Abstract
The present study examined changes in maternal blood parameters, particularly those related to blood coagulation, as well as alterations in blood coagulation-related gene expression in the liver during gestation in rats. Fibrinogen concentration and platelet count increased as pregnancy progressed whereas prothrombin time and overall activity of vitamin-K–dependent coagulation factors decreased before delivery, suggesting a physiologic response to prevent prolonged bleeding at parturition. Conversely, compared with values for nonpregnant rats, activated partial thromboplastin time was prolonged before delivery and antithrombin time was significantly higher during fetal organogenesis and thereafter, indicating a mechanism to prevent the development of deep tissue thrombosis in dams. DNA microarray analysis revealed no differences in coagulation-related gene expression in the liver on gestation day 13 between pregnant and nonpregnant rats, whereas the gene expression of various fibrinogen-related factors, coagulation factors II and X, and the anticoagulation factor-related factor leuserpin 2 were increased on gestational day 19. In addition, changes similar to those reported previously in pregnant rats were confirmed. The data obtained from the present study can be used as background data for effective evaluation of reproductive toxicology in rats, and they suggest that the rat is a useful animal model for investigating the mechanisms of disorders in the blood coagulation system that can occur during late pregnancy in women.
Abbreviation: GD, gestation day
The rat is commonly used as a model in studies on embryology and reproductive toxicology. During pregnancy, rats show significant changes in blood parameters that are similar to those in pregnant women.3,7,9,19 Therefore, meaningful interpretation of data from toxicology studies using pregnant animals can be difficult without the benefit of historical control data. In addition, precisely assessing the fetotoxicity of the compound administered requires that the secondary effects of maternal toxicity on the fetus are taken into consideration. However, few data regarding changes in maternal blood parameters during pregnancy in rats have been reported.8,10,19,25 In particular, detailed reports on changes in blood coagulation-related parameters in pregnant rats are sparse. This area is important because hemostatic changes associated with increased risk of thrombosis are frequent during late pregnancy in humans.3,4,7,9,11 Pregnant women also show increased susceptibility to drugs due to decreased levels of drug-binding proteins.22
This study was performed to clarify how hematologic and biochemical parameters, especially those related to blood coagulation, change during the course of gestation in rats and how well pregnant rats serve as models of pregnant women in research pertaining to clinical pathology and gestation. In addition, blood coagulation-related gene expression in rat liver was analyzed by using DNA microarrays.
Materials and Methods
Animals.
Male and female specific pathogen-free rats of the Sprague–Dawley strain were purchased from Charles River Laboratories Japan (Kanagawa, Japan) at approximately 13 to 16 wk of age. The animals were housed individually in bracket-type stainless steel wire-mesh cages (width, 254 mm; depth, 350 mm; height, 170 mm) in an animal room under controlled conditions (temperature, 21 to 26 °C; relative humidity, 42% to 64%; ventilation, 10 to 15 changes per hour; 12:12-h light:dark cycle). For mating, 1 male and 1 female rat were caged together for as long as 1 wk. Daily vaginal smears were obtained, and gestational day (GD) 0 was assigned based on obtaining a sperm-positive vaginal smear. A total of 50 pregnant animals (P group) were used for this study. In addition, 20 nonpregnant virgin female rats comprised a control group. All animals were given standard pelletted food (NMF, Oriental Yeast, Tokyo, Japan) and municipal tap water ad libitum throughout the acclimation and experimental periods.
The experimental procedures were conducted according to the Animal Welfare Guidelines of Bozo Research Center.13
Experimental designs.
All rats were euthanized by exsanguination from the abdominal aorta under ether anesthesia. Subsets of 10 pregnant rats were euthanized at GD7 (implantation), GD13 and GD17 (period of organogenesis), and GD20 (immediately before parturition). In the nonpregnant group, 10 animals were euthanized at the start of experiment (GD0). In addition, 5 animals each from the P and nonpregnant groups were euthanized at GD13 and GD19 for DNA microarray analysis on blood coagulation-related genes in the liver.
The animals were checked daily for clinical signs and were weighed immediately before euthanasia.
Hematology.
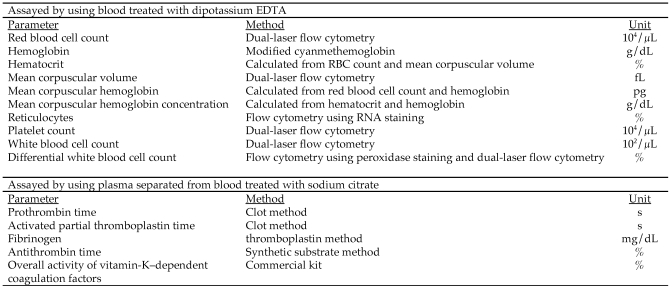
At euthanasia of each animal, blood (approximately 1 mL) was collected from the abdominal aorta into blood collection tubes (SB41, Sysmex Corporation, Hyogo, Japan) containing dipotassium EDTA. The RBC count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, reticulocytes, platelets, WBC count, and differential leukocyte count were assayed by using an automated hematology system (Advia 120, Bayer Corporation, Tarrytown, NY; Figure 1).
Figure 1.
Parameters, methods, and units of measure for hematology.
In addition, blood (approximately 0.9 mL) was collected in the same way into blood collection tubes containing 3.8% (w/v) sodium citrate (Wako Pure Chemical Industries, Tokyo, Japan) and centrifuged at 1600 × g for 10 min to separate plasma. The plasma sample obtained was examined for prothrombin time, activated partial thromboplastin time, and fibrinogen content by using an automated coagulometer (ACL 100 (Instrumentation Laboratory, Tokyo, Japan), for antithrombin time (ATIII) by using an automatic clinical chemistry analyzer (TBA120FR, Toshiba, New York, NY), and for overall activity of vitamin-K–dependent coagulation factors by using a commercial assay (Thrombo Test, Owren Axis-shield, Oslo, Norway).
Blood biochemistry.
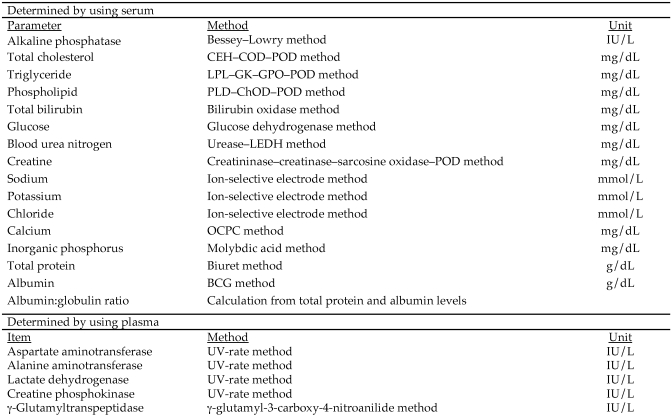
Blood (approximately 4 mL) was collected from each rat as described earlier, put into test tubes containing coagulant (Venoject II-Autosep, Terumo Corporation, Tokyo, Japan), and centrifuged at 1600 × g for 10 min to separate sera. The sera samples obtained were evaluated for alkaline phosphatase, total cholesterol, triglycerides, phospholipids, total bilirubin, glucose, blood urea nitrogen, creatine, sodium, potassium, chloride, calcium, inorganic phosphorus, total protein, and albumin by using an automatic clinical chemistry analyzer (TBA120FR, Toshiba; Figure 2). Additional blood (approximately 2.0 mL) from each rat was collected into tubes containing heparin sodium (20 U heparin per 1 mL blood) and centrifuged at 1600 × g for 10 min to separate plasma. The plasma sample obtained was examined for aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, creatine phosphokinase, and γ-gutamyltranspeptidase by using an automatic clinical chemistry analyzer (TBA120FR, Toshiba Corporation).
Figure 2.
Parameters, methods, and units of measure for blood biochemistry.
RNA extraction, microarray analysis, and microarray data analysis.
The liver slices obtained on GD13 and GD19 were submerged immediately in RNA stabilization reagent (RNAlater, Qiagen, Valencia, CA). After being incubated at 4 °C overnight, samples were stored at –80 °C until total RNA was prepared. Total RNA was extracted and purified by using a commercial kit (RNeasy Mini Kit, Qiagen) according to the manufacturer's instructions. The integrity of the purified total RNA was determined by denaturing agarose gel electrophoresis.
Microarray analysis was performed according to the protocol provided by the manufacturer of the microarray chip (Affymetrix, Tokyo, Japan). Briefly, 10 μg total RNA from each rat was used for cDNA synthesis using the T7-(dT)24 primer [5′ GGC CAG TGA ATT GTA ATA CGA CTC ACT ATA GGG AGG CGG (dT)24 3′]. The cDNA was resuspended in RNase-free water and used to synthesize biotinylated cRNA (3′ Amplification Reagents for IVL Labeling Kit, Affymetrix, Santa Clara, CA). After a 16-h incubation at 37 °C, the resultant biotin-labeled cRNA was fragmented and stored at –20 °C until hybridization. The hybridization solution was prepared by using a commercial kit (GeneChip Eukaryotic Hybridization Control Kit, Affymetrix) and was hybridized to the microarray chip (Rat Expression Array 230A, Affymetrix) at 45 °C for 16 h in a hybridization oven (GeneChip Hybridization Oven 640, Affymetrix). The chips were washed and stained automatically (Fluidics Station, Affymetrix) and scanned (Gene Array Scanner, Affymetrix).
Images from the scanned chips were processed and analyzed (Microarray Suite 5.0, Affymetrix, and Excel, Microsoft, Redmond, WA). For analysis of the different liver tissue target RNA samples (GD13 and GD19), values for gene expression in the samples of nonpregnant virgin controls were set as the baseline.
After global normalization was performed for each experimental datum, the fold change was derived as the ratio of average differences from an experimental array compared with a control array. Statistical analysis was performed by Student t and Welch t tests (Excel, Microsoft, Redmond, WA) as appropriate. Genes with low reliability (detection P value greater than 0.05) were excluded from the analysis.
Statistical analysis.
The mean (± 1 SD) was calculated for each of the parameters examined for each animal, and these results were analyzed for differences between the nonpregnant and pregnant groups by using F tests (Excel, Microsoft). Further analysis was performed by using Student or Aspin–Welch t tests (Excel, Microsoft). A P value of less than 0.05 indicated statistically significant differences.
Results
Clinical signs and pathologic findings
No abnormal clinical signs or pathology were observed in any animals.
Changes in hematologic parameters
The results of hematology assays are shown in Table 1. Compared with those of nonpregnant rats, the RBC count, hemoglobin concentration, and hematocrit of pregnant rats were significantly lower on GD7 and continued to decrease through GD20 (Table 1). On the other hand, reticulocytes began to significantly increase on GD7, reached a plateau from GD13 to GD17, and returned to the control level on GD20 (Table 1). The WBC count was significantly increased in pregnant animals throughout the gestation period (Table 2). Relative to differential leukocyte counts in nonpregnant rats, neutrophils in pregnant rats were increased on and after GD13 and the monocyte count was increased from GD7 to GD17, but lymphocyte and eosinophil counts were decreased on and after GD13 (Table 2).
Table 1.
Changes in hematology values (mean ± 1 SD) in pregnant rats
| RBC count (×104/µL) | hemoglobin (g/dL) | hematocrit (%) | Mean corpuscular volume (fL) | Mean corpuscular hemoglobin (pg) | Mean corpuscular hemoglobin concentration (g/dL) | Reticulocytes (%) | |
| Nonpregnant | 823 ± 32 | 16.0 ± 0.4 | 40.8 ± 1.2 | 49.6 ± 1.2 | 19.5 ± 0.5 | 39.3 ± 0.7 | 1.5 ± 0.2 |
| GD7 | 773 ± 32a | 15.2 ± 0.5a | 39.1 ± 1.1a | 50.7 ± 1.9 | 19.7 ± 0.7 | 38.8 ± 0.6 | 2.9 ± 0.5d |
| GD13 | 677 ± 22a | 13.6 ± 0.6a | 35.1 ± 1.6a | 51.9 ± 2.2b | 20.1 ± 0.8c | 38.7 ± 0.4b | 4.3 ± 0.6d |
| GD17 | 679 ± 29a | 13.4 ± 0.4a | 34.1 ± 1.0a | 50.2 ± 1.5 | 19.8 ± 0.5 | 39.4 ± 0.5 | 3.8 ± 0.8d |
| GD20 | 614 ± 32a | 12.0 ± 0.6a | 30.8 ± 1.6a | 50.2 ± 1.0 | 19.6 ± 0.5 | 39.0 ± 0.4 | 1.4 ± 0.6 |
P < 0.01 compared with value from nonpregnant animals (Student t test; n = 10 per group)
P < 0.05 compared with value from nonpregnant animals (Aspin–Welch t test; n = 10 per group)
P < 0.05 compared with value from nonpregnant animals (Student t test; n = 10 per group)
P < 0.01 compared with value from nonpregnant animals (Aspin–Welch t test; n = 10 per group)
Table 2.
Changes in WBC parameters (mean ± 1 SD) in pregnant rats
| Differential leukocyte counts (%) |
|||||||
| WBC count (×102/µL) | Lymphocytes | Neutrophils | Eosinophils | Basophils | Monocytes | Large unstained cells | |
| Nonpregnant | 81.4 ± 14.7 | 79.0 ± 4.7 | 15.3 ± 4.1 | 1.8 ± 0.6 | 0.3 ± 0.1 | 2.5 ± 0.8 | 1.0 ± 0.3 |
| GD7 | 107.9 ± 19.7a | 74.4 ± 6.2 | 19.3 ± 6.3 | 1.6 ± 0.3 | 0.4 ± 0.1 | 3.3 ± 0.6b | 1.1 ± 0.4 |
| GD13 | 126.0 ± 27.1c | 67.1 ± 4.1a | 27.6 ± 3.7a | 1.0 ± 0.3c | 0.4 ± 0.1 | 3.2 ± 0.5b | 0.7 ± 0.3 |
| GD17 | 109.7 ± 27.0d | 58.4 ± 5.8a | 35.1 ± 6.2a | 1.3 ± 0.3d | 0.3 ± 0.1 | 4.0 ± 0.7a | 0.9 ± 0.3 |
| GD20 | 123.7 ± 28.4c | 55.0 ± 7.2a | 39.5 ± 7.4c | 0.9 ± 0.3c | 0.3 ± 0.1 | 3.2 ± 0.9 | 1.2 ± 0.7 |
P < 0.01 compared with value from nonpregnant animals (Student t test; n = 10 per group)
P < 0.05 compared with value from nonpregnant animals (Student t test; n = 10 per group)
P < 0.01 compared with value from nonpregnant animals (Aspin–Welch t test; n = 10 per group)
P < 0.05 compared with value from nonpregnant animals (Aspin–Welch t test; n = 10 per group)
Changes in blood coagulation-related parameters
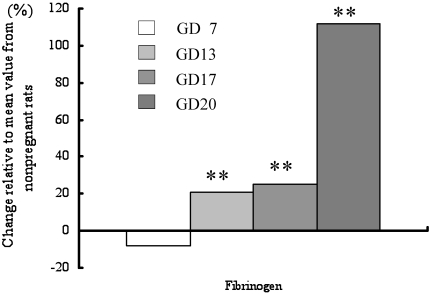
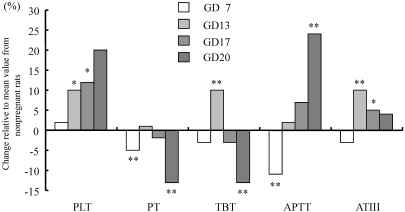
Compared with values in the nonpregnant group, fibrinogen (Figure 3) and platelet levels gradually increased from GD13 onward in pregnant rats (Table 3). In addition, the prothrombin time and overall activity of vitamin-K–dependent coagulation factors were significantly shortened on GD20 (Figure 4). The activated partial thromboplastin time on GD7 was shortened, after which it was significantly higher increased on GD20. The antithrombin time was increased on GD13 and GD17 and even more so on GD20.
Figure 3.
Changes in blood coagulation-related parameters in pregnant rats. Data are presented as differences from the levels in nonpregnant rats. *, P < 0.05; **, P < 0.01 compared with value from control group.
Table 3.
Changes in blood coagulation-related parameters (mean ± 1 SD) in pregnant rats
| Fibrinogen (mg/dL) | Platelets (×104/µL) | Prothrombin time (s) | Overall activity of vitamin-K–dependent coagulation factors (s) | APTT (s) | Antithrombin time (%) | |
| Nonpregnant | 217 ± 14 | 114.2 ± 13.7 | 13.0 ± 0.5 | 28.91 ± 1.47 | 18.8 ± 1.1 | 147 ± 6 |
| GD7 | 201 ± 23 | 116.8 ± 12.0 | 12.3 ± 0.3a | 28.15 ± 1.55 | 16.8 ± 1.4b | 142 ± 7 |
| GD13 | 263 ± 40a | 125.8 ± 5.1c | 13.1 ± 0.6 | 31.68 ± 1.35b | 19.2 ± 1.7 | 162 ± 12a |
| GD17 | 271 ± 35a | 128.1 ± 12.5d | 12.7 ± 0.4 | 27.96 ± 1.06 | 20.2 ± 2.5 | 155 ± 8d |
| GD20 | 460 ± 44a | 137.7 ± 40.2 | 11.3 ± 0.3a | 25.19 ± 0.98b | 23.4 ± 1.5b | 154 ± 9 |
APTT, activated partial thromboplastin time
P < 0.01 compared with value from nonpregnant animals (Aspin–Welch t test; n = 10 per group)
P < 0.01 compared with value from nonpregnant animals (Student t test; n = 10 per group)
P < 0.05 compared with value from nonpregnant animals (Aspin–Welch t test; n = 10 per group)
P < 0.05 compared with value from nonpregnant animals (Student t test; n = 10 per group)
Figure 4.
Changes in blood coagulation-related parameters in pregnant rats. Data are presented as differences from the levels in nonpregnant rats. *, P < 0.05; **, P < 0.01 compared with value from control group. PLT, platelet count; PT, prothrombin time; TBT, overall activity of vitamin-K–dependent coagulation factors; APTT, activated partial thromboplastin time; ATIII , antithrombin time.
Changes in blood biochemical parameters.
Compared with those in the nonpregnant rats, lactate dehydrogenase was higher throughout the gestation period, and alkaline phosphatase was decreased on GD20 in pregnant animals (Table 4). Creatine phosphokinase showed higher values on and after GD13 (Table 4). The total cholesterol and phospholipids showed significantly lower values on GD13 but showed significantly higher values on GD20 (Table 5). Triglyceride levels were significantly higher on GD17 and GD20, whereas creatine was decreased on GD13 (Table 5). In addition, sodium and chloride in pregnant animals were decreased throughout the gestational period, especially on GD20 (Table 6). In addition, the calcium levels on GD17 and GD20 were decreased whereas phosphorus was increased from GD7 to GD17 (Table 6). Albumin and total protein levels were significantly decreased from GD13 to GD20, and the albumin:globulin ratio showed lower values on and after GD13 (Table 6).
Table 4.
Changes in blood chemistry parameters (IU/L; mean ± 1 SD) in pregnant rats
| Aspartate aminotransferase | Alanine aminotransferase | Lactate dehydrogenase | γ-glutamyl transpeptidase | Alkaline phosphatase | Creatine phosphokinase | |
| Nonpregnant | 66 ± 13 | 42 ± 13 | 39 ± 5 | 1 ± 0 | 407 ± 108 | 59 ± 7 |
| GD7 | 59 ± 6 | 40 ± 5 | 47 ± 6a | 1 ± 0 | 524 ± 207 | 65 ± 8 |
| GD13 | 71 ± 9 | 50 ± 8 | 52 ± 8a | 1 ± 0 | 500 ± 171 | 97 ± 17b |
| GD17 | 75 ± 10 | 65 ± 15a | 62 ± 14b | 1 ± 1 | 440 ± 55 | 92 ± 17b |
| GD20 | 62 ± 9 | 49 ± 6 | 61 ± 14b | 1 ± 0c | 244 ± 62a | 88 ± 12a |
P < 0.01 compared with value from nonpregnant animals (Student t test; n = 10 per group)
P < 0.01 compared with value from nonpregnant animals (Aspin–Welch t test; n = 10 per group)
P < 0.05 compared with value from nonpregnant animals (Student t test; n = 10 per group)
Table 5.
Changes in blood chemistry parameters (mg/dL; mean ± 1 SD) in pregnant rats
| Total cholesterol | Triglycerides | Phospholipids | Total bilirubin | Glucose | Blood urea nitrogen | Creatine | |
| Nonpregnant | 64 ± 6 | 39 ± 25 | 135 ± 13 | 0.1 ± 0.1 | 167 ± 7 | 21 ± 3 | 0.26 ± 0.02 |
| GD7 | 65 ± 7 | 52 ± 24 | 138 ± 16 | 0.1 ± 0.1 | 170 ± 15 | 19 ± 2 | 0.26 ± 0.04 |
| GD13 | 43 ± 7a | 55 ± 29 | 99 ± 13a | 0.1 ± 0.0 | 136 ± 14c | 19 ± 3 | 0.21 ± 0.03a |
| GD17 | 71 ± 12 | 181 ± 64c | 154 ± 17b | 0.0 ± 0.1 | 122 ± 10a | 19 ± 3 | 0.24 ± 0.02b |
| GD20 | 96 ± 11a | 255 ± 99c | 202 ± 21a | 0.1 ± 0.0 | 108 ± 12c | 19 ± 1 | 0.26 ± 0.03 |
P < 0.01 compared with value from nonpregnant animals (Student t test; n = 10 per group)
P < 0.05 compared with value from nonpregnant animals (Student t test; n = 10 per group)
P < 0.01 compared with value from nonpregnant animals (Aspin–Welch t test; n = 10 per group)
Table 6.
Changes in blood chemistry parameters (mean ± 1 SD) in pregnant rats
| Na (mmol/L) | K (mmol/L) | Cl (mmol/L) | Ca (mg/dL) | P (mg/dL) | Total protein (g/dL) | Albumin (g/dL) | Albumin:globulin ratio | |
| Nonpregnant | 142 ± 1 | 4.4 ± 0.4 | 109 ± 2 | 9.9 ± 0.3 | 5.9 ± 0.3 | 6.3 ± 0.3 | 3.0 ± 0.2 | 0.94 ± 0.07 |
| GD7 | 141 ± 1a | 4.2 ± 0.3 | 107 ± 1b | 10.1 ± 0.3 | 6.4 ± 0.6c | 6.1 ± 0.3 | 3.0 ± 0.2 | 0.93 ± 0.04 |
| GD13 | 138 ± 2a | 4.5 ± 0.3 | 104 ± 2b | 10.0 ± 0.5 | 7.5 ± 0.5b | 5.8 ± 0.3a | 2.7 ± 0.1b | 0.86 ± 0.05b |
| GD17 | 139 ± 1a | 4.6 ± 0.4 | 102 ± 2b | 10.1 ± 0.2 | 6.8 ± 0.6a | 5.9 ± 0.3d | 2.8 ± 0.2b | 0.88 ± 0.05 |
| GD20 | 138 ± 1a | 4.7 ± 0.4 | 102 ± 1a | 9.2 ± 0.3b | 5.9 ± 0.7 | 5.3 ± 0.3b | 2.3 ± 0.1b | 0.74 ± 0.03a |
P < 0.01 compared with value from nonpregnant animals (Aspin–Welch t test; n = 10 per group)
P < 0.01 compared with value from nonpregnant animals (Student t test; n =0 10 per group)
P < 0.05 compared with value from nonpregnant animals (Aspin–Welch t test; n = 10 per group)
P < 0.05 compared with value from nonpregnant animals (Student t test; n = 10 per group)
Changes in blood coagulation-related genes expression.
The expression of blood coagulation-related genes in the liver on GD13 was unchanged from that of nonpregnant animals (Table 7). In contrast, the expression of genes related to fibrinogen, coagulation factors (FII, X), and Leuserpin 2 clearly were increased on GD19.
Table 7.
Modified genes involved in blood coagulation
| Accession number | GeneBank definition and comment | Fold changea on GD19 |
| NM_012559.1 | Fibrinogen B beta chain mRNA | 1.57 |
| U05675.1 | Fibrinogen B beta chain mRNA | 1.57 |
| AI072045 | Fibrinogen, A alpha polypeptide | 1.52 |
| AA875097 | Fibrinogen, A alpha polypeptide | 2.30 |
| NM_017143.1 | Coagulation factor X | 1.68 |
| NM_022924.1 | Coagulation factor II (F2) | 1.41 |
| NM_024382.1 | Leuserpin 2 (Serpind1) | 1.74 |
Compared with value from nonpregnant animals; no difference between pregnant and nonpregnant rats on GD13.
Discussion
In this study, we examined changes in blood parameters, especially those related to blood coagulation, as well as alterations in the expression of blood coagulation-related genes in the liver during gestation in rats. As noted in the introduction, hemostatic changes in pregnant women are remarkable, especially in late pregnancy,3,4,7,9,11 and the risk of thromboembolism becomes 5 times greater.5 However, in pregnant women, platelet counts change only slightly or remain constant,5,21 and the prothrombin time may not change.5,21 Therefore, changes in factors VII through X rather than those in thrombocytes and prothrombin are thought to increase the risk of thromboembolism.5 In this regard, 1 study3 reported increases in coagulation factors II and X in pregnant women.
Fibrinogen and platelet counts in pregnant rats increased during the course of pregnancy, and the prothrombin time and activity of vitamin-K–dependent coagulation factors decreased before delivery. These changes could be a physiologic reaction to prevent prolonged bleeding at parturition. In contrast, the activated partial thromboplastin time clearly was prolonged before delivery, and the antithrombin time was higher during fetal organogenesis (GD13) and thereafter. These changes may indicate a mechanism to prevent the development of deep tissue thrombosis in dams. Microarray analysis of blood coagulation-related genes in the liver indicated no changes in expression on GD13, whereas those of the fibrinogen-related factors coagulation factors (II and X) and the anticoagulation factor-related gene leuserpin 2 (Serpind1) were elevated on GD19, in good correspondence with the results of the hematologic examination. Leuserpin 2 is a serine proteolytic enzyme that rapidly destroys thrombin in the presence of heparin.18
As previously reported,8 RBC, hematocrit, and hemoglobin in pregnant rats were significantly decreased compared with those of nonpregnant animals, and the degree of effect progressed through gestation in the present study. This pattern is considered to be secondary to hemodilution reported in both pregnant women and rats.5,8 In pregnant women, hemodilution is considered to begin around midorganogenesis, and the increase in plasma volume during the second trimester is thought to be the cause of the ‘physiological anemia of pregnancy,’ which is the progressive decrease in hemoglobin and hematocrit concentrations that occurs until approximately gestational week 30 in women.23
In pregnant rats, reticulocytes began to increase on GD7, reached a plateau during organogenesis (GD13 and GD17), and then returned to the level of the nonpregnant group before delivery. The increase in reticulocytes is said to be related to the increased demand for RBC during organogenesis.8 Moreover, the increase in serum progesterone concentration during organogenesis is thought to be a trigger of erythropoiesis.2,8 There are conflicting reports regarding changes in reticulocyte levels in pregnant women.12,16
In the present study, WBC counts increased during the course of gestation. Neutrophil and monocyte counts increased from GD13 to GD20 and from GD7 to GD17, respectively, whereas lymphocytes and eosinophils decreased from GD13 to GD20. Such changes in WBC, monocyte, and lymphocyte counts are similar to those reported previously for rats.8 In contrast, WBC parameters increase throughout gestation in pregnant women.16,20
Similarly to reports for pregnant rabbits,25 rats,8,17 and women,15 total protein and albumin levels in pregnant ratss decreased from the beginning of organogenesis, and the drop in total protein likely reflects the decreased serum albumin levels. In addition, glucose decreased significantly from early organogenesis (GD13) through the fetal growth stage (GD20), and the decrease in serum glucose concentration may be partially due to the increased demand for glucose by developing fetuses.
All varieties of lipids decreased throughout the gestation period in previous reports of rats,8,14 whereas total cholesterol, triglycerides, and phospholipids on GD20 increased in the present study. The reason underlying these differences is unknown. Reports regarding changes in serum lipid levels in pregnant women are similarly conflicting.1,6
As reported previously,8 Na and Cl levels decreased through gestation, and Ca level was lower on GD20 in pregnant rats in the present study, whereas their P concentration was higher from GD7 to GD17 compared with that of nonpregnant animals, in contrast to previous findings in rats.8 In pregnant women, changes in electrolytes similar to those observed in the present pregnant rats were thought to reflect the increased demand for skeletal mineralization during fetal growt,24 and to be responsible for increased water retention induced by plasma volume expansion.5,23 Alkaline phosphatase, an enzyme leaked from the liver, decreased remarkably on GD20 in rats, as reported for pregnant women19 and pregnant rabbits,25 in which it was considered to be related to the increased estrogen level or increased estrogen:progesterone ratio.
The data shown in this paper can be used as background information for effective evaluation of reproductive toxicology in rats. Moreover, the rat seems to be a useful animal model for investigating the mechanisms of disorders in the blood coagulation system which are reported to occur during late pregnancy in women.
Acknowledgments
The authors thank Dr Kunio Doi (Professor Emeritus, University of Tokyo) for critical evaluation of this manuscript. We also express our heartfelt gratitude to Dr Yuzo Asano, Dr Kazutoshi Tamura, and Mr Kazuhisa Hatayama (Bozo Research Center) for their assistance in this research.
References
- 1.Bacq Y, Zarka O. 1994. [Liver in normal pregnancy]. Gastroenterol Clin Biol 18:767–774 [Article in French] [PubMed] [Google Scholar]
- 2.Beischer NA, MacKay EV. 1978. Obstetrics and the newborn. London (UK): WB Saunders and Company [Google Scholar]
- 3.Bremme KA. 2003. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 16:153–168 [DOI] [PubMed] [Google Scholar]
- 4.Brenner B. 2004. Haemostatic changes in pregnancy. Thromb Res 114:409–414 [DOI] [PubMed] [Google Scholar]
- 5.Burtis CA, Ashwood ER. 1994. Clinical chemistry of pregnancy, p 2107-2148. In: Burtis CA, Ashwood ER. Tietz textbook clinical chemistry. Philadelphia (PA): WB Saunders and Company [Google Scholar]
- 6.Chiang AN, Yang ML, Hung JH, Chou P, Shyn SK, Ng HT. 1995. Alterations of serum lipid levels and their biological relevance during and after pregnancy. Life Sci 56:2367–2375 [DOI] [PubMed] [Google Scholar]
- 7.Clark P. 2003. Changes of hemostasis variables during pregnancy. Semin Vasc Med 3:13–24 [DOI] [PubMed] [Google Scholar]
- 8.De Rijk EP, van Esch E, Flik G. 2002. Pregnancy dating in the rat: placental morphology and maternal blood parameters. Toxicol Pathol 30:271–282 [DOI] [PubMed] [Google Scholar]
- 9.Franchini M. 2006. Haemostasis and pregnancy. Thromb Haemost 95:401–413 [DOI] [PubMed] [Google Scholar]
- 10.González-Mariscal G, Díaz-Sánchez V, Melo AI, Beyer C, Rosenblatt JS. 1994. Maternal behavior in New Zealand white rabbits: quantification of somatic events, motor patterns, and steroid plasma levels. Physiol Behav 55:1081–1089 [DOI] [PubMed] [Google Scholar]
- 11.Holmes VA, Wallace JM. 2005. Haemostasis in normal pregnancy: a balancing act? Biochem Soc Trans 33:428–432 [DOI] [PubMed] [Google Scholar]
- 12.Howells MR, Jones SE, Napier JA, Saunders K, Cavill I. 1986. Erythropoiesis in pregnancy. Br J Haematol 64:595–599 [DOI] [PubMed] [Google Scholar]
- 13.Institute of laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 14.LaBorde JB, Wall KS, Bolon B, Kumpe TS, Patton R, Zheng Q, Kodell R, Young JF. 1999. Haematology and serum chemistry parameters of the pregnant rat. Lab Anim 33:275–287 [DOI] [PubMed] [Google Scholar]
- 15.Lind T. 1980. Clinical chemistry of pregnancy, p 1-24. In: Latner A, Schwartz M, Advances in clinical chemistry. London (UK): Academic Press; [DOI] [PubMed] [Google Scholar]
- 16.Mercelina-Roumans PE, Ubachs JM, van Wersch JW. 1994. Leucocyte count and leucocyte differential in smoking and non-smoking females during pregnancy. Eur J Obstet Gynecol Reprod Biol 55:169–173 [DOI] [PubMed] [Google Scholar]
- 17.Naismith DJ, Richardoson DP, Ritchie CD. 1976. Dietary protein and energy as determinants of foetal growth in the rat. Proc Nutr Soc 35:124A–125A [PubMed] [Google Scholar]
- 18.NCBI [Internet]. Entrez Gene: 2008update [20 Dec 2008]. Available at http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene&cmd=Retrieve&dopt=full_report&list_uids=79224#refseq
- 19.Papworth TA, Clubb SK. 1995. Clinical pathology in the female rat during the pre- and postnatal period. Comparative Haematology International 5:13–24 [Google Scholar]
- 20.Peck TM, Arias F. 1979. Hematologic changes associated with pregnancy. Clin Obstet Gynecol 22:785–798 [DOI] [PubMed] [Google Scholar]
- 21.Pitkin RM, de Witte DL. 1979. Platelet and leukocyte counts in pregnancy. JAMA 242:2696–2698 [PubMed] [Google Scholar]
- 22.Roberts JS, Silbergeld EK. 1995. Pregnancy, lactation, and menopause: how physiology and gender affect the toxicity of chemicals. Mt Sinai J Med 62:343–355 [PubMed] [Google Scholar]
- 23.Stock M, Metcalfe J. 1994. Maternal physiology during gestation, p 947–983. In: Knobil E, Neill J. The physiology of reproduction. New York (NY): Raven Press [Google Scholar]
- 24.Watney PJ, Rudd BT. 1974. Calcium metabolism in pregnancy and in the newborn. J Obstet Gynaecol Br Commonw 81:210–219 [DOI] [PubMed] [Google Scholar]
- 25.Wells MY, Decobecq CP, Decouvelaere DM, Justice C, Guttin P. 1999. Changes in clinical pathology parameters during gestation in the New Zealand white rabbit. Toxicol Pathol 27:370–379 [DOI] [PubMed] [Google Scholar]