Abstract
Spontaneous tumors are reported to occur in 45% to 71% of Sprague-Dawley rats, yet few studies have considered the effect of the sedentary condition of standard laboratory cages on tumorigenesis. Tumor profiles and tumor promoting hormone prolactin were compared in female Sprague-Dawley rats (108) that were allocated into 3 groups: those housed without outside activity (SED group), with twice-weekly 1-h sessions of physical activity in large box (PA group), and with regular voluntary running-wheel exercise (EX). Compared with the EX group, SED rats had more and larger tumors throughout most of their lifespan; tumor profiles of PA rats were similar to those of the SED group. A larger percentage of animals in the SED group had tumors (54%), compared with EX rats (38%). At 64 wk, tumors in SED animals included thyroid carcinoma, malignancy, mammary fibroadenoma, cystadenoma, and granuloma, whereas benign mammary gland cysts were most common in EX. Prolactin levels were highest in SED animals at 24 and 52 wk. In conclusion, increased tumor number, increased tumor size, type of spontaneous tumor, and increased prolactin in rats were associated with standard laboratory housing, which limited physical activity, and were not primarily due to aging.
Knowledge about the underlying causes of mammary tumor development has been advanced by the use of rodent models for various reasons, including their relatively short lifespan and the ability to experimentally induce tumorigenesis. Most studies have investigated experimentally induced tumor growth, but such experiments are unlikely to predict the incidence of spontaneous tumor development. After the first year of age, spontaneous tumor development typically occurs at the rate of 45% to 71% in laboratory rats, in which the percentage of females with tumors is almost double that in males.7,13 This high percentage of tumor-bearing rats probably reflects the effects of sex, age, and tumor-promoting hormones on tumorigenesis. However, a potentially overlooked factor is the sedentary cage environment of virtually all laboratory animals. The detrimental effect of the chronic, low-activity environment typical of a standard cage on aging and disease is supported indirectly by research indicating that regular exercise mitigates tumor growth.1,8,10,11,15,17 An environment whereby an animal engages in extremely low levels of physical activity over a long period of time is likely to have a strong influence-more than has been previously appreciated- on spontaneous tumor development. The influence of residing in a standard cage on spontaneous tumor development in laboratory rats has not been studied systematically over a typical lifespan.
The paradigm in which animals that reside solely in standard caging serve as referent controls in experiments has recently been questioned because of research indicating health consequences associated with long-term physical inactivity.1,3 Nevertheless, providing laboratory animals access to physical activity is complicated in some experimental designs due to different feeding regimens, surgical interventions, and so on. The effect of regular access to exercise on tumor number and size in laboratory animals depends on whether the exercise is forced (increases tumors) or voluntary (decreases tumors).9,15,17 Prolactin (PRL) secretion plays an important regulatory role in tumorigenesis. Reducing PRL secretion in animals through voluntary exercise may attenuate tumor development.1 In contrast, when exercise is forced, stress-related hormones and tumor development increase.10 The danger in not providing laboratory animals access to physical activity and, in effect, forcing animals to be sedentary is that the types of hormonal and metabolic changes associated with being sedentary can affect the spontaneous development of tumors.6 Long-term studies that assess the influence of housing on spontaneous tumor development are sparse. Furthermore, why the rate of tumor development in female rats is typically twice that of male rats is unclear.7,13,16 A systematic long-term investigation on the environment influences tumor development throughout the life of female rats is needed.
The purpose of this study was to determine how spontaneous tumor growth in female Sprague–Dawley rats residing in standard cages compared with that in animals that had access to regular voluntary exercise or to twice-weekly 1-h access to voluntary physical activity outside a standard cage. Unlike most previous studies in which tumors were chemically induced in exercise and sedentary groups, this study addressed naturally occurring or spontaneous tumors.
Materials and Methods
Animals.
Female Sprague–Dawley rats (n = 108; age, 3 wk; Charles River,Wilmington, MA) were weighed and divided randomly into 3 equal groups in standard cages (0.454 m × 0.238 m × 0.20 m) and receiving: 1) no additional opportunities for exercise (SED group); 2) two 1-h sessions of physical activity in a large box each week (PA group); and 3) access every other day for 24 h to a wheel for voluntary exercise. Corncob bedding (Bed-O-Cobs, Maumee, OH) was used in all cages. Animals were housed in pairs within their designated group in climate-controlled rooms (24 ± 2 °C) with a 12:12-h light:dark cycle on a normal day cycle, with free access to water and food (LabDiet 5001 Rodent Chow, Purina, St Louis, MO). The heath surveillance program included comprehensive serology: samples were sent quarterly from each rat colony to the Research Animal Diagnostic Laboratory at the University of Missouri (Columbia, MO). Parasitology was done inhouse. All test results were negative. The study was conducted in accordance with ethical procedures and policies and was approved by the institutional animal care and use committee.
Activity and exercise protocols and measurements.
Handling procedures were similar for all animals, with weekly weighing, numbering, tumor measurements, and cage changes and biweekly blood pressure measurements. Systolic blood pressure, diastolic blood pressure, and heart rate were measured by a noninvasive tail cuff method while each animal was restrained in a plastic tube that was placed on a warming mat (38 °C). A photoelectric sensor in the tail cuff, an amplifier (model 31 NIBP, IITC Life Sciences, Woodland Hills, CA), and software (IITC Life Sciences) were used for pulse detection. Once the initial threshold was reached, systolic blood pressure was determined. All animals completed the technique and yielded informative recordings within approximately 5 min.
Physical activity [distance (in meters) travelled in 24 h) was monitored in all groups. The SED group was placed under 24-h surveillance once monthly and an infrared camera (model VDI-2000BI B/W, PI Manufacturing, Walnut, CA) used to record movement within a standard cage. Two animals were randomly selected each month for tracking. Recorded videos were evaluated to measure total distance covered in 24 h. The PA and EX groups also underwent 24-h surveillance monthly when they resided in a standard cage.
Animals in the PA group were removed from their standard cages and placed in a large plastic activity box (1.21 m × 0.59 m × 0.45 m) for 1 h of voluntary physical activity twice each week. The activity box was equipped with 4 small platforms of different heights (0.15 and 0.19 m) in each corner and 2 plastic tubes (0.66 m) along the lengths of the walls, to encourage movement. Six animals were placed in a box at a time. Two animals were selected randomly and marked for tracking purposes. The distance covered by PA rats in the physical activity box were tracked monthly by recording with an overhead high-speed 16-mm digital camera (shutter speed, 60 frames per second; Sony, San Diego, CA) followed by manual tracing of the animals' movement on a computer screen. Total distance covered in meters per hour was averaged.
Animals in the EX group had free access to running wheels for 24 h on alternate days. On days when they did not have access to the wheel, rats in the EX group were housed in pairs like the animals in both other groups. Voluntary running wheels (Nalgene, Rochester, NY) for the EX group were connected to magnetic switches that recorded the number of revolutions per day, translated into meters per day.
Food consumption measurements.
Food intake over 24 h was measured twice during the study (when the animals were 24 and 64 wk old). The weight of food remaining in the food tray was subtracted from the weight of food placed in the food tray 24 h before.
Trunk fat measurement.
When 64 wk old, a subset of animals (n = 10) from each group was euthanized by decapitation and exsanguination. Fat from the abdominal cavity was removed and weighed and its volume measured by water displacement.
Tumor measurements.
Once palpable tumors appeared (typically beginning at 59 wk of age), the position, date of appearance, and size of tumors were recorded weekly for each rat. The percentage of animals with palpable tumors was recorded for each group. The mean number of tumors per animal (tumor multiplicity) was calculated. We used calipers to measure tumor size (in millimeters) at initial discovery of the mass and weekly thereafter.
At 64 wk, a subset of 12 animals from each group was euthanized by decapitation and exsanguination, and tumors were excised from animals. Tumors were weighed and measured for volume and immediately placed in 10% formalin solution for 24 h, sliced, and replaced in formalin. A random subsample of tumors was sent to the Animal Disease Diagnostic Laboratory (Reynoldsburg, OH) for histopathology. Animals remaining in the experiment after 64 wk were monitored as described earlier. Tumors were excised from the remaining animals only after the veterinarian determined that the rat should be euthanized.
PRL assay.
Blood was collected for evaluation of serum PRL by making a small incision in the tail and by exsanguination after decapitation. Serum PRL was measured at 24, 48, and 64 wk with a double-antibody radioimmunoassay using reagents from Dr AF Parlow, Scientific Director, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA. Radioiodinated rat RP3PRL was purchased from Perkin-Elmer, Waltham, MA.
Statistics.
Analysis of variance with and without repeated measures and Bonferonni and Dunn post hoc tests were used to compare data. SuperANOVA (Abacus Concepts, Berkeley, CA) was used to run the analyses. Differences were considered to be statistically significant when the P value was less than 0.05.
Results
Daily physical activity and exercise.
The average distance covered during 24 h did not differ among SED, PA, and EX over 64 wk. The distance covered by SED and PA animals peaked at 24 wk of age, with a daily distance of 272 ± 30 m/d. From 24 wk until death, the distance averaged only 70 ± 8 m/d. Access to hourly sessions in a large box twice weekly only added approximately 60 m to a typical 24-h day (135 ± 21 m/d) for the PA group. In contrast, the mean distance covered by the EX group peaked at 10 wk (16,667 ± 817 m/d), averaged 4157 ± 767 m/d, and then decreased to 135 ± 57 m/d by 120 wk.
Effects of aging on body weight and trunk fat at 64 wk.
Body weight increased in age for all groups but was not significantly different among groups at any time during the lifetime study.
At 64 wk, a sample of animals from each group was euthanized, and visceral fat was removed from the trunk area. Mean visceral fat mass was 20.9 ± 1.2 g in the SED group, 23.3 ± 1.4 g in the PA group, and 19.9 ± 1.2 g for the EX group. Mean visceral fat volume was 22.8 ± 1.3 mL for SED animals, 26.1 ± 1.7 mL for PA animals, and 22.0 ± 1.5 mL in EX rats. Differences in fat mass and volume were significant (P < 0.050) only between the PA and EX groups.
Food consumption.
Food consumption over 24 h was measured twice during a 2-wk period when the animals were 24 wk old. Consumption was higher in the EX group than in the PA or SED groups (P < 0.05).
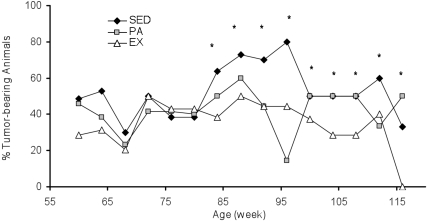
Percentage of tumor-bearing animals among aging rats.
The percentage of animals in each group with palpable tumors beginning at age 60 wk, when palpable tumors were detected in all groups, is displayed in Figure 1. During weeks 60 to 120, the EX group on average had the smallest percentage of tumor-bearing animals (38%) and the SED group the largest (54%). The percentage of tumor-bearing animals in the PA group was 42%. Bonferonni and Dunn analyses of the percentage of tumor-bearing animals in each of the 3 groups over time indicated significant differences between SED and EX rats (P = 0.005) and between SED and PA animals (P = 0.023).
Figure 1.
Percentage of rats in the SED, PA, and EX groups with tumors during weeks 60 through 120. *, Percentage of tumor-bearing animals was greater (P < 0.05) in the SED group than among EX rats.
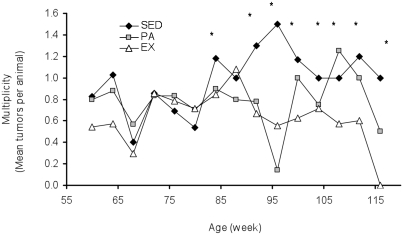
Tumor multiplicity in aging rats.
The mean number of palpable tumors per animal (multiplicity) is shown in Figure 2. After 88 wk, tumor multiplicity was 0.69 for EX animals, 0.75 for PA rats, and 0.96 for SED animals. Bonferonni and Dunn analyses of mean tumor multiplicity showed significantly lower mean tumor multiplicity among EX rats than SED animals (P = 0.012) and among SED rats compared with PA animals (P = 0.048).
Figure 2.
Tumor multiplicity (mean number of tumors per animal) during weeks 60 through 120 in SED, PA, and EX rats. *, Tumor multiplicity was greater (P < 0.05) in SED rats than EX animals.
Average tumor size in aging rats.
Compared with animals in the SED and PA groups, the rats in the EX group had the smallest tumors at almost every age (Figure 3). Bonferonni and Dunn analyses of mean tumor size over time showed significantly slower tumor growth in EX rats compared with SED (P = 0.013) and PA (P = 0.014) animals.
Figure 3.
Tumor size (mean ± SEM) for SED, PA, and EX rats during weeks 60 through 120. Tumors were smaller (P < 0.05) in EX rats than in PA (a) and SED (b) animals.
Tumor analyses at 64 wk.
Only tumors that were palpable and located ventrally on the rat were excised for analysis. Histologic evaluation on all tumors removed from 6 animals per group at age 64 wk (Table 1) was performed by a licensed veterinarian trained in rat tumor pathology. Most tumors were benign. None of 6 tumors from the EX group were malignant, compared with 2 of 6 tumors from the SED group and 2 of 7 tumors from the PA group. In this study, spontaneous tumors included epithelial and connective tissue components that varied from adenoma to sarcoma. Virtually all of the tumors were embedded in mammary glands, although 3 of the 4 malignant tumors were thyroid. Some tumors displayed secretions (for example, blood, milky solution), whereas others were dense with no noticeable secretory fluids. There were several instances of infiltrating ductal carcinomas, in which tumors spread into surrounding skeletal muscles.
Table 1.
Number, type, and size of tumors in 64-wk-old female Sprague–Dawley rats (n = 36 per group)
| SED | PA | EX | |
| Number of rats with tumors | 23 | 22 | 14 |
| Total number of tumors | 66 | 52 | 36 |
| Tumor size (mm, mean ± SEM) | 22 ± 6 | 28 ± 10 | 12 ± 5a |
| Types (number) of tumors | Granuloma (1) Mammary cystadenoma (1) Mammary cyst (4) Neoplastic invasion of adjacent skeletal muscle (malignant; 1) Ovarian cyst (1) Thyroid carcinoma (malignant; 1) | Mammary cystadenoma (1) Mammary fibroadenoma (1) Mammary gland cyst (4) Thyroid carcinoma (malignant; 2) | Endometrial polyp (2) Mammary fibroadenoma (1) Mammary gland cyst (4) |
Value for EX rats significantly (P < 0.05) lower than that for SED and PA rats.
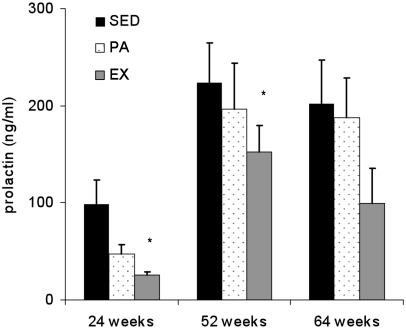
Prolactin levels at different ages.
Mean serum PRL levels at 24 and 52 wk were lowest in the EX rats and highest in the SED group (P < 0.05; Figure 4). PRL at 64 wk did not differ among the 3 groups, although the trend was EX < PA < SED.
Figure 4.
Serum prolactin levels (mean ± SEM) of SED, PA, and EX rats at 24, 52, and 64 wk of age. *, Serum prolactin was lower (P < 0.05) in EX rats than in either PA or SED animals.
Discussion
The development of tumors in adult Sprague–Dawley rats has been attributed to normal aging as well as specific hormones, stress, and forced exercise.15 What has not been investigated in a systematic way is the effect of standard laboratory housing over a typical lifespan on spontaneous tumor growth and development in laboratory rodents. The environment in which the animal lives may be important—more so than previously thought— in determining the rate and type of tumor development. The animals' environment, particularly housing conditions (which in turn determine access to physical activity or exercise), may play a greater role in spontaneous mammary tumorigenesis than does sex or aging.
During the first 60 wk of the present study, tumor number and size were similar among all groups. However at 64 wk, with few exceptions, tumor growth and development differed in several important ways depending on how animals were housed (Figure 3). Previous reports of percentage of animals bearing spontaneous tumors ranged from 45% to 71%.7,13,14 In the present study, the SED group exceeded this range, with percentage tumor-bearing animals peaking at 80% at 104 wk. The percentage of rats with tumors peaked at 60% for PA and 50% for EX at 88 wk. These results indicate that adult rats with no access to exercise beyond standard caging throughout their lifespan had a higher rate of tumor formation in adulthood than did animals with free access to exercise.
In a previous study of female rats, all of which were housed in standard cages, more than 95% of tumors in female Sprague–Dawley rats were mammary tumors classified histologically as adenoma, adenofibroma, fibroma, or adenocarcinoma and approximately 66% of the mammary tumors were benign rather than malignant.16 In the present study, 64-wk-old SED and PA female rats had malignant tumors but the EX group did not. Histologic analyses of tumors from SED and PA rats were similar and included malignant types such as thyroid carcinoma and neoplastic invasion of adjacent skeletal muscle, as well as benign tumors such as mammary gland cyst, granuloma cyst, and mammary cystadenoma. In contrast, tumors in the EX group included mammary gland cysts, polyps, and adenomas, all of which were benign.
Results from the current study suggest that the tumorigenesis and malignancy that have been considered as part of typical aging processes in laboratory rats are exacerbated by standard laboratory housing conditions. There is little evidence available to confirm breeding history (for example, recorded number of litters, number of newborns, age of the mother at the time of her first litter, preweanling mortality) influences tumor development in rats. An important finding from the current study is that animals that resided solely in their cages without opportunity of increased physical exercise showed higher percentage tumor-bearing, multiplicity, size, and malignancy with age, especially during the second half of their lifespan, when compared with animals that had access to a running wheel. Results from the PA group were surprising in some respects, given that tumor results were virtually indistinguishable from those of the SED group. On further examination, however, the results for the PA group support previous studies,4,10 in which a minimal daily distance (at least 4000 m) and a minimal intensity (moderate) associated with regular exercise were necessary to observe significant differences in tumor size and growth. In this study, neither the PA nor SED animals came close to that minimal daily distance, but the EX animals, which voluntarily ran on a running wheel more than 4000 m/d on average, apparently did.
Tumor size remained attenuated in EX over the course of their lifespan, despite the decline in daily running. Compared with EX rats, SED and PA animals both participated in very little daily physical activity and demonstrated larger tumors and higher multiplicity, especially in old age. These results strongly suggest that the spontaneous tumor profile of animals provided access to exercise over their lifespan is likely a more typical age-related model of tumor development than are animals denied access to regular physical activity or exercise—a condition that should not be considered as a referent control but instead should be seen as abnormal.
Energy balance is associated with cancer risk.12 Body weight did not differ among the 3 groups throughout life, supporting previous studies that exercise-induced body weight changes occur in male but not female rats.5 Interestingly, visceral fat weight and volume only differed between EX and PA rats, with SED animals indistinguishable from EX. This result is difficult to explain, and the use of more technically advanced body composition methods may elucidate the basis for these results.
Prolactin, a peptide hormone synthesized and secreted by lactotroph cells, is considered to be a stress marker.15 The PRL level also increases as a result of a sedentary environment, nonvoluntary, acute exercise,1 and forced restraint6 and is directly associated with tumor growth.2,13 Prolactin levels were lower in EX animals than SED and PA rats. This result was expected due to the well-accepted stress-reducing benefits of regular exercise. With age, PRL increased in all groups, but PRL levels seemed to show a stepwise pattern: lowest in the EX rats, intermediate for the PA animals, and highest in the SED group. The beneficial effects of regular exercise diminished over time. Despite the stepwise pattern, after 64 wk, PRL levels were statistically similar among the 3 experimental groups. This result likely implicates the convergence of age and reduced physical activity on PRL levels.
In summary, animals that had no access to physical activity or exercise outside a standard cage demonstrated higher percentage tumor-bearing, multiplicity, average tumor size, and malignancy with age compared with animals provided regular access to exercise. These phenotypes were accompanied by increased PRL levels and implicate the sedentary environment inherent in a typical laboratory cage, more so than sex or age, as a main contributor to spontaneous tumor development in rats. Long-term animal experiments should take into account the powerful effect of environment, in particular the laboratory housing and access to physical activity and exercise, on the health of the animal.
Acknowledgments
The authors acknowledge support from the National Institutes on Aging (grant R15 AG 20526-01A1) and Linda Zehler, Director of Laboratory Animal Resources.
References
- 1.Alessio HM, Hagerman AE, Nagy S, Philip BN, Byrnes RN, Woodward JL, Callahan P, Wiley RL. 2005. Exercise improves biomarkers of health and stress in animals fed ad libitum. Physiol Behav 84:65–72 [DOI] [PubMed] [Google Scholar]
- 2.Ben-Jonathan N, Liby K, McFarland M, Zinger M. 2002. Prolactin as an autocrine–paracrine growth factor in human cancer. Trends Endocrinol Metab 13:245–250 [DOI] [PubMed] [Google Scholar]
- 3.Booth FW, Lees SJ. 2006. Physically active subjects should be the control group. Med Sci Sports Exerc 38:405–406 [DOI] [PubMed] [Google Scholar]
- 4.Colbert LH, Davis JM, Essig DA, Ghaffar A, Mayer EP. 2000. Exercise and tumor development in a mouse predisposed to multiple intestinal adenomas. Med Sci Sports Exerc 32:1704–1708 [DOI] [PubMed] [Google Scholar]
- 5.Cortright RN, Chandler MP, Lemon PWR, DiCarlo SE. 1997. Daily exercise reduces fat, protein, and body mass in male but not female rats. Physiol Behav 62:105–111 [DOI] [PubMed] [Google Scholar]
- 6.Demarest KT, Moore KE, Riegle GD. 1985. Acute restraint stress decreases dopamine synthesis and turnover in the median eminence: a model for the study of the inhibitory neuronal influence on tuberoindfundibular dopaminergic neurons. Neuroendocrinology 41:504–508 [DOI] [PubMed] [Google Scholar]
- 7.Durbin PW, Williams MH, Jeung N, Arnold JS. 1966. Development of spontaneous mammary tumors over the life span of the female Charles River Sprague–Dawley rat: the influence of ovariectomy, thyroidectomy, and adrenalectomy–ovariectomy. Cancer Res 26:400–411 [PubMed] [Google Scholar]
- 8.Frisch RE, Wyshak G, Albright NL, Albright TE, Schiff I, Jones KP, Witschi J, Shiang E, Koff E, Marguglio M. 1985. Lower prevalence of breast cancer and cancers of the reproductive system among college athletes compared to nonathletes. Br J Cancer 52:885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman-Goetz L. 2003. Physical activity and cancer prevention: animal–tumor models. Med Sci Sports Exerc 35:1828–1833 [DOI] [PubMed] [Google Scholar]
- 10.Jonsdottir IH, Hoffman P. 2000. The significance of intensity and duration of exercise on natural immunity in rats. Med Sci Sports Exerc 32:1908–1912 [DOI] [PubMed] [Google Scholar]
- 11.Lee IM, Sesso HD, Chen JJ, Paffenbarger RS., Jr 2001. Does physical activity play a role in the prevention of prostate cancer? Epidemiol Rev 23:132–137 [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee P, Sotnikov AV, Mangian HJ, Zhou JR, Visek WJ, Clinton SK. 1999. Energy intake and prostate tumor growth, angiogenesis, and vascular endothelial growth factor expression. J Natl Cancer Inst 91:512–523 [DOI] [PubMed] [Google Scholar]
- 13.Okada M, Takeuchi J, Sobue M, Kataoka K, Inagaki Y, Shigemura M, Chiba T. 1981. Characteristics of 106 spontaneous mammary tumours appearing in Sprague–Dawley female rats. Br J Cancer 43:689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prejean JD, Peckham JC, Casey AE, Griswold DP, Weisburger EK, Weisburger JH. 1973. Spontaneous tumors in Sprague–Dawley rats and Swiss mice. Cancer Res 33:2768–2773 [PubMed] [Google Scholar]
- 15.Saez Mdel D, Barriga C, Garcia JJ, Rodriguez AB, Ortego E. 2007. Exercise-induced stress enhances mammary tumor growth in rats: beneficial effects of the hormone melatonin. Mol Cell Biochem 294:19–24 [DOI] [PubMed] [Google Scholar]
- 16.Westerlind KC. 2003. Physical activity and cancer prevention—mechanisms. Med Sci Sports Exerc 35:1834–1840 [DOI] [PubMed] [Google Scholar]
- 17.Zinger M, McFarland M, Ben Jonathan N. 2003. Prolactin expression and secretion by human breast glandular and adipose tissue explants. J Clin Endocrinol Metab 88:689–696 [DOI] [PubMed] [Google Scholar]