Abstract
Effects of acute stress exposure on learning and memory have been frequently studied in both animals and humans. However, only a few studies have focused specifically on working memory performance and the available data are equivocal. The present study examined working memory performance during the Sternberg item recognition task after exposure to a predominantly adrenergic stressor. Twenty four healthy subjects were randomly assigned to a stress group or a control group. The stress group was exposed to the cold pressor stress test (CPS; i.e. insertion of the dominant hand into ice water for 60s), while 37 °C warm water was used with the control group. Twenty minutes after the stress exposure, working memory performance was tested with the Sternberg item recognition task with three levels of cognitive load. Sympathetic nervous system and hypothalamic pituitary adrenocortical (HPA) axis activation during CPS, were assessed by measuring heart rate and salivary cortisol before and during (heart rate) or 30 min after (cortisol) the stress procedure. Exposure to the CPS test was associated with a significant increase in heart rate but no increase in salivary cortisol. Participants exposed to the stress procedure showed significantly shorter reaction times during trials with higher cognitive load but tended to show higher false alarm rates than control subjects. The present results indicate that exposure to CPS can be associated with signs of both enhanced and impaired working memory performance. The observed behavioral pattern might represent a form of streamlined information processing advantageous in a threatening situation.
Keywords: Stress, Working memory, Reaction time, Cortisol, Arousal
1. Introduction
Stress response can be characterized as behavioral and neuroendocrine activation associated with exposure to a threatening stimulus. Hormones and neurotransmitters released during stress are believed to be responsible for behavioral changes associated with stress exposure including effects on learning and memory (de Kloet, Joels, & Holsboer, 2005). The effect of stress exposure on learning and memory is complex and is determined by factors such as length of stress exposure, nature of the stress stimulus, specific cognitive function examined, age and gender (Sandi & Pinelo-Nava, 2007). Acute stress exposure can have positive effect on cognitive functions, particularly in paradigms when circulating cortisol does not reach very high concentrations (Andreano & Cahill, 2006; Jelici, Geraerts, Merckelbach, & Guerrieri, 2004; Steidl, Mohiuddin, & Anderson, 2006).
Cold pressor stress is an experimental stress paradigm based on a short term painful stimulation by immersing the hand into ice-cold water. This paradigm has been frequently used in stress research and is known to be associated with substantial activation of the autonomic nervous system as well as mild to moderate activation of the hypothalamic pituitary adrenocortical (HPA) axis (McRae et al., 2006; Schwabe, Haddad, & Schachinger, 2008). Our previous findings showed that while the cold pressor stress procedure used in our lab caused only mild HPA axis activation, it was associated with an enhancing effect on learning performance with indices to a possible involvement of working memory (Duncko, Cornwell, Cui, Merikangas, & Grillon, 2007).
Working memory represents a cognitive function responsible for holding a limited amount of information active for short time period (Repovs & Baddeley, 2006) and is also important for optimal functioning of the executive system involved in gating and filtering out irrelevant information (Kane & Engle, 2002), a function that seems to be particularly important under stressful conditions. Literature shows that working memory performance after acute stress exposure can be impaired (Elzinga & Roelofs, 2005; Kuhlmann, Piel, & Wolf, 2005; Oei, Everaerd, Elzinga, van Well, & Bermond, 2006; Robinson, Sunram-Lea, Leach, & Owen-Lynch, 2008), as well as improved or not affected (Kuhlmann et al., 2005; Vedhara, Hyde, Gilchrist, Tytherleigh, & Plummer, 2000).
The present study was designed to test the hypothesis that exposure to a predominantly adrenergic stressor such as cold pressor stress can be associated with enhanced working memory performance in healthy human subjects.
2. Methods
2.1. Participants
Twenty four physically and mentally healthy volunteers (12 women) with the age of 28.1 years (±1.6) participated in the study. Physical and mental health of the participants was determined by a physical examination, the structured clinical interview for DSM-IV (First, Spitzer, Williams, & Gibbon, 1995) and urine toxicology analysis. All participants were required to be free of a medical condition, past or current psychiatric disorder and current use of drugs or psychoactive medication. Participants were randomly assigned to the control (N = 13, women = 7) or the stress group (N = 11, women = 5). All volunteers signed a written informed consent approved by the National Institute of Mental Health (NIMH) Human Investigation Review Board.
2.2. Stress procedure
All testing was performed in the afternoon (12–4 pm). After being explained the procedure and signing the informed consent the participants were seated in the testing room and the electrodes for heart rate recording were attached. After the 10 min baseline HR recording was performed, the participants provided the base-line saliva sample and underwent the cold pressor test or the control procedure. During the cold pressor test, the participants were asked to immerse their dominant hand up to the wrist into room temperature water (23 °C) for 5 min and immediately after that to immerse the same hand for 1 min into ice water (0–2 °C) produced by mixing 2 L of tap water with 2 L of crushed ice. The 5 min room temperature immersion was used to ensure that all participants had similar skin temperature when inserting their hands into the ice water. Participants assigned to the control group were asked to immerse their dominant hand for 5 min into luke-warm water (37 °C).
2.3. Sternberg item recognition task
To test the working memory performance, the Sternberg task (Sternberg, 1966) was administered 20 min after the end of the cold pressor test or the control procedure. The task consisted of presenting a target screen with 2, 3 or 4 uppercase letters for 750 msec, followed by a 750 msec period during which the participant tried to remember the letters and a recognition screen with 2, 3 or 4 uppercase letters. The participants were instructed to respond, by pushing a yes or no button, whether any of the target letters were present on the recognition screen. The feedback was provided by a word “Wrong!” or “Correct!” showing on the screen for 200 msec after each trial. Only one target letter was present on the recognition screen in target present trials and no target letter was present on the recognition screen in target absent trials. Three types of trials with 2, 3 or 4 letters presented on both the target and recognition screen represented the three levels of processing capacity load. A total of 120 trials were randomly presented in one session with 20 trials per each level of processing capacity load for both target absent and target present condition. The duration of the test was 6.6 ± 1.4 min.
2.4. Heart rate
Heart rate was recorded by a Psylab BioAmplifier (Contact Precision Instruments Inc., Boston, MA) with two electrodes placed on each side of the chest. Baseline heart rate was calculated as average of the last minute of the baseline recording. Stress heart rate was calculated as average from the 1 min of ice water immersion (stress group) or the last minute of warm water immersion (control group).
2.5. Salivary cortisol
Saliva samples were obtained after the 10 min of baseline recording and at 30 min after the end of the cold pressor stress or the control procedure with the use of plain cotton swab Salivettes (Sarstedt, Leicester, UK). The selection of timepoints for saliva collection was based on our previous findings showing the peak in cortisol concentration in saliva at 30 min after stress exposure (Duncko et al., 2007; Jezova, Makatsori, Duncko, Moncek, & Jakubek, 2004). After collection, samples were frozen and stored at −70 °C. Before the assay, samples were thawed at room temperature and centrifuged at 3000 g for 10 min. The concentration of cortisol was measured by a commercially available radioimmunoassay (Coat-a-Count, DPC, Los Angeles, CA).
2.6. Statistics
A Repeated Measures ANOVA with time as within subject factor (two levels) and group and gender as between subject factors was used to analyze the effect of stress exposure on heart rate and cortisol concentrations in saliva. Due to technical problems during hear rate recording and insufficient amount of saliva collected, HR data from one participant and cortisol data from three participants were excluded from the analysis. Repeated measures ANOVA analyses with processing load as within subject (three levels) and group and gender as between subject factors were used to test the effect of stress on working memory performance. Test of within subjects contrasts was used to identify the within subject level with statistically significant difference between groups. These analyses were performed separately for reaction time and accuracy in target absent and target present trials.
3. Results
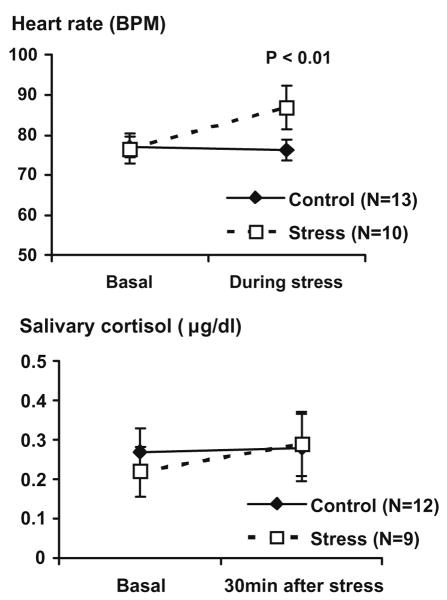
The analysis of heart rate at baseline and after water immersion revealed a significant main effect of time (F = 14.3, p < 0.01) and a time × group interaction (F = 14.4, p < 0.01). The test of within subject contrasts showed that the exposure to the ice water (stress) but not the warm water condition (control) was associated with immediate significant increase in heart rate. On the other hand, the analysis of cortisol concentrations in saliva at baseline and after water immersion showed no effect of time (F < 1, NS) or stress (F < 1, NS) (Fig. 1). There was no significant effect of gender on changes in heart rate (F < 1, NS) or cortisol concentrations (F < 1, NS).
Fig. 1.
Heart rate (BPM) and the concentration of cortisol in saliva (μg/dl) before and during/after the exposure to the cold pressor stress or control procedure. Data are expressed as mean ± SEM. P-value reflects the statistical significance of the time × group interaction.
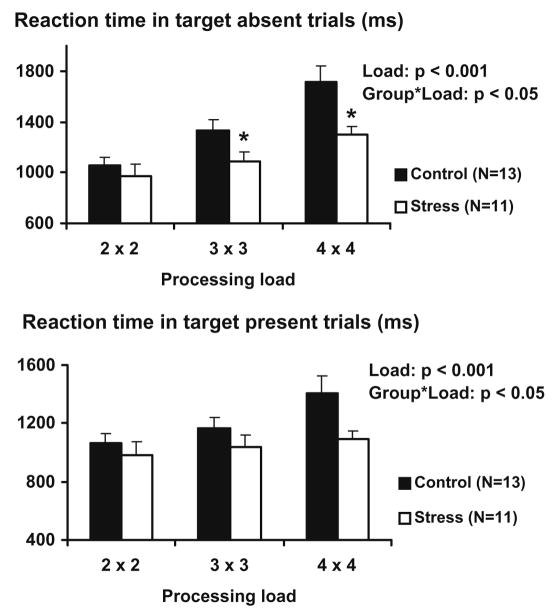
The analysis of reaction times during the Sternberg item recognition task showed significant effect of cognitive load for both target absent (F = 34.5, p < 0.001) and target present trials (F = 13.2, p < 0.001), with reaction time gradually increasing from 2 × 2 to 3 × 3 and 4 × 4 load trials (Fig. 2). The load × group interaction was significant for both target absent (F = 3.9, p < 0.05) and target present trials (F = 3.4, p < 0.05). The test of within subject contrast revealed that in target absent trials, the control group had significantly larger increase in reaction times from cognitive load 2 × 2 to 3 × 3 (F = 4.5; p < 0.05) and 4 × 4 (F = 4.4; p < 0.05) than the stressed group. In target present trials, the within subject contrast in increase in reaction times from cognitive load 2 × 2 to 3 × 3 (F = 0.3, NS) and from 2 × 2 to 4 × 4 (F = 3.5, p < 0.1) did not reach statistical significance.
Fig. 2.
Reaction time during target absent and target present trials of the Sternberg item recognition task. Data are expressed as mean ± SEM. Statistical significance: P-values reflect the statistical significance of the main effect of processing load and the processing load × group interaction; * reflects p < 0.05 in the within subject contrast for that particular processing load.
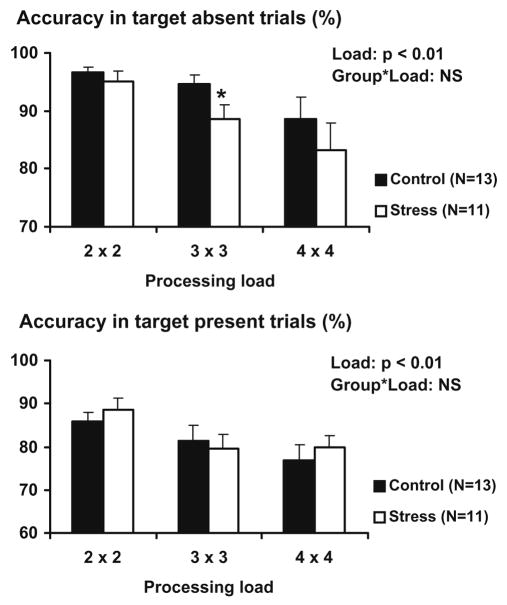
The analysis of accuracy during the Sternberg item recognition task revealed a main effect of cognitive load for both target absent (F = 9.2, p < 0.01) and target present trials (F = 7.6, p < 0.01), with accuracy gradually decreasing from 2 × 2 to 3 × 3 and 4 × 4 load trials. The overall load × group interaction did not reach statistical significance (absent: F < 1, NS; present: F < 1, NS). However, the test of within subject contrasts of the cognitive load × group interaction revealed that in target absent trials, the decrease in accuracy from 2 × 2 to 3 × 3 load trials was significantly larger in the stress group compared to the no stress group (F = 4.9, p < 0.05). The test of within subject contrasts of cognitive load × group interaction for target present trials was not significant (Fig. 3). There was no significant effect of gender on reaction time (target absent: F = 1.4, NS; target present: F < 1, NS) or accuracy (target absent: F = 1.8, NS; target present: F < 1, NS) during the Sternberg item recognition task.
Fig. 3.
Accuracy during target absent and target present trials of the Sternberg item recognition task. Data are expressed as mean ± SEM. Statistical significance: P-values reflect the statistical significance of the main effect of processing load and the processing load × group interaction; * reflects p < 0.05 in the within subject contrast for that particular processing load.
4. Discussion
The present data show that acute exposure to the cold pressor stress can be associated with signs of enhanced working memory performance represented by shorter reaction times in trials with higher cognitive load. These results are in accordance with our previous findings that had pointed out the possible involvement of working memory in improved learning performance after exposure to cold pressor stress test (Duncko et al., 2007) and extend the current literature examining the effect of stress on learning.
The finding of shorter reaction times observed in high cognitive load trials in the stress group is in contrast with longer reaction times reported in studies testing working memory performance after speech stress exposure (Elzinga & Roelofs, 2005; Kuhlmann et al., 2005; Oei et al., 2006) or glucocorticoid treatment (Lupien, Gillin, & Hauger, 1999). Our interpretation is that this contradiction is related to the different level of HPA axis activation during stress paradigms applied in these studies. In accordance with our previous findings (Duncko et al., 2007), the cold pressor stress test exposure in the present study was associated with no significant changes in cortisol concentrations in saliva. On the contrary, other studies testing the effect of acute stress exposure on working memory used the Trier Social Stress Test (TSST) or intense naturalistic stressors known to be associated with HPA axis activation at substantially higher level than the cold pressor stress test (McRae et al., 2006; Robinson et al., 2008). Studies using the TSST reported significant increase in cortisol concentrations and either impaired (Elzinga & Roelofs, 2005; Oei et al., 2006) or unaffected (Kuhlmann et al., 2005) working memory performance in the stressed group. Interestingly, the study of (Elzinga & Roelofs, 2005) investigated the role of individual variability in cortisol response and found that individuals with no increase in cortisol concentrations after stress showed improved working memory performance. Similar dose effect was reported by some of the pharmacological studies where high dose of cortisol was associated with impaired performance (Lupien et al., 1999; Wolf et al., 2001) but lower doses did not have an effect or even tended to improve performance (Lupien et al., 1999; Monk & Nelson, 2002).
While participants in the stressed group showed faster responses during trials with higher cognitive load, they also tended to show higher number of false recognitions in target absent trials with higher cognitive load. No such trend has been found in target present trials which might be related to target absent trials representing a slightly higher cognitive challenge than target present trials. Similar findings have been reported in the literature and it has been suggested that the higher rate of false alarms in subjects exposed to stress is related to impaired ability to remember details belonging to a specific context (Nadel & Payne, 2002). The present findings indicate that under specific stress conditions the impaired remembering of details can be associated with shorter reaction time. We suggest that even with the higher number of false alarms, the shorter reaction time can be advantageous in a threatening situation and that this behavioral pattern might represent a form of streamlined information processing occurring in certain type of stress conditions.
Several mechanisms could play a role in the effect of stress on working memory performance observed in the present study. One major mechanism thought to be involved in behavioral effects of stress is arousal mediated through the central noradrenergic pathways (Berridge, 2007). Increased central noradrenergic activity can be associated with impairment of prefrontal cortex functions involved in working memory performance (Ramos & Arnsten, 2007) and could play a role in the lower accuracy observed in the stressed group. However, our previous findings (Duncko et al., 2007) document that the CPS procedure as administered in our laboratory is not associated with autonomic activation 15–30 min post stress which points to a low probability of massive central noradrenergic activation during the learning task. It is more likely that the effect of cold pressor stress exposure on learning performance is related to changes initiated during the stress exposure and that these changes last long enough to affect learning performance 15–30 min post stress. Another neurotransmitter system thought to be involved in mediating behavioral and endocrine effects of stress is the extrahypothalamic corticotropin releasing hormone (CRH) system (de Kloet et al., 2005). In addition to its role in regulation of the central noradrenergic system, CRH receptors are abundant in both hippocampus and prefrontal cortex (Orozco-Cabal, Pollandt, Liu, Shinnick-Gallagher, & Gallagher, 2006) and CRH injection has been reported to affect hippocampal function in a way that could result in enhanced working memory performance (Kortekaas, Costall, & Smythe, 1999; Pieretti, Ortolani, Di Giannuario, & Loizzo, 1990). It has to be noted that the central CRH function has not been assessed in the present study and further studies will be necessary to investigate the possible involvement of this mechanism. Other possible mechanisms involved in the observed effects of stress exposure include neuromodulators such as brain-derived neurotrophic factor (Hall, Thomas, & Everitt, 2000), the opioid system (Bodnar, 2008), and neuropeptide Y (Thorsell et al., 2000).
Neither neuroendocrine nor behavioral response to the stress procedure in the present study was affected by gender. This is in contrast to the literature reporting the stress response being modulated by sex hormones (Wolf, 2003) and should be interpreted with caution since we did not collect the information on menstrual cycle of our female participants. Lack of this information limits our ability to conclude on possible effects of sex hormones on working memory during stress. Another limitation of the present study is that we only used two sampling timepoints to asses the HPA axis activity and that the single timepoint at 30 min post stress does not cover the whole response curve. However, the knowledge on kinetics of the HPA axis activation (de Kloet et al., 2005), as well as data reported on this specific stress procedure (Duncko et al., 2007; McRae et al., 2006) allow the assumption that the timepoint 30 min post stress correlated with the peak of cortisol concentration in saliva. Moreover, the cortisol concentration in saliva sampled after the Sternberg item recognition task was expected to reflect the concentration of cortisol in blood during the task and is relevant to asses the nongenomic effects of stress induced cortisol release on the working memory performance.
In conclusion, the present study reports that exposure to a predominantly adrenergic stress procedure such as cold pressor stress can be associated with signs of both enhanced and impaired working memory performance. The observed behavioral pattern includes shorter reaction times and a trend for higher number of false alarms and might represent a form of streamlined information processing advantageous in a threatening situation.
Acknowledgments
This research was supported in part by the Intramural Program of the National Institute of Mental Health, National Institutes of Health.
Footnotes
Disclaimer
The viewpoints expressed in this article do not necessarily represent those of the National Institutes of Health or the Department of Health and Human Services.
References
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychological Science. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Research Reviews. 2007 doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2007. Peptides. 2008;29:2292–2375. doi: 10.1016/j.peptides.2008.09.007. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Duncko R, Cornwell B, Cui L, Merikangas KR, Grillon C. Acute exposure to stress improves performance in trace eyeblink conditioning and spatial learning tasks in healthy men. Learning & Memory. 2007;14:329–335. doi: 10.1101/lm.483807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K. Cortisol-induced impairments of working memory require acute sympathetic activation. Behavioral Neuroscience. 2005;119:98–103. doi: 10.1037/0735-7044.119.1.98. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RI, Williams JBW, Gibbon M. Structured clinical interview for DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- Hall J, Thomas K, Everitt B. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nature Neuroscience. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Jelici M, Geraerts E, Merckelbach H, Guerrieri R. Acute stress enhances memory for emotional words, but impairs memory for neutral words. International Journal of Neuroscience. 2004;114:1343–1351. doi: 10.1080/00207450490476101. [DOI] [PubMed] [Google Scholar]
- Jezova D, Makatsori A, Duncko R, Moncek F, Jakubek M. High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Progress in NeuroPsychopharmacology and Biological Psychiatry. 2004;28:1331–1336. doi: 10.1016/j.pnpbp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kortekaas R, Costall B, Smythe JW. Changes in hippocampal theta following intrahippocampal corticotropin-releasing hormone (CRH) infusions in the rat. Brain Research Bulletin. 1999;48:603–607. doi: 10.1016/s0361-9230(99)00039-8. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience. 2005;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose–response study in humans. Behavioral Neuroscience. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA. Stress reactivity: Biological and subjective responses to the cold pressor and Trier Social stressors. Human Psychopharmacology. 2006;21:377–385. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson CA. The effects of hydrocortisone on cognitive and neural function: A behavioral and event-related potential investigation. Neuropsychopharmacology. 2002;26:505–519. doi: 10.1016/S0893-133X(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Nadel L, Payne JD. The relationship between episodic memory and context: Clues from memory errors made while under stress. Physiological Research. 2002;51(Suppl 1):S3–S11. [PubMed] [Google Scholar]
- Oei NY, Everaerd WT, Elzinga BM, van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress. 2006;9:133–141. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP. Regulation of synaptic transmission by CRF receptors. Reviews Neuroscience. 2006;17:279–307. doi: 10.1515/revneuro.2006.17.3.279. [DOI] [PubMed] [Google Scholar]
- Pieretti S, Ortolani E, Di Giannuario A, Loizzo A. Effects of endorphin derivatives on the EEG alterations induced by corticotropin releasing factor in the rabbit hippocampus. Pharmacological Research. 1990;22:627–633. doi: 10.1016/s1043-6618(05)80055-6. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacology & Therapeutics. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Robinson SJ, Sunram-Lea SI, Leach J, Owen-Lynch PJ. The effects of exposure to an acute naturalistic stressor on working memory, state anxiety and salivary cortisol concentrations. Stress. 2008;11:115–124. doi: 10.1080/10253890701559970. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plasticity. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33:890–895. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Steidl S, Mohi-uddin S, Anderson AK. Effects of emotional arousal on multiple memory systems: Evidence from declarative and procedural learning. Learning & Memory. 2006;13:650–658. doi: 10.1101/lm.324406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, et al. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proceedings of the National Academy of Sciences. 2000;97:12404–12852. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedhara K, Hyde J, Gilchrist ID, Tytherleigh M, Plummer S. Acute stress, memory, attention and cortisol. Psychoneuroendocrinology. 2000;25:535–549. doi: 10.1016/s0306-4530(00)00008-1. [DOI] [PubMed] [Google Scholar]
- Wolf OT. HPA axis and memory. Best Practice & Research Clinical Endocrinology & Metabolism. 2003;17:287–299. doi: 10.1016/s1521-690x(02)00101-x. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Convit A, McHugh PF, Kandil E, Thorn EL, De Santi S, et al. Cortisol differentially affects memory in young and elderly men. Behavioral Neuroscience. 2001;115:1002–1011. doi: 10.1037//0735-7044.115.5.1002. [DOI] [PubMed] [Google Scholar]