Abstract
Introduction
Although the aging of the population is causing a dramatic rise in the incidence of lumbar spinal stenosis, the indications and options for surgical treatment are not clearly defined.
Methods
In an attempt to aid clinical decision making, a selective literature review was conducted, taking into account the guidelines of the Association of the Scientific Medical Societies in Germany (AWMF).
Results
In degenerative lumbar spinal stenosis hypertrophy of the facet joints and yellow ligaments brings about constriction of the spinal canal, leading to back pain and activity-dependent lower limb symptoms (neurogenic claudication). If conservative treatment fails, an imaging study, usually magnetic resonance imaging, is required. In the case of severe symptoms the progressive underlying degeneration necessitates surgical treatment. Minimally invasive fenestration techniques are usually employed to decompress the spinal canal; in the presence of instability, fusion is indicated.
Discussion
Despite the proven superiority of surgery in the management of refractory lumbar spinal stenosis, there is a lack of evidence-based data regarding the different surgical treatment options. The evaluation of modern, minimally invasive techniques is thus difficult.
Keywords: lumbar spinal stenosis, neurogenic claudication, surgery, laminectomy, fenestration, fusion
Ubiquitous degeneration associated with aging may lead to stenoses of the spinal canal, especially along the lumbar spine. As a result of the changing societal age structure, the incidence of symptomatic lumbar spinal stenosis is increasing exponentially. In patients older than 60, lumbar spinal stenosis is found on magnetic resonance imaging in more than 20% of cases (1). Older patients’ desires for mobility and functionality, combined with improved perioperative management, have resulted in a situation where surgical intervention is being increasingly considered. In 1990, 60 in 100 000 people in this age group were treated surgically (2). In the United States, the incidence of surgery has increased eight-fold from 1979 to 1992 (2). Lumbar spinal stenosis is therefore increasingly gaining in importance generally and for neurosurgeons and orthopedic surgeons. The indication for surgery and the choice of surgical method are made more difficult by the simultaneous development of new therapeutic approaches, especially since evidence-based decision making aids for the treatment of patients are lacking. We present an overview of current strategies for the diagnosis and treatment of degenerative lumbar spinal stenosis.
Methods
This review article is based on a comprehensive selective literature review, taking into consideration the guidelines of the Association of the Scientific Medical Societies in Germany (AWMF).
Pathophysiology
Lumbar spinal stenosis is defined as a circumscribed, osteoligamentous narrowing of the spinal canal. The clinical burden includes backaches and symptoms in the legs that deteriorate upon standing and walking (neurogenic claudication) (3). According to the sagittal diameter of the spinal canal, distinction is made between relative lumbar spinal stenosis (10 to 14 mm) and absolute lumbar spinal stenosis (<10 mm), although this does not do justice to the complex pathoanatomy of this pathology. This parameter takes into consideration only central stenosis and not lateral stenosis in the area of the lateral recess and neuroforamen. Usually, combinations of both forms are present.
Lumbar spinal stenosis is categorized into primary (congenital) and secondary (degenerative, post-traumatic, etc) forms. Because of its enormous predominance, this article will deal with the degenerative variant only. Etiologically, three main factors are responsible for the development of degenerative lumbar spinal stenosis:
Degeneration of the disk of a motion segment results in disk protrusion with ventral narrowing of the canal and a loss in the height of the segment. The height loss automatically narrows the lateral recess and neuroforamen. Biomechanically, it effects ligamentous laxity with subsequently increased segmental mobility, which in turn results in additional strain especially on the facet joints.
The bone structures react to this subclinical instability of the segment with osseous hypertrophy, which presents especially as hypertrophy of the facet joints, whereas the ligamentum flavum shows fibrotic hypertrophy in addition to folding inwards subsequent to height loss.
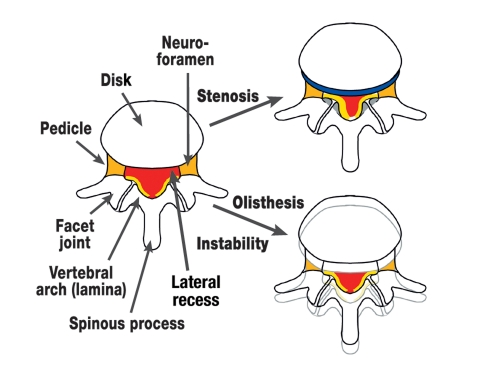
If these reactive processes do not succeed in stabilizing the segment, disk degeneration and laxity of capsules and ligaments may result in the manifest instability of spondylolisthesis (figure 1).
Figure 1.
Schematic axial representation of the degenerative changes to the lumbar spine. Narrowing of the spinal canal (red) develops subsequent to disk protrusion (blue), hypertrophy of the facet joints (gray), and hypertrophy or folding in of the ligamentum flavum (yellow). Depending on the location of the changes, the lateral recess and/or neuroforamina may also undergo narrowing (orange). Lumbar olisthesis or instability can also result in a narrowing of the spinal canal and especially the neuroforamina.
These pathoanatomical changes result in nerve root compression, which is affected by the position of the spine. The strain imposed by standing is in itself sufficient to result in hyperlordosis of the affected segment, with further protrusion of the ligamentum flavum into the spinal canal. This seems to be due not only to purely mechanical irritation of the nerve roots but also to vascular compression. Pathophysiologically, arterial ischemia as well as venous congestion are under discussion (4). The strain and weight imposed by walking results in decompensation of the vascular flow to the spinal nerves, which is mostly sufficient during rest.
Clinical symptoms
The pathophysiology results in the characteristic overall clinical picture: the dependence on weight bearing and bodily position. The patients mostly complain of long-term, slowly progressing back pain, which radiates into the legs upon standing or walking, and result in unspecific complaints such as fatigue and a sensation of heaviness. Further on, neurological deficits such as hypesthesias or pareses, as well as permanent symptoms during rest are possible, including the very rare cauda equine syndrome. As a result of the pain occurring mainly during walking, the patients’ walking distance is typically limited. By eliminating the described hyperlordosis (see pathophysiology) in a bent position, cycling or leaning on to a shopping trolley results in alleviation of the symptoms. In contrast to peripheral arterial occlusive disease, however, stopping walking/standing still is not sufficient for recovery. The patients have to sit down having walked a certain distance. The peripheral pulse should obviously be considered in the differential diagnosis between vascular and neurogenic claudication.
Problems in the clinical assessment of the often elderly patients are posed on the one hand by comorbidities – such as polyneuropathy in diabetes and coxarthrosis – and on the other hand by the general degeneration of the spinal column. Because of the latter, some patients simultaneously develop symptomatic arthrosis in the facet joints, instability of the affected segment, or osteochondrosis. Depending on how pronounced these degenerative sequelae are, the patients primarily complain of back pain and not the leg pain that is typical of stenosis. Additionally, a concomitant disk prolapse may complicate the situation, or unilateral symptoms may predominate. The table provides an overview of differential diagnoses (3).
Table. Most common differential diagnoses of lumbar spinal stenosis (adapted from [3]).
| Lumbar spine | Skeleton | Other |
|---|---|---|
| Disk prolapse | Cervical/thoracic stenosis with myelopathy | Peripheral arterial occlusive disease |
| Spondylolisthesis | Ankylosing spondylitis | Leriche’s syndrome |
| Facet joint syndrome | Arthrosis of the iliosacral joint | Abdominal aortic aneurysm |
| Spinal fractures | Coxarthrosis | Neuropathies |
| Spinal tumors | Tendopathies | |
| Inflammations (spondylodiscitis, epidural abscess, borreliosis) |
The patients’ complaints should not be underestimated under any circumstances. Although patients may present without pain during rest, i.e., during the medical examination, the limitations on their mobility and quality of life are often enormous.
Diagnosis
Lumbar spinal stenosis is a diagnosis that is derived from the patient’s medical history. Because the symptoms are dependent on activity and body position, a neurological examination will yield findings only at an advanced stage of disease, in the form of manifest root compression. Results from electrophysiological tests are equally uncharacteristic, for the same reason. However, the value of such tests lies in differential diagnostic considerations – for example, in polyneuropathy – much in the same way as laboratory analysis in inflammatory processes.
If very severe pain prevails or the pain is treatment resistant, then imaging methods are indicated. The method of choice is magnetic resonance imaging (MRI). However, the extent of the radiological findings does not correlate with the intensity of clinical symptoms (1). To plan surgery or clarify questions about the condition of the bones, computed tomography (CT) is also used as a secondary procedure. Both procedures ignore the dynamic component of the stenosis. Potential instability in the sense of spinal misalignment or spondylolisthesis can be made visible using lateral radiography in flexion and extension. Radiographs in two planes are often prepared as an initial diagnostic test in clinical practice. Its value lies in the differential diagnosis of fractures, tumors, spondylodiscitis, and scoliosis.
In case surgery is indicated and the information gleaned from the MRI scan is insufficient or MRI is contraindicated, lumbar myelography with postmyelo-CT is applied. Imaging in particular the contrast medium filled dural sac in functional positions allows exact assessment of dynamic stenosis, and the correct surgical procedure can therefore be selected.
Treatment
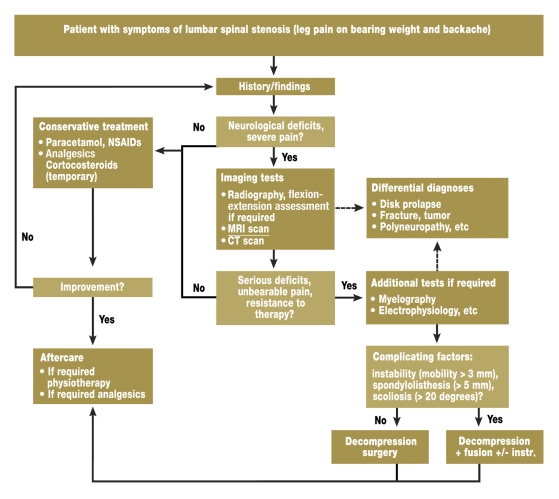
The decision of whether to use conservative or surgical treatment depends crucially on the spontaneous disease course. In lumbar spinal stenosis, this has not been sufficiently investigated. In most patients (60% to 70%), the pain seems to stagnate in the medium term. In patients with pronounced symptoms, a high degree of stenosis, and spondylolisthesis, a progressive disease course may be assumed (5). A treatment algorithm for lumbar spinal stenosis is described in figure 2 (3).
Figure 2.
Algorithm for the treatment of lumbar spinal stenosis (adapted from [3]). NSAIDs, non-steroidal anti-inflammatory drugs
Non-randomized comparative studies have suggested that surgical treatment is superior (6), and recently, two prospective studies showed the advantage of surgical compared with conservative treatment with evidence levels I and II. This proves the benefit of surgery in lumbar spinal stenosis (7, 8). A prospective cohort study with 125 consecutive patients showed significant improvement in more than 60% of patients after two years, compared with only 25% after conservative treatment (7). Malmivaara et al. randomized 94 patients to surgical or conservative treatment and showed a significant advantage for surgery in terms of disability, leg pain, and backache. For example, surgery reduced activity-dependent backache on a 10 point pain scale from a score of 6.9 to 2.7. After conservative treatment, an intensity of backache of 5.1 has been reported. This corresponds to a dramatic clinical difference for patients (8) and is consistent with clinical experience.
A recent publication in the New England Journal of Medicine, the Spine Patient Outcomes Research Trial (SPORT) from the US, supported these results in a larger group of 289 patients in a randomized cohort and 365 patients in an observational cohort. Surgery resulted in faster and significantly better alleviation of complaints than conservative treatment. Interestingly, patients who did not have surgery also experienced a reduction in symptoms, albeit at a slower rate. However, this study showed that surgery is superior to conservative treatment in the longer term (9).
Conservative treatment
Because spinal complaints often present in a wave pattern, conservative treatment is initially indicated, except for cases of very severe pain and pronounced neurological deficits. In clinical practice, many measures are deployed; a combination of drug treatment, physiotherapy, and physical strategies is ideally recommended as a multimodal therapeutic concept. Pain relieving and anti-inflammatory drugs such as non-steroidal anti-inflammatory drugs (NSAIDs), a short course of corticosteroids, opioids if required, and muscle relaxants are the main treatments. Physiotherapeutically, delordosing flexion exercises, medical training therapy to strengthen the stabilizing stomach and back muscles, and treadmill and ergometer training are used (8). Electrotherapy and transcutaneous electrical nerve stimulation (TENS), passive measures, and delordosing ortheses are used.
In principle, any treatment aims to relieve and stabilize the affected segments and to promote patients’ general physical fitness (10). The efficacy of these measures has neither been shown nor disproved thus far. Only calcitonin administration has proved to be ineffective (11). After diagnostic imaging, therapeutic infiltrations are given by epidural or periradicular administration, or in the area of the facet joints, but their effect may be merely temporary and they may cause complications. Especially in patients with predisposing conditions, such as diabetic patients, and in repeated infiltrations, infections are possible, which may have severe consequences (12). Since most patients have chronic, stagnating, or slowly progressive symptoms that are controllable but to an insufficient degree by conservative measures, causal treatment of spinal canal stenosis is often required. Especially neurogenic claudication can barely be influenced conservatively. In contrast to disk prolapse, which tends to regress spontaneously, the causative degenerative changes associated with spinal stenosis can be expected to progress slowly.
Surgery
An indication for surgery exists in cases of consistent clinical and radiological findings after adequate conservative therapeutic measures have failed for a time span of at least three months. Caution is advised in questionable findings, mild clinical symptoms, or unrealistic expectations on the patient’s part.
Laminectomy
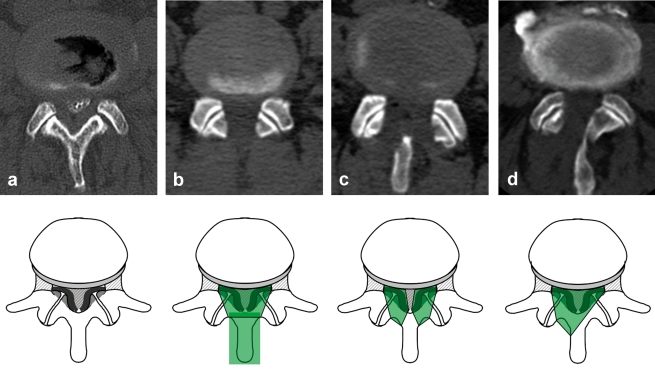
The traditional standard operation in lumbar spinal stenosis is decompression laminectomy. Spinous processes, vertebral lamina, ligamenta flava, and parts of the facet joints are ablated during this removal of the roof of the spinal canal (figure 3).
Figure 3.
Postmyelographic computed tomography scan before (a) and immediately after decompressing lumbar spinal stenosis by means of laminectomy (b), bilateral fenestration (c), and unilateral fenestration with undercutting contralateral decompression (d). The decompression techniques are illustrated with corresponding schematic representations of access (green). Laminectomy of the spinal canal decompresses a longer section of the spine, whereas fenestration techniques are limited to the level of the intervertebral space and the hypertrophied facet joints.
In a meta-analysis, the success rate of this procedure has been shown to be merely 64% (13); the lack of success was partly attributed to the development of postoperative instabilities. Nerve compression is usually limited to the height of the intervertebral space in the area of the hypertrophied joint facets and the ligamentum flavum. Removing long sections is therefore not necessary, which has – aided by enormously increasing numbers of surgical procedures – resulted in the development of newer, less invasive techniques.
Fenestration
Modified interspinal and partial laminectomy techniques have been developed in the same way as decompression techniques directly through the spinous process. As an alternative to laminectomy, interlaminar fenestration techniques have become established that spare the midline structures and thus the dorsal tension band. All these procedures aim to decompress the nerve roots, by resecting the ligamentum flavum and parts of the medial facet joint. Encouraging results have been shown for bilateral fenestration on the one hand, and unilateral fenestration with undercutting contralateral decompression on the other hand (14, 15). Recently, unilateral endoscopic interventions have been publicized, but no advantage compared with microscopic techniques has been shown. The authors’ own (Thomé et al.’s) randomized study showed that microsurgical bilateral fenestration was superior to unilateral access and laminectomy (16). We thus favor this method in typical bilateral stenosis symptoms, and use a high speed bur for exact ablation of the bone structures. Depending on the underlying pathology, e.g., in unilateral symptoms or lateral stenosis, unilateral access is the method of choice.
In principle, in a scenario of adequate decompression with microsurgical techniques, all methods can yield success rates with regard to the leg pain component. However, residual backache is common.
Stabilization
Stenosis surgery aims primarily to relieve the nerve roots by widening the spinal canal. The assumption that lumbar spinal stenosis develops as a result of segmental instability often leads physicians to advocate stabilization in addition to decompression (17). Biomechanically, stenosis-related hypertrophy constitutes a reaction to segmental hypermobility (see pathophysiology). Resecting these ligamentous and bone structures in the context of decompression could potentially cause renewed instability. Considerations along these lines have resulted in the development of the minimally invasive decompression techniques mentioned earlier. Current guidelines reject stabilization by default, on the basis of an extensive literature search (18). The reactive degenerative changes obviously prevent manifest segmental instability in the sense of spondylolisthesis. However, the possibility of segmental instability should always be considered.
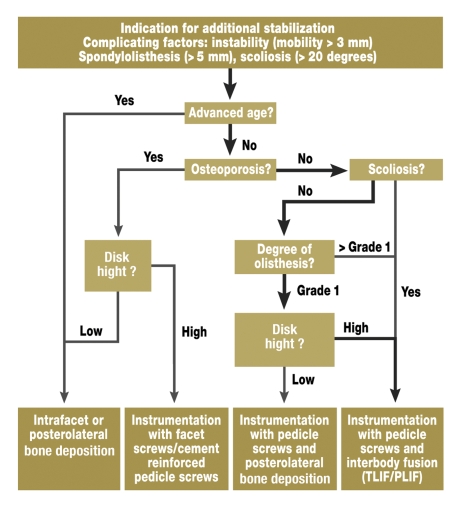
In patients in whom factors are present that imply compromised stability of the segment to be decompressed (figure 2), many authors recommend additional stabilization (19). Even though the criteria of instability are still the subject of much controversy, this situation has to be assumed in serious spondylolisthesis or scoliosis. Of particular importance is flexion-extension radiography to identify pathological hypermobility. Whether stabilization should be undertaken without instrumentation – only by deposition of bone – or with instrumentation – e.g., with pedicle screws – is also under discussion. While instrumentation increases the fusion rate, studies thus far have not shown an effect on the clinical result (19). In recent years, fusion methods using different interbody cages have increasingly been undertaken (10). In view of the scanty evidence relating to the kind of additional stabilization (18), we have set out our own treatment algorithm for the purpose of orientation (figure 4). We have to repeat at this point that this algorithm should be used only when signs of instability are present. Most patients require merely decompression.
Figure 4.
Algorithm for additional stabilization in the subgroup of patients with lumbar spinal stenosis and complicating factors in the sense of instability (TLIF/PLIF: transforaminal/posterior lumbar interbody fusion). An orientation aid is given with regard to the selection of the stabilization procedure on the basis of different individual variables. In the authors’ experience, most patients follow the highlighted treatment pathway.
To avoid the sequelae of fusion, dynamic stabilization systems have been introduced. The clinical results are currently assessed as being comparable to those of fusion, especially as implant failures and degeneration of adjacent joints were observed no less often (20).
Interspinous spacers
In recent years, interspinous implants have increasingly been used in the treatment of lumbar spinal stenosis. The rationale behind this minimally invasive intervention is that of delordosing the segment and thus a widening of the spinal canal in the upright position. Opponents of the technology fear increased degeneration of the lumbar spine as a result of segmental kyphosis in the long term. Prospective studies in patients with moderate symptoms have shown improvements of 45% after spacer implantation after two years, compared with 7% after conservative treatment (21). These encouraging results, however, have not been replicated without limitations (22). Further, no comparison has been made between spacer surgery and decompression surgery. Although no conclusive assessment is currently possible, we speculate that interspinous spacers are an intermediate option between conservative and surgical treatment. This makes them eligible for patients with mild symptoms or as a temporary solution. The hope that they offer a clinically relevant stabilizing function has thus far not been fulfilled (23).
Conclusions
Although only few evidence-based insights into the treatment options of lumbar spinal stenoses exist, surgical treatment makes sense, and is indicated, in relevant and therapy resistant symptoms. The manifold surgical techniques enable surgeons to address the individual patient’s situation. Long-term results relating to modern techniques are often lacking.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 2.Ciol MA, Deyo RA, Howell E, Kreif S. An assessment of surgery for spinal stenosis: time trends, geographic variations, complications, and reoperations. J Am Geriatr Soc. 1996;44:285–290. doi: 10.1111/j.1532-5415.1996.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 3.AWMF-Leitlinie. Leitlinien der Deutschen Gesellschaft für Neurochirurgie „Lumbale Spinalkanalstenose“ http://www.uni-duesseldorf.de/awmf/ll-na/008-022.htm. 2005.
- 4.Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21:2046–2052. doi: 10.1097/00007632-199609010-00024. [DOI] [PubMed] [Google Scholar]
- 5.Benoist M. The natural history of lumbar degenerative spinal stenosis. Joint Bone Spine. 2002;69:450–457. doi: 10.1016/s1297-319x(02)00429-3. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y, Singer DE, Wu YA, Keller RB, Atlas SJ. The effect of surgical and nonsurgical treatment on longitudinal outcomes of lumbar spinal stenosis over 10 years. J Am Geriatr Soc. 2005;53:785–792. doi: 10.1111/j.1532-5415.2005.53254.x. [DOI] [PubMed] [Google Scholar]
- 7.Athiviraham A, Yen D. Is Spinal Stenosis Better Treated Surgically or Nonsurgically? Clin Orthop Relat Res. 2007;458:90–93. doi: 10.1097/BLO.0b013e31803799a9. [DOI] [PubMed] [Google Scholar]
- 8.Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32:1–8. doi: 10.1097/01.brs.0000251014.81875.6d. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulte TL, Bullmann V, Lerner T, et al. Lumbale Spinalkanalstenose. Orthopäde. 2006;35:675–694. doi: 10.1007/s00132-006-0971-5. [DOI] [PubMed] [Google Scholar]
- 11.Podichetty VK, Segal AM, Lieber M, Mazanec DJ. Effectiveness of salmon calcitonin nasal spray in the treatment of lumbar canal stenosis: a double-blind, randomized, placebo-controlled, parallel group trial. Spine. 2004;29:2343–2349. doi: 10.1097/01.brs.0000143807.78082.7f. [DOI] [PubMed] [Google Scholar]
- 12.Hooten WM, Mizerak A, Carns PE, Huntoon MA. Discitis after lumbar epidural corticosteroid injection: A case report and analysis of the case report literature. Pain Med. 2006;7:46–51. doi: 10.1111/j.1526-4637.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 13.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine. 1992;17:1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kleeman TJ, Hiscoe AC, Berg EE. Patient outcomes after minimally destabilizing lumbar stenosis decompression: the „Port-Hole" technique. Spine. 2000;25:865–870. doi: 10.1097/00007632-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 15.Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006;59:1264–1269. doi: 10.1227/01.NEU.0000245616.32226.58. [DOI] [PubMed] [Google Scholar]
- 16.Thome C, Zevgaridis D, Leheta O, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine. 2005;3:129–141. doi: 10.3171/spi.2005.3.2.0129. [DOI] [PubMed] [Google Scholar]
- 17.Irwin ZN, Hilibrand A, Gustavel M, et al. Variation in surgical decision making for degenerative spinal disorders. Part I: lumbar spine. Spine. 2005;30:2208–2213. doi: 10.1097/01.brs.0000181057.60012.08. [DOI] [PubMed] [Google Scholar]
- 18.Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 10: fusion following decompression in patients with stenosis without spondylolisthesis. J Neurosurg Spine. 2005;2:686–691. doi: 10.3171/spi.2005.2.6.0686. [DOI] [PubMed] [Google Scholar]
- 19.Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: fusion in patients with stenosis and spondylolisthesis. J Neurosurg Spine. 2005;2:679–685. doi: 10.3171/spi.2005.2.6.0679. [DOI] [PubMed] [Google Scholar]
- 20.Schnake KJ, Schaeren S, Jeanneret B. Dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolisthesis. Spine. 2006;31:442–449. doi: 10.1097/01.brs.0000200092.49001.6e. [DOI] [PubMed] [Google Scholar]
- 21.Zucherman JF, Hsu KY, Hartjen CA, et al. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine. 2005;30:1351–1358. doi: 10.1097/01.brs.0000166618.42749.d1. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui M, Smith FW, Wardlaw D. One-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine. 2007;32:1345–1348. doi: 10.1097/BRS.0b013e31805b7694. [DOI] [PubMed] [Google Scholar]
- 23.Kim KA, McDonald M, Pik JH, Khoueir P, Wand MY. Dynamic intraspinous spacer technology for posterior stabilization: case-control study on the safety, sagittal angulation, and pain outcome at 1-year follow-up evaluation. Neurosurg Focus. 2007;22 [PubMed] [Google Scholar]