Abstract
Introduction
Pregnancy, birth, and the puerperium are associated with significant physiological changes and adaptations in the cardiovascular system, which pose a significant risk to pregnant women with congenital heart disease (CHD). Thanks to advances in pediatric cardiac surgery and cardiology the majority of children with CHD survive to adulthood, and an increasing number eventually become pregnant. In fact, cardiac disease – mostly congenital – is now a leading cause of maternal death in western industrialized countries.
Methods
Selective literature review.
Results and discussion
Optimal care of women with CHD before, during, and after pregnancy requires a multidisciplinary team including obstetricians, cardiologists, and anaesthetists. Successful pregnancy at a minimum risk is feasible for most women with CHD when appropriate counseling and optimal care are provided.
Keywords: maternity, congenital heart disease, multidisciplinary care
Introduction
The advances achieved in pediatric cardiology and cardiac surgery over the last five decades have significantly improved the prospects for survival of children with congenital heart disease (CHD), with at least 85% of affected newborns now reaching adulthood (1, 2). In Germany, the number of adults with CHD is currently estimated as 150 000 and is expected to rise by about 5000 patients annually (3). The fact that the majority of CHD patients now survive into adulthood does not mean, however, that their heart disease is cured. In fact, in most cases CHD cannot be cured. Rather, a surgical correction is a repair which results in residual and secondary lesions. Especially patients with complex heart defects must be regarded as chronically sick and their quality of life is often compromised by arrhythmia, heart failure, the prospect of more surgery, and premature death (e1). For women with this condition, pregnancy often represents a major additional stress factor.
As the prognosis of women with CHD has improved and the incidence of rheumatic heart disease has declined, CHD is now a leading cause of maternal death in the western industrialized countries (4, e2). To improve the care of these patients, it is therefore increasingly important for all those involved in medical care provision to be informed about the risks to which these women are exposed during pregnancy. Only on this basis can timely counseling and optimal care be assured during pregnancy, childbirth, and the puerperium (5, 6).
In this review article the authors outline what the comprehensive antenatal counseling and care of women with CHD should ideally comprise as a basis for correctly assessing and, whenever possible, preventing foreseeable problems during pregnancy.
Method
The authors conducted a comprehensive literature search in PubMed (www.ncbi.nlm.nih.gov) using the following search terms: "pregnancy," "congenital heart disease," "heart disease," "valve disease," "risk," "recurrence," "outcome," "pharmacotherapy," "anticoagulation," and "drugs." The choice of articles was based on a subjective estimation of their clinical relevance. In addition, relevant text books and the authors’ personal literature archives were consulted. This article is an updated version of a review published in the British Medical Journal in 2006 (6).
Early counseling
To prevent unplanned and potentially high-risk pregnancies in patients with severe CHD, counseling and education about potential risks should already be included in the pediatric cardiological care of these patients (e3). Pregnancy counseling appropriate to these patients’ individual situation comprises an estimation of the risk to which they are exposed as expectant mothers and of the risks for their child.
The maternal risk
Pregnancy is associated with significant physiological changes which can considerably overburden the cardiovascular system of women with CHD and can lead to cardiac complications (table 1). Blood volume and cardiac output increase by about 30% to 45% up to the end of the second trimester. Women with stenotic valves and limited cardiac output therefore tolerate pregnancy poorly. The increase in blood volume can promote the development of heart failure in women with impaired ventricular function. The fall in systemic vascular resistance during pregnancy can lead to an increase in a right-left shunt and thereby intensify cyanosis.
Table 1. Cardiovascular changes during pregnancy.
| Parameter | Change during pregnancy |
|---|---|
| Blood volume | ↑ 35% |
| Cardiac output | ↑ 40–43% |
| Stroke volume | ↑ 30% |
| Heart rate | ↑ 15–17% |
| Systemic vascular resistance | ↓ 15–21% |
| Mean arterial blood pressure | No substantial change |
| Systolic blood pressure | ↓; 3–5 mm Hg |
| Diastolic blood pressure | ↓ 5–10 mm Hg |
| Central venous pressure | No substantial change |
| Colloid osmotic pressure | ↓ 14% |
| Hemoglobin | ↓ 2,1 g/dL |
| Oxygen consumption | ↑ 30% |
| Right ventricle (end diastole) | ↑ 18% |
| Right atrium | ↑ 19% |
| Left ventricle (end diastole) | ↑ 6% |
| Left atrium | ↑ 12% |
From: Abbas Amr E, Lester Steven J, Connolly H: Pregnancy and the cardiovascular system. International Journal of Cardiology, 2005; 98: 11. With the kind permission of Elsevier Ltd.
Independent risk factors for cardiac complications valid for all heart diseases have been defined in the Canadian CARPREG studies (retrospective analysis of 252 pregnancies and prospective multicenter study on 599 pregnancies of women with a heart defect) (table 2) (7, 8, 10). Based on an analysis of 90 pregnancies in women with CHD only, the risk factors of these studies were confirmed for this patient population, and the presence of a dysfunction of the subpulmonary right ventricle and/or severe pulmonary valve insufficiency were included as additional risk factors (table 2) (21). The frequency of cardiovascular complications in women with CHD was 19.4% (21). For women with CHD, the individual estimation of the risk associated with pregnancy and childbirth should also include an exact knowledge of their heart defect, medical history, and hemodynamic status (table 3, e-table) (6, 9).
Table 2. General risk factors for maternal and fetal complications independent of the diagnosis.
| Risk factors for maternal complications (heart failure, symptomatic arrhythmia, cerebral stroke/transient ischemic attack [TIA], death) | Risk factors for fetal complications (intrauterine growth retardation, premature birth, intracranial bleeding, spontaneous abortion, neonatal demise, stillbirth) |
|---|---|
| NYHA (New York Heart Association) class > II heart failure before onset of pregnancy or cyanosis | NYHA (New York Heart Association) class > II heart failure before onset of pregnancy or cyanosis |
| Impaired function of systemic ventricle (ejection fraction <40%) | Impaired function of systemic ventricle (ejection fraction <40%) |
| Left heart obstruction (mitral valve area <2 cm2, aortic valve area <1.5 cm2), peak pressure gradient in the left ventricular outflow tract >30 mm Hg (measured by Doppler echocardiography) before pregnancy | Left heart obstruction (mitral valve area <2 cm2, aortic valve area <1.5 cm2), peak pressure gradient in the left ventricular outflow tract >30 mm Hg (measured by Doppler echocardiography) before pregnancy |
| History of cardiac complications such as symptomatic cardiac arrhythmia, cerebral stroke/TIA or pulmonary edema | Maternal age <20 or >35 years |
| Dysfunction of the subpulmonary right ventricle +/– severe pulmonic valve insufficiency | Maternal smoking |
| Maternal treatment with anticoagulants |
Table 3. Risk stratification of women with congenital heart defect according to diagnosis (9).
| Experience shows low risk Estimated risk of cardiac complications or death: >1‰ and < 1% | Experience shows medium risk Estimated risk of cardiac complications or death: 1% to 5% | Experience shows high risk Estimated risk of cardiac complications or death: >5% |
|---|---|---|
|
|
|
D-TGA, simple transposition of the great arteries
E-Table. Specific risks of individual heart conditions in the event of pregnancy and advice on their management.
| Relative risk | Lesion | Exclude before pregnancy | Potential hazards | Recommended treatment during pregnancy and peripartum |
|---|---|---|---|---|
| Low | Ventricular septal defects |
|
|
|
| Atrial septal defects (unoperated) |
|
|
|
|
| Coarctation (repaired) |
|
|
|
|
| Tetralogy of Fallot |
|
|
|
|
| Moderate | Mitral stenosis |
|
|
|
| Aortic stenosis |
|
|
|
|
Systemic right ventricle
|
|
|
|
|
| Cyanotic lesions without pulmonary hypertension |
|
|
|
|
| Fontan-type circulation |
|
|
|
|
| High | Marfan syndrome |
|
|
|
| Eisenmenger syndrome; pulmonary hypertension |
|
|
|
VSD, ventricular septum defect; ASD, atrial septum defect; D-TGA, dextro-transposition of the great arteries; ccTGA, congenitally corrected transposition of the great arteries; LMH, low molecular weight heparin; ASA, acetylsalicylic acid; RVOT, right ventricular outflow tract.
From: Uebing A, Steer PJ, Yentis SM, Gatzoulis MA: Pregnancy and congenital heart disease. BMJ 2006; 332:401-6. With kind permission of the BMJ Publishing Group Ltd.
Every risk stratification must be based on a full examination of the patient, the taking of a medical history, a physical examination, echocardiography and, if appropriate, collection of blood samples. In some cases exercise testing, MRI or cardiac catheterization may be necessary.
The risk for the child
The risk of fetal and neonatal complications is higher in pregnant women with CHD compared to healthy women (18% versus 7% in healthy women) (table 2) (7, 10). In the presence of maternal cyanosis, a heart defect with left heart obstruction or impairment of ventricular function, the maternal cardiovascular system may not be capable of adequately supplying the fetus with nutrients, resulting in a slowing of fetal growth making premature termination of pregnancy necessary or leading to spontaneous abortion. Regular biometric and Doppler sonographic evaluations provide an impression of the potential risks for the fetus.
If treatment of the maternal circulatory conditions fails to stabilize the supply of nutrients to the fetus, a decision must be taken regarding the optimal timing of delivery. Depending on the clinical situation, monitoring of fetal growth by Doppler sonographic evaluations of placental function is required at intervals of two to four weeks (e4).
The risk for a woman with CHD of having a child which also has a structural heart defect depends on the type of maternal heart disease and varies between about 3% and 12% (11, e5). For lesions with an autosomal dominant transmission pattern (such as Noonan syndrome and Marfan syndrome) the risk rises to as much as 50%. In such cases, genetic screening by chorionic villous biopsy can be offered in the 12th week of pregnancy. In mothers with interrupted aortic arch, common arterial trunk or tetralogy of Fallot, special attention should be paid to the possibility of a deletion syndrome at chromosome 22q11 (box 1) (e6).
Box 1. Deletion syndromechromosome 22q11 (e21).
A hemizygotic deletion on band 11 of the long arm of chromosome 22 (22q11) is the genetic basis in the majority of patients with a DiGeorge syndrome, velocardiofacial syndrome (Sphrintzen syndrome) or conotruncal anomaly face syndrome.
Deletion 22q11 exhibits great phenotypic heterogeneity. Principal findings are
conotruncal heart defect (common arterial trunk +/– interrupted aortic arch, tetralogy of Fallot, pulmonary atresia)
Immunodeficiency
Hypoparathyroidism
Developmental retardation
Facial dysmorphism
Palatal abnormality.
The deletion can be detected in up to 50% of patients with a conotruncal malformation by fluorescence in situ hybridization (FISH). The deletion is inherited by autosomal dominant transmission and usually shows a more pronounced phenotypic manifestation in the children of affected parents than in the parents themselves. This requires a genetic diagnostic program and, if appropriate, counseling before pregnancy if there is clinically suspected deletion in a patient with CHD.
Because of their increased risk of giving birth to a child with heart disease, all women with CHD should be offered differentiated fetal echocardiography in the 19th to 22nd week of pregnancy (12, e4). To keep the incidence of false positive findings as low as possible, this examination should be carried out by specialists trained in prenatal diagnostic procedures. Measurement of nuchal fold thickness in the 12th to 13th week of pregnancy is to be regarded as an early screening test. The sensitivity of a nuchal fold thickened above the 99th percentile for the presence of a significant heart defect is 40%, while the specificity of the method is 99%. The incidence of congenital heart disease with normal nuchal fold thickness is about 1/1000 (13).
Contraception and termination of pregnancy
Timely counseling about the possibilities offered by the different methods of contraception and their potential problems is itself an important feature of the care of adolescent women with heart disease and is aimed at preventing unintended and potentially hazardous pregnancies. Despite the wide range of available contraceptive methods, they present a variety of problems (box 2, e4).
Box 2. Contraception and termination of pregnancy.
"Natural methods of contraception" and "barrier methods" offer the comparatively lowest contraceptive safety and cannot be recommended if a pregnancy represents a substantial risk.
Estrogen-containing oral contraceptives ("combination pill") should not be taken because of the thrombophilic properties of estrogen if there is an increased thromboembolic risk because of the heart defect (cyanotic heart defect, pulmonary hypertension, poor cardiac function, atrial arrhythmias, Fontan circulation, prosthetic heart valve).
The "minipill" which contains only progesterone does not increase the thromboembolic risk, but is less safe than the "combination pill" (Pearl Index 0.4 to 3 compared with 1.0 to 0.9) and can lead to irregular uterine bleeding.
Progesterone depot injections are an alternative for adolescents whose reliability of contraceptive intake is doubtful. One injection is effective for 6 to 12 weeks, sometimes leads to amenorrhea, but can also cause heavy vaginal bleeding.
Progesterone coated intrauterine devices are an effective and safe contraceptive instrument. Devices of this kind can be left in place for up to five years, reduce the extent of menstrual bleeding and have a lower infectious risk.
Laparoscopic tubal sterilization is the most lastingly effective contraceptive method and should be considered when the risk of pregnancy is estimated as very high (Eisenmenger syndrome, pulmonary hypertension). However, the risk associated with the procedure must be taken into account.
Termination of pregnancy is considered medically indicated for moderate or high risk pregnancies. However, the risk associated with the procedure increases with the gestational age, and termination should be performed immediately after the decision to do so. Suction curettage under regional anesthesia is the method of first choice. Medicinal termination of pregnancy cannot be generally recommended also because of the unpredictable hemodynamic risks of the medications used (oral antiprogesterones, vaginally administered prostaglandins); the procedure must always be performed in the hospital setting.
Patient care during pregnancy
Ideally, a patient management plan should already be drawn up before the onset of pregnancy by a pediatric cardiologist or cardiologist trained in the care of these patients (14). If this has not been done, it should be carried out as soon as possible after pregnancy has been confirmed.
The extent and location of care provision should be determined according to the presumed risk for the patient (table 4). Every examination of the pregnant woman should include screening for symptoms of maternal heart failure and arrhythmia. Symptoms of pre-eclampsia are important because they represent a vital danger especially for patients with a complex heart defect and particularly for women with Eisenmenger syndrome.
Table 4. Extent and location of care of patients with CHD depending on the estimated maternal and fetal risk.
| Estimated risk | Extent and location of care |
|---|---|
| Low |
|
| Medium or high |
|
The intervals between maternal examinations are to be determined on a risk-adapted and thus individual basis. For pregnant women with a medium and high risk (table 3, e-table), examinations at two week intervals are recommended up to the 24th week of pregnancy (e4). Regular echocardiographic evaluations are especially indicated in subjects with dilatation of the aorta (Marfan syndrome, bicuspid aortic valve), left heart obstruction (aortic and mitral stenosis), impaired ventricular function, clinical deterioration or new onset heart murmur. Radiographic examinations are possible during pregnancy but, because of the radiation exposure, should only be carried out if strictly indicated (15). Since the fetal risk associated with magnetic resonance imaging during pregnancy cannot yet be conclusively assessed, such examinations – especially in the first trimester – should only be performed if cardiac imaging is essential for the patient’s further care (16, e7).
For cyanotic patients in the last trimester, bed rest and oxygen administration are often indicated (6).
Cardiac medications during pregnancy
Hardly any cardiac drugs are formally approved for use in pregnancy and lactation (17). Most heart drugs cross the placenta. Pharmacotherapy in women with CHD during pregnancy should thus always be approached critically.
Antiarrhythmic agents such as adenosine, digitalis, lidocaine, flecainide, sotalol, and calcium channel blockers are considered safe in pregnancy (e8). Beta blockers can also be administered during pregnancy. Amiodarone, however, should not be used unless strictly indicated because of the risk of infantile thyroid dysfunction or adverse CNS effects (e8). Electric cardioversion during pregnancy is considered safe (e9).
Diuretics are necessary if the mother develops signs of heart failure or pulmonary edema (e10). Loop diuretics like furosemide are suitable for use during pregnancy. Due to the risk of feminization of a male fetus, amiloride can be given instead of spironolactone as a potassium sparing diuretic. ACE inhibitors and angiotensin receptor blockers are contraindicated in pregnancy since they lead to tubular dysgenesis with oligohydramnion and anuria in newborns as well as cranial ossification abnormalities (17, e8, e9, e11).
Since many drugs are excreted in breast milk, as a general rule they should preferably be taken after rather than before breast feeding.
Thromboembolism and anticoagulation
Thromboembolic events are also six times more common in healthy pregnant women than in non-pregnant women. Patients with cyanosis or slow blood flow resulting from the Fontan circulation (direct connection between the systemic veins with the pulmonary arteries for palliation of a univentricular heart) are at even greater risk (18, e4). In patients with left-right shunt, the risk of paradoxical embolisms increases during pregnancy. The administration of low dose acetylsalicylic acid is therefore useful in these patient populations. Anticoagulation in pregnant women with mechanical heart valves represents a particular challenge.
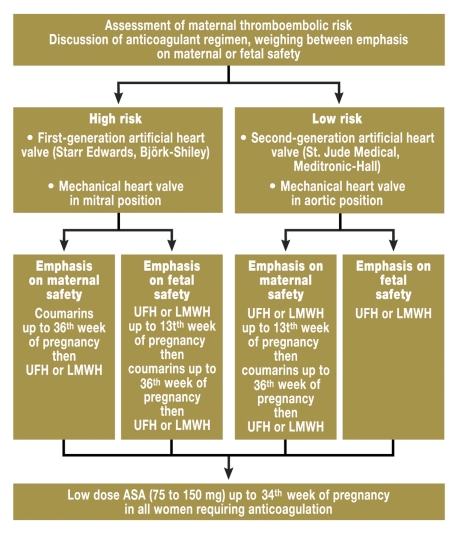
Coumarin derivatives (phenprocoumon, warfarin) are effective oral anticoagulants but harbor risks for the fetus because they cross the placenta. They are teratogenic in early pregnancy and increase the risk of fetal bleeding. Heparins, on the other hand, are not subject to diaplacental transfer and thus represent no problem for the fetus, but may be associated with an increased rate of maternal thromboembolisms especially after prosthetic heart valve replacement (19, e12). Every decision regarding anticoagulation during pregnancy must therefore take account of the risks for the mother and child. The figure presents a possible algorithm for anticoagulation during pregnancy based on available recommendations (5, 19, e13). The following factors should be considered when selecting the anticoagulant and its dosage: coumarins (INR ideally as before pregnancy) are ubject to the risk of embryopathy, although this appears dose dependent (no embryopathies have so far been observed at a warfarin dose <5 mg, equivalent to 3 mg phenprocoumon) (22, 23, e17). Nevertheless, their use only appears justified in patients with a high thromboembolic risk and who request maximum maternal safety after the patient briefing.
Figure.
Algorithm for anticoagulation in pregnancy. UFH, unfractionated heparin; LMWH, low molecular weight heparin (with kind permission of BMJ Publishing Group, Ltd.)
Unfractionated heparin (UFH) should be dosed according to the activated partial thromboplastin time (aPTT). A 2.5 to 3.0 fold increase in aPTT should be achieved (19). Obtaining an effective increase in aPTT is often difficult, however, and sometimes requires a continuous intravenous infusion (e12).
Low molecular weight heparin (LMWH) is distinguished by better bioavailability than UFH. It requires very careful dosing, however, since complications observed during LMWH therapy in pregnant women with mechanical heart valve may have been the result of insufficient dosage (19, e18). So far, experience with LMWH during pregnancy has only been reported for very small series (24). Even on the basis of this limited experience a clear recommendation can be given to base the dosage on the antifactor Xa level. A sufficient dose may be assumed at an antifactor Xa level of 1.0 to 1.2 U/mL about four to six hours after the injection and a trough level of 0.6 to 0.7 U/mL (19). The dosage should be re-evaluated at one to two week intervals and adjusted as necessary.
Assuming good monitoring of efficacy and dose adjustment, both LMWH and UFH may therefore be considered as possible anticoagulation options for these patients.
Low dose (60 to 150 mg/day) acetylsalicylic acid (ASA) up to the 34th week of pregnancy is regarded as safe for mother and child (e18). Since the addition of ASA to oral anticoagulation reduces the incidence of arterial thromboembolism in non-pregnant women with mechanical heart valves, it can also be considered as a supplement to therapy in pregnant women (25, e20).
Planning of labor phase and birth
The timely planning of the labor phase and birth is part of the clinical care program during pregnancy. The timing and mode of delivery should be based on a consensus between all the disciplines involved and the patient. The plan must be clearly documented in a form accessible to all departments at all times (e-box). The obstetrics department and responsible cardiologist in the patient’s town of residence should also be informed, especially if the patient lives some distance from the center. It is recommended to provide the patient with a copy of the birth schedule and the physicians’ letters so that all information is definitively available on admission.
E-Box.
Delivery management plan for women with cardiac disease
Cardiac diagnosis: ………………………………………………………………………………………………
If admitted to labor ward, please inform:
Gynecologist (name): ………………………………………………………………………………………………
Anesthetist (name): ………………………………………………………………………………………………
Cardiologist (name): ………………………………………………………………………………………………
Antenatal admission from ……………………………………… weeks.
Mode of delivery: Elective lower caesarean section/trial of vaginal delivery
Caesarean section
| Anesthetic technique: | Epidural / spinal / general / other | |
| Maternal monitoring: | ECG / SaO2 / non-invasive blood pressure / invasive blood pressure | |
| Postpartum phase: | No oxytocin bolus / continuous oxytocin infusion (dose: ……….) |
Vaginal delivery – dilation phase
| Thromboembolic deterrent stockings in labor / cardiac medication to be continued ……… | |
| Endocarditis prophylaxis: Elective / if operative delivery | |
| Epidural for analgesia: none / when requested / as soon as in established labor | |
| Comments re anesthetic: ………………………………………… | |
| Maternal monitoring: ECG / SaO2 / non-invasive blood pressure / invasive blood pressure |
Vaginal delivery – expulsion phase
| Normal / early assistance |
Vaginal delivery – postpartum phase
| Normal management / no oxytocin bolus / continuous oxytocin infusion (dose: …………………………) |
Puerperium
| Intensive care ward (for at least ……………….. days) / LMWH (for: ……………….. days) / | |
| Postpartum cardiac medication ……………………………………………………………………… |
The clinical situation may require practitioners to deviate from the preferred course of events.
Amended with kind permission of Prof. Philip J. Steer, High Risk Obstetric Unit, Chelsea and Westminster Hospital, Imperial. ECG, electrocardiogram; SaO2, oxygen saturations; LMWH, low molecular weight heparin.
Delivery
The decision regarding the mode of childbirth is usually taken by the obstetricians. Vaginal delivery appears definitely too hazardous under cardiological aspects if there is a risk of aortic rupture (for example in patients with Marfan syndrome, bicuspid aortic valve or operated aortic isthmus stenosis) (table 3) (20). Otherwise, vaginal delivery involves fewer complications for the mother and child than caesarian section since it causes fewer volume fluctuations, lower blood loss, and a smaller risk of thromboembolism and infection (e4). The strain on the cardiovascular system due to the labor and expulsion phase can be limited by early and judicious administration of epidural anesthesia; this relieves the mother of pain and fear and consequently reduces the increase in cardiac output during labor and delivery. To keep the expulsion phase short and less stressful for the mother, depending on the individual situation, childbirth may be assisted at an early stage by the use of a vacuum extractor cup or forceps (e4). The maximum duration of the expulsion phase is discussed and documented beforehand as a strategy for minimizing the stress on the maternal cardiovascular system (e-box).
An important factor for successful childbirth is continuous monitoring of the fetal and maternal state. Monitoring of maternal cardiovascular function is guided by the severity of the patient’s heart disease and should be carried out in accordance with the (documented) recommendations of the team responsible for the patient’s management (e-box). In most cases, non-invasive blood pressure measurement, electrocardiography, and pulse oximetry are sufficient. Especially in patients with left heart obstruction or aortopathy (such as Marfan syndrome, postoperative status after aortic isthmus stenosis with aortic aneurysm), however, invasive arterial blood pressure measurement is valuable to detect rapid changes in blood pressure. In patients with a right-left shunt, an air filter must be used to prevent systemic air embolisms. In pregnant women with a heart defect, compression of the vena cava with a fall in blood pressure (vena cava compression syndrome) may occur in supine position, and can be prevented by consistently maintaining slight left lateral positioning (15°) (caution: inverse position and abnormal course of the lower vena cava especially in women with complex heart defects).
Not only the direct stress resulting from labor and delivery is potentially hazardous for women with CHD, the phase directly after delivery also involves risks. On the one hand, additional blood volumes rapidly enter the heart due to the post partum uterine contraction and the decompression of the lower vena cava, potentially resulting in volume overload, and on the other hand the blood loss following delivery can cause a fall in blood pressure. Uterine contraction inducing agents such as oxytocin and ergometrine also exert considerable circulatory effects. Oxytocin can cause hypotension because of its vasodilator properties. Ergometrine, on the other hand, can have hypertensive actions. The effects may then be particularly intense and hazardous if these agents are administered too rapidly or in excessively large doses. Oxytocin should therefore be given as a long-term infusion and not as a bolus. Combination preparations of oxytocin and ergometrine should in principle be avoided because of their unpredictable cardiovascular effects (e13).
Puerperium
Close monitoring of cardiovascular functions is also required during the puerperium in women exposed to a high risk (table 3). Especially in patients with pulmonary hypertension, the increased mortality risk for the mother continues until the 10th day after delivery (e14). The thromboembolic risk is particularly increased during the puerperium. Most thromboembolic mortalities occur during this phase (4). Thromboembolic prophylaxis with low molecular weight heparin is therefore invariably indicated in women with a moderate and high risk (table 3). It can be initiated six to twelve hours after delivery (risk of secondary bleeding) and the mother can breast feed without misgivings. The mother can also breast feed while receiving coumarins (e8, e10).
Conclusion
Pregnancy, delivery, and puerperium are associated with a substantial risk for women with CHD and their children, but this risk is usually acceptable if optimal care is provided. Early and thorough examination and counseling and the timely planning of care at a suitable center by a specialized team comprising obstetricians, prenatologists, cardiologists, and anesthetists are the key to a favorable outcome with a minimum of risk.
CASE.
A 31-year-old woman with complex CHD (functionally single ventricle, status post Fontan operation with creation of a total cavopulmonary anastomosis by lateral intraatrial tunnel) first attended the clinic in the 16th week of pregnancy. She had not had a cardiological examination for the previous eight years.
History and results of clinical examination
The patient did not report having reduced physical capabilities, dyspnea, palpitations or dizziness. The examination revealed elevated jugular venous pressure and a quiet systolic heart murmur. The physical examination otherwise showed no abnormalities; arterial oxygen saturation 97%.
Diagnosis
Normal sinus rhythm in electrocardiography; documentation of good ventricular function; exclusion of significant valve stenoses or insufficiencies and of thrombi in the Fontan pathways by echocardiography and MRI.
Pregnancy care plan
Continuation of pregnancy with close monitoring of cardiac findings
If signs of heart failure or increased cyanosis: bed rest with anticoagulation and oxygen administration
Close monitoring of fetal growth
Fetal echocardiography between the 16th and 20th week of pregnancy
Initiation of acetylsalicylic acid (75 mg/day) for thromboembolism prophylaxis
Elective admission to the gynecological clinic at the start of the 36th week of pregnancy to ensure optimal management of delivery
Course of pregnancy
The patient did not develop cardiac arrhythmia, heart failure, cyanosis or hypertension. Ventricular function remained unimpaired. Fetal growth proceeded normally, a fetal heart defect was excluded.
On admission, the acetylsalicylic acid medication was terminated and replaced by low molecular weight heparin. In the 39th week of pregnancy, delivery was induced to ensure care provision on a workday. Heparin was suspended from the day of induction until after delivery. During childbirth the patient received epidural anesthesia and endocarditis prophylaxis. Delivery was uncomplicated, resulting in the birth of a healthy boy.
The patient rapidly recovered and after four days’ observation was discharged home in a good general condition. On the day of discharge the heparin medication was replaced again with aspirin. An examination three months after delivery showed the patient to be still in good condition (sinus rhythm, good ventricular function).
Acknowledgments
Translated from the original German by mt-g.
Footnotes
Conflict of interst statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Nieminen HP, Jokinen EV, Sairanen HI. Late results of pediatric cardiac surgery in Finland: a population-based study with 96% follow-up. Circulation. 2001;104:570–575. doi: 10.1161/hc3101.093968. [DOI] [PubMed] [Google Scholar]
- 2.Thorne S, Deanfield J. Long-term outlook in treated congenital heart disease. Arch Dis Child. 1996;75:6–8. doi: 10.1136/adc.75.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaemmerer H, Hess J. Adult patients with congenital heart abnormalities: present and future. Dtsch Med Wochenschr. 2005;130:97–101. doi: 10.1055/s-2005-837381. [DOI] [PubMed] [Google Scholar]
- 4.Lewis G, Drife JO. Why mothers die 2000-2002 - the sixth report of confidential enquiries into maternal deaths in the United Kingdom. London: RCOG Press; 2004. [Google Scholar]
- 5.Task force on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology. Expert consensus document on management of cardiovascular diseases during pregnancy. Eur Heart J. 2003;24:761–781. doi: 10.1016/s0195-668x(03)00098-8. [DOI] [PubMed] [Google Scholar]
- 6.Uebing A, Steer PJ, Yentis SM, Gatzoulis MA. Pregnancy and congenital heart disease. BMJ. 2006;332:401–406. doi: 10.1136/bmj.332.7538.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–521. doi: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 8.Siu SC, Sermer M, Harrison DA, et al. Risk and predictors for pregnancy-related complications in women with heart disease. Circulation. 1997;96:2789–2794. doi: 10.1161/01.cir.96.9.2789. [DOI] [PubMed] [Google Scholar]
- 9.Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol. 2007;49:2303–2311. doi: 10.1016/j.jacc.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Siu SC, Colman JM, Sorensen S, et al. Adverse neonatal and cardiac outcomes are more common in pregnant women with cardiac disease. Circulation. 2002;105:2179–2184. doi: 10.1161/01.cir.0000015699.48605.08. [DOI] [PubMed] [Google Scholar]
- 11.Nora JJ. From generational studies to a multilevel genetic-environmental interaction. J Am Coll Cardiol. 1994;23:1468–1471. doi: 10.1016/0735-1097(94)90393-x. [DOI] [PubMed] [Google Scholar]
- 12.Steer PJ, Gatzoulis MA, Baker P. Consensus views arising from the 51st study group: heart disease and pregnancy. In: Steer PJ, Gatzoulis MA, Baker P, editors. Heart Disease and Pregnancy. London: Royal College of Obstetricians and Gynaecologists Press; 2006. pp. 327–332. [Google Scholar]
- 13.Hyett J, Perdu M, Sharland G, Snijders R, Nicolaides KH. Using fetal nuchal translucency to screen for major congenital cardiac defects at 10 to 14 weeks of gestation: population based cohort study. BMJ. 1999;318:81–85. doi: 10.1136/bmj.318.7176.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess J, Bauer U, de Haan F, et al. Empfehlungen für Erwachsenen- und Kinderkardiologen zum Erwerb der Zusatz-Qualifikation "Erwachsene mit angeborenen Herzfehlern" (EMAH) Clin Res Cardiol 2007; 2. (Suppl 1):19–26. [Google Scholar]
- 15.ACOG Committee Opinion. Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004;104:647–651. doi: 10.1097/00006250-200409000-00053. Number 299, September 2004 (replaces No. 158, September 1995) [DOI] [PubMed] [Google Scholar]
- 16.Leyendecker JR, Gorengaut V, Brown JJ. MR-imaging of maternal diseases of the abdomen and pelvis during pregnancy and the immediate postpartum period. Radiographics. 2004;24:1301–1316. doi: 10.1148/rg.245045036. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer C, Weber-Schöndorfer C. Medikamentöse Therapie in der Schwangerschaft. Dtsch Arztebl. 2005;102(37):A 2480–A 2489. [Google Scholar]
- 18.Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, Spray TL. Thrombus formation after the fontan operation. Ann Thorac Surg. 2001;71:1990–1994. doi: 10.1016/s0003-4975(01)02472-9. [DOI] [PubMed] [Google Scholar]
- 19.Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: the Seventh ACCP. Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):627–644. doi: 10.1378/chest.126.3_suppl.627S. [DOI] [PubMed] [Google Scholar]
- 20.Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg. 2003;76:309–314. doi: 10.1016/s0003-4975(03)00169-3. [DOI] [PubMed] [Google Scholar]
- 21.Khairy P, Ouyang DW, Fernandes SM, Lee-Parritz A, Economy KE, Landzberg MJ. Pregnancy outcomes in women with congenital heart disease. Circulation. 2006;113:517–524. doi: 10.1161/CIRCULATIONAHA.105.589655. [DOI] [PubMed] [Google Scholar]
- 22.Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med. 2000;160:191–196. doi: 10.1001/archinte.160.2.191. [DOI] [PubMed] [Google Scholar]
- 23.Vitale N, De Feo M, De Santo LS, Pollice A, Tedesco N, Cotrufo M. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol. 1999;33:1637–1641. doi: 10.1016/s0735-1097(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 24.Oran B, Lee-Parritz A, Ansell J. Low molecular weight heparin for the prophylaxis of thromboembolism in women with prosthetic mechanical heart valves during pregnancy. Thromb Haemost. 2004;92:747–751. doi: 10.1160/TH04-06-0337. [DOI] [PubMed] [Google Scholar]
- 25.Cappelleri JC, Fiore LD, Brophy MT, Deykin D, Lau J. Efficacy and safety of combined anticoagulant and antiplatelet therapy versus anticoagulant monotherapy after mechanical heart-valve-replacement: a metaanalysis. Am Heart J. 1995;130:547–552. doi: 10.1016/0002-8703(95)90365-8. [DOI] [PubMed] [Google Scholar]
- e1.Warnes CA. The adult with congenital heart disease: born to be bad? J Am Coll Cardiol. 2005;46:1–8. doi: 10.1016/j.jacc.2005.02.083. [DOI] [PubMed] [Google Scholar]
- e2.Welsch H, Krone HA, Wisser J. Maternal mortality in Bavaria between 1983 and 2000. Am J Obstet Gynecol. 2004;191:304–308. doi: 10.1016/j.ajog.2003.12.031. [DOI] [PubMed] [Google Scholar]
- e3.Kafka H, Johnson MR, Gatzoulis MA. The team approach to pregnancy and congenital heart disease. Cardiol Clin. 2006;24:587–605. doi: 10.1016/j.ccl.2006.08.009. [DOI] [PubMed] [Google Scholar]
- e4.Steer PJ. Pregnancy and contraception. In: Gatzoulis MA, Swan L, Therrien J, Pantley GA, editors. Adult congenital heart disease: A practical guide. Oxford: BMJ Publishing Group, Blackwell Publishing Ltd; 2005. pp. 16–35. [Google Scholar]
- e5.von Obernitz N, Strauss A. Fetal echocardiography - see with new eyes. Geburtsh Frauenheilk. 2001;61:364–374. [Google Scholar]
- e6.Bassett AS, Chow EW, Husted J, et al. Clinical features of 78 adults with 22q11-deletion-syndrome. Am J Med Genet A. 2005;138:307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.De Wilde JP, Rivers AW, Price DL. A review of the current use of magnetic resonance imaging in pregnancy and safety implications for the fetus. Prog Biophys Mol Biol. 2005;87:335–353. doi: 10.1016/j.pbiomolbio.2004.08.010. [DOI] [PubMed] [Google Scholar]
- e8.Qasqas SA, McPherson C, Frishman WH, Elkayam U. Cardiovascular pharmaco-therapeutic considerations during pregnancy and lactation. Cardiol Rev. 2004;12:201–221. 240–261. doi: 10.1097/01.crd.0000102420.62200.e1. [DOI] [PubMed] [Google Scholar]
- e9.Siu SC, Colman JM. Heart disease and pregnancy. Heart. 2001;85:710–715. doi: 10.1136/heart.85.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.James PR. Drugs in pregnancy. Cardiovascular disease. Best Pract Res Clin Obstet Gynaecol. 2001;15:903–911. doi: 10.1053/beog.2001.0237. [DOI] [PubMed] [Google Scholar]
- e11.Elkayam U. Pregnancy through a prosthetic heart valve. J Am Coll Cardiol. 1999;33:1642–1645. doi: 10.1016/s0735-1097(99)00088-1. [DOI] [PubMed] [Google Scholar]
- e12.Warnes C. Prosthetic heart valves. In: Steer PJ, Gatzoulis MA, Baker P, editors. Heart disease and pregnancy. London: Royal College of Obstetrician and Gynaecologists Press; 2006. pp. 157–168. [Google Scholar]
- e13.Lupton M, Oteng-Ntim E, Ayida G, Steer PJ. Cardiac disease in pregnancy. Curr Opin Obstet Gynecol. 2002;14:137–143. doi: 10.1097/00001703-200204000-00006. [DOI] [PubMed] [Google Scholar]
- e14.Monnery L, Nanson J, Charlton G. Primary pulmonary hypertension in pregnancy; a role for novel vasodilators. Br J Anaesth. 2001;87:295–298. doi: 10.1093/bja/87.2.295. [DOI] [PubMed] [Google Scholar]
- e15.Abbas AE, Lester SJ, Connolly H. Pregnancy and the cardiovascular system. Int J Cardiol. 2005;98:179–189. doi: 10.1016/j.ijcard.2003.10.028. [DOI] [PubMed] [Google Scholar]
- e16.Schaefer C, Hannemann D, Meister R, et al. Vitamin-K-antagonists and pregnancy outcome. A multi-centre prospective study. Thromb Haemost. 2006;95:949–957. doi: 10.1160/TH06-02-0108. [DOI] [PubMed] [Google Scholar]
- e17.Elkayam U, Singh H, Irani A, Akhter MW. Anticoagulation in pregnant women with prosthetic heart valves. J Cardiovasc Pharmacol Ther. 2004;9:107–115. doi: 10.1177/107424840400900206. [DOI] [PubMed] [Google Scholar]
- e18.Imperiale TF, Petrulis AS. A meta-analysis of low-dose aspirin for the prevention of pregnancy-induced hypertensive disease. Jama. 1991;266:260–264. [PubMed] [Google Scholar]
- e19.Klein LL, Galan HL. Cardiac disease in pregnancy. Obstet Gynecol Clin North Am. 2004;31:429–459. doi: 10.1016/j.ogc.2004.03.001. [DOI] [PubMed] [Google Scholar]
- e20.Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome; DiGeorge syndrome: the chromosome 22q11 - two deletion syndromes. Lancet. 2007;370:1443–1452. doi: 10.1016/S0140-6736(07)61601-8. [DOI] [PubMed] [Google Scholar]