Abstract
The B cell antigen receptor (BCR) is a multiprotein complex consisting of the membrane-bound Ig molecule and the Ig-α/Ig-β heterodimer. On BCR engagement, Ig-α and Ig-β become phosphorylated not only on tyrosine residues of the immunoreceptor tyrosine-based activation motif but also on serine and threonine residues. We have mutated all serine and threonine residues in the Ig-α tail to alanine and valine, respectively. The mutated Ig-α sequence was expressed either as a single-chain Fv/Ig-α molecule or in the context of the complete BCR. In both cases, the mutated Ig-α showed a stronger tyrosine phosphorylation than the wild-type Ig-α and initiated increased signaling on stimulation. These findings suggest that serine/threonine kinases can negatively regulate signal transduction from the BCR.
The members of the multisubunit immune recognition receptor family have similarities in their structure and share signal transduction pathways (1, 2). Prominent members of this family are the B cell antigen receptor (BCR), the T cell antigen receptor (TCR), and the high-affinity IgE receptor (FcɛRI). These receptors consist of a ligand-binding part and signaling subunits carrying an immunoreceptor tyrosine-based activation motif (ITAM; refs. 1 and 3). Studies on the BCR suggest that, already in the absence of the ligand, the receptor is associated with a preformed transducer complex comprising intracellular kinases, phosphatases, and adaptor molecules (4). Engagement of the receptor results in the activation of protein tyrosine kinases (PTKs), which phosphorylate several intracellular substrate proteins including the adapter protein SLP-65 (also called BLNK or BASH; refs. 5–7) and the Ig-α/Ig-β heterodimer (8). The ITAM sequence of Ig-β, however, is less efficiently tyrosine phosphorylated than that of Ig-α, and the reason for this difference is not known thus far (9, 10). After phosphorylation, the ITAM tyrosines become binding targets for proteins with Src homology 2 (SH2) domains (11–14). The best studied interaction is that of the two tandem-arranged SH2 domains of the PTK Syk with the phosphorylated ITAM tyrosines of CD3-ɛ (15). The binding of Syk to the ITAM results in an increased kinase activity (16–18). The Syk-associated BCR is efficiently internalized and transported to endosomal compartments where antigen processing occurs (19). Syk binding and activity is also required for endocytosis of ITAM-containing Fc receptors in macrophages (20, 21).
BCR engagement results not only in tyrosine phosphorylation but also in increased serine and threonine phosphorylation. Because of the lack of universal anti-phosphoserine or anti-phosphothreonine antibodies, the latter events are poorly studied and require laborious biochemical techniques for their detection. Phosphorylation of serine/threonine residues occurs in the cytoplasmic sequence of many receptors or their signal-transducing elements and can have either positive or negative effects on signal transduction through these receptors (22–26). The cytoplasmic tails of Ig-α and Ig-β are phosphorylated on the ITAM tyrosines as well as on serine and threonine (9, 27, 28). We have mutated all serine/threonine residues in the cytoplasmic sequence of Ig-α and show herein that they negatively regulate phosphorylation of the ITAM tyrosines.
Materials and Methods
Vector Construction.
The cassette expression vector pANP8n-cy was designed to express different cytoplasmic sequences of signaling molecules as membrane-bound single chain (scFv) molecules. The scFv molecules are expressed under the control of the ubiquitously active human β-actin promoter. For the construction, we subcloned a 730-bp PstI/NotI fragment containing the scFv B1–8 cDNA from the plasmid pHEN-FvB1-8 (kindly provided by T. Simon, Amersham Pharmacia, Freiburg, Germany) into the plasmid pLVHLT-Xh (H. Voegele and M.R., unpublished work), which contains the B1-8 heavy chain leader sequence 5′ of the PstI site. The scFv consists of the VH and VL domains of the B1-8 antibody, which recognize the hapten 4-hydroxy-5-iodo-3-nitrophenylacetyl (29) and are linked via a (Gly4Ser)3 linker (30). Next, we introduced SalI and EcoRI cloning sites 5′ and 3′ of the scFv cDNA, respectively. The internal BamHI site of the scFv fragment was destroyed by site-directed mutagenesis. This modified scFv fragment was cloned as a 880-bp SalI/EcoRI fragment into the expression vector Fv-CD8-5OM (kindly provided by K. Karjalainen and T. Brocker, Basel Institute for Immunology, Basel), which encodes the extracellular hinge region of the CD8α molecule followed by the transmembrane and intracellular portion of the TCR-ζ chain (31). The resulting construct pANP8Z codes for scFv/TCR-ζ and was used as a template for amplifying the CD8α and the transmembrane region. The PCR product was recloned as an EcoRI/BamHI fragment into pANP8Z, thereby deleting the cytoplasmic sequence of TCR-ζ. Finally, a short BamHI/HindIII/EcoRV polylinker sequence was introduced 3′ of the transmembrane region. The expression vector contains the ampicillin and neomycin resistance genes.
The coding sequence of the cytoplasmic tail of Ig-α and the A1,2V-mutated Ig-α tail were obtained as BamHI fragments by PCR with the vector pRP261mb1 (10) as template and cloned into pANP8n-cy to yield the expression vectors pANP8mb1 and pANP8mb1/A1,2V, respectively. To obtain the pANP8tl expression vector, pANP8n-cy was cut with BamHI, blunted, and re-ligated, and this vector codes for a scFv receptor containing only five cytoplasmic amino acids (RRIDP).
Site-directed mutagenesis of the mb1 c-DNA and subsequent cloning into the Ig-α expression vector pEVmb1neo (32) was performed as described (10) and yielded the expression vectors pEVmb1/A1,2V, pEVmb1/F1A1,2V, pEVmb1/F2A1,2V, and pEVmb1/F1,2A1,2V.
Cell Culture.
The J558L myeloma cell line (33) and its transfectants were maintained in RPMI medium 1640 supplemented with 10% (vol/vol) heat-inactivated FCS (Vitromex, Vilshofen, Germany), 2 mM l-glutamine, 50 units/ml penicillin, 50 μg/ml streptomycin, 10 mM Hepes buffer, and 50 μM β-mercaptoethanol at 37°C and 5% CO2. J558Lμm cells, which intracellularly express membrane-bound μm heavy chain, λ light chain, and Ig-β, were cultured in mycophenolic acid selection medium (34). The cell lines J558Lμm/mb1 (wild-type Ig-α), J558Lμm/mb1-M4 [Y1 > F1, (= F1)], J558Lμm/mb1-M3 [Y2 > F2, (= F2)], and J558Lμm/mb1-M1 [Y1 > F1 and Y2 > F2, (= F1,2)] expressing wild-type BCR or BCRs with ITAM tyrosine mutated Ig-α on the surface were a gift from H. Flaswinkel (Max-Planck-Institut for Immunology, Freiburg, Germany) and were maintained under mycophenolic acid and neomycin coselection (10). J558L and J558Lμm cells were transfected with linearized plasmids by electroporation and maintained under selection with neomycin and neomycin/mycophenolic acid as described (10).
Stimulation of Cells.
The preparation of pervanadate for stimulation of cells was performed as described (4). A total of 2.5 × 106 to 3 × 106 cells was suspended in 1 ml of RPMI medium 1640 without supplements and stimulated at 37°C with 8 or 20 μM pervanadate/H2O2 or with 10 μg/ml goat anti-mouse IgM antibodies (Sigma). Cells were pelleted and lysed on ice for 20–30 min in 1 ml of lysis buffer consisting of 1% (vol/vol) detergent (Triton X-100 or Nonidet P-40), 137 mM NaCl, 50 mM Tris⋅HCl (pH 7.8), 10% (vol/vol) glycerol, 1 mM sodium orthovanadate, 2 mM EDTA (pH 8.0), plus protease inhibitors leupeptin and aprotinin (10 μg/ml each), and 1 mM phenylmethylsulfonyl fluoride. Cell debris was spun down for 15 min at 16,500 × g at 4°C. Proteins of cleared cellular lysates were size separated by SDS/10% PAGE. Phosphorylated substrate proteins were detected by anti-phosphotyrosine (4G10, Upstate Biotechnology, Lake Placid, NY) immunoblotting as described (12).
Results
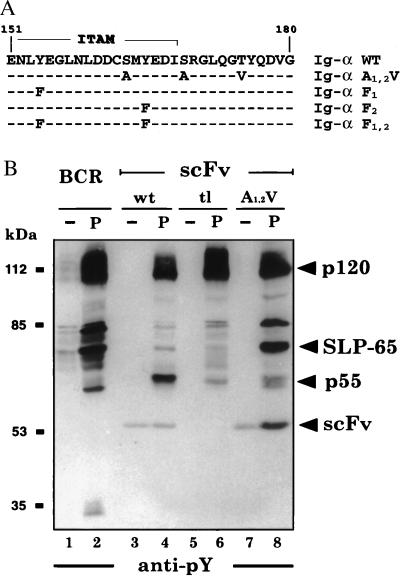
In the Ig-α cytoplasmic tail sequence, two serines are flanking the second ITAM tyrosine, which is followed by one threonine (Fig. 1A). To test whether phosphorylation of these amino acids plays a role in BCR function, we changed the two serines to alanines and the threonine to valine (A1,2V; Fig. 1A). These mutations generated an Ig-α cytoplasmic tail that can no longer be phosphorylated by serine/threonine kinases. The signaling function of the wild-type and the mutated Ig-α tail was first tested in the context of a chimeric antigen receptor. Extracellularly, this receptor carries a scFv fragment with specificity for the hapten nitrophenyl, followed by a CD8 linker and the transmembrane part of the TCR-ζ chain. The cytoplasmic part consists of wild-type Ig-α tail, a truncated tail of only five amino acids (RRIDP), or the A1,2V-mutated Ig-α tail. The three different scFv molecules were expressed on J558L cells, and their signaling function was compared with that of the complete BCR. Exposure of BCR-positive J558L cells to the phosphatase inhibitor pervanadate results in an increased PTK substrate phosphorylation (4). After treatment with 20 μM pervanadate, all J558L transfectants showed increased phosphorylation of a 120-kDa protein (Fig. 1B). In addition, the BCR-positive J558L cells also showed phosphorylation of 80-kDa and 65-kDa substrates, which we previously have identified as HS1 and SLP-65, respectively (4). Interestingly, these two substrates were phosphorylated weakly in cells expressing the scFv/Ig-α molecule (Fig. 1B, lanes 3 and 4). In these cells, the dominant substrate was a 55-kDa protein (p55), which was not phosphorylated in cells expressing the complete BCR. The phosphorylation of p55 required the Ig-α tail, because it was phosphorylated weakly in the scFv/tl-transfectant of J558L (Fig. 1B, lanes 5 and 6). Cells expressing the scFv/Ig-α-A1,2V mutated molecule did not show prominent p55 phosphorylation but displayed strong phosphorylation of HS1 and SLP-65 (Fig. 1B, lanes 7 and 8). The identity of p55 is yet unclear, but we have excluded the possibility that it is Lyn or another Src family kinase (data not shown).
Figure 1.
Mutation of the serine (S) and threonine (T) residues in the Ig-α tail increases the signal transduction from chimeric scFv/Ig-α molecules. (A) Position of the two serines and the threonine in the cytoplasmic sequence of Ig-α. The ITAM sequence is overlined. The mutations are shown in bold, and dashes indicate the identity to the wild-type Ig-α sequence. (B) PTK substrate phosphorylation in activated J558L B cells expressing the complete BCR (lanes 1 and 2) or chimeric scFv/Ig-α molecules with the wild-type Ig-α tail sequence (wt, lanes 3 and 4), a cytoplasmic sequence of only five amino acids, RRIDP (tl, lanes 5 and 6), or the A1,2V-mutated Ig-α tail (A1,2V, lanes 7 and 8). The cells were either left untreated (lanes 1, 3, 5, and 7) or exposed for 2 min to 20 μM pervanadate (lanes 2, 4, 6, and 8). Proteins of total cellular lysates were size-separated by SDS/10% PAGE and analyzed by Western blotting with the anti-pY antibody 4G10.
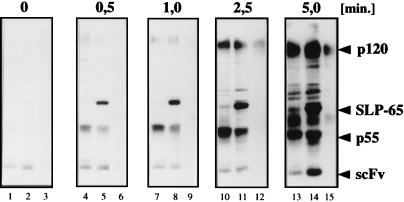
After pervanadate treatment, the Ig-α tail of the scFv/Ig-αA1,2V but not that of the scFv/Ig-α became tyrosine phosphorylated (Fig. 1B, lanes 8 and 4). To analyze the signaling behavior of the three different scFv molecules in more detail, we exposed the scFv transfectants of J558L for increasing times to 8 μM pervanadate (Fig. 2). In this time course experiment, the scFv/Ig-αA1,2V-expressing cells displayed rapid SLP-65 phosphorylation. At 0.5 and 1 min, SLP-65 was the dominant PTK substrate protein in scFv/Ig-αA1,2V transfectant (Fig. 2, lanes 5 and 8) but not at all in the scFv/Ig-α transfectant, which instead showed an increased p55 phosphorylation (Fig. 2, lanes 4 and 7). At later time points, the p55 substrate was also phosphorylated in the scFv/Ig-αA1,2V transfectant. The phosphorylation of ITAM tyrosines in the scFv/Ig-αA1,2V molecule was seen only after 5 min of stimulation (Fig. 2, lane 14). Thus, the rapid phosphorylation of SLP-65 and p55 does not require prior ITAM phosphorylation. Cells expressing the scFv/tl molecule displayed only a delayed p120 phosphorylation (Fig. 2, lane 15). Together, these experiments suggest that the serine/threonine residues in the Ig-α tail negatively regulate SLP-65 and ITAM phosphorylation. At the same time, these residues promote the tyrosine phosphorylation of p55, an as-yet unidentified PTK substrate.
Figure 2.
Time course analysis of pervanadate-induced tyrosine phosphorylation of PTK substrate proteins in scFv transfectants of J558L expressing scFv molecules with the wild-type Ig-α tail (lanes 1, 4, 7, 10, 13), the A1,2V-mutated Ig-α tail (lanes 2, 5, 8, 11, 14), or the tail truncation (lanes 3, 6, 9, 12, 15). Cells were stimulated for the indicated times with 8 μM pervanadate. The proteins of total cellular lysates were size-separated by SDS/10% PAGE and analyzed by 4G10 immunoblotting.
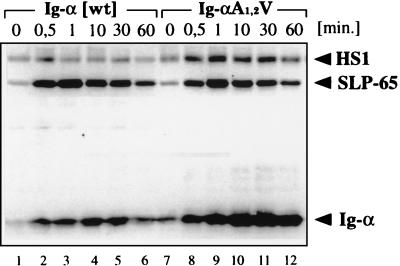
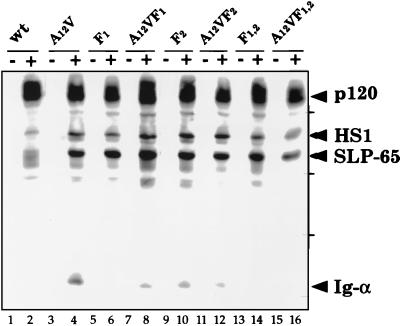
We next analyzed the effect of the serine/threonine mutations of Ig-α in the context of the complete BCR (Fig. 3). J558Lμm cells were transfected with a vector encoding either wild-type Ig-α or the A1,2V-mutated Ig-α protein. The BCR-positive transfectants J558Lμm/mb-1 and J558Lμm/mb-1A1,2V expressing similar amounts of the BCR on the cell surface were stimulated for different times with goat anti-mouse IgM antibodies. Both transfectants responded to BCR ligation with a rapid SLP-65 phosphorylation, but they differed from each other in their Ig-α phosphorylation. The tyrosine phosphorylation of the A1,2V-mutated Ig-α is stronger and more prolonged than that of the wild-type Ig-α. Thus, the serine and threonine residues in the Ig-α tail also negatively regulate ITAM phosphorylation in the context of the complete BCR. We have previously shown that on BCR engagement both ITAM tyrosines of Ig-α (Y1, Y2) become phosphorylated but the phosphorylation of the first tyrosine is more prominent. To analyze the effect of the A1,2V mutation on the phosphorylation of the two ITAM tyrosines, we exchanged these tyrosines in the Ig-αA1,2V sequence with phenylalanines. We analyzed in parallel J558Lμm transfectants expressing either Ig-α A1,2V or Ig-α proteins with these ITAM tyrosine mutations (Y1 > F1, Y2 > F2, and Y1,2 > F1,2) in the context of the complete BCR (Fig. 4). Transfectants expressing equal amounts of BCR on the surface (data not shown) were stimulated with 20 μM pervanadate. The PTK substrate phosphorylation in these cells was then analyzed by SDS/PAGE and Western blotting. This analysis showed that after the mutation of the first ITAM tyrosine, the second ITAM tyrosine is phosphorylated only weakly (Fig. 4, lane 6). The additional A1,2V mutation of the Ig-α tail (F1A1,2V) showed an increase in the phosphorylation of the second (Y2) ITAM tyrosine (Fig. 4, lane 8). The same analysis of the Y2 > F2 mutations in the different Ig-α tails did not show drastic differences in Ig-α phosphorylation (Fig. 4, lanes 10 and 12). Therefore, this analysis suggests that the serine/threonine residues mostly regulate tyrosine phosphorylation of the second tyrosine (Y2) in the ITAM of Ig-α. Indeed, the second ITAM tyrosine is flanked by the two serines and is closer to the threonine than the first ITAM tyrosine.
Figure 3.
The time course analysis of PTK substrate phosphorylation in stimulated J558L cells expressing either a wild-type (wt) BCR (lanes 1–6) or a BCR with an A1,2V-mutated Ig-α protein (lanes 7–12). Cells were stimulated for the indicated times with 10 μg/ml of goat anti-mouse IgM antibodies. The proteins of total cellular lysates were size-separated by SDS/10% PAGE and analyzed by 4G10 immunoblotting.
Figure 4.
Influence on the A1,2T mutation of the Ig-α tail sequence on the phosphorylation of the two ITAM tyrosines. Western blot analysis of PTK substrate phosphorylation in J558L/Ig-α transfectants expressing either wild-type (wt) Ig-α (lanes 1 and 2) or mutations of Ig-α (lanes 3–16). The cells either were left unstimulated or were stimulated for 3 min with 20 μM pervanadate. The proteins of total cellular lysates were size separated by SDS/10% PAGE and analyzed by 4G10 immunoblotting.
Discussion
Little is known about the function of serine/threonine phosphorylation that occurs on the signaling subunits of multisubunit immune recognition receptor family receptors (9, 27). We found that a mutated Ig-α protein lacking all serine/threonine residues in its cytoplasmic tail displays an increased ITAM phosphorylation. This finding suggests that serine/threonine kinases are negatively regulating the signaling output from the BCR. Serine/threonine residues are found in cytoplasmic sequences of many receptors containing an ITAM (3, 35). Interestingly, serine/threonine residues are more frequently found around the second ITAM tyrosine, and our mutational analysis suggests that it is the second tyrosine that becomes more strongly phosphorylated after the serine/threonine to alanine/valine mutation. In the case of the FcɛRIβ chain, direct biochemical evidence suggested that phosphorylation occurs prominently at S229 in the YSAL sequence of the second ITAM tyrosine (36). The TCR signaling subunits CD3-ɛ, CD3-δ, and CD3-γ all contain a serine at this position (3). The second ITAM tyrosine is bound by the N-terminal SH2 domain of either ZAP70 or Syk, and this binding is stronger than that of the C-terminal SH2 domain to the first ITAM tyrosine (15). A serine/threonine kinase, phosphorylating residues close to the second ITAM tyrosine and thereby inhibiting the ITAM phosphorylation could therefore efficiently regulate the coupling between ZAP70/Syk to multisubunit immune recognition receptor family receptors. The Ig-β signaling subunit of the BCR becomes tyrosine phosphorylated only weakly on receptor engagement, and the reduced tyrosine phosphorylation may be due to the fact that both ITAM tyrosines of Ig-β are surrounded by serine/threonine residues. Indeed, after an in vitro kinase reaction of purified BCR complexes, Ig-β is phosphorylated more strongly than Ig-α on serine/threonine residues (9).
The exposure of J558L B cells to either antigen or pervanadate results in an increased phosphorylation of PTK substrates (4, 37). The tyrosine phosphorylation of the Ig-α/Ig-β heterodimer, however, is not increased drastically by pervanadate (see Fig. 4, lane 2). It is possible that pervanadate blocks not only tyrosine but also serine/threonine phosphatases. Thus, pervanadate treatment is expected to result in an increased serine/threonine phosphorylation of the Ig-α/Ig-β heterodimer. The stronger serine/threonine phosphorylation could prevent the subsequent phosphorylation of the ITAM tyrosines as suggested by our finding that, on pervanadate treatment, the A1,2V mutated Ig-α proteins become more strongly tyrosine phosphorylated (see Fig. 4, lane 4).
The identity of the serine/threonine kinases that phosphorylate the Ig-α/Ig-β heterodimer is not known thus far. A serine/threonine kinase activity was copurified with the cytoplasmic tail of either Ig-α or Ig-β (9). In another report, a serine/threonine kinase was described to associate with membrane-bound Ig molecules lacking the Ig-α/Ig-β heterodimer (38). It is also not clear under which condition the serine/threonine phosphorylation of Ig-α and/or Ig-β occurs. The unligated BCR may already be serine/threonine phosphorylated, and this phosphorylation could prevent its nonspecific activation or modify the postulated BCR maintenance signal (39, 40). Alternatively, serine/threonine phosphorylation may occur only after BCR engagement and may be part of a negative feedback loop to terminate BCR signal transduction. Our finding that T cells expressing the A1,2V mutated scFv/Ig-α molecule have a constitutive NF-AT activation would be in line with the first possibility (data not shown).
How serine/threonine phosphorylation can inhibit the ITAM tyrosine phosphorylation is not known. However, several examples have now been reported whereby phosphorylation of serine/threonine residues allows protein–protein interactions (41), which uncouple a receptor from its signal transducer (42, 43). The rapid phosphorylation of p55 observed on stimulation of the scFv/Ig-α wild-type, but not of its serine/threonine mutant, may reflect such a mechanism. Indeed, apart from its positive, ITAM-dependent signaling function, the Ig-α tail also has been implicated in BCR signal inhibition, because B cells expressing a BCR with an Ig-α truncation are hyperactive and can be stimulated more easily (44, 45). Moreover, an Ig-α-associated protein of 52 kDa (alpha-4) has been identified as the adapter protein for the protein-serine/threonine phosphatase PP2a (46–48). In another report, a 55-kDa protein was found to be associated with Ig-α but not with Ig-β (49). Further analysis is required to test whether p55 associates with the BCR and directly participates in signal inhibition.
In summary, our data suggest that the signal transduction from the BCR is under a multilevel control involving a positive and a negative feedback regulation. The latter seems to involve the phosphorylation of highly conserved serine/threonine residues in the cytoplasmic tail of Ig-α and Ig-β.
Acknowledgments
We thank Drs. P. J. Nielsen and L. Leclercq for critically reading the manuscript and U. Braun for expert technical help. This work was supported by the Deutsche Forschungsgemeinschaft through SFB 364 and the Leibniz Price.
Abbreviations
- BCR
B cell antigen receptor
- ITAM
immunoreceptor tyrosine-based activation motif
- TCR
T cell antigen receptor
- PTK
protein tyrosine kinase
- SH2
Src homology 2
- scFv
variable fragment single chain
References
- 1.Cambier J C. J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 2.DeFranco A L. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 3.Reth M. Nature (London) 1989;338:383. [Google Scholar]
- 4.Wienands J, Larbolette O, Reth M. Proc Natl Acad Sci USA. 1996;93:7865–7870. doi: 10.1073/pnas.93.15.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wienands J, Schweikert J, Wollscheid B, Jumaa H, Nielsen P J, Reth M. J Exp Med. 1998;188:791–795. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu C, Turck C W, Kurosaki T, Chan A C. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 7.Goisuka R, Fujimura Y-i, Mamada H, Umeda A, Morimura T, Uetsuka K, Doi K, Tsuji S, Kitamura D. J Immunol. 1998;161:5804–5808. [PubMed] [Google Scholar]
- 8.Gold M R, Matsuuchi L, Kelly R B, DeFranco A L. Proc Natl Acad Sci USA. 1991;88:3436–3440. doi: 10.1073/pnas.88.8.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark M R, Campbell K S, Kazlauskas A, Johnson S A, Hertz M, Potter T A, Pleiman C, Cambier J C. Science. 1992;258:123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- 10.Flaswinkel H, Reth M. EMBO J. 1994;13:83–89. doi: 10.1002/j.1460-2075.1994.tb06237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Songyang Z, Shoelson S E, McGlade J, Olivier P, Pawson T, Bustelo X R, Barbacid M, Sabe H, Hanafusa H, Yi T, et al. Mol Cell Biol. 1994;14:2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann G, Maier D, Freuler F, Tschopp C, Baudisch K, Wienands J. Eur J Immunol. 1994;24:1799–1807. doi: 10.1002/eji.1830240812. [DOI] [PubMed] [Google Scholar]
- 13.Cambier J C, Johnson S A. Immunol Lett. 1995;44:77–80. doi: 10.1016/0165-2478(94)00196-x. [DOI] [PubMed] [Google Scholar]
- 14.Labadia M E, Ingraham R H, Schembri-King J, Morelock M M, Jakes S. J Leukocyte Biol. 1996;59:740–746. [PubMed] [Google Scholar]
- 15.Futterer K, Wong J, Grucza R A, Chan A C, Waksman G. J Mol Biol. 1998;281:523–537. doi: 10.1006/jmbi.1998.1964. [DOI] [PubMed] [Google Scholar]
- 16.Rowley R B, Burkhardt A L, Chao H G, Matsueda G R, Bolen J B. J Biol Chem. 1995;270:11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- 17.Kurosaki T, Johnson S A, Pao L, Sada K, Yamamura H, Cambier J C. J Exp Med. 1995;182:1815–1823. doi: 10.1084/jem.182.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vely F, Nunes J A, Malissen B, Hedgecock C J. Eur J Immunol. 1997;27:3010–3014. doi: 10.1002/eji.1830271138. [DOI] [PubMed] [Google Scholar]
- 19.Cassard S, Salamero J, Hanau D, Spehner D, Davoust J, Fridman W H, Bonnerot C. J Immunol. 1998;160:1767–1773. [PubMed] [Google Scholar]
- 20.Crowley M T, Costello P S, Fitzer-Attas C J, Turner M, Meng F, Lowell C, Tybulewicz V L, DeFranco A L. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strzelecka A, Kwiatkowska K, Sobota A. FEBS Lett. 1997;400:11–14. doi: 10.1016/s0014-5793(96)01359-2. [DOI] [PubMed] [Google Scholar]
- 22.Mufson R A. FASEB J. 1997;11:37–44. doi: 10.1096/fasebj.11.1.9034164. [DOI] [PubMed] [Google Scholar]
- 23.Gandino L, Longati P, Medico E, Prat M, Comoglio P M. J Biol Chem. 1994;269:1815–1820. [PubMed] [Google Scholar]
- 24.Gold M R, Chiu R, Ingham R J, Saxton T M, van Oostveen I, Watts J D, Affolter M, Aebersold R. J Immunol. 1994;153:2369–2380. [PubMed] [Google Scholar]
- 25.Lim C P, Cao X. J Biol Chem. 1999;274:31055–31061. doi: 10.1074/jbc.274.43.31055. [DOI] [PubMed] [Google Scholar]
- 26.Li J, DeFea K, Roth R A. J Biol Chem. 1999;274:9351–9356. doi: 10.1074/jbc.274.14.9351. [DOI] [PubMed] [Google Scholar]
- 27.Van Noesel C J, Borst J, De Vries E F, Van Lier R A. Eur J Immunol. 1990;20:2789–2793. doi: 10.1002/eji.1830201238. [DOI] [PubMed] [Google Scholar]
- 28.Leprince C, Draves K E, Ledbetter J A, Torres R M, Clark E A. Eur J Immunol. 1992;22:2093–2099. doi: 10.1002/eji.1830220820. [DOI] [PubMed] [Google Scholar]
- 29.Bothwell A L M, Pasking M, Reth M, Imanishi-Kari T, Rajewsky K, Baltimore D. Cell. 1981;24:625–632. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- 30.Huston J S, Levinson D, Mudgett-Hunter M, Tai M-S, Novotny J, Margolies M N, Ridge R J, Bruccoleri R E, Haber E, Crea R, et al. Proc Natl Acad Sci USA. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brocker T, Peter A, Traunecker A, Karjalainen K. Eur J Immunol. 1993;23:1435–1439. doi: 10.1002/eji.1830230705. [DOI] [PubMed] [Google Scholar]
- 32.Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M. Nature (London) 1990;343:760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- 33.Oi V T, Morrison S L, Herzenberg L A, Berg P. Proc Natl Acad Sci USA. 1983;80:825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hombach J, Leclercq L, Radbruch A, Rajewsky K, Reth M. EMBO J. 1988;7:3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cambier J C. Immunol Today. 1995;16:110. doi: 10.1016/0167-5699(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 36.Pribluda V S, Pribluda C, Metzger H. J Biol Chem. 1997;272:11185–11192. doi: 10.1074/jbc.272.17.11185. [DOI] [PubMed] [Google Scholar]
- 37.Lin J, Brown V K, Justement L B. J Immunol. 1992;149:3182–3190. [PubMed] [Google Scholar]
- 38.Williams G T, Peaker C J, Patel K J, Neuberger M S. Proc Natl Acad Sci USA. 1994;91:474–478. doi: 10.1073/pnas.91.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Law D A, Chan V W F, Datta S K, DeFranco A. Curr Biol. 1993;3:645–657. doi: 10.1016/0960-9822(93)90062-s. [DOI] [PubMed] [Google Scholar]
- 40.Neuberger M S. Cell. 1997;90:971–973. doi: 10.1016/s0092-8674(00)80362-1. [DOI] [PubMed] [Google Scholar]
- 41.Yaffe M B, Cantley L C. Nature (London) 1999;402:30–31. doi: 10.1038/46925. [DOI] [PubMed] [Google Scholar]
- 42.Bunemann M, Hosey M M. J Physiol (London) 1999;517:5–23. doi: 10.1111/j.1469-7793.1999.0005z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeVine H., III Mol Neurobiol. 1999;19:111–149. doi: 10.1007/BF02743657. [DOI] [PubMed] [Google Scholar]
- 44.Kraus M, Saijo K, Torres R M, Rajewsky K. Immunity. 1999;11:537–545. doi: 10.1016/s1074-7613(00)80129-6. [DOI] [PubMed] [Google Scholar]
- 45.Torres R M, Hafen K. Immunity. 1999;11:527–536. doi: 10.1016/s1074-7613(00)80128-4. [DOI] [PubMed] [Google Scholar]
- 46.Inui S, Kuwahara K, Mizutani J, Maeda K, Kawai T, Nakayasu H, Sakaguchi N. J Immunol. 1995;154:2714–2723. [PubMed] [Google Scholar]
- 47.Inui S, Sanjo H, Maeda K, Yamamoto H, Miyamoto E, Sakaguchi N. Blood. 1998;92:539–546. [PubMed] [Google Scholar]
- 48.Nanahoshi M, Tsujishita Y, Tokunaga C, Inui S, Sakaguchi N, Hara K, Yonezawa K. FEBS Lett. 1999;446:108–112. doi: 10.1016/s0014-5793(99)00189-1. [DOI] [PubMed] [Google Scholar]
- 49.Clark M R, Johnson S A, Cambier J C. EMBO J. 1994;13:1911–1919. doi: 10.1002/j.1460-2075.1994.tb06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]