Abstract
Introduction
New anticancer treatments have increased survival rates for cancer patients but often at the cost of sterility. One way of preserving fertility in these patients is the use of cryopreservation of ovarian tissue with subsequent retransplantation following a period of recurrence-free survival. We report the follow-up of the first case of retransplantation of ovarian tissue in Germany.
Methods
Immediately following the diagnosis of anal cancer, ovarian tissue was removed laparoscopically, and cryopreserved. The patient was then treated with combined radiochemotherapy, which resulted in iatrogenic premature ovarian failure, and was associated with inhibin B serum levels lower than 10 ng/L. After the 2.5 year period of cancer remission, the cryopreserved ovarian tissue was retransplanted orthotopically.
Results
Five months later estradiol serum levels had risen from lower than 20 pg/mL to 436 pg/mL. Three ovarian follicles were detected ultrasonographically in the pelvic side wall. Finally the patient reported her first menstruation after the intervention. The endocrine activity of the transplanted cryopreserved tissue has demonstrated viability, and the ability to develop.
Discussion
Cycle monitoring and timed intercourse should now help to acheive conception. These first results from Germany for retransplantation of cryopreserved ovarian tissue clearly show its potential for preserving fertility.
Keywords: fertility protection, ovarian tissue, cryopreservation, transplantation, cancer
Aggressive chemotherapy and radiotherapy, carried out as part of the treatment for malignant disease, often result in loss of gonadal function. The ensuing infertility causes suffering for large numbers of patients. Cyclophosphamide is particularly likely to cause loss of ovarian function, along with radiotherapy at a dose of over 8 Gy (1) In recent years a number of strategies have been developed to enable these patients to have children using their own gametes. With radiotherapy alone, it is possible to transpose the ovaries outside the radiation field. Where chemotherapy can be postponed, it is possible to carry out ovarian stimulation and recover eggs, which can be frozen in either a fertilized or unfertilized state (2, 3). Cryopreservation of ovarian tissue is possible where chemotherapy cannot be postponed. The advantage of the cryopreservation of ovarian tissue lies in the fact that it can be carried out immediately without ovarian stimulation and that as many as 500 to 1000 primordial follicles are present in even small biopsies (3).
In principle, there are three ways in which a pregnancy could be achieved via the use of this tissue. In the first, oocytes are matured in vitro and used for in vitro fertilization (IVF). This has shown no success to date with human ovarian tissue, for technical reasons (4). Another method is the xenotransplantation of the tissue into immune deficient animals, for example SCID mice (severe immunodeficiency mice). It is possible to mature human oocytes in SCID mice, but mature oocytes generated in this way have not been transplanted into humans, to date (6–9). The only method which has so far shown success is the retransplantation of ovarian tissue into the patient. A review article by Donnez et al. presents the various options for autologous transplantation of cryopreserved tissue (3). Three live births have been reported to date following the retransplantation of cryopreserved ovarian tissue (10, 11, 12), as well as two further pregnancies which resulted in miscarriage (13, 14). An additional five pregnancies have been described following the transplantation of fresh ovarian tissues between monovular twins (15).
We present here an account of the first retransplantation of cryopreserved ovarian tissue carried out with a view to facilitating pregnancy, in Germany.
Methods
In 2004, the 28-year-old patient had a pea-sized, invasive anal carcinoma diagnosed. An endoscopic biopsy confirmed the diagnosis histologically. She had no significant past gynecological history. The patient reported regular cycles of around 28 days. An organ conserving approach was used to treat the anal cancer, in conformity with the German national guideline (AWMF-Leitlinien-Register; 32/020) in the form of combined radiotherapy and chemotherapy. The patient received two courses of chemotherapy with mitomycin C and 5-fluorouracil and a primary, curative, precutaneous, small volume radiotherapy of the tumor region to a dose of 59.4 Gy and of the inguinal lymphatic drainage area to a dose of 50.4 Gy respectively (6/15 MV photons; 15/21 MeV electrons; single dose 1.8 Gy; computer-aided multiple field radiation technique with individual field collimation [CT-based tumor-specific radiation field definition]). The treatment was well tolerated by the patient and she remains recurrence free. Following treatment, the patient became amenorrheic, with raised gonadotropin levels suggesting primary ovarian failure, along with low estradiol and inhibin B levels (<10 ng/L). Cyclical hormone replacement therapy was commenced with estradiol valerate and levonorgestrel for a two-year period. During this time she had regular periods. On discontinuing the hormone replacement therapy, amenorrhea returned.
Prior to retransplantation, humane menopausal gonadotropin was administered for two weeks (HMG 225 IU/day). There was no resulting rise in estradiol level or ultrasonographically follicular growth.
Removal of ovarian tissue
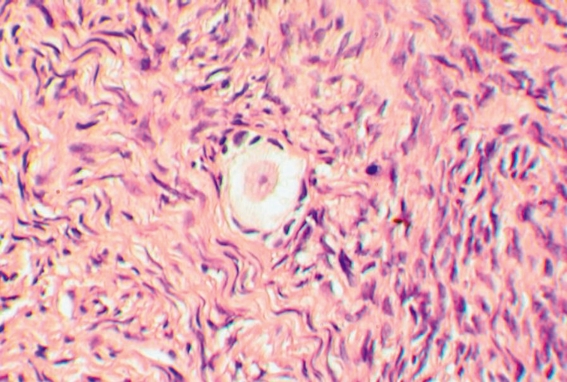
Prior to radiotherapy and chemotherapy around two-thirds of the ovarian cortex was removed laparoscopically from the right ovary. A histological reference sample showed numerous primordial and primary follicles (figure 1).
Figure 1.
Histological section of a reference sample of the removed ovarian tissue with a clearly visible primordial follicle (200x magnification)
Cryopreservation
The biopsy specimens were cut into pieces of around 1 x 2 x 1 mm, and equilibrated in 1.5 M DMSO (dimethyl sulphoxide)/propanediol solution in increasing steps of 0.25 M. The pieces of ovarian tissue remained in each concentration for 7 minutes, and then for 30 minutes in the 1.5 M solution. The tissue was then frozen in standard cryopreservation containers, and in an open freezing system which allows the initiation of auto-crystallization. It was later thawed in a bath of warm water. The tissue fragments were released/retrieved from the protective cryopreservation medium in reverse order with the addition of 0.25 M of saccharose, and then cultured in the medium for 60 minutes prior to transplantation (16).
Retransplantation
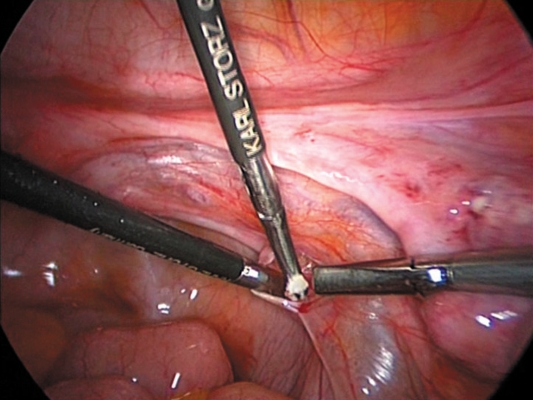
Using bipolar diathermy to maintain hemostasis, a 1 cm opening was made in the peritoneum in the region of the broad ligament, below the right tube, and anterior to the ovarian ligament, and a 1.5 cm deep pocket created using blunt dissection. Into this pocket were introduced six 1 to 2 mm sized fragments of ovarian tissue (figure 2) and the pocket was closed with vicryl. The transplanted ovarian tissue could easily be identified through the peritoneum, anteriorly. Additional ovarian fragments were transplanted to the region of the right pelvic side wall anterior to the right ureter in a second similarly prepared pocket (figure 3).
Figure 2.
Thawed ovarian tissue prior to retransplantation
Figure 3.
Introduction of the fragments of ovarian tissue into the peritoneal pocket
Hormone assay
Hormone levels were determined using immunoassay. Inhibin B was measured using a dimeric inhibin B-specific ELISA.
Results
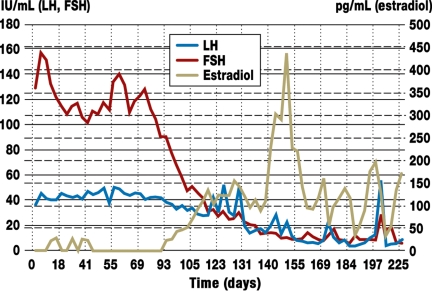
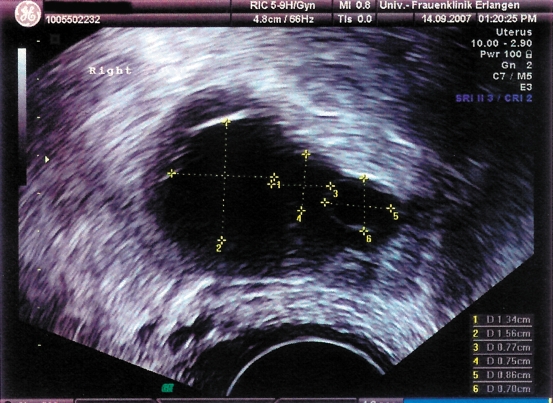
The laparoscopic retransplantation was uneventful. HMG was administered in similar dosages for seven days postoperatively. Beginning on the day before surgery, blood was taken daily for FSH, LH, estradiol, and progesterone assay (graph). While the preoperative FSH and LH levels were high and estradiol levels low, a fall in gonadotropins was measured at two and a half months postoperatively, and a rise in estradiol to 155 pg/mL at four months, increasing further after a further four weeks, to 436 pg/mL. One large and two small follicles were seen on ultrasound in the region of the transplanted tissue (figure 4). The endometrial thickness was approximately 6 mm. The progesterone level rose to 2.1 ng/mL. The patient thereafter reported a first menstrual period following retransplantation.
Graph.
Sequential serum levels of FSH, LH, and estradiol. The restoration of ovarian endocrine function was first detected from three months following transplantation; day 0 is the day prior to transplantation of the cryopreserved ovarian tissue
Figure 4.
Sonographic image of the follicles in the transplanted tissue
Discussion
Technological progress in assisted reproduction offers the realistic promise of preserving fertility in women threatened with its loss through cytotoxic treatment, premature menopause or surgical removal of the ovaries (3). In general, young women facing chemotherapy or radiotherapy should be offered fertility preserving treatment. Survival rates have increased for individual cancers, and such women have the right to fulfil a potential subsequent wish to have children. One fertility preserving treatment is the cryopreservation of ovarian tissue with retransplantation.
The authors report the first transplantation of ovarian tissue following cryopreservation in Germany, in a patient with two and a half year’s remission following radiotherapy and chemotherapy for anal carcinoma. Cryopreservation used the so-called slow freezing technique, which is currently considered the best approach (18, 19). Both Donnez and Meirow used the same technique in the published cases (10, 11). Before using this technique in humans, we first validated the freezing and retransplantation techniques in a series of animal studies. Vitality studies, in which living and dead follicles were easily distinguishable, showed a follicular survival rate of over 80% (8).
The cryopreservation of ovarian tissue works well. It is less clear how successfully these egg cells will lead to pregnancy. Of the methods on offer, only retransplantation has so far led to three live births. Retransplantation can also meet the additional goal of restoring gonadal function and hormonal status, thereby improving quality of life for these women, at least for some considerable time period (10).
Opinions have differed among the various groups involved in this technique as to the surgical approach to retransplantation; is orthotopic, or heterotopic transplantation the treatment of choice? And should gonadotrophin stimulation be used, or, on the other hand, gonadotrophin suppression with gonadotropin-releasing hormone (GnRH) analogs (3)? Transplantation into the area of the original ovarian site appears to have benefits (3). Not least, in that it allows for ease of monitoring of the tissue via transvaginal ultrasonography. We chose this site for transplantation for this reason, and in the hope that transplantation into this region might facilitate a spontaneous pregnancy (10). Anastomotic reconnection of the small tissue strips is not feasible, but unnecessary as ovarian tissue with a thickness of around 1mm survives transplantation well (20).
The appearances of the transplanted tissue on transvaginal ultrasonography showed antral follicles with a maximal follicular diameter of 15.6 mm. The development of the sonographically demonstrated antral follicles can only have derived from primordial or primary follicles, since only these can survive the process of freezing and thawing, and the hypoxic ischemic insult of retransplantation (3, 22). The maturation and growth phase from primary to antral follicle takes around 85 days, growth from primordial follicles still longer (21). This corresponds with the observed time lag of three months from retransplantation to the first rise in estradiol, and around five months to the first ovulation.
For the pharmacological management of the development of the transplanted tissue, we chose to support neovascularization by the administration of gonadotropins. Gonadotropins can promote new vessel formation (9, 23). Long-term stimulation with gonadotropins in animal studies resulted in premature follicle loss, however (9), and was therefore avoided. We cannot support the administration of GnRH-analogs at the time of transplantation, as suggested by Donnez et al. Our own investigations showed no benefit for the survival of primordial follicles with GnRH-analog administration (7), which was therefore not used.
A further danger is the reintroduction of malignant disease via a theoretical reintroduction of tumor cells via the transplanted tissue. This risk seems in general to be higher for patients with leukemias and lymphomas than for women with solid tumors, especially those with low metastatic potential, such as the patient in this case study with anal carcinoma (24). The retransplantation was experienced as positive by the patient we describe. At her own wishes, close monitoring of hormone levels has been introduced, along with recommendations as to the ideal time for sexual intercourse around the time of ovulation.
Around 100 ovarian tissue samples are cryopreserved each year in Germany. Many patients remain unaware of this possibility. With improved education, all women facing cytotoxic treatment for malignant disease should be advised about the possibility of ovarian preservation (25). A new German nationwide network founded in 2006, the Netzwerk Fertiprotekt, founded in 2006 by members of university departments of gynecology, is concerned with fertility protection in female cancer patients, and is helpful both for patients and doctors (see the German-language website www.fertiprotekt.de). In summary, Germany’s first retransplantation of ovarian tissue carried out to enable pregnancy was successful insofar as it has demonstrated hormonal activity in the retransplanted tissue. Cryopreservation of ovarian tissue for the preservation of fertility in women with malignant disease has therefore moved from being a purely experimental technique to offering patients realistic hope of restored fertility.
Acknowledgments
Translated from the original German by Dr. Sandra Goldbeck-Wood.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors
References
- 1.Beckmann MW, Binder H, Dittrich R, et al. Fertility preservations of patients at risk in german reproductive university centres - a concept paper. Geburtsh Frauenheilk. 2006;66:241–251. [Google Scholar]
- 2.Binder H, Dittrich R, Müller A, Cupisti S, Beckmann MW. Fertilitätserhaltung bei onkologischen Therapien. Geburtsh und Frauenheilk. 2006;66:199–226. [Google Scholar]
- 3.Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:19–35. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 4.Ksiazkiewicz LK. Recent achievements in in vitro culture and preservation of ovarian follicles in mammals. Reprod Biol. 2006;6:3–16. [PubMed] [Google Scholar]
- 5.Gook DA, Edgar DH, Borg J, et al. Diagnostic assessment of the developmental potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Hum Reprod. 2005;1:72–78. doi: 10.1093/humrep/deh550. [DOI] [PubMed] [Google Scholar]
- 6.Maltaris T, Boehm D, Dittrich R, Seufert R, Koelbl H. Reproduction beyond cancer: a message of hope for young women. Gynecol Oncol. 2006;103:1109–1121. doi: 10.1016/j.ygyno.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Maltaris T, Beckmann MW, Binder H, et al. The effect of a GnRH-agonist on cryopreserved human ovarian grafts in severe combined immunodeficient mice. Reproduction. 2007;133:503–509. doi: 10.1530/REP-06-0061. [DOI] [PubMed] [Google Scholar]
- 8.Maltaris T, Kaya H, Hoffmann I, Mueller A, Beckmann MW, Dittrich R. Comparison of xenografting in SCID-mice and LIVE/DEAD assay as a predictor of the developmental potential of cryopreserved ovarian tissue. In Vivo. 2006;20:11–16. [PubMed] [Google Scholar]
- 9.Maltaris T, Beckmann MW, Mueller A, Hoffmann I, Kohl J, Dittrich R. Significant loss of primordial follicles after prolonged gonadotropin stimulation in xenografts of cryopreserved human ovarian tissue in severe combined immunodeficient mice. Fertil Steril. 2007;87:195–197. doi: 10.1016/j.fertnstert.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 10.Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 11.Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 12.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for hodgkin’s disease. Oncologist. 2007;12:1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod. 2005;20:3539–3546. doi: 10.1093/humrep/dei250. [DOI] [PubMed] [Google Scholar]
- 14.Demeestere I, Simon P, Buxant F, et al. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod. 2006;21:2010–2014. doi: 10.1093/humrep/del092. [DOI] [PubMed] [Google Scholar]
- 15.Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med. 2007;356:1382–1384. doi: 10.1056/NEJMc066574. [DOI] [PubMed] [Google Scholar]
- 16.Dittrich R, Maltaris T. A simple freezing protocol for the use of an open freezing sytem for cryopreservation of ovarian tissue. Cryobiology. 2006;52:166. doi: 10.1016/j.cryobiol.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Oktay K, Sonmezer M, Oktem O, Fox K, Emons G, Bang H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12:1055–1066. doi: 10.1634/theoncologist.12-9-1055. [DOI] [PubMed] [Google Scholar]
- 18.Dittrich R, Maltaris T, Mueller A, et al. Successful uterus cryopreservation in an animal model. Horm Metab Res. 2006;38:141–145. doi: 10.1055/s-2006-925175. [DOI] [PubMed] [Google Scholar]
- 19.Dittrich R, Mueller A, Hoffmann I, Beckmann MW, Maltaris T. Cryopreservation of complex systems: slow freezing has not had its day yet. Rejuvenation Res. 2007;10:101–102. doi: 10.1089/rej.2006.9095. [DOI] [PubMed] [Google Scholar]
- 20.Dittrich R, Recabarren S, Mitze M, Jaeger W. Role of gonadotropins in malignant progression of sex cord stromal tumors produced by sequential auto- and isogenic transplantation of ovarian tissue in ovariectomized rats. J Cancer Res Clin Oncol. 2001;127:495–501. doi: 10.1007/s004320100251. [DOI] [PubMed] [Google Scholar]
- 21.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–87. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- 22.Kim SS, Hwang IT, Lee HC. Heterotopic autotransplantation of cryobanked human ovarian tissue as a strategy to restore ovarian function. Fertil Steril. 2004;82:930–932. doi: 10.1016/j.fertnstert.2004.02.137. [DOI] [PubMed] [Google Scholar]
- 23.Dissen GA, Lara HE, Fahrenbach WH, Costa ME, Ojeda SR. Immature rat ovaries become revascularized rapidly after autotransplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology. 1994;134:1146–1154. doi: 10.1210/endo.134.3.8119153. [DOI] [PubMed] [Google Scholar]
- 24.Shaw JM, Bowles J, Koopman P, Wood EC, Trounson AO. Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum Reprod. 1996;11:1668–1673. doi: 10.1093/oxfordjournals.humrep.a019467. [DOI] [PubMed] [Google Scholar]
- 25.von Otte S, Friedrich M, Diedrich K, Kupka M. Fertilitätserhalt bei onkologischen Patientinnen: Stand und Perspektiven. Dtsch Arztebl. 2006;103:2149–2153. [Google Scholar]