M210313200. [DOI] [PubMed] [Google Scholar]
- 36.Agrez MV, Bates RC, Boyd AW, Burns GF. Arg-Gly-Asp-containing peptides expose novel collagen receptors on fibroblasts: implications for wound healing. Cell. Regul. 1991;2:1035–1044. doi: 10.1091/mbc.2.12.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaff M, Aumailley M, Specks U, Knolle J, Zerwes HG, Timpl R. Integrin and Arg-Gly-Asp dependence of cell adhesion to the native and unfolded triple helix of collagen type VI. Exp. Cell Res. 1993;206:167–176. doi: 10.1006/excr.1993.1134. [DOI] [PubMed] [Google Scholar]
- 38.Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem. Biophys. Res. Commun. 1992;182:1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 39.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 40.Nieswandt B, Brakebusch C, Bergmeier W, Schulte V, Bouvard D, Mokhtari-Nejad R, Lindhout T, et al. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20:2120–2130. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holtkotter O, Nieswandt B, Smyth N, Muller W, Hafner M, Schulte V, Krieg T, et al. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J. Biol. Chem. 2002;277:10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 42.Siljander PR, Munnix IC, Smethurst PA, Deckmyn H, Lindhout T, Ouwehand WH, Farndale RW, et al. Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood. 2004;103:1333–1341. doi: 10.1182/blood-2003-03-0889. [DOI] [PubMed] [Google Scholar]
- 43.Sarratt KL, Chen H, Zutter MM, Santoro SA, Hammer DA, Kahn ML. GPVI and alpha2beta1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow. Blood. 2005;106:1268–1277. doi: 10.1182/blood-2004-11-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mavaddat N, Mason DW, Atkinson PD, Evans EJ, Gilbert RJ, Stuart DI, Fennelly JA, et al. Signaling lymphocytic activation molecule (CDw150) is homophilic but self-associates with very low affinity. J. Biol. Chem. 2000;275:28100–28109. doi: 10.1074/jbc.M004117200. [DOI] [PubMed] [Google Scholar]
- 45.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nice EC, Catimel B. Instrumental biosensors: new perspectives for the analysis of biomolecular interactions. Bioessays. 1999;21:339–352. doi: 10.1002/(SICI)1521-1878(199904)21:4<339::AID-BIES11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]