Abstract
Telomeres are repetitive DNA sequences that cap and protect the ends of chromosomes; critically short telomeres may lead to cellular senescence or carcinogenic transformation. Previous findings suggest a link between psychosocial stress, shorter telomeres, and chronic disease risk. This cross-sectional study examined relative telomere length in relation to perceived stress and urinary stress hormones in a sample of participants (n = 647) in the National Institute of Environmental Health Sciences Sister Study, a cohort of women ages 35 to 74 years who have a sister with breast cancer. Average leukocyte telomere length was determined by quantitative PCR. Current stress was assessed using the Perceived Stress Scale and creatinine-adjusted neuroendocrine hormones in first morning urines. Linear regression models estimated differences in telomere length base pairs (bp) associated with stress measures adjusted for age, race, smoking, and obesity. Women with higher perceived stress had somewhat shorter telomeres [adjusted difference of −129bp for being at or above moderate stress levels; 95% confidence interval (CI), −292 to 33], but telomere length did not decrease monotonically with higher stress levels. Shorter telomeres were independently associated with increasing age (−27bp/year), obesity, and current smoking. Significant stress-related differences in telomere length were seen in women ages 55 years and older (−289bp; 95% CI, −519 to −59), those with recent major losses (−420bp; 95% CI, −814 to −27), and those with above-average urinary catecholamines (e.g., epinephrine: −484bp; 95% CI, −709 to −259). Although current perceived stress was only modestly associated with shorter telomeres in this broad sample of women, our findings suggest the effect of stress on telomere length may vary depending on neuroendocrine responsiveness, external stressors, and age.
Introduction
Telomeres are repetitive DNA sequences that cap and protect chromosomal integrity. Average leukocyte telomere length is thought to provide a cumulative marker of cellular aging that integrates across multiple pathways, including DNA damage due to oxidative stress and the replicative history of various populations of WBC. A cross-sectional study showed shorter telomere length was associated with perceived stress among mothers of chronically ill children and control mothers (1), suggesting a novel link between psychosocial stress and chronic disease outcomes typically associated with aging. Shorter telomere length has been associated with early mortality and outcomes such as cardiovascular and metabolic disease (2–4). Critically short telomeres and resulting genome instability may also increase risk of carcinogenic transformation (5–9). Much of this work has focused on telomere length in tumor tissue, but studies suggest correlation between telomere length in different tissues, include leukocytes (10).
Broadly defined, stress is a condition that occurs when environmental demands require physiologic or behavioral changes to maintain balance and homeostasis. The Perceived Stress Scale is a global measure commonly used to evaluate the internalized experience of stress and reflect the extent to which life situations are perceived to be stressful (11). Although perceived stress may be affected by external circumstances, it also reflects personality, learned coping skills, and environmental resources. Although the Perceived Stress Scale typically refers to a short-term period (e.g., the past 30 days), perceived stress is often used as a measure of generalized stress response in epidemiologic studies. Epel et al. (1) found that that shorter telomere length was related to higher perceived stress scores in both caregivers and controls and posited that shorter telomeres were not simply due to the severe stress experienced by many of the caregivers, but rather that this relationship exists across the continuum of normative stress levels.
Telomere length was also associated with elevations in urinary catecholamines and cortisol in a subset of the caregiver subjects (12). The neuroendocrine hormones, cortisol, epinephrine, norepinephrine, and dopamine are released into plasma during activation of the hypothalamic-pituitary-adrenal axis, sympathetic nervous system, and adrenal medulla. All serve to regulate the physiologic stress response, and their urinary levels are often used as a cumulative measure of plasma levels and physiologic stress responsiveness (13). In addition to the traditional “fight or flight” stress hormones, norepinephrine and epinephrine, activation of the sympathetic nervous system can also lead to dopamine secretion (14), and increased urinary dopamine has been reported women with posttraumatic stress disorder (15). Elevated or lower urinary cortisol levels (16), along with elevated epinephrine and norepinephrine, contribute to a composite score known as allostatic load, which also includes measures of inflammation, and cardiovascular and metabolic dysfunction (17). Elevations in urinary catecholamines have also been linked to early mortality (18).
Telomere length may represent a novel intermediate along the pathway linking chronic stress and the physiologic stress response to disease pathogenesis. The present cross-sectional study investigated the association of current perceived stress and telomere length using baseline data from a cohort of women who have a sister with breast cancer (19). Neuroendocrine hormones in first morning urine specimens were also examined in relation to telomere length, overall and as potential modifiers of the association of telomere length and perceived stress levels. We hypothesized perceived stress would have the strongest relationship to telomere length in women with higher urinary stress hormones, and also evaluated the possible modifying effects of external stressors and potential confounding by covariates.
Materials and Methods
Study Sample
The National Institute of Environmental Health Sciences Sister Study is a prospective study of a volunteer cohort of women ages 35 to 74 years with at least one sister diagnosed with breast cancer, who themselves had no prior diagnosis of breast cancer (19). The Sister Study was approved by the Institutional Review Board of the National Institute of Environmental Health Science, NIH. Recruitment began in 2004 and is expected to be completed in 2008. A sample for telomere analyses was derived from participants enrolled in the cohort and included in the first annual health update follow-up as of June 2005. Of 2,086 identified women, 295 were ineligible due to one or more of the following reasons: missing or inadequate blood or urine sample, urine sample that was not a first morning void, major dental procedure or surgery within the past week, current shift work, chemotherapy or radiation treatment for cancer, or missing value for race, perceived stress, or smoking status. A further 18 women were excluded due to known breast cancer diagnosis before establishment of the sampling frame (November 2005), leaving 1,773 eligible for sampling. Because stress levels were low, we oversampled women with higher perceived stress scores. The sample was also enriched to provide power to examine an expected association of smoking and shorter telomeres, and to maximize the number of non-Whites represented. Thus, we included all women known at the time to meet one of three criteria: high or very high perceived stress (perceived stress score of 6 to 16), non-White race, and current smokers. We then derived random sample of everyone else (women with lower stress levels, nonsmokers, and Caucasians) for a final study sample size of 740. Because the sampling frame was determined using preliminary data, some women had race and smoking status reclassified and would have met the above criteria but were not included based on known data at the time.
Characteristics of eligible participants and the final study sample are shown in Table 1.
Table 1.
Characteristics of eligible and selected study participants
| Weighted, random sample |
Eligible for sampling* |
|
|---|---|---|
| n = 740 | n = 1,773 | |
| n (%) | n (%) | |
| Perceived stress | ||
| Very low (0) | 134 (18) | 427 (24) |
| Low (1–2) | 182 (25) | 590 (33) |
| Moderate (3–5) | 133 (18) | 465 (26) |
| High (6–7) | 166 (22) | 166 (9) |
| Very high (8–16) | 125 (17) | 125 (7) |
| Age (y) | ||
| 35–44 | 134 (18) | 263 (15) |
| 45–54 | 279 (38) | 649 (37) |
| 55–64 | 212 (29) | 565 (32) |
| 65–74 | 115 (16) | 295 (17) |
| Race/ethnicity † | ||
| Non-Hispanic White | 624 (84) | 1,641 (93) |
| African-American | 50 (7) | 50 (3) |
| Hispanic | 17 (2) | 33 (2) |
| Other | 49 (7) | 49 (3) |
| Smoking ‡ | ||
| Never | 346 (47) | 918 (52) |
| Former | 216 (29) | 672 (38) |
| Current | 178 (24) | 183 (10) |
| BMI§ | ||
| <25 | 309 (42) | 783 (44) |
| 25–30 | 209 (28) | 536 (30) |
| 30–35 | 141 (19) | 295 (17) |
| >35 | 77 (10) | 150 (8) |
| History of cardiovascular disease and/or diabetes | ||
| 137 (19) | 289 (16) | |
Sampling frame determined using preliminary data on race (not ethnicity) and smoking. Age at enrollment missing on one eligible participant not sampled for telomere study.
Hispanic ethnicity includes all non-Black women who reported Hispanic ethnicity.
Occasional smokers classified as never-smokers (n = 38 eligible, all sampled).
Measured BMI, missing data on nine eligibles, four of whom were sampled.
Data Collection
Data on stress and covariates were collected through computer assisted telephone interview. Biological specimens, and measured height and weight were collected during a home visit.
Perceived Stress
The short version of the Perceived Stress Scale consists of 4 questions that assess the subject’s internal appraisal of stress in the past 30 d (11); for each question, response categories ranged from a score of 0 (low) to 4 (high), which were summed to a total of 0 to 16 points. The distribution of responses in the cohort was highly skewed, with a median Perceived Stress Scale score of 2; so, 5 categories were constructed [very low (0 points), low (1–2 points), moderate (3–5 points), high (6–7 points), and very high (8–16 points); Table 1].
External Stressors
The baseline study questionnaire did not include a life events scale. However, we constructed a summary variable, major life losses, enumerating occurrence of premature deaths of first degree relatives across three categories: death of a child of any age or premature death (i.e., before age 65 y) of a parent or sibling. Another variable, recent major stressors, identified the occurrence of one or more major losses in the past two y, including death of a child, parent, or sibling, and loss of marital partnership (divorced, separated, or widowed). Family breast cancer history was parameterized to reflect potential stress due to increased burden of familial breast cancer risk; for this purpose, we created a variable summing the number of breast cancer cases in first degree relatives (total n = mother, sisters, and daughters with breast cancer) plus the sum of occurrences of early onset breast cancer across the three generations (total n = 0–3 for a mother, sister, and/or daughter diagnosed younger than age 45 y). Missing data on age at diagnosis (n = 30 sisters) were imputed as later onset to reflect the distribution of the majority and based on the idea that younger onset disease identified by participants would be most relevant to the experience of chronic stress related to familial breast cancer risk.
Covariates
Demographic and socioeconomic variables included age (continuous and categorical), race (White, African-American, and other, including Hispanic), education (4-levels), and marital status (currently married or living as if married). Physiologic factors and health behaviors included body mass index (BMI— continuous and categorical; calculated based on measured height and weight), smoking history (never regularly smoked for 6 mo or longer, former smoker, or currently smoke at least 1 cigarette per day for the past 12 mo), average sleep duration in the past 6 wk (hours per night), regular exercise in the past 12 mo (yes/no), menopausal status, and hormone replacement (ever/never). Medical history included self-reported health status, diabetes diagnosed after age 25 y, cardiovascular disease (including heart attack, bypass surgery, angioplasty, congestive heart failure, cardiac arrhythmic, medicated angina, stroke/TIA) and history of clinical depression (ever/never).
Telomere Length
Whole blood was collected during the home visit, and shipped and stored at −80°C until DNA extraction by standard methods. Total leukocyte DNA was used as a template for PCR-based assays of relative telomere length conducted according to methodology previously described (1, 20). Briefly, each sample was amplified for telomeric DNA and a single copy gene using 1 µL aliquots containing 100 to 200 ng template DNA. Cycle threshold was transformed into nanograms of DNA based on a standard curve. The quantitative assay determines the amount of telomeric DNA (T) relative to the amount of single-copy control gene DNA (S) to obtain a relative ratio (T/S ratio). Of the 740 specimens submitted for telomere assays, most were run on duplicate plates including three additional known controls per plate (one each at high, medium, and low T/S ratio), which were subsequently used to adjust sample T/S ratios for the variation in known control values across plates. The coefficient of variation across adjusted replicates (sample T/S ratios after adjusting for plate to plate variation) was 8.5% for 647 samples run on duplicate plates. Averaged T/S ratios of these adjusted replicates were used for the final analysis sample; sensitivity analyses examined associations for unadjusted values and including samples without complete assay replicates. The mean T/S ratio value for replicates (n = 647; 1.32; SD, 0.25) was normally distributed. The remaining 93 specimens assayed included 10 specimens missing replicates (mean T/S ratio, 1.33; SD, 0.19), 29 with failed PCR-reactions, and the initial batch (plate) of pilot assays, which did not include plate controls and therefore was not combined with the adjusted replicates in the final analysis sample.
Urine Catecholamines and Cortisol
First morning urine specimens were collected by subjects the morning of the blood draw. Women were instructed to collect the first urine of the day and record times of sleep onset, last awakening, first morning void, and last urination before the collected specimen. Most specimens were collected soon after waking (median, 5 min) and reflected overnight sampling with a median of 6.5 h since time of last urination (interquartile range, 4–8 h). Specimens did not include preservative, but subjects were asked to refrigerate specimens until they were collected by the examiner. Samples were transported by examiners, who prepared and shipped them on ice, and were subsequently aliquotted on receipt in the laboratory and stored at −80°C.
Quantitative assessment of epinephrine, norepinephrine, and dopamine was conducted by Enzyme Immunoassay (Total CAT EIA; Alpco Diagnostics), with results shown to be comparable with high performance liquid chromatography (e.g., r = 0.88 for norepinephrine) in pilot studies. Each specimen was run once, and each assay plate included two known controls at high and low concentrations. The coefficient of variation for low and high controls across 11 plates ranged from 12% and 19% for epinephrine, to 19% and 28% for norepinephrine, to 19% and 26% for dopamine. Despite these apparently high coefficient of variations, all controls performed within expected ranges based on kit specifications, e.g., the observed range of the epinephrine low control was 6.9 to 11.1 ng/mL versus 5 to 14 ng/mL expected. Urine samples were aliquoted, refrozen, shipped overnight, and maintained at −80°C before evaluation of urinary free cortisol by RIA (21). Each specimen was run once; known controls at high, medium, and low concentration were included on each plate with percent coefficient of variation (across 16 plates) ranging from 14% (high) to 31% (medium) and 29% (low) for the controls.
Concentrations of urinary hormones are typically adjusted based on creatinine values to control for urinary flow rate. Creatinine values were obtained on fresh urines upon initial receipt using a semiquantitative method (dip stick) providing categorical values (10, 50, 100, 200, and 300 mg/dl). We also determined exact creatinine values on frozen samples shipped for catecholamine assays using the automatic analyzer Cobras Mira (reagents from Roche). At the low end, dip stick estimates tended to be lower than exact values; whereas at the high end, they were higher. For specimens lacking exact creatinine values due to insufficient sample (n = 64), quantitative values were imputed based on dipstick readings using the midpoint in the other measured specimens (dipstick reading 10 = 32.5, 50 = 52, 100 = 88, 200 = 148, and 300 = 218 mg/dl). Urinary hormone values were directly adjusted for creatinine. Values were obtained on the majority of the study sample, and distributions were similar when limited to the final analysis sample with complete telomere data (data not shown). As expected, the individual stress hormones were somewhat correlated with each other. For example, log-transformed epinephrine values were significantly associated with cortisol levels (Pearson correlation coefficient, r = 0.36; P < 0.0001) and with norepinephrine levels (r = 0.40; P < 0.0001). However, stress hormone levels were not strongly or significantly correlated with perceived stress scores (data not shown). Because of the high assay percent coefficient of variation, creatinine-adjusted values were subsequently grouped using quartile cut-points across the full sample for epinephrine, norepinephrine, and dopamine. Cortisol values were grouped into quintiles to allow examination of low and high extremes compared with the middle of the distribution.
Analyses
Our final analysis sample was restricted to the 647 women for whom duplicate telomere data were available. Telomere length in bp was estimated by multiplying 1 TS ratio unit by 4,270bp (mean, 5,618; bp, SD1069; ref. 20). Statistical analyses were conducted using SAS (version 9.1). Tables included 95% confidence intervals (CI), which were considered to indicate statistical significance at a P value of <0.05 when they excluded the null (odds ratio, 1.0). Supporting P values were generated for tests of trend and interaction.
Linear regression models were constructed to examine age-adjusted associations of telomere length with perceived stress and covariates. Negative β coefficients correspond to associations with shorter telomere length. Main effects examined included perceived stress level, urinary stress hormones, external stressors, and covariates. Little evidence of confounding was observed, and the final model adjusted for sample selection criteria (non-White race, current smoking), age, and BMI (continuous). We also ran models excluding eight pairs of sisters in the data set but saw no effect of these correlated data.
We constructed stratified models to explore potential modifiers of the association between perceived stress and telomere length. These included urinary stress hormone levels, external stressors, demographic factors, and health status. In stratified models, median cut-points were used to group urinary hormone levels and perceived stress scores. For analyses suggesting a difference in effects, models were constructed including both variables and a product term to test significance of the interaction on a multiplicative scale. Finally, a generalized linear model was used to estimate the adjusted mean relative telomere lengths for selected categorical covariates (race, smoking, and BMI) and perceived stress stratified across the four age categories.
Results
Compared with low or very low perceived stress, higher perceived stress (moderate, high, or very high) was nonsignificantly associated with shorter telomeres (age-adjusted difference in telomere length, −129bp; 95% CI, −290 to 33; Table 2). Shorter telomere length was observed primarily for the moderately elevated stress categories with no additional shortening in the highest stress category. The significant association of moderately elevated perceived stress and shorter telomeres (−290bp) was somewhat attenuated in models adjusted for race, smoking, and BMI. Urinary stress hormones seemed unrelated to telomere length, with the exception of a trend toward shorter telomeres at higher and lower cortisol levels. None of the external stressors examined (recent major stressors, major life losses, and familial breast cancer risk) were significantly associated with shorter telomeres.
Table 2.
Difference in telomere length associated with perceived stress and covariates
| n = 647 | Age adjusted | Fully adjusted | |||
|---|---|---|---|---|---|
| n | β* | 95% CI (lower, upper) | β† | 95% CI (lower, upper) | |
| Perceived stress level | |||||
| Very low/low (0–2) | 283 | Reference | Reference | ||
| Moderate/high/very high (≥3) | 364 | −129 | −290, 33 | −129 | −292, 33 |
| Very low (0) | 120 | Reference | Reference | ||
| Low (1–2) | 163 | −127 | −372, 118 | −92 | −338, 154 |
| Moderate (3–5) | 115 | −290 | −557, −23 | −214 | −483, 55 |
| High (6–7) | 139 | −211 | −465, 44 | −220 | −476, 36 |
| Very high (8–16) | 110 | −100 | −369, 170 | −101 | −372, 170 |
| Urinary stress hormones ‡ | |||||
| Epinephrine | |||||
| Q1 | 168 | Reference | Reference | ||
| Q2 | 160 | 45 | −181, 271 | 53 | −172, 279 |
| Q3 | 163 | 0 | −224, 225 | −8 | −237, 220 |
| Q4 | 152 | 32 | −261, 197 | −22 | −255, 211 |
| Norepinephrine | |||||
| Q1 | 159 | Reference | Reference | ||
| Q2 | 163 | 235 | 8, 462 | 231 | 4, 458 |
| Q3 | 158 | 184 | −45, 414 | 181 | −49, 411 |
| Q4 | 164 | 153 | −76, 382 | 198 | −35, 430 |
| Dopamine | |||||
| Q1 | 158 | Reference | Reference | ||
| Q2 | 169 | −135 | −360, 91 | −180 | −406, 45 |
| Q3 | 168 | 42 | −184, 268 | 18 | −209, 244 |
| Q4 | 149 | −28 | −261, 205 | −43 | −276, 189 |
| Cortisol | |||||
| Q1 | 133 | −171 | −420, 78 | −157 | −409, 95 |
| Q2 | 128 | −74 | −326, 177 | −65 | −319, 189 |
| Q3 | 135 | Reference | Reference | ||
| Q4 | 119 | −53 | −310, 204 | −97 | −354, 161 |
| Q5 | 124 | −160 | −416, 95 | −158 | −414, 98 |
| Major life events | |||||
| Recent major stressors§ | |||||
| None | 516 | Reference | Reference | ||
| 1 or more | 131 | 181 | −19, 380 | 214 | 13, 414 |
| Major life losses∥ | |||||
| None | 287 | Reference | Reference | ||
| 1 | 280 | −128 | −301, 46 | −90 | −266, 85 |
| 2 or more | 80 | 71 | −191, 333 | 89 | −173, 352 |
| Familial breast cancer risk¶ | |||||
| 1 | 282 | Reference | Reference | ||
| 2 | 263 | −42 | −224, 140 | −28 | −210, 154 |
| 3 | 80 | −69 | −330, 193 | −6 | −268, 256 |
| 4 or more | 22 | −105 | −560, 350 | −92 | −557, 373 |
| Demographic factors | |||||
| Age groups (y) | |||||
| 35–44 | 120 | Reference | Reference | ||
| 45–54 | 238 | −365 | −595, −135 | −358 | −588, −127 |
| 55–64 | 182 | −517 | −759, −276 | −520 | −763, −278 |
| 65–74 | 107 | −763 | −1,036, −491 | −838 | −1,118, −558 |
| Race | |||||
| White | 539 | Reference | Reference | ||
| African-American | 47 | 19 | −292, 330 | 68 | −248, 383 |
| Other | 61 | −100 | −377, 176 | −98 | −376, 180 |
| Education | |||||
| PhD, MS | 119 | Reference | Reference | ||
| BA | 163 | −46 | −292, 199 | −56 | −302, 190 |
| AA/tech. | 97 | −121 | −400, 158 | −103 | −385, 179 |
| Some college | 166 | −89 | −334, 156 | 8 | −244, 259 |
| ≤High school | 102 | −303 | −580, −26 | −205 | −491, 82 |
| Currently living with a marital partner | |||||
| Yes | 484 | Reference | Reference | ||
| No | 163 | 20 | −165, 205 | 51 | −140, 241 |
| Health status/behaviors | |||||
| BMI (Measured) | |||||
| <25 | 259 | Reference | Reference | ||
| 25–30 | 184 | −199 | −395, −2 | −196 | −394, 1 |
| 30–35 | 128 | −247 | −467, −27 | −225 | −447, −3 |
| >35 | 73 | −259 | −528, 11 | −263 | −538, 13 |
| Smoking | |||||
| Never | 305 | Reference | Reference | ||
| Former | 187 | 53 | −137, 243 | 55 | −137, 247 |
| Current | 155 | −293 | −494, −92 | −303 | −508, −99 |
| Cardiovascular disease/diabetes | |||||
| No | 524 | Reference | Reference | ||
| Yes | 123 | −342 | −549, −134 | −281 | −495, −68 |
| Ever diagnosed with clinical depression | |||||
| No | 508 | Reference | Reference | ||
| Yes | 139 | −126 | −321, 70 | −78 | −281, 126 |
| Current health | |||||
| Excellent | 201 | Reference | Reference | ||
| Very Good/Good | 395 | −24 | −200, 153 | 70 | −113, 253 |
| Fair | 38 | −41 | −401, 319 | 114 | −267, 495 |
| Poor | 13 | −705 | −1,286, −121 | −538 | −1,139, 64 |
Linear regression model with telomere length as outcome, estimated β coefficient and 95% CI corresponding to difference in bp average telomere length, adjusting for age in years (continuous). Effects of age groups not further adjusted for age. Missing data on 3 for BMI.
Fully adjusted linear regression models (total n = 644) included stress (5 levels), smoking (current, former), non-White race (2 categories), age (continuous), and BMI (4 levels).
Creatinine-adjusted values for hormones measured in first morning urines, by quartiles (epinephrine, norepinephrine, and dopamine) and quintiles (cortisol); Q1 = low. Urinary assay values were missing for n = 4, 4, 3, and 8, respectively.
Recent major stressors in past 24 mo, including death of first degree relative or death of spouse, divorce, or separation.
Major life losses (premature death of first degree relative). Sum of occurrences across categories: including death of a child (any age), death of parent or a sibling before age 65.
Summation: total number of first degree relatives diagnosed with breast cancer + occurrences of diagnoses before age 45 in a mother, sister, or daughter.
Analyses confirmed expected associations of shorter telomeres with increasing age (−27 bp per year) with a clear dose-effect across decades, and with higher BMI, current smoking, lower education, poor health, and cardiovascular or metabolic disease (Table 2). History of clinical depression was not associated shorter telomeres in the fully adjusted model. Other factors examined, but not significantly associated with telomere length, included average sleep duration, regular exercise, high blood pressure/cholesterol or borderline diabetes, and use of hormone replacement therapy (data not shown).
Stratified analyses are shown in Table 3. Higher perceived stress (moderate, high, or very high) was associated with shorter telomeres in women with higher (above the median) epinephrine, norepinephrine, and dopamine but not cortisol. For epinephrine, the interaction was significant (interaction P < 0.0001), with shorter telomeres in women with higher urinary epinephrine (−484 bp for higher versus lower perceived stress) but not in those with lower epinephrine (210 bp for higher versus lower stress). Higher perceived stress was also significantly associated with shorter telomeres among women with a recent history of major stressors (−420 bp for higher versus lower perceived stress) and trended toward a gradient difference in telomere length across increasing stress levels (Ptrend = 0.07; data not shown). History of major life losses and familial breast cancer risk did not seem to modify the telomere stress association. Significant stress-related differences in telomere length (−289 bp) were seen in women age 55 years and older (interaction P = 0.10). Nonsignificant stress-related differences in telomere length were also seen in women reporting CVD/diabetes or poor health, history of clinical depression, and absence of a marital partnership.
Table 3.
Association of higher perceived stress and telomere length: stratified analyses
| Stratification factor | n | Perceived stress | Difference in telomere length | |
|---|---|---|---|---|
| % moderate, high, or very high stress level | β* | 95% CI lower, upper | ||
| Urinary hormone levels | ||||
| Epinephrine | ||||
| Low | 328 | 59 | 210 | −23, 442 |
| High | 315 | 54 | −484 | −709, −259 |
| Norepinephrine | ||||
| Low | 322 | 55 | −27 | −263, 208 |
| High | 322 | 58 | −246 | −475, −17 |
| Dopamine | ||||
| Low | 327 | 57 | 12 | −230, 254 |
| High | 317 | 56 | −257 | −478, −37 |
| Cortisol | ||||
| Low | 319 | 57 | −206 | −441, 29 |
| High | 320 | 56 | −101 | −331, 129 |
| Major life events/stress | ||||
| Recent major losses † | ||||
| 0 | 515 | 55 | −73 | −253, 106 |
| 1 or more | 131 | 61 | −420 | −814, −27 |
| Major life losses ‡ | ||||
| 0 | 287 | 60 | −143 | −390, 105 |
| 1 | 280 | 52 | −93 | −349, 163 |
| 2 or more | 80 | 60 | −146 | −600, 308 |
| Familial breast cancer§ | ||||
| 1 | 282 | 52 | −2 | −250, 247 |
| 2 | 263 | 61 | −368 | −635, −102 |
| 3 or more | 102 | 57 | 101 | −298, 501 |
| Demographics and health | ||||
| Age (y) | ||||
| Under age 55 | 358 | 59 | −8 | −238, 222 |
| 55 and older | 289 | 53 | −289 | −519, −59 |
| Current marital partnership | ||||
| Yes | 484 | 57 | −69 | −260, 123 |
| No | 163 | 53 | −290 | −594, 15 |
| Poor health, cardiovascular disease, or diabetes | ||||
| No | 519 | 55 | −97 | −273, 80 |
| Yes | 128 | 62 | −183 | −594, 227 |
| Ever diagnosed with clinical depression | ||||
| No | 508 | 51 | −87 | −271, 96 |
| Yes | 139 | 76 | −216 | −611, 187 |
Linear regression models with telomere length as outcome for being at or above high-average levels of perceived stress; estimated β coefficient and 95% CIs corresponding to difference in bp average telomere length, adjusting for age and BMI (continuous), non-White race, and smoking history. Stratified using median cut-points for creatinine-adjusted urinary hormones.
Recent major losses, including death of parent, sister, or spouse, divorce, or separation in past 24 mo.
Life losses (premature death of first degree relative), sum of occurrences across categories, including death of a child (any age), death of parent or sibling before age 65 y.
Summation of primary relatives diagnosed with breast cancer + occurrences of diagnoses before age 45 in a mother, sister, or daughter.
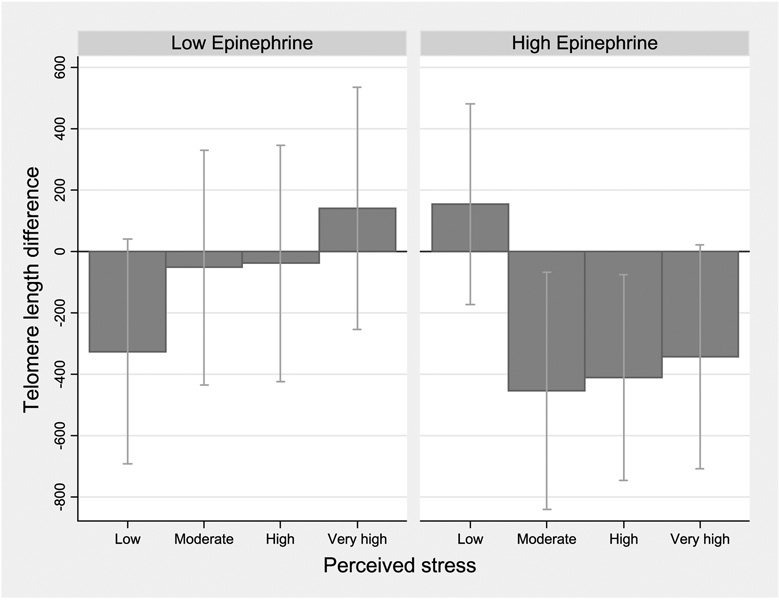
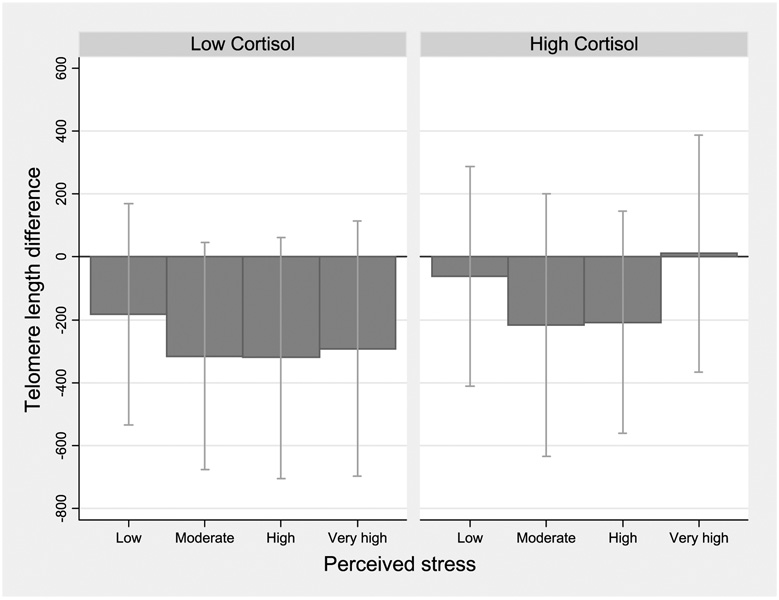
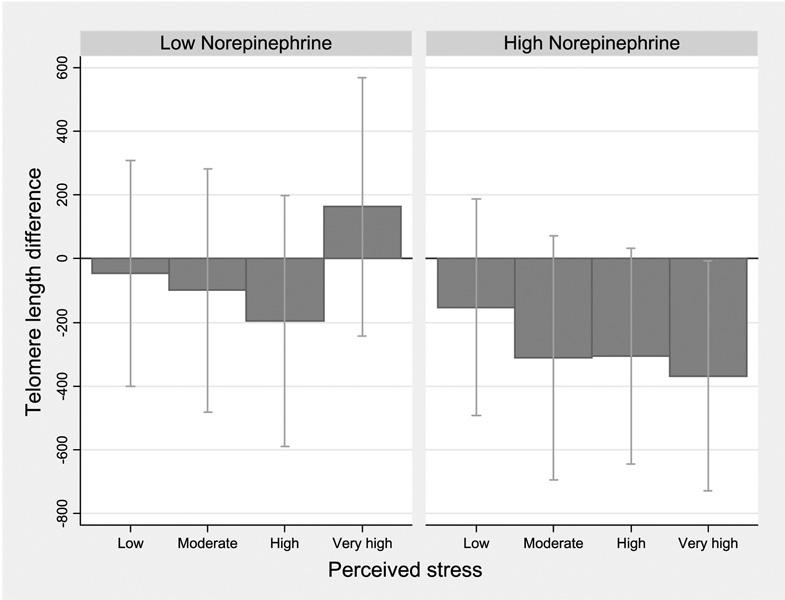
Figure 1 to Figure 3 show telomere length differences associated with 4 levels of perceived stress compared with the lowest category, stratified by stress hormone levels. Among women with higher epinephrine, shorter telomeres were seen for moderate and higher stress categories (Ptrend = 0.001; Fig. 1). Although the effect difference for norepinephrine corresponded to a nonsignificant interaction (P = 0.24), women with higher norepinephrine showed a clear gradient with shorter telomere length across increasing perceived stress categories (Ptrend = 0.03; Fig. 2). Results for dopamine were similar, with no significant interaction but a gradient shortening of telomere length across the increasing stress categories (Ptrend = 0.02; data not shown). In women with lower cortisol levels, nonsignificantly shorter telomeres were seen for moderate and high perceived stress (Ptrend = 0.09; Fig. 3).
Figure 1.
Estimated telomere length difference in association with higher perceived stress: stratified by urinary epinephrine levels. Linear regression models with telomere length as outcome for higher levels of perceived stress compared with the lowest category; estimated β coefficient and 95% CI corresponding to difference in bp average telomere length, adjusting for age and BMI (continuous),non-White race, and smoking history. Stratified using median cut-point for creatinine-adjusted urinary epinephrine; high, above the median.
Figure 3.
Estimated telomere length difference in association with higher perceived stress: stratified by urinary cortisol levels. Linear regression models with telomere length as outcome for higher levels of perceived stress compared with the lowest category; estimated β coefficient and 95% CI corresponding to difference in bp average telomere length, adjusting for age and BMI (continuous),non-White race, and smoking history. Stratified using median cut-point for creatinine-adjusted urinary cortisol.
Figure 2.
Estimated telomere length difference in association with higher perceived stress: stratified by urinary norepinephrine levels. Linear regression models with telomere length as outcome for higher levels of perceived stress compared with the lowest category; estimated β coefficient and 95% CI corresponding to difference in bp average telomere length, adjusting for age and BMI (continuous),non-White race,and smoking history. Stratified using median cut-point for creatinine-adjusted urinary norepinephrine.
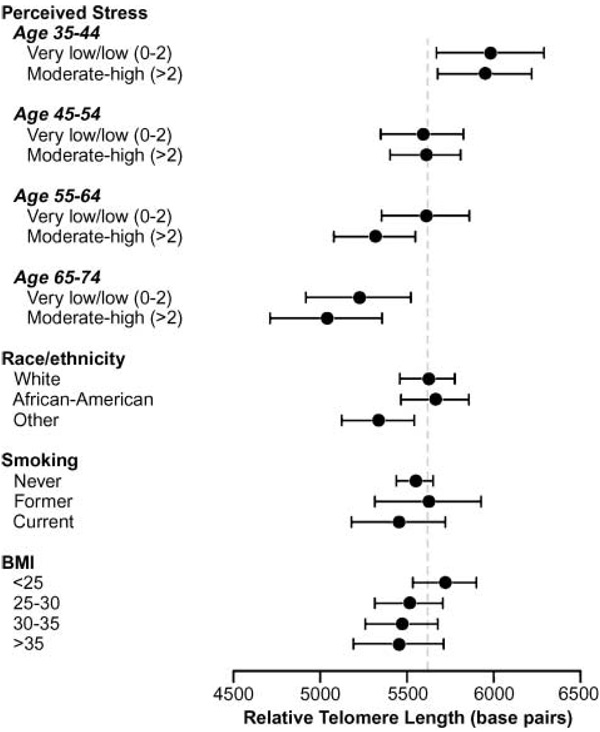
Figure 4 shows estimated relative telomere length in women with higher and lower perceived stress across four age categories, juxtaposed with means across different categories of BMI, smoking, and race.
Figure 4.
Estimated mean relative telomere length for perceived stress across age categories and covariates. Estimated means and 95% CIs in a general linear model including categories of stress (above and below the median) by 10-y age groups, and adjusted for race, smoking, and BMI categories.
Discussion
Our findings suggest a modest overall relationship between current perceived stress and shorter telomere length in the study sample. Stratified analyses suggested significant associations of perceived stress and shorter telomeres in some subgroups, namely women with higher urinary catecholamines, those experiencing recent major stressors, and women age 55 years and older. History of major life losses and degree of familial breast cancer risk were not associated with telomere length overall, and did not seem to clearly modify the association of perceived stress and telomere length.
Based on the observations by Epel et al. (1), we expected to see a stronger association and dose-response relationship between perceived stress and telomere length. Differences in the present study include a larger sample size, an older and broader age distribution, and lower perceived stress scores. Because of the wide distribution of telomere length in the population, cross-sectional studies require a large sample size to detect differences (22); compared with Epel et al. (1), our sample size should have been sufficient to detect a difference of similar magnitude. Despite a lack of additional specific stress scales, we identified groups of objective stressors based on existing data (e.g., recent death of a first-degree relatives or spouse, divorce, or separation), which modified the association between telomere length and perceived stress.
There are few published studies on psychosocial stress and telomere length. A recent study reported shorter telomere length in caregivers of Alzheimer patients compared with age and sex-matched controls (23). Caregivers in this study tended to be older (average age 65 years) and were more likely than controls to report depressive symptoms. Another study reported shorter telomeres in patients with major depression (24); in our data, history of depression was associated with higher perceived stress but not significantly related to shorter telomeres. Confidence in the present findings is increased by the replication of expected covariate associations with telomere length, such as age, obesity, smoking, and cardiovascular disease (25). The observed association of shorter telomeres and lower education supports an effect of socioeconomic status described by others (26), although this association was attenuated in the fully adjusted model, suggesting socioeconomic factors and other covariates may operate along a shared causal pathway.
Urinary catecholamines and cortisol are often considered markers of current physiologic stress response. Although overnight or first morning urines can be considered an “integrative” measure of general stress levels (13), neuroendocrine hormones may vary with circadian and seasonal rhythms and are influenced by acute and chronic stressors. We saw no clear associations of urinary stress hormones with telomere length, in contrast to Epel’s previous findings showing a significant association between elevated cortisol, epinephrine, and norepineprine and telomere length in the caregiver study (12). The relatively high coefficient of variation in our assays could have led to nondifferential misclassification, reducing the likelihood of observing associations. Nevertheless, expected correlations between urinary hormones were observed and adjustment for plate variation did not seem to alter the overall lack of main effects (data not shown). Significant interactions between perceived stress and neuroendocrine hormones were observed, however, supporting our premise that telomere length may be affected by elevated perceived stress primarily in the context of an elevated physiologic stress response, and consistent with a hypothesis that telomere length may represent an intermediate on the pathway linking the biological stress response with disease outcomes.
Interpreting urinary cortisol data is complicated by the typical daily rhythm and rapid increase in cortisol during and after awakening. First morning values may reflect basal hypothalamic-pituitary-adrenal activity and responsiveness, minimizing the influence of daytime activities. The U-shaped pattern showing shorter telomeres at lower and higher cortisol levels is suggestive given evidence that chronic, traumatic, or early life stress may be associated with increased or blunted hypothalamic-pituitary-adrenal activity (27–29). Dopamine has not been previously studied in relation to telomere length. Although not traditionally considered a stress hormone, dopamine is a precursor of norepinephrine and is released in response to stress (14). Dopamine may also influence the immune system via paracrine or autocrine pathways, along with the other catecholamines (30, 31). In our data, epinephrine and norepinephrine levels were generally higher in women with higher dopamine levels. However, these hormones did not explain the association of perceived stress and shorter telomeres seen in women with elevated dopamine.
We observed no overall dose-response for telomere length and higher perceived stress, except in analyses stratified by urinary catecholamines and recent major stressors. The perceived stress scale reflects the influence of contemporary or recent stressors, general stress responsiveness, and coping resources, so women with high scores likely included some experiencing atypical or transient stressors, as well as those with a more typical high stress response to chronic or daily stressors. We had limited data on external stressors or psychosocial determinants of perceived stress. Although we saw no apparent main effects or confounding by recent major stressors, higher perceived stress was significantly associated with shorter telomeres in women with a history of a recent major stressor. This does not necessarily imply that brief, intense stressors effect telomere length, however, as these events may have been preceded by years of chronic stress. This finding may simply represent our ability to observe an effect in women with more homogeneous levels of external stressors.
Cross-sectional data on perceived stress is a crude proxy for the lifetime burden of acute and chronic stress. The low perceived stress scores in this cohort are likely due to the age-distribution and psychosocial characteristics of these volunteer participants. Although older women on average reported lower perceived stress, the stronger association with shorter telomeres in older women may reflect the cumulative burden of stress over time. Alternatively, aging may be related to differences in the physiologic stress response. Hormonal changes with aging might remove a buffer against stress-related effects on telomere length, for example, as suggested by experimental research showing altered negative feedback of the hypothalamic-pituitary-adrenal-axis to acute stress in older women, and potentially modulating effects of estrogen replacement therapy on feedback sensitivity (32). In our data, hormone replacement therapy was not independently associated with telomere length and did not modify the association of perceived stress and telomere length (data not shown). Our ability to examine these effects was limited by sample size and the close relationship between age, menopausal status, and hormone replacement therapy.
The high-throughput PCR-based assay of relative telomere length has attained popularity in epidemiologic studies, and is thought to provide a reasonable estimate of average telomere length in whatever tissue was used for DNA. Our study determined telomere length in total leukocytes compared with peripheral blood mononuclear cells in the caregiver studies (1). These studies did not show an influence of lymphocyte phenotypes that explained the associations with telomere length (1, 23). Differences in our findings might reflect differential effects of stress on granulocytes (the predominant population of total leukocytes) compared with lymphocytes (the majority of peripheral blood mononuclear cell). Effects in shorter lived leukocytes may reflect to a greater extent the effects of stress on hematopoetic stem cells rather than effects on the replication and expansion of longer lived lymphocytes. Stress has been linked to systemic inflammation, i.e., induction of the acute phase response or inflammatory cytokines (33, 34) but may have either stimulatory or suppressive effects on lymphocytes depending on whether stress is acute or chronic (35) and the specific mechanisms examined (36). Further studies examining immune phenotype and inflammatory biomarkers might help elucidate pathways relating stress to leukocyte and lymphocyte telomere length.
Our data showed that age-related differences in telomere length surpassed the differences associated with perceived stress and modifiable factors such as obesity and smoking. The biological impact of effect sizes observed (e.g., older women with higher perceived stress had 5% shorter telomeres than low stress women) is also unclear given the broad range of variation of telomere length in the population, and is part of the larger question relating telomere length to aging and disease outcomes.
Although our cohort consists of volunteers with a sister with breast cancer, participants reported a diverse range of health risk factors and conditions (data not shown), and most had only one affected sister. The proportion with known breast cancer genes is expected to be low, and most of the excess risk associated with family history is likely due to shared environment and multiple gene polymorphisms that individually confer low risk. The fact that we saw expected associations of telomere length with age, obesity, and smoking provides further reassurance about the generality of our findings. We hypothesized that breast cancer family history would comprise a potential stressor, but reported stress levels were low, so the sample was enriched for higher perceived stress. The sample was also enriched for non-White race and smoking, neither of which confounded the association of perceived stress and telomere length. Smoking has inflammatory and immunosuppressive effects and may also be associated with psychosocial factors and neuroendocrine stress responsiveness (37–39). However, post hoc analyses of the telomere/stress association did not show effect modification by current smoking status.
In sum, although we did not observe a dramatic overall association of telomere length with current stress measures, our findings suggest perceived stress effects on telomere length may vary depending on neuroendocrine responsiveness and exposure to environmental stressors, and may be stronger in older women. The observed subgroup findings may be notable. In older women, for example, the difference in telomere length associated with a relatively small elevation in perceived stress score was comparable with differences associated with being obese, current smoking, or diagnosis of cardiovascular disease. Thus, even moderately elevated stress levels in a low-stress population could have substantial effect on telomere length.
Acknowledgments
We thank Teresa Stepanek for laboratory work on telomere assays conducted in Dr. Cawthon’s laboratory, and Feng Ma and other personnel in the laboratories of Dr. Diane Miller and Clemens Kirschbaum who did the urinary catecholamine and cortisol assays.
Grant support: NIH, National Institute for Environmental Health Sciences (Z01 ES04400509), and by Department of Defense Breast Cancer Research Concept Award (BC045286).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [see comment]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 3.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 4.Tentolouris N, Nzietchueng R, Cattan V, et al. White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care. 2007;30:2909–2915. doi: 10.2337/dc07-0633. [DOI] [PubMed] [Google Scholar]
- 5.Meeker AK, Argani P. Telomere shortening occurs early during breast tumorigenesis: a cause of chromosome destabilization underlying malignant transformation? J Mammary Gland Biol Neoplasia. 2004;9:285–296. doi: 10.1023/B:JOMG.0000048775.04140.92. [DOI] [PubMed] [Google Scholar]
- 6.Meeker AK, Hicks JL, Iacobuzio-Donahue CA, et al. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res. 2004;10:3317–3326. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- 7.Tabori U, Nanda S, Druker H, Lees J, Malkin D. Younger age of cancer initiation is associated with shorter telomere length in Li-Fraumeni syndrome. Cancer Res. 2007;67:1415–1418. doi: 10.1158/0008-5472.CAN-06-3682. [DOI] [PubMed] [Google Scholar]
- 8.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 9.Deng Y, Chang S. Role of telomeres and telomerase in genomic instability, senescence and cancer. Lab Invest. 2007;87:1071–1076. doi: 10.1038/labinvest.3700673. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich U, Griese E, Schwab M, Fritz P, Thon K, Klotz U. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;119:89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983:385–396. [PubMed] [Google Scholar]
- 12.Epel ES, Lin J, Wilhelm FH, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Fibiger W, Singer G, Miller AJ, Armstrong S, Datar M. Cortisol and catecholamines changes as functions of time-of-day and self-reported mood. Neurosci Biobehav Rev. 1984;8:523–530. doi: 10.1016/0149-7634(84)90009-5. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc J, Ducharme MB. Plasma dopamine and noradrenaline variations in response to stress. Physiol Behav. 2007;91:208–211. doi: 10.1016/j.physbeh.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Friedman MJ, Jalowiec J, McHugo G, Wang S, McDonagh A. Adult sexual abuse is associated with elevated neurohormone levels among women with PTSD due to childhood sexual abuse. J Trauma Stress. 2007;20:611–617. doi: 10.1002/jts.20221. [DOI] [PubMed] [Google Scholar]
- 16.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 17.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Reuben DB, Talvi SL, Rowe JW, Seeman TE. High urinary catecholamine excretion predicts mortality and functional decline in high-functioning, community-dwelling older persons: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2000;55:M618–M624. doi: 10.1093/gerona/55.10.m618. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg CR, Shore DL, Umbach DM, Sandler DP. Using risk-based sampling to enrich cohorts for endpoints, genes, and exposures. Am J Epidemiol. 2007;166:447–455. doi: 10.1093/aje/kwm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirschbaum C, Strasburger CJ, Jammers W, Hellhammer DH. Cortisol and behavior: 1. Adaptation of a radioimmunoassay kit for reliable and inexpensive salivary cortisol determination. Pharmacol Biochem Behav. 1989;34:747–751. doi: 10.1016/0091-3057(89)90269-4. [DOI] [PubMed] [Google Scholar]
- 22.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;35:1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 23.Damjanovic AK, Yang Y, Glaser R, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lung FW, Chen NC, Shu BC. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet. 2007;17:195–199. doi: 10.1097/YPG.0b013e32808374f6. [DOI] [PubMed] [Google Scholar]
- 25.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 26.Cherkas LF, Aviv A, Valdes AM, et al. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 27.Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 29.Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59:161–179. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- 30.Basu S, Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol. 2000;102:113–124. doi: 10.1016/s0165-5728(99)00176-9. [DOI] [PubMed] [Google Scholar]
- 31.Meredith EJ, Chamba A, Holder MJ, Barnes NM, Gordon J. Close encounters of the monoamine kind: immune cells betray their nervous disposition. Immunology. 2005;115:289–295. doi: 10.1111/j.1365-2567.2005.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Kirschbaum C. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology. 1999;70:422–430. doi: 10.1159/000054504. [DOI] [PubMed] [Google Scholar]
- 33.Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 34.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 36.Lucas RM, Ponsonby AL, Dear K. Mid-life stress is associated with both up- and down-regulation of markers of humoral and cellular immunity. Stress. 2007;10:351–361. doi: 10.1080/10253890701379023. [DOI] [PubMed] [Google Scholar]
- 37.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 38.Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- 39.Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006;59:236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]